Effect of Organic Cation on Optical Properties of [A]Mn(H2POO)3 Hybrid Perovskites
Abstract
1. Introduction
2. Results and Discussion
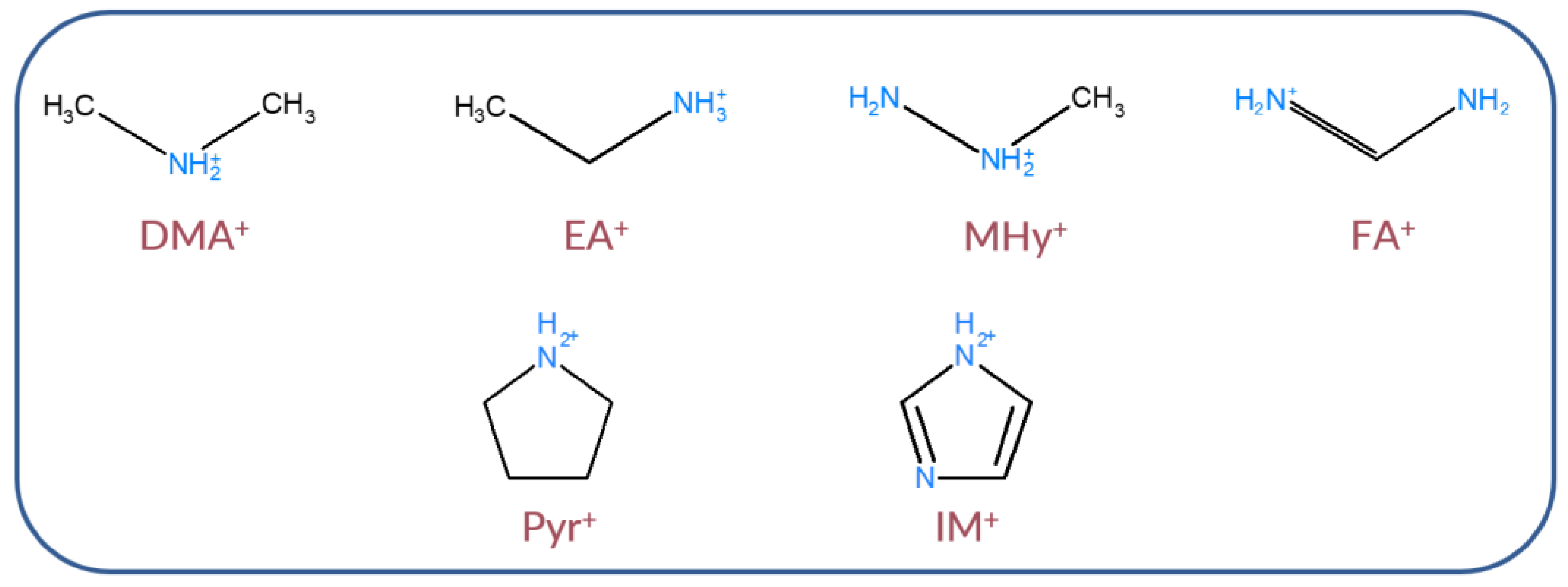
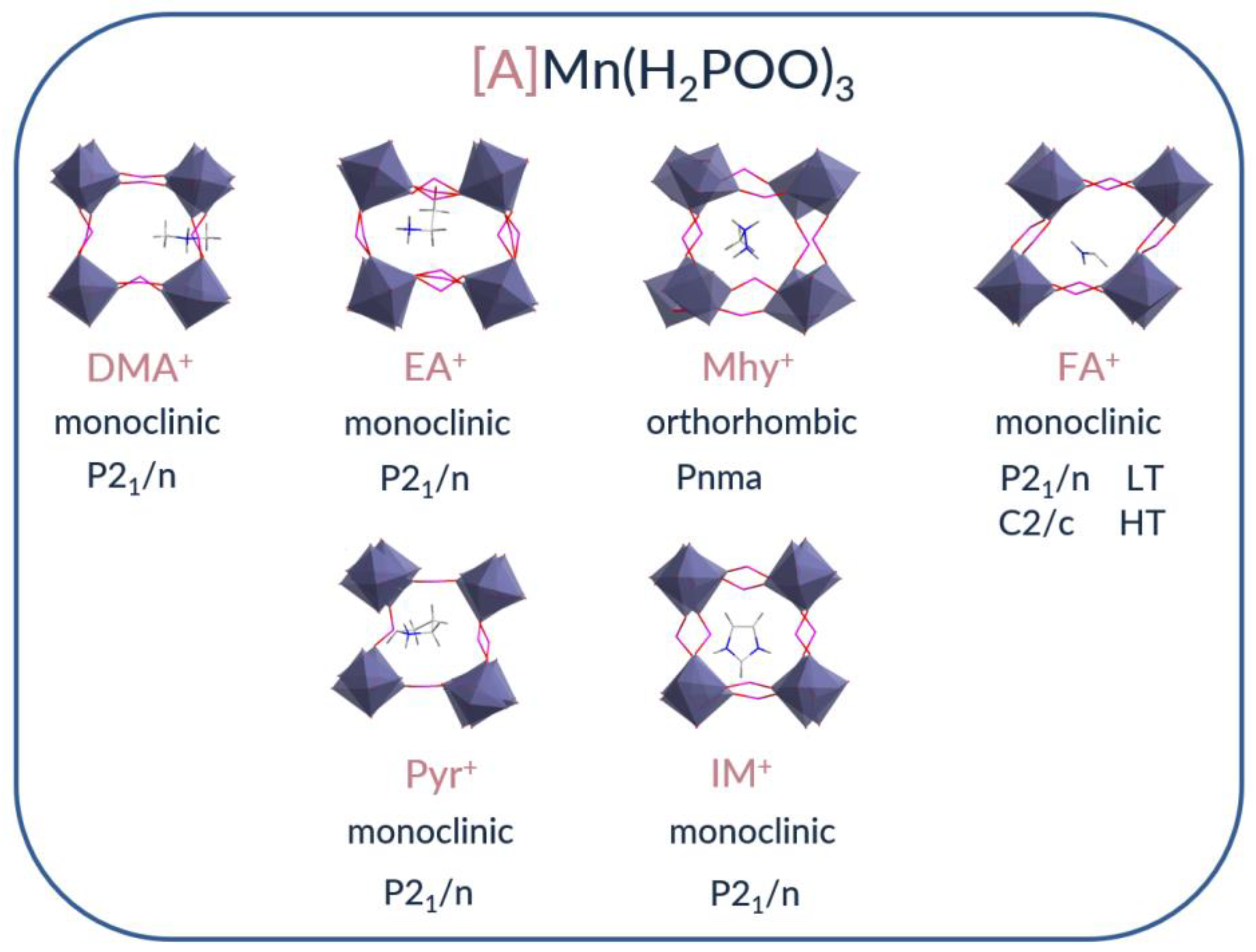
2.1. Crystal Structure
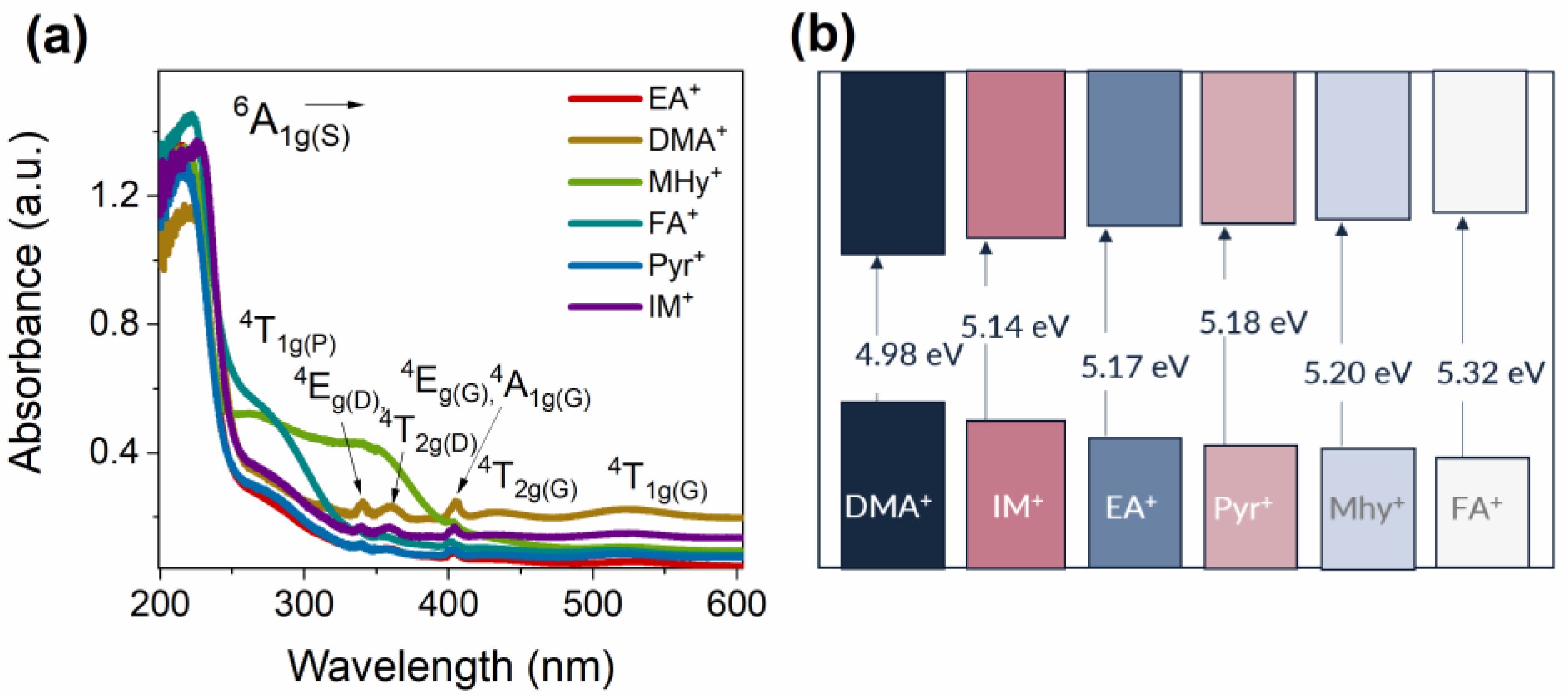
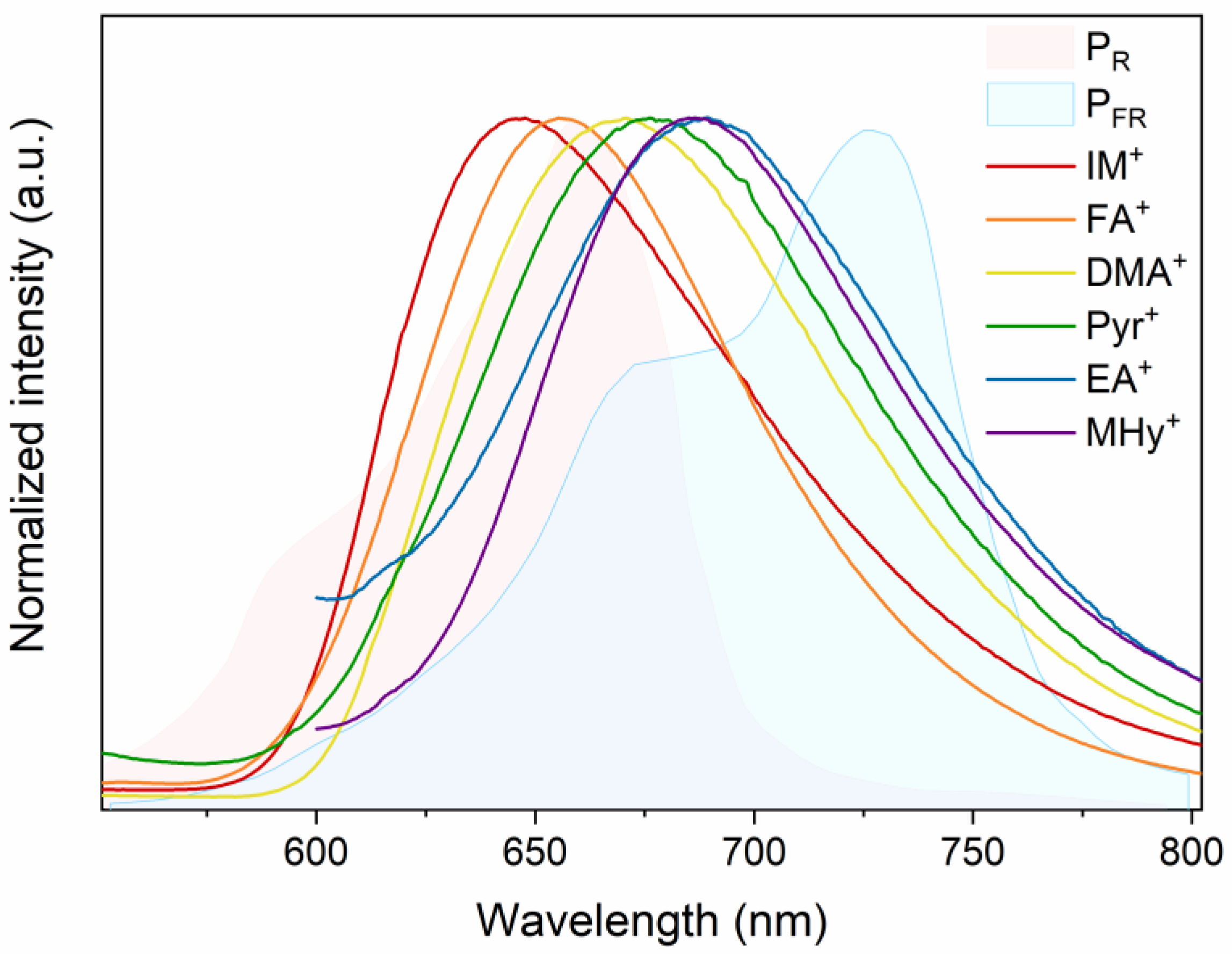
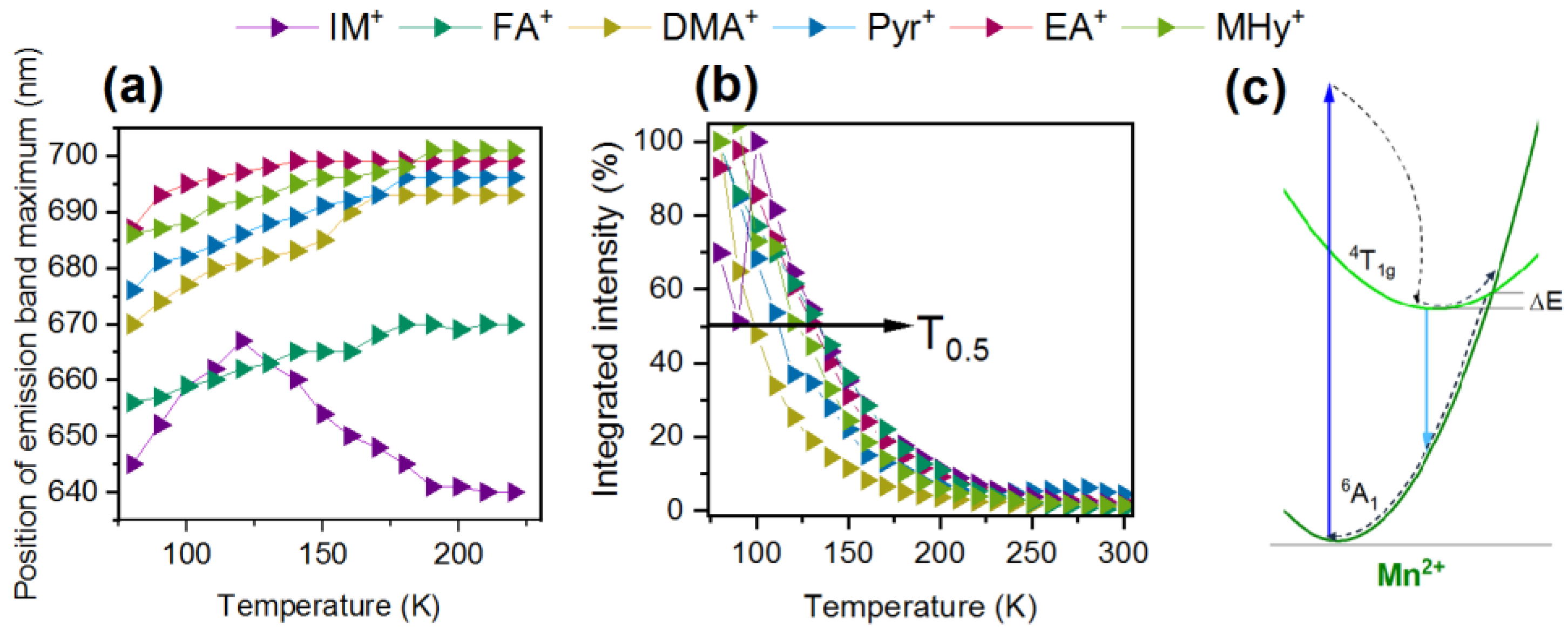
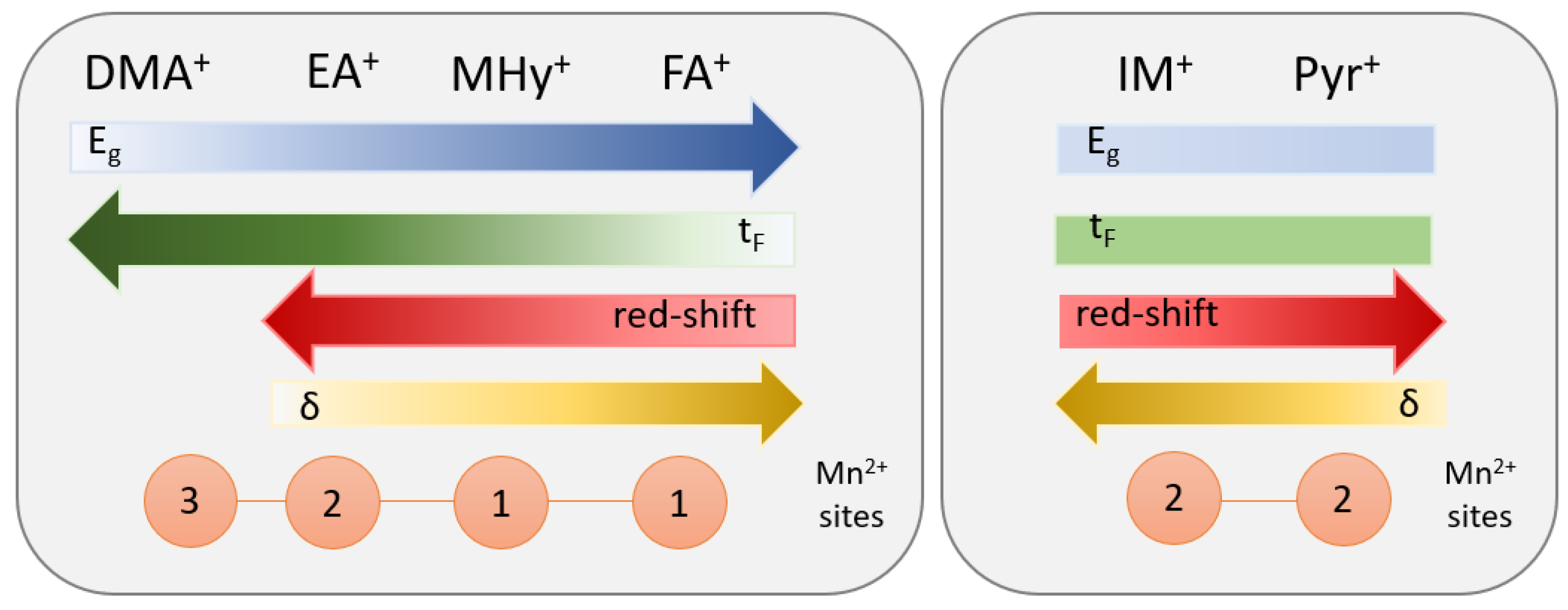
2.2. Spectroscopic Properties
| DMA+ | EA+ | MHy+ | FA+ | IM+ | Pyr+ | |
|---|---|---|---|---|---|---|
| IR (pm) | 272 | 344 | 264 | 253 | 320 | 258 |
| tF | 0.90 | 0.905 | 0.891 | 0.858 | 0.869 | 0.90 |
| Eg (eV) | 4.98 | 5.17 | 5.20 | 5.32 | 5.14 | 5.18 |
| λmax (nm) | 670 | 688 | 686 | 656 | 646 | 675 |
| δ (%) | 0.17 | 0.39 | 0.44 | 10.6 | 1.35 | 0.18 |
| T0.5 (K) | 100 | 130 | 122 | 135 | 135 | 115 |
3. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, L.; Zhang, X.; Yu, Y.; Xu, X.; Tang, J.; He, X.; Wu, J.; Lan, Z. Efficient Planar Perovskite Solar Cells Based on High-Quality Perovskite Films with Smooth Surface and Large Crystal Grains Fabricated in Ambient Air Conditions. Sol. Energy 2017, 155, 942–950. [Google Scholar] [CrossRef]
- Ptak, M.; Sieradzki, A.; Šimėnas, M.; Maczka, M. Molecular Spectroscopy of Hybrid Organic–Inorganic Perovskites and Related Compounds. Coord. Chem. Rev. 2021, 448, 214180. [Google Scholar] [CrossRef]
- Ptak, M.; Gągor, A.; Sieradzki, A.; Bondzior, B.; Dereń, P.; Ciupa, A.; Trzebiatowska, M.; Mączka, M. The Effect of K+ Cations on the Phase Transitions, and Structural, Dielectric and Luminescence Properties of [Cat][K0.5Cr0.5(HCOO)3], Where Cat Is Protonated Dimethylamine or Ethylamine. Phys. Chem. Chem. Phys. 2017, 19, 12156–12166. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Gągor, A.; Zaręba, J.K.; Stefańska, D.; Drozd, M.; Balciunas, S.; Šimenas, M.; Banys, J.; Sieradzki, A. Three-Dimensional Perovskite Methylhydrazinium Lead Chloride with Two Polar Phases and Unusual Second-Harmonic Generation Bistability above Room Temperature. Chem. Mater. 2020, 32, 4072–4082. [Google Scholar] [CrossRef]
- Gao, H.Q.; Wei, W.J.; Tan, Y.H.; Tang, Y.Z. Phase Transition and Negative Thermal Expansion in Guanidinium Magnesium-Hypophosphite Hybrid Perovskite. Chem. Mater. 2020, 32, 6886–6891. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic-Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Trzebiatowska, M.; Mączka, M.; Ptak, M.; Giriunas, L.; Balciunas, S.; Simenas, M.; Klose, D.; Banys, J. Spectroscopic Study of Structural Phase Transition and Dynamic Effects in a [(CH3)2NH2][Cd(N3)3] Hybrid Perovskite Framework. J. Phys. Chem. C 2019, 123, 11840–11849. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.H.; Lee, B.R.; Jung, E.D.; Yu, J.C.; Di Nuzzo, D.; Friend, R.H.; Song, M.H. Amine-Based Passivating Materials for Enhanced Optical Properties and Performance of Organic-Inorganic Perovskites in Light-Emitting Diodes. J. Phys. Chem. Lett. 2017, 8, 1784–1792. [Google Scholar] [CrossRef]
- Bermúdez-García, J.M.; Sánchez-Andújar, M.; Señarís-Rodríguez, M.A. A New Playground for Organic-Inorganic Hybrids: Barocaloric Materials for Pressure-Induced Solid-State Cooling. J. Phys. Chem. Lett. 2017, 8, 4419–4423. [Google Scholar] [CrossRef]
- Xu, W.J.; Du, Z.Y.; Zhang, W.X.; Chen, X.M. Structural Phase Transitions in Perovskite Compounds Based on Diatomic or Multiatomic Bridges. CrystEngComm 2016, 18, 7915–7928. [Google Scholar] [CrossRef]
- Boström, H.L.B.; Hill, J.A.; Goodwin, A.L. Columnar Shifts as Symmetry-Breaking Degrees of Freedom in Molecular Perovskites. Phys. Chem. Chem. Phys. 2016, 18, 31881–31894. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhou, X.; Jiang, S.; Xiang, G.; Li, L.; Li, Y.; Jing, C.; Ling, F.; Wang, Y.; Xiao, P. Dual-Mode Luminescence Temperature Sensing Performance of Manganese (II) Doped CsPbCl3 Perovskite Quantum Dots. Ceram. Int. 2022, 48, 33645–33652. [Google Scholar] [CrossRef]
- Wang, M.; Zang, Z.; Yang, B.; Hu, X.; Sun, K.; Sun, L. Performance Improvement of Perovskite Solar Cells through Enhanced Hole Extraction: The Role of Iodide Concentration Gradient. Sol. Energy Mater. Sol. Cells 2018, 185, 117–123. [Google Scholar] [CrossRef]
- Ptak, M.; Mączka, M. Phonon Properties and Mechanism of Order-Disorder Phase Transition in Formamidinium Manganese Hypophosphite Single Crystal. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118010. [Google Scholar] [CrossRef]
- Li, M.; Smetana, V.; Wilk-Kozubek, M.; Mudryk, Y.; Alammar, T.; Pecharsky, V.K.; Mudring, A.V. Open-Framework Manganese(II) and Cobalt(II) Borophosphates with Helical Chains: Structures, Magnetic, and Luminescent Properties. Inorg. Chem. 2017, 56, 11104–11112. [Google Scholar] [CrossRef]
- Bermúdez-García, J.M.; Yáñez-Vilar, S.; García-Fernández, A.; Sánchez-Andújar, M.; Castro-García, S.; López-Beceiro, J.; Artiaga, R.; Dilshad, M.; Moya, X.; Señarís-Rodríguez, M.A. Giant Barocaloric Tunability in [(CH3CH2CH2)4N]Cd[N(CN)2]3 Hybrid Perovskite. J. Mater. Chem. C 2018, 6, 9867–9874. [Google Scholar] [CrossRef]
- Mączka, M.; Gągor, A.; Ptak, M.; Stefańska, D.; Macalik, L.; Pikul, A.; Sieradzki, A. Structural, Phonon, Magnetic and Optical Properties of Novel Perovskite-like Frameworks of TriBuMe[M(Dca)3] (TriBuMe = Tributylmethylammonium; Dca = Dicyanamide; M = Mn2+, Fe2+, Co2+, Ni2+). Dalt. Trans. 2019, 48, 13006–13016. [Google Scholar] [CrossRef]
- Trzebiatowska, M.; Gągor, A.; Macalik, L.; Peksa, P.; Sieradzki, A. Phase Transition in the Extreme: A Cubic-to-Triclinic Symmetry Change in Dielectrically Switchable Cyanide Perovskites. Dalt. Trans. 2019, 48, 15830–15840. [Google Scholar] [CrossRef]
- Xu, W.J.; Li, P.F.; Tang, Y.Y.; Zhang, W.X.; Xiong, R.G.; Chen, X.M. A Molecular Perovskite with Switchable Coordination Bonds for High-Temperature Multiaxial Ferroelectrics. J. Am. Chem. Soc. 2017, 139, 6369–6375. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Bai, X.; Wu, H.; Shen, X.; Zhang, Y.; Zhang, W.; Zheng, W.; Song, H.; Yu, W.W.; et al. Spontaneous Silver Doping and Surface Passivation of CsPbI3 Perovskite Active Layer Enable Light-Emitting Devices with an External Quantum Efficiency of 11.2%. ACS Energy Lett. 2018, 3, 1571–1577. [Google Scholar] [CrossRef]
- Zhao, X.; Ng, J.D.A.; Friend, R.H.; Tan, Z.K. Opportunities and Challenges in Perovskite Light-Emitting Devices. ACS Photonics 2018, 5, 3866–3875. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, T.; Leng, C.; Zang, Z.; Wang, M.; Hu, W.; Tang, X.; Lu, S.; Fang, L.; Zhou, M. Performance Improvement of Perovskite Solar Cells by Employing a CdSe Quantum Dot/PCBM Composite as an Electron Transport Layer. J. Mater. Chem. A 2017, 5, 17499–17505. [Google Scholar] [CrossRef]
- Kore, B.P.; Das, S.; Sarma, D.D. Temperature-Dependent Anomalous Mn2+ Emission and Excited State Dynamics in Mn2+-Doped MAPbCl3-XBrx Nanocrystals. J. Chem. Sci. 2021, 133, 64. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Gągor, A.; Stefańska, D.; Sieradzki, A. Layered Lead Iodide of [Methylhydrazinium]2PbI4 with a Reduced Band Gap: Thermochromic Luminescence and Switchable Dielectric Properties Triggered by Structural Phase Transitions. Chem. Mater. 2019, 31, 8563–8575. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Sieradzki, A. Methylhydrazinium Lead Bromide: Noncentrosymmetric Three-Dimensional Perovskite with Exceptionally Large Framework Distortion and Green Photoluminescence. Chem. Mater. 2020, 32, 1667–1673. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Grätzel, M. The Light and Shade of Perovskite Solar Cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-Halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2020, 592, 381–385. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.P.; et al. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Drozdowski, D.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Fedoruk, K.; Mączka, M.; Sieradzki, A. Three-Dimensional Methylhydrazinium Lead Halide Perovskites: Structural Changes and Effects on Dielectric, Linear, and Nonlinear Optical Properties Entailed by the Halide Tuning. J. Phys. Chem. C 2022, 126, 1600–1610. [Google Scholar] [CrossRef]
- Kieslich, G.; Sun, S.; Cheetham, A.K. An Extended Tolerance Factor Approach for Organic-Inorganic Perovskites. Chem. Sci. 2015, 6, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, G.; Sun, S.; Cheetham, A.K. Solid-State Principles Applied to Organic–Inorganic Perovskites: New Tricks for an Old Dog. Chem. Sci. 2014, 5, 4712–4715. [Google Scholar] [CrossRef]
- Boström, H.L.B. Tilts and Shifts in Molecular Perovskites. CrystEngComm 2020, 22, 961–968. [Google Scholar] [CrossRef]
- Wu, Y.; Shaker, S.; Brivio, F.; Murugavel, R.; Bristowe, P.D.; Cheetham, A.K. Mn(H2POO)3: A New Family of Hybrid Perovskites Based on the Hypophosphite Ligand. J. Am. Chem. Soc. 2017, 139, 16999–17002. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Gągor, A.; Stefańska, D.; Zaręba, J.K.; Pikul, A. Structural, Magnetic and Photoluminescence Properties of New Hybrid Hypophosphites: Discovery of the First Noncentrosymmetric and Two Cobalt-Based Members. Dalt. Trans. 2022, 51, 9094–9102. [Google Scholar] [CrossRef]
- Mączka, M.; Stefańska, D.; Gągor, A.; Pikul, A. The Cation-Dependent Structural, Magnetic and Optical Properties of a Family of Hypophosphite Hybrid Perovskites. Dalt. Trans. 2022, 51, 352–360. [Google Scholar] [CrossRef]
- Mączka, M.; Gągor, A.; Pikul, A.; Stefańska, D. Novel Hypophosphite Hybrid Perovskites of [CH3NH2NH2][Mn(H2POO)3] and [CH3NH2NH2][Mn(H2POO)2.83(HCOO)0.17] Exhibiting Antiferromagnetic Order and Red Photoluminescence. RSC Adv. 2020, 10, 19020–19026. [Google Scholar] [CrossRef]
- Mączka, M.; Stefańska, D.; Ptak, M.; Gągor, A.; Pikul, A.; Sieradzki, A. Cadmium and Manganese Hypophosphite Perovskites Templated by Formamidinium Cations: Dielectric, Optical and Magnetic Properties. Dalt. Trans. 2021, 50, 2639–2647. [Google Scholar] [CrossRef]
- Xue, J.; Li, F.; Liu, F.; Noh, H.M.; Lee, B.R.; Choi, B.C.; Park, S.H.; Jeong, J.H.; Du, P. Designing Ultra-Highly Efficient Mn2+-Activated Zn2GeO4 Green-Emitting Persistent Phosphors toward Versatile Applications. Mater. Today Chem. 2022, 23, 100693. [Google Scholar] [CrossRef]
- Mączka, M.; Gągor, A.; Ptak, M.; Stefańska, D.; Sieradzki, A. Temperature-Dependent Studies of a New Two-Dimensional Cadmium Dicyanamide Framework Exhibiting an Unusual Temperature-Induced Irreversible Phase Transition into a Three-Dimensional Perovskite-like Framework. Phys. Chem. Chem. Phys. 2018, 20, 29951–29958. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Dai, H.; Li, S.; Li, Z.; Li, J.; Xin, S.; Wang, C.; Zhu, G.; Dong, B. Novel Rare Earth Free Phosphors CsMg2P3O10: Mn2+ with Efficient and Ultra-Broadband Red Emission for Plant Growth LEDs. J. Am. Ceram. Soc. 2022, 105, 4719–4730. [Google Scholar] [CrossRef]
- Song, E.; Chen, M.; Chen, Z.; Zhou, Y.; Zhou, W.; Sun, H.T.; Yang, X.; Gan, J.; Ye, S.; Zhang, Q. Mn2+-Activated Dual-Wavelength Emitting Materials toward Wearable Optical Fibre Temperature Sensor. Nat. Commun. 2022, 13, 2166. [Google Scholar] [CrossRef]
- Bermúdez-García, J.M.; Sánchez-Andújar, M.; Castro-García, S.; López-Beceiro, J.; Artiaga, R.; Señarís-Rodríguez, M.A. Giant Barocaloric Effect in the Ferroic Organic-Inorganic Hybrid [TPrA][Mn(Dca)3] Perovskite under Easily Accessible Pressures. Nat. Commun. 2017, 8, 15715. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefańska, D. Effect of Organic Cation on Optical Properties of [A]Mn(H2POO)3 Hybrid Perovskites. Molecules 2022, 27, 8953. https://doi.org/10.3390/molecules27248953
Stefańska D. Effect of Organic Cation on Optical Properties of [A]Mn(H2POO)3 Hybrid Perovskites. Molecules. 2022; 27(24):8953. https://doi.org/10.3390/molecules27248953
Chicago/Turabian StyleStefańska, Dagmara. 2022. "Effect of Organic Cation on Optical Properties of [A]Mn(H2POO)3 Hybrid Perovskites" Molecules 27, no. 24: 8953. https://doi.org/10.3390/molecules27248953
APA StyleStefańska, D. (2022). Effect of Organic Cation on Optical Properties of [A]Mn(H2POO)3 Hybrid Perovskites. Molecules, 27(24), 8953. https://doi.org/10.3390/molecules27248953







