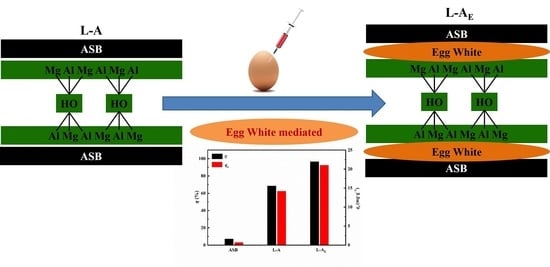
Egg White-Mediated Fabrication of Mg/Al-LDH-Hard Biochar Composite for Phosphate Adsorption
Abstract
1. Introduction
2. Results
2.1. Comparison of Adsorption Performance of the Materials
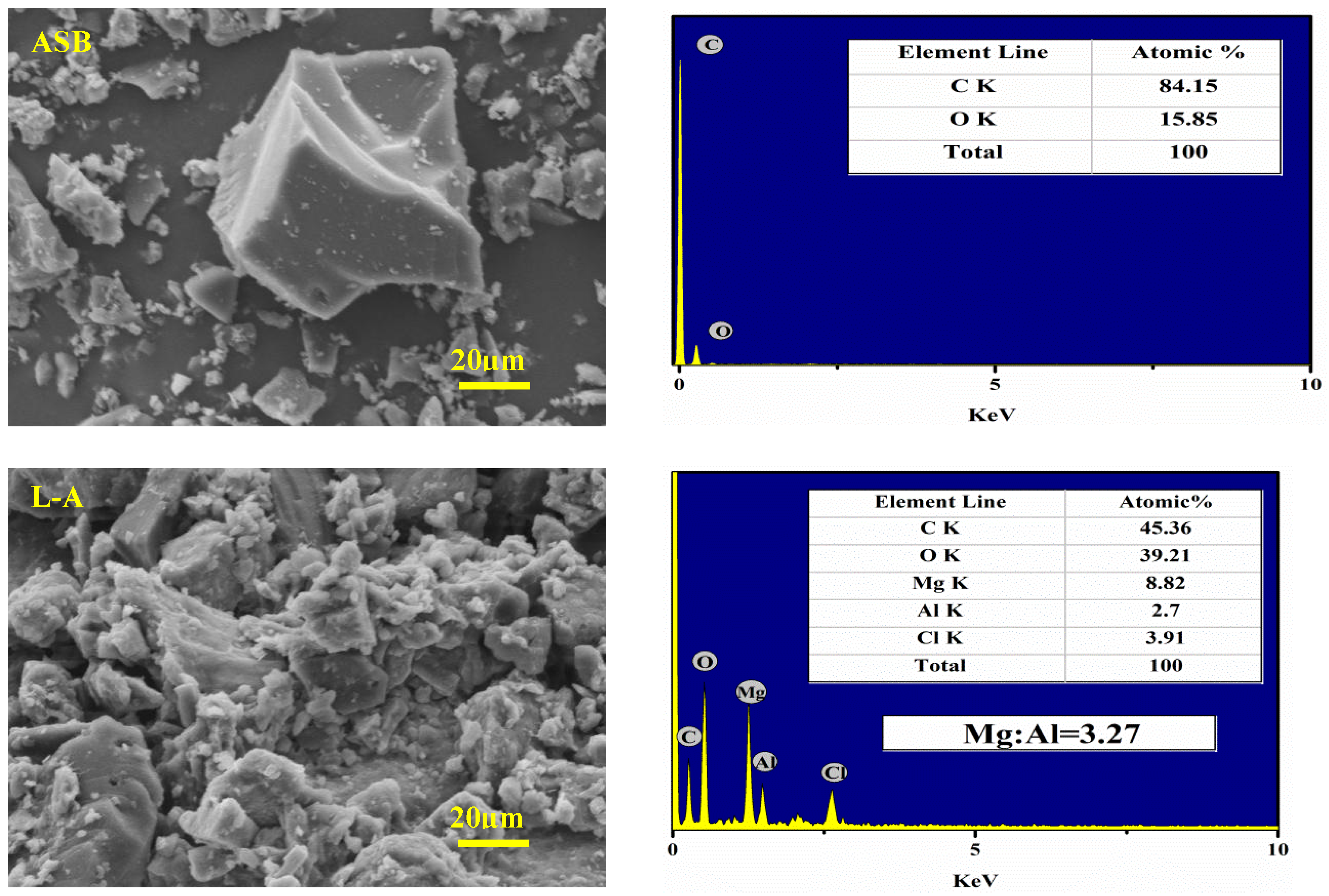
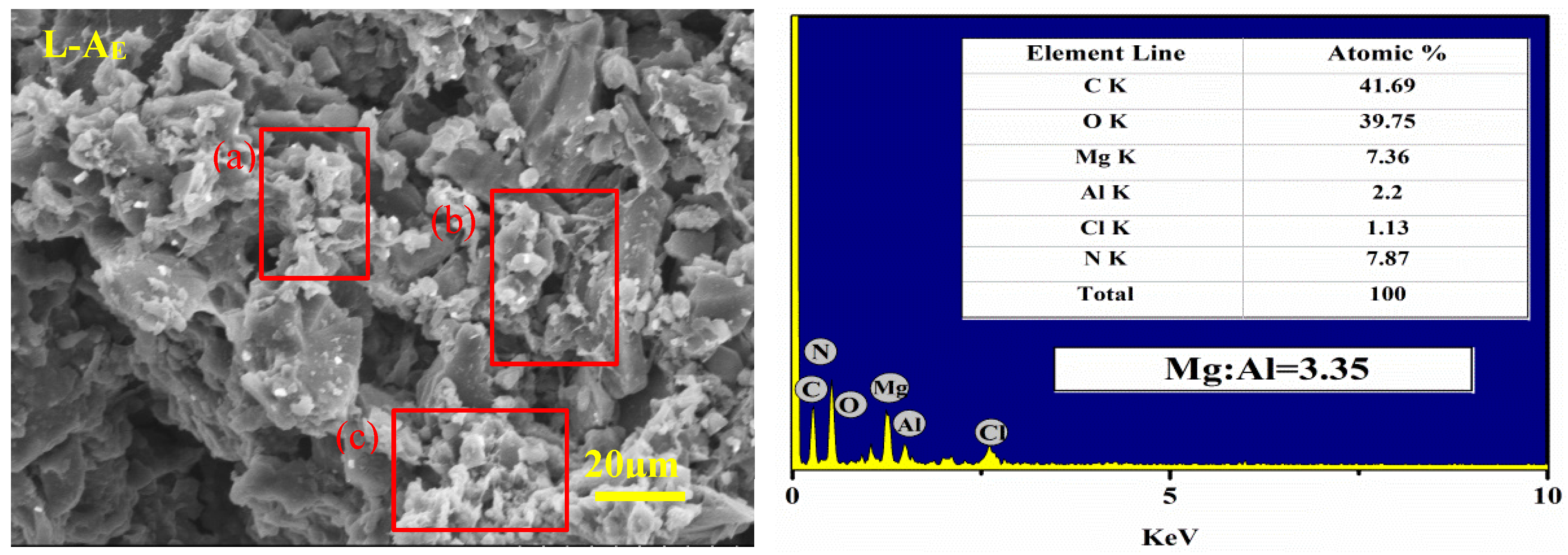
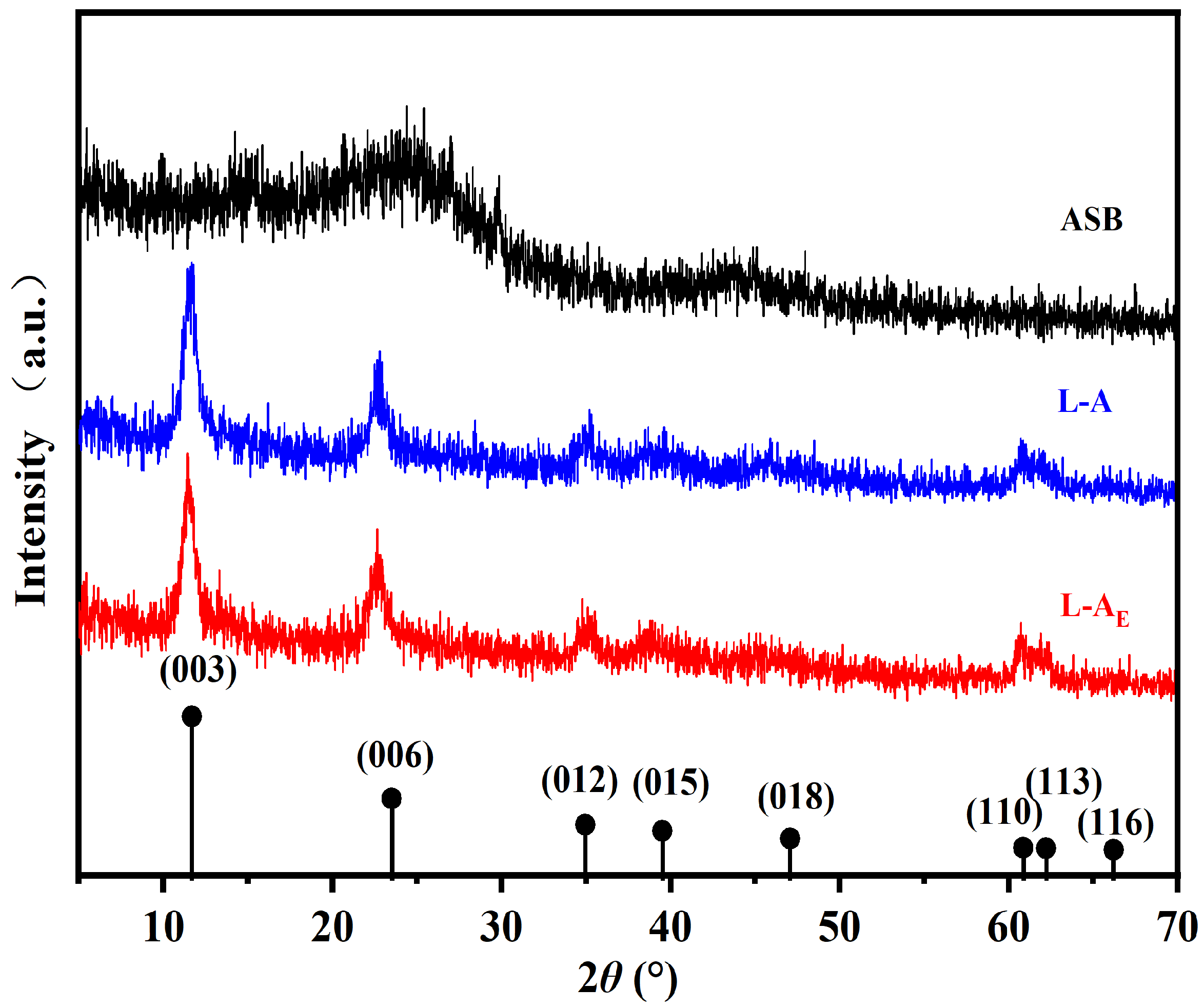
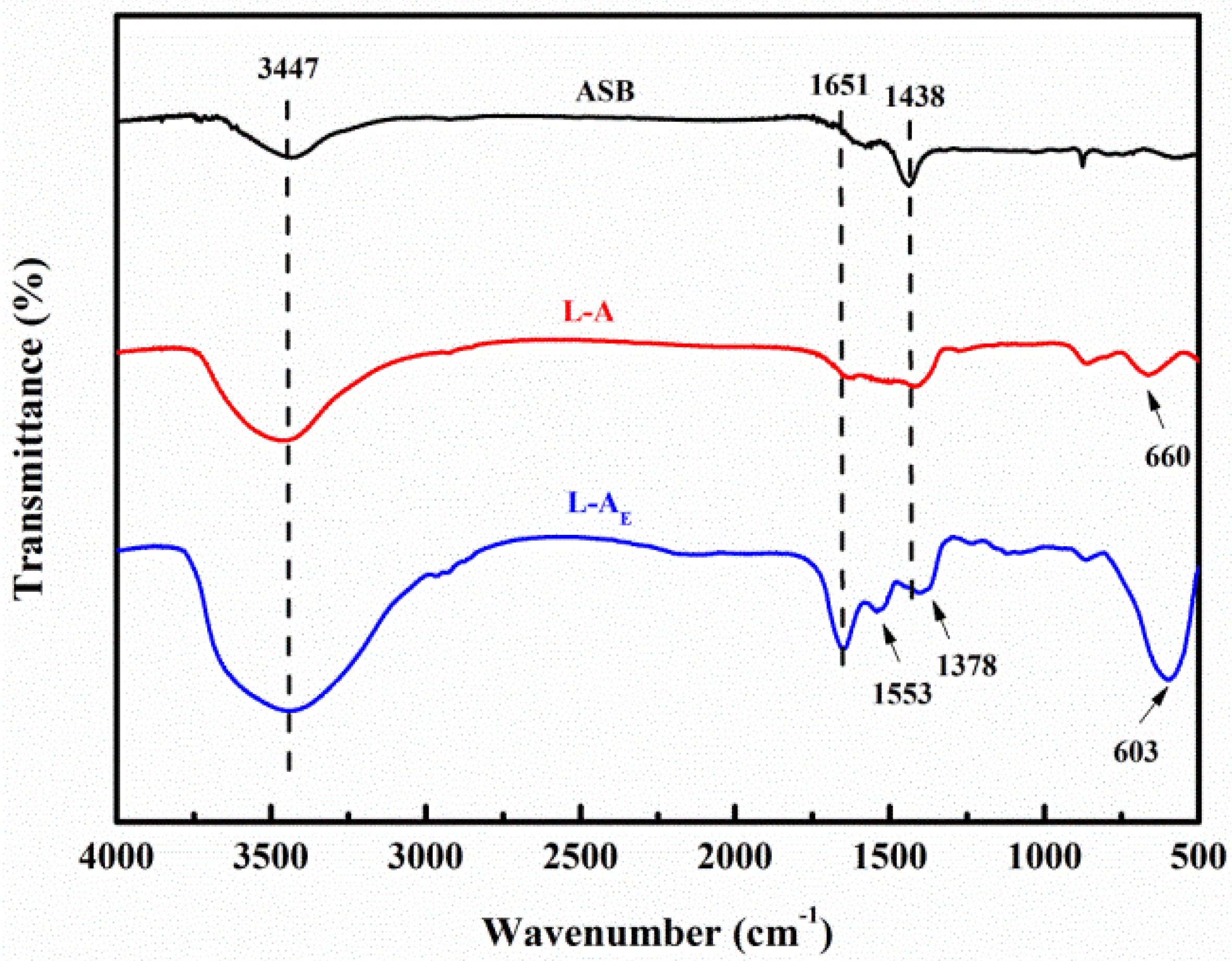
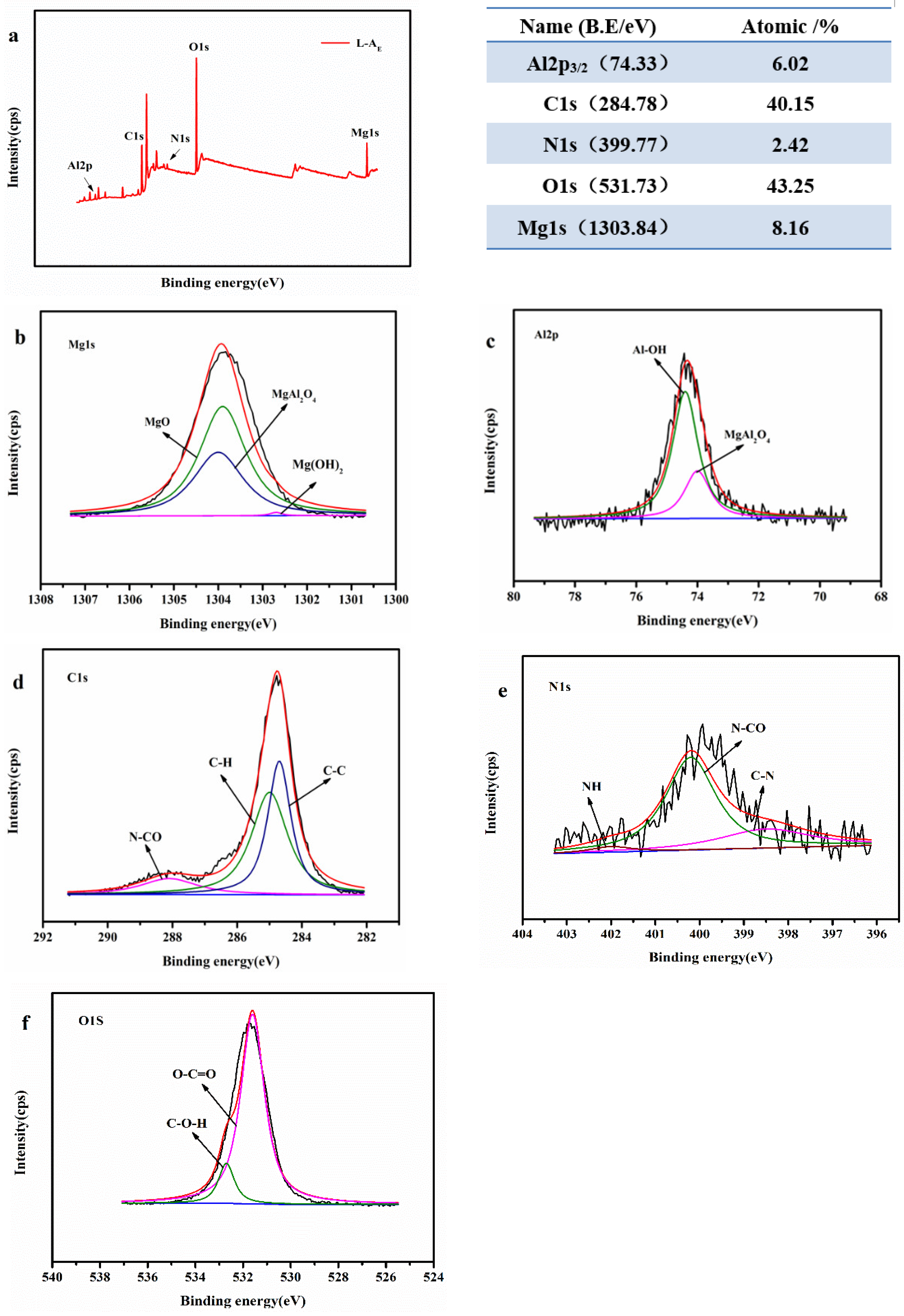
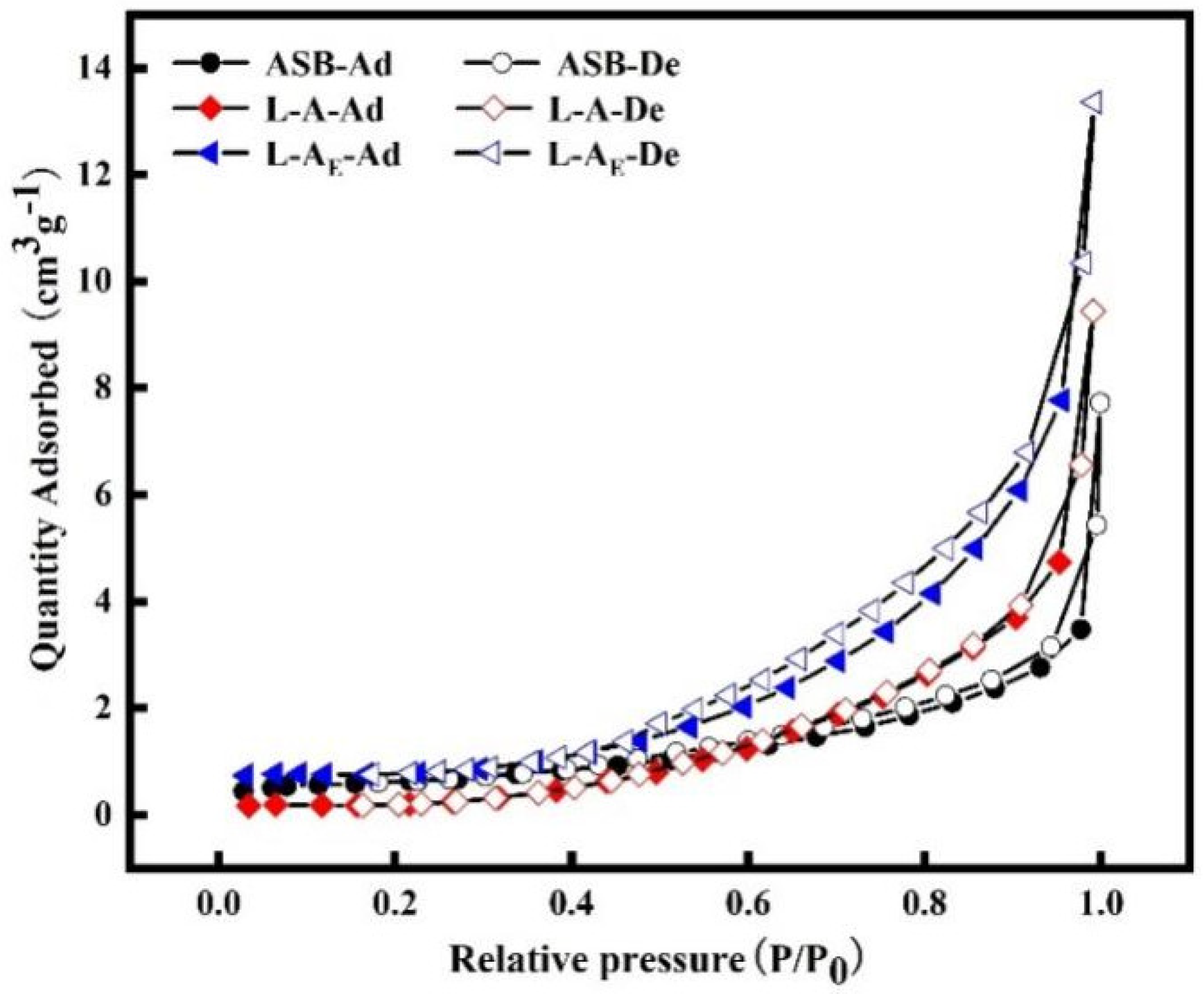
2.2. Basic Characterization of the Materials
2.3. Adsorption Kinetics and Isothermal Adsorption of Phosphate on L-AE
3. Experimental
3.1. Materials
3.2. Preparation of Adsorbents
3.3. Characterization of Adsorbents
3.4. Batch Adsorption Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Zeng, F.; Jiang, H.; Yu, H. Total recovery of nitrogen and phosphorus from three wetland plants by fast pyrolysis technology. Bioresour. Technol. 2011, 102, 3471–3479. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Ylmen, R.; Gustafsson, A.M.K.; Camerani-Pinzani, C.; Steenari, B.-M. Recovery of phosphorous from industrial waste water by oxidation and precipitation. Environ. Technol. 2018, 39, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, C.; Chen, S.; Ma, L.; Li, Y.; Lu, Y. Phosphate adsorption characteristics of La(OH)3-modified, canna-derived biochar. Chemosphere 2022, 286, 131773. [Google Scholar] [CrossRef]
- Vohla, C.; Koiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, U. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Beaudry, J.W.; Sengupta, S. Phosphorus recovery from wastewater using pyridine-based ion-exchange resins: Role of impregnated iron oxide nanoparticles and preloaded Lewis acid (Cu2+). Water Environ. Res. 2021, 93, 774–786. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, M.-J.; Lee, S.-J.; Noh, W.; Kwon, J.M.; Choi, J.S.; Kang, C.-M. Efficient recovery of nitrate and phosphate from wastewater by an amine-grafted adsorbent for cyanobacterial biomass production. Bioresour. Technol. 2016, 205, 269–273. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Chen, X.; Lei, Z.; Yuan, T.; Shimizu, K.; Zhang, Z.; Lee, D.-J. Insight into aerobic phosphorus removal from wastewater in algal-bacterial aerobic granular sludge system. Bioresour. Technol. 2022, 352, 127104. [Google Scholar] [CrossRef]
- Yalcuk, A.; Ugurlu, A. Comparison of horizontal and vertical constructed wetland systems for landfill leachate treatment. Bioresour. Technol. 2009, 100, 2521–2526. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Zaghlool, E.; Khalil, M.; Kotp, Y.H. Surface and internal modification of composite ion exchange membranes for removal of molybdate, phosphate, and nitrate from polluted groundwater. Arab. J. Chem. 2022, 15, 103747. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, J.P. Application of Zirconium/PVA Modified Flat-Sheet PVDF Membrane for the Removal of Phosphate from Aqueous Solution. Ind. Eng. Chem. Res. 2016, 55, 6835–6844. [Google Scholar] [CrossRef]
- Li, Y.; Fu, F.; Cai, W.; Tang, B. Synergistic effect of mesoporous feroxyhyte nanoparticles and Fe(II) on phosphate immobilization: Adsorption and chemical precipitation. Powder Technol. 2019, 345, 786–795. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Tsang, D.C.W.; Cheng, K.; Ok, Y.S. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery. J. Hazard. Mater. 2019, 365, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Yang, K.; Li, Z.; Zhang, H.; Qian, J.; Chen, G. Municipal wastewater phosphorus removal by coagulation. Environ. Technol. 2010, 31, 601–609. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Meenakshi, S. Fabrication of hybrid chitosan encapsulated magnetic-kaolin beads for adsorption of phosphate and nitrate ions from aqueous solutions. Int. J. Biol. Macromol. 2021, 168, 750–759. [Google Scholar] [CrossRef]
- Wendling, L.A.; Blomberg, P.; Sarlin, T.; Priha, O.; Arnold, M. Phosphorus sorption and recovery using mineral-based materials: Sorption mechanisms and potential phytoavailability. Appl. Geochem. 2013, 37, 157–169. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, H.; Yang, H. Phosphate Removal from Wastewater by Magnetic Amorphous Lanthanum Silicate Alginate Hydrogel Beads. Minerals 2022, 12, 171. [Google Scholar] [CrossRef]
- Cui, M.; Wang, D.; Huang, T.; Liu, F. Adsorption Characteristics of Phosphorus Wastewater on the Synthetic Ferrihydrite. Huan Jing Ke Xue 2016, 37, 3498–3507. [Google Scholar]
- Sun, J.; Norouzi, O.; Masek, O. A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Ma, Z.; Dong, X.; Wei, Z.; Liu, X.; Zhu, L. Mg/Al-layered double hydroxide modified biochar for simultaneous removal phosphate and nitrate from aqueous solution. Environ. Technol. Innov. 2021, 23, 101771. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Six, J.; Parikh, S.J. The effects of walnut shell and wood feedstock biochar amendments on greenhouse gas emissions from a fertile soil. Geoderma 2013, 200, 90–98. [Google Scholar] [CrossRef]
- Oginni, O.; Yakaboylu, G.A.; Singh, K.; Sabolsky, E.M.; Unal-Tosun, G.; Jaisi, D.; Khanal, S.; Shah, A. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources. J. Environ. Chem. Eng. 2020, 8, 103723. [Google Scholar] [CrossRef]
- Jiao, G.; Ma, J.; Li, Y.; Jin, D.; Ali, Z.; Zhou, J.; Sun, R. Recent advances and challenges on removal and recycling of phosphate from wastewater using biomass-derived adsorbents. Chemosphere 2021, 278, 130377. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Dweiri, F.; Almanassra, I.; Chatla, A.; Atieh, M. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Yang, D.; Xiang, J.; Chen, Y. Removal and recovery of phosphorus from secondary effluent using layered double hydroxide-biochar composites. Sci. Total Environ. 2022, 844, 156802. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Song, K.; Choi, K.; Lee, Y.; Jung, K. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with mg/al-calcined layered double hydroxides via co-pyrolysis. Compos. B 2019, 176, 107209. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Zhang, Z.; Liu, Q.; Jiang, K.; Tian, J.; Zhang, Y.; Ni, F. Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar. J. Environ. Chem. Eng. 2021, 9, 105079. [Google Scholar] [CrossRef]
- Shin, E.W.; Karthikeyan, K.G.; Tshabalala, M.A. Orthophosphate sorption onto lanthanum-treated lignocellulosic sorbents. Environ. Sci. Technol. 2005, 39, 6273–6279. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Ding, H.; Wan, Y.; Tang, Z.; Gao, J. Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method. Int. J. Mol. Sci. 2018, 19, 3055. [Google Scholar] [CrossRef]
- Elleuch, A.; Boussetta, A.; Yu, J.; Halouani, K.; Li, Y. Experimental investigation of direct carbon fuel cell fueled by almond shell biochar: Part I. Physicochemical characterization of the biochar fuel and cell performance examination. Int. J. Hydrogen Energy 2013, 38, 16590–16604. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Inyang, M. Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere 2013, 92, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, J.; Jiang, S.; Li, H.; Ren, Z.; Xu, Q.; Jiang, Q.; Liu, W.; Li, R.; Zhang, Y. A composite of Ni-Fe-Zn layered double hydroxides/biochar for atrazine removal from aqueous solution. Biochar 2020, 2, 455–464. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.; Ma, Y.; Liu, L.; Meng, R.; Yao, J. Highly efficient adsorption and recycle of phosphate from wastewater using flower-like layered double oxides and their potential as synergistic flame retardants. J. Colloid Interface Sci. 2020, 562, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Athreya, A.G.; Shareef, M.I.; Gopinath, S.M. Antibacterial Activity of Silver Nanoparticles Isolated from Cow’s Milk, Hen’s Egg White and Lysozyme: A Comparative Study. Arab. J. Sci. Eng. 2019, 44, 6231–6240. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Morphological modification and corrosion response of MgO and Mg3(PO4)2 composite formed on magnesium alloy. Compos. Part B 2019, 176, 107225. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Weng, B.; Atrens, A.; Ma, S.; Liu, L.; Pan, F. Sealing of anodized magnesium alloy AZ31 with MgAl layered double hydroxides layers. RSC Adv. 2018, 8, 2248–2259. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Andrzejczuk, M.; Dolata, M.; Lewandowska, M.; Janik-Czachor, M. Characterization of a calcium phosphate-TiO2 nanotube composite layer for biomedical applications. Mater. Sci. Eng. 2011, 31, 906–914. [Google Scholar] [CrossRef]
- Nogueira, K.A.B.; Cecilia, J.A.; Santos, S.O.; Aguiar, J.E.; Vilarrasa-Garcia, E.; Rodriguez-Castellon, E.; Azevedo, D.C.S.; Silva, I.J.J. Adsorption behavior of bovine serum albumin on Zn-Al and Mg-Al layered double hydroxides. J. Sol-Gel Sci. Technol. 2016, 80, 748–758. [Google Scholar] [CrossRef]
- Amirthavalli, C.; Manikandan, A.; Prince, A.A.M. Effect of zinc precursor ratio on morphology and luminescent properties of ZnO nanoparticles synthesized in CTAB medium. Ceram. Int. 2018, 44, 15290–15297. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Meenakshi, S. Development of sodium alginate@ZnFe-LDHs functionalized beads: Adsorption properties and mechanistic behaviour of phosphate and nitrate ions from the aqueous environment. Environ. Chem. Ecotoxicol. 2021, 3, 42–50. [Google Scholar] [CrossRef]
- Yan, L.; Yang, K.; Shan, R.; Yan, T.; Wei, J.; Yu, S.; Yu, H.; Du, B. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core-shell Fe3O4@LDHs composites with easy magnetic separation assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zheng, Y.; Wang, Y.; Shen, L.; Wang, H.; Zheng, Y.; He, N.; Li, Q. Highly selective adsorption of phosphate by pyromellitic acid intercalated ZnAl-LDHs: Assembling hydrogen bond acceptor sites. Chem. Eng. J. 2015, 260, 809–817. [Google Scholar] [CrossRef]
- Nuryadin, A.; Imai, T.; Kanno, A.; Yamamoto, K.; Sekine, M.; Higuchi, T. Phosphate adsorption and desorption on two-stage synthesized amorphous-ZrO2/Mg–Fe layered double hydroxide composite. Mater. Chem. Phys. 2021, 266, 124559. [Google Scholar] [CrossRef]
- Hao, H.; Wang, Y.; Shi, B. NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study. Water Res. 2019, 155, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Q.; Chen, J.; Zhang, L.; Chang, N. Phosphate adsorption on hydroxyl–iron–lanthanum doped activated carbon fiber. Chem. Eng. J. 2013, 215, 859–867. [Google Scholar] [CrossRef]
- Wu, L.; Wei, C.; Zhang, S.; Wang, Y.; Kuzyakov, Y.; Ding, X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean. Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]


| Models | Parameters | Adsorbents | |
|---|---|---|---|
| L-AE | L-A | ||
| Pseudo-first-order | qe (mg·g−1) | 51.70 | 43.58 |
| K1 (min−1) | 0.255 | 0.306 | |
| R2 | 0.9158 | 0.8966 | |
| Pseudo-second-order | qe (mg·g−1) | 55.01 | 46.09 |
| K2 (g·mg−1·min−1) | 0.008 | 0.012 | |
| R2 | 0.9956 | 0.9815 | |
| Langmuir | qm (mg·g−1) | 133.13 | 44.71 |
| KL (L·mg−1) | 0.0038 | 0.013 | |
| R2 | 0.9936 | 0.9548 | |
| Freundlich | KF (mg·g−1) (L·mg−1)1/n | 2.73 | 4.62 |
| 1/n | 0.56 | 0.35 | |
| R2 | 0.9531 | 0.7967 | |
| Adsorbents | pH | Temperature (K) | qm (mg·g−1) | Ref. |
|---|---|---|---|---|
| Fe3O4@Zn–Al–LDH | - | 298 | 36.9 | [42] |
| Fe3O4@Mg–Al–LDH | - | 298 | 31.7 | [42] |
| Fe3O4@Ni–Al–LDH | - | 298 | 26.5 | [42] |
| pyromellitic acid intercalated ZnAl-LDHs | - | 293 | 57.05 | [43] |
| ZnFe-LDHs@Alg | - | 303 | 94.64 | [41] |
| 20% Mg-BC | 6.0 | 298 | 56.12 | [28] |
| 20% Al-BC | 6.0 | 298 | 38.71 | [28] |
| amorphous-ZrO2/Mg–Fe layered double hydroxide composite | 7.0 | 290 | 66.08 | [44] |
| La(OH)3-modified, canna-derived biochar | 7.0 | 298 | 37.37 | [4] |
| NaLa(CO3)2/Fe3O4 composites | 6.8 | 298 | 77.85 | [45] |
| MgCo2O4 | 5.0 | 303 | 58.69 | [46] |
| MgO-biochar | 6.0 | 298 | 18.94 | [47] |
| L-A | 6.5 | 303 | 44.71 | This study |
| L-AE | 6.5 | 303 | 133.13 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, S.; Ren, H.; Zhang, Y.; Ma, Z. Egg White-Mediated Fabrication of Mg/Al-LDH-Hard Biochar Composite for Phosphate Adsorption. Molecules 2022, 27, 8951. https://doi.org/10.3390/molecules27248951
Ma X, Li S, Ren H, Zhang Y, Ma Z. Egg White-Mediated Fabrication of Mg/Al-LDH-Hard Biochar Composite for Phosphate Adsorption. Molecules. 2022; 27(24):8951. https://doi.org/10.3390/molecules27248951
Chicago/Turabian StyleMa, Xiaolong, Shuqi Li, He Ren, Yin Zhang, and Zichuan Ma. 2022. "Egg White-Mediated Fabrication of Mg/Al-LDH-Hard Biochar Composite for Phosphate Adsorption" Molecules 27, no. 24: 8951. https://doi.org/10.3390/molecules27248951
APA StyleMa, X., Li, S., Ren, H., Zhang, Y., & Ma, Z. (2022). Egg White-Mediated Fabrication of Mg/Al-LDH-Hard Biochar Composite for Phosphate Adsorption. Molecules, 27(24), 8951. https://doi.org/10.3390/molecules27248951