Abstract
Three-dimensional lead halide perovskites are known for their excellent optoelectronic properties, making them suitable for photovoltaic and light-emitting applications. Here, we report for the first time the Raman spectra and photoluminescent (PL) properties of recently discovered three-dimensional aziridinium lead halide perovskites (AZPbX3, X = Cl, Br, I), as well as assignment of vibrational modes. We also report diffuse reflection data, which revealed an extended absorption of light of AZPbX3 compared to the MA and FA counterparts and are beneficial for solar cell application. We demonstrated that this behavior is correlated with the size of the organic cation, i.e., the energy band gap of the cubic lead halide perovskites decreases with the increasing size of the organic cation. All compounds show intense PL, which weakens on heating and shifts toward higher energies. This PL is red shifted compared to the FA and MA counterparts. An analysis of the PL data revealed the small exciton binding energy of AZPbX3 compounds (29–56 meV). Overall, the properties of AZPbX3 are very similar to those of the well-known MAPbX3 and FAPbX3 perovskites, indicating that the aziridinium analogues are also attractive materials for light-emitting and solar cell applications.
1. Introduction
Hybrid organic–inorganic compounds (HOIPs) have been extensively investigated in recent years due to their attractive functional properties [1,2,3,4]. In particular, three-dimensional (3D) lead halide perovskites of general formula APbX3 (A = organic cation, X = Cl−, Br−, I−) have recently emerged as candidates for photovoltaic, light-emitting, lasing and scintillating applications [2,4,5,6,7,8,9,10]. Their attractiveness stems from high one- and two-photon absorption coefficients, long carrier diffusion lengths and tunable band gaps [2,8,11,12]. Unfortunately, the small size of the perovskite cavity should allow accommodation of only the smallest organic cations. Moreover, many lead halides comprising small cations, such as hydroxylammonium, hydrazinium, azetidinium and imidazolium, do not crystallize in the 3D perovskite structure [13,14,15,16]. As a result, 3D structures were found only for methylammonium (CH3NH3+, MA+) [17], formamidinium (NH2CHNH2+, FA+) [17,18] and methylhydrazinium (CH3NH2NH2+, MHy+) cations [8,9]. It is worth adding that MHyPbX3 perovskites (X = Br−, Cl−) differ from their centrosymmetric MA+ and FA+ counterparts since their low-temperature (LT) phases are polar; that is, they crystallize in chiral space group P21 [8,9,10]. Therefore, these compounds exhibit second harmonic generation (SHG) and pyroelectric properties [8,9,10].
In order to understand the optical properties of lead halide perovskites and obtain information on exciton binding energy, it is important to study their electronic absorption and PL in a broad temperature range. PL studies of MAPbX3 and FAPbX3 have been reported in many papers, and these spectra consist of narrow bands near 402–413, 532–567 and 756–830 nm for the Cl, Br and I analogues, respectively, which were attributed to free excition (FE) recombination [19,20,21,22,23,24,25]. It is worth adding that, in many cases, additional narrow bands were observed at slightly lower energies, which were attributed to defects [21,23]. A recent study of MAPbI3 suggested, however, that for this compound, the lower energy band near 830 nm corresponds to FE recombination, while surface defects lead to appearance of a higher energy and weaker band near 780 nm [25]. In contrast to MAPbX3 and FAPbX3 compounds, MHyPbX3 analogues also showed the presence of additional broad and highly Stokes-shifted bands, which could be attributed to self-trapped excitons (STEx) [8,9,10]. The presence of these bands is consistent with large distortion and off-center displacement of Pb2+ in these compounds [8,9,10], leading to a large increase of the electron–phonon interaction [10].
It is also important to understand phonon properties since a key phenomenon relevant for the optoelectronic applications of hybrid perovskites is electron–phonon coupling, which depends on vibrational energies and lattice dynamics [26,27,28]. Therefore, MA-, FA- and MHy-based perovskites have been the subject of numerous Raman studies [8,27,29,30,31,32,33,34,35]. In some cases, IR and THz spectra were also reported [32,34,36,37]. These studies showed that internal modes of organic cations are usually observed above 300 cm−1, and these bands are much weaker than the majority of lattice modes, which appear below 250 cm−1 [8,27,29,30,31,32,33,34,35].
It has been very recently reported that 3D perovskites can also be obtained by using aziridinium (CH2CH2NH2+, AZ+) cation [38]. At room temperature (RT), these compounds crystallize in the cubic symmetry (space group Pmm), and their band gaps were estimated as 2.99, 2.27 and 1.52 eV for the Cl, Br and I analogue, respectively [38]. This report also showed IR spectra, but no analysis of the obtained spectra and assignment of bands was proposed [38].
Herein, we report the synthesis of these compounds through the use of a different method than previously reported, reinvestigate electron absorption of these compounds and report temperature-dependent PL data to obtain information on exciton binding energy. We also report RT Raman spectra and propose the assignment of the observed bands to respective vibrations of atoms.
2. Results and Discussion
2.1. Raman Spectra
Vibrational modes of the 3D perovskites can be subdivided into vibrations of inorganic framework and lattice modes of organic cations (translational and librational modes) and internal modes of organic cations [8,27,29,30,31,32,33,34,35,39,40,41]. The former Raman studies of 3D perovskites showed that the vibrations of the inorganic frameworks give rise to very strong bands in the low-wavenumber region [8,27,29,30,31,32,33,34,35,39,40,41]. We assign, therefore, the very strong and asymmetric band at 139–122 cm−1 to Pb-X stretch (Figure 1 and Supplementary Table S1). The second low-wavenumber band is very sensitive to the type of halogen anion; that is, it shifts from 468 cm−1 for AZPbCl3 to 308 cm−1 for AZPbBr3 and 240 cm−1 for AZPbI3 (Figure 1 and Supplementary Table S1). Very similar behavior was previously reported for the MA-, FA- and MHy-based 3D perovskites, which showed the corresponding bands at 472 and 481 cm−1 for MAPbCl3 and MHyPbCl3, respectively; at 323, 307 and 309 cm−1 for MAPbBr3, FAPbBr3 and MHyPbBr3, respectively; and at 241 and 237 cm−1 for MAPbI3 and FAPbI3, respectively [34]. Studies of MAPbX3 perovskites showed that this mode, often assigned to the torsional mode of MA+ [32,39], is not a pure torsion but a mixed mode that also involves breathing or rocking motions of hydrogen atoms [42]. Another rationale why this mode cannot be attributed to a pure torsion is its very large width of the Raman band and the fact that it should be Raman-inactive [32,41]. Furthermore, this mode was shown to couple with the inorganic cage through N−H·X hydrogen bonds [34,41,42]. Due to special character of this mode, Nakada et al. proposed to call it MA-cage mode [29]. It is worth adding that theoretical calculations performed for an isolated MHy+ cation also showed the mixed character of the lowest wavenumber modes near 250 cm−1, with large contribution of the NH2 torsion [43]. Although no theoretical data reporting vibrational modes of AZ+ cation are available, the fact that the discussed modes of AZPbX3 compounds have similar frequencies to MA-, FA- or MHy-cage modes; they strongly depend on the type of halogen anion; and the Raman bands are very broad strongly support the assignment of the 468–240 cm−1 bands to the AZ-cage modes.
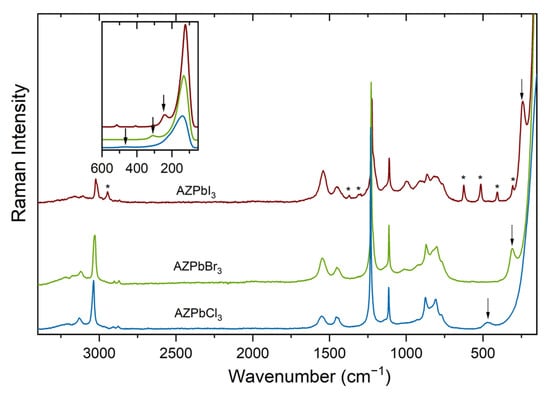
Figure 1.
Raman spectra of AZPbX3 perovskites. Inset shows details of the low-wavenumber region, and asterisks (*) indicate bands from an impurity phase. Arrows indicate bands very sensitive to the type of halogen anion.
The remaining modes can be attributed to internal vibrations of AZ+ cation. Free AZ+ cation has 21 vibrational degrees of freedom. Six of them correspond to symmetric stretching (νsNH2), antisymmetric stretching (νasNH2), scissoring (δNH2), rocking (ρNH2), wagging (ωNH2) and torsion or twisting (τNH2) modes of the NH2 groups. The remaining 15 modes can be subdivided into symmetric stretching (2νsCH2), antisymmetric stretching (2νasCH2), scissoring (2δCH2), rocking (2ρCH2), wagging (2ωCH2) and torsion or twisting (2τCH2) modes of the CH2 groups, as well as ring stretch and two ring deformation modes.
The vibrational modes of aziridine molecule were discussed in a few papers, which reported IR and Raman spectra of vapor and liquid aziridine, as well as ab initio calculations [44,45,46]. Based on these studies, we can unambiguously assign the narrow Raman bands near 3010–3130 cm−1 to the νCH2 modes (Figure 1 and Supplementary Table S1). The Raman spectra also show a few weak and broad bands in the 3150–3230 cm−1 range (Supplementary Table S1). Their large width and the fact that they correspond to very broad and strong IR bands [38] indicate that they correspond to the νNH2 modes. It is worth noting that these bands are observed at higher wavenumbers than the corresponding bands of MAPbX3 perovskites (3100–3190 cm−1; see Reference [34]), indicating that AZ+ cations form weaker hydrogen bonds with the halide anions than MA+ cations. The δNH2 mode is observed as a broad band near 1540–1550 cm−1 (Figure 1 and Supplementary Table S1). The band near 1460 cm−1 with a shoulder near 1440 cm−1 corresponds to the δCH2 modes, and the most intense band near 1220–1230 cm−1 can be attributed to the ring stretch [46]. Bands in the 760–1220 cm−1 range are more difficult to assign since the assignment of modes proposed in the literature is based on studies of aziridine molecule, not aziridinium cation, and therefore the energy of some modes may be significantly modified after protonation of the molecule. Nevertheless, we propose a tentative assignment of these bands in Supplementary Table S1.
In order to check if the samples are stable in air, we repeated the Raman measurements after 59–62 days (Supplementary Figure S1). These data show that the Raman spectrum of AZPbCl3 shows the presence of only two new and very weak bands at 2962 and 655 cm−1 that could be related to an impurity phase. In the case of AZPbBr3, the additional bands, which appear at 2962, 1317, 677, 573, 429 and 338 cm−1, are much stronger. The Raman spectra of AZPbI3 show that the additional bands at 2944, 1372, 1302, 625, 513, 407 and 309 cm−1 are observed already for the freshly synthesized sample, and their intensity strongly increases with time (Supplementary Figure S1). The Raman data indicate, therefore, that the air stability of the AZPbX3 compounds decreases in the order Cl > Br > I. As already reported by Petrosova et al., the new phases appear most likely due to the opening of the AZ+ ring and formation of low-dimensional perovskites based on 2-haloethylammonium cations [38].
2.2. Optical Properties
The RT diffuse reflectance spectra of AZPbCl3 and AZPbBr3 show the presence of sharp bands located at 415 and 553 nm, respectively (Figure 2a), which can be attributed to excitonic absorption. The obtained results were used to estimate the energy band gap (Eg) of the investigated materials by using the Kubelka–Munk equation [47]:
where R is a reflectance. Eg values were determined by plotting [F(R)·hν]2 versus energy (hν) (Supplementary Figures S2–S4). The excitonic peaks of AZPbCl3 and AZPbBr3 were subtracted from the Kubelka–Munk function to estimate the band gap with reasonable accuracy. The diagram of the determined energy band gaps of the investigated compounds is shown in Figure 2b. As can be seen, the smallest optical band gap equal to 1.51 eV is observed for AZPbI3, and it increases to 2.24 eV and 3.01 eV for AZPbBr3 and AZPbCl3, respectively. The determined values are in good agreement with those reported by Petrosova et al. [38]. It is worth adding that former studies revealed that 3D FAPbX3 perovskites have a slightly smaller Eg (3.03, 2.12–2.26 and 1.36–1.51 eV for FAPbCl3, FAPbBr3 and FAPbI3, respectively) than MAPbX3 analogues (2.88–3.177, 2.15–2.392 and 1.44–1.63 eV for MAPbCl3, MAPbBr3 and MAPbI3, respectively) [48,49,50,51,52]. Significantly larger band gaps were, however, reported for MHy-based perovskites (3.4 and 2.58 eV for MHyPbCl3 and MHyPbBr3, respectively [8,9]). Since the values reported in the literature for the FA and MA perovskites are scattered, we measured the absorption spectra of available MA and FA analogues and compared them with those obtained for AZPbX3. This comparison clearly shows that both the absorption edges and excitonic bands of AZPbX3 compounds are significantly red shifted compared to MAPbX3 (Supplementary Figure S5). A small red shift is also evident for AZPbBr3 when compared to FAPbBr3 (Supplementary Figure S5). Thus, the energy band gap of lead halide perovskites increases in the order AZ+ < FA+ < MA+ < MHy+. The largest band gaps of MHyPbX3 analogues can be attributed to the large distortion of their inorganic subnetworks [8,9]. The MA, FA and AZ lead halides crystallize, however, in the ideal cubic perovskites structure at RT (except for MAPbI3, which, at RT, is tetragonal). Therefore, different band gaps of these compounds cannot be attributed to different degrees of octahedral distortion. Our inspection of the crystallographic data shows that MAPbX3 analogues have much smaller lattice parameters than the corresponding FAPbX3 analogues and that these parameters increase slightly when going to AZPbX3. Therefore, it is evident that Eg decreases with the increasing size of the organic cation, thus leading to an increased lattice parameter and Pb-X bond length.
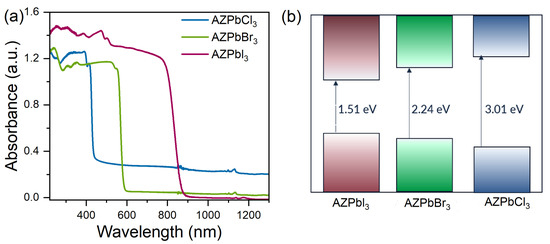
Figure 2.
(a) Diffuse absorption spectra of the investigated samples and (b) scheme of the energy band gap estimated by using the Kubelka–Munk function.
The LT emission spectra of AZPbCl3 under 266 nm consist of an intense and narrow band at 410 nm and a broad band at 548 nm. (Figure 3a). The full width at half maximum (FWHM) of the narrow band is only 44 meV (6 nm) at 80 K. Its position overlaps with the excitonic absorption (Figure 3a). A small Stokes shift, as well as a narrow PL, is characteristic of FE recombination. Recent studies concerning MHyPbCl3 showed the FE band at 362 nm [9], while for MAPbCl3 and FAPbCl3, the excitonic band was located at 404 nm and near 410 nm [13,19,21]. The very large FWHM of the broad band (145 nm, 589 meV) and its large Stokes shift (133 nm) are characteristic features of STEx emission [53,54]. In the case of 3D lead halides perovskites, this type of broadband PL was reported for MHyPbCl3 at 512 nm [9] and for CsPbCl3 at 653 nm [55].
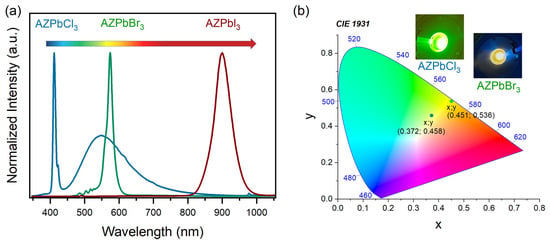
Figure 3.
(a) Photoluminescence spectra of AZPbX3, where X = Cl, Br, and I were recorded at 80 K; and (b) CIE coordinates, together with pictures of glowing samples, of AZPbCl3 and AZPbBr3, respectively.
The PL spectrum of AZPbBr3 is red shifted compared to AZPbCl3 and has a band maximum at 574 nm (Figure 3). Its FWHM is 71 meV (19.9 nm). The observed band is asymmetric and consists of two narrower bands with maxima at 560 nm and 574 nm. The presence of two or even more bands was often reported for 3D lead halide perovskites, and additional bands were assigned either to the presence of trap states (bound excitons, BEs) or presence of domains with different symmetries [20,56,57,58,59,60]. For instance, studies of FAPbBr3 showed that, at RT, the cubic and tetragonal domains show PL at 537 and 557 nm, respectively [58], while studies of MAPbBr3 single crystals revealed that the spectrum at 60 K consists of a band near 544 nm attributed to FE recombination, as well as bands at 551 and 556 nm attributed to BE emission [56]. The small energy separation between the two emission bands of AZPbBr3 and the linear behavior of the emission intensity as a function of the laser power (Supplementary Figure S6) is consistent with assignment of the higher energy band to FE recombination in the cubic phase and the lower energy band either to BE emission from the cubic phase or FE emission from a lower symmetry phase. It is worth adding that the emission of AZPbBr3 is red shifted compared to FAPbBr3, in agreement with the red shift of the excitonic absorption.
Based on the PL spectra, the CIE chromaticity coordinates of the investigated compounds were calculated and presented in Figure 3b, together with photographs of the glowing samples. These CIE coordinates correspond to yellow-green and yellow color for AZPbCl3 and AZPbBr3, respectively.
PL of AZPbI3 is shifted toward the infrared region. The observed band is located at 899 nm, and its FWHM is 86 meV (56.3 nm). The emission intensity of this band changes linearly with the excitation power density (Supplementary Figure S7), allowing us to assign this PL to FE recombination. Similar bands were observed at 830 and 847 nm (at RT) for good-quality MAPbI3 and FAPbI3 single crystals [25,61], and near 780 and 820 nm (at 100 K) for MAPbI3 and FAPbI3 thin films, respectively, [57]. These data from the literature show that PL bands exhibit a red shift with the improving quality of the samples and when MA+ is replaced by FA+. As can be seen, the PL of AZPbI3 is even more red shifted than the PL of the FA analogue.
The temperature-dependent emission spectra of all aziridinium 3D lead halide perovskites show a strong influence of temperature on the position of the PL bands and their intensities (Figure 4). In the case of the chloride, the shape of the FE and STEx bands does not change upon heating, and the emission is quite stable with T0.5 = 150 K (Figure 4a and Figure 5). The energy activation (Ea) for thermal quenching was estimated, using the Boltzmann relation, to be 56 meV (Supplementary Figure S8). The emission of AZPbBr3 is less stable with the Ea of 29 meV and T0.5 of 159 K (Figure 4b and Figure 5 and Supplementary Figure S9). As can be seen, a separation between the two PL bands becomes more pronounced with the increasing temperature. Furthermore, the higher energy band exhibits a significant shift toward higher energies as the temperature increases (Supplementary Figure S10). It can be observed that the PL intensity of AZPbI3 decreases rapidly with an increasing temperature. The energy activation for thermal quenching is 40 meV, while T0.5 = 126 K (Figure 4c and Figure 5 and Supplementary Figure S11). Similar to the bromide analogue, the FE band shifts to higher energies on heating; that is, the band maximum moves from 899 nm at 80 K to 860 nm at 300 K (Supplementary Figure S12).
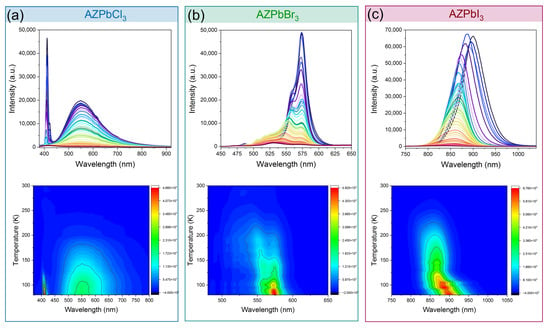
Figure 4.
Temperature-dependent emission spectra and PL intensity contour maps of (a) AZPbCl3 under 266 nm excitation, (b) AZPbBr3 under 450 nm excitation and (c) AZPbl3 under 450 nm excitation.
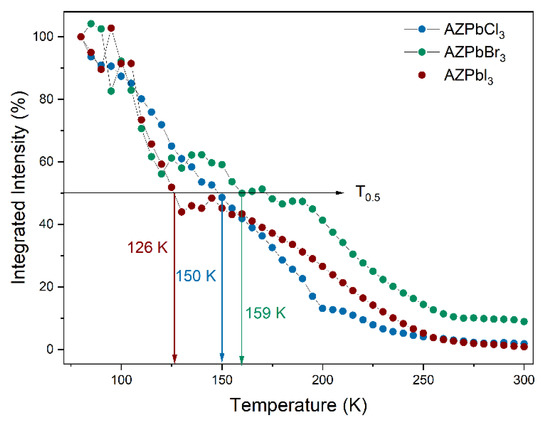
Figure 5.
Integrated emission intensity of the investigated samples at various temperatures.
Studies of AZPbX3 samples show that, in all cases, FE band exhibits shift to higher energies upon heating and that the Ea values are small and fall within the 29–56 meV range. This behavior is similar to that reported for the MAPbX3 and FAPbX3 counterparts, which also showed a significant blue shift of PL upon heating [20,57,62] and small activation energies (105.21, 13–53.35, 21.67, 35 and 45 eV for MAPbCl3, MAPbBr3, FAPbBr3, FAPbI3 and MAPbI3, respectively [20,62,63,64].
3. Experimental Section
3.1. Synthesis of Single Crystals
Due to very high cost of aziridine and its low stability, aziridine was prepared by reacting cheap 2-bromoethylammonium hydrobromide (99%, Sigma-Aldrich, St. Louis, MO, USA) with potassium hydroxide (90%, Sigma-Aldrich), a method that was reported for the first time by Gabriel [65]. First, 15 mmol of KOH was placed in a plastic vial and dissolved in 3 mL of distilled water. Then a solution of 2-bromoethylammonium hydrobromide in 3 mL of water and 6 mL of acetonitrile (AcCN) was added. The vial was closed, shaken a few times and left at RT for 20 h (Scheme 1, Solution A).
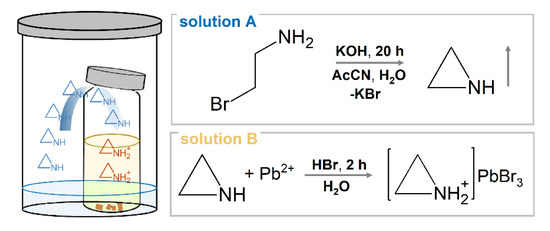
Scheme 1.
Schematic diagram of in situ synthesis of aziridine (Solution A in the large vial), which is used as a diffusing agent necessary for AZPbBr3 crystallization (Solution B in the smaller vial).
In order to synthesize AZPbBr3, 2 mmol of PbBr2 (98%, Sigma-Aldrich) was placed in a small glass vial and dissolved in 8 mL of hydrobromic acid (48%, Sigma-Aldrich). This glass vial was then placed in the plastic vial containing the abovementioned rection mixture, and the lid of the plastic vial was closed (Scheme 1). Orange crystallites were separated from the solution after 2 h (Scheme 1, Solution B).
AZPbI3 was prepared in a similar way, but the solution in a small vial contained 2 mmol of PbI2 (99%, Sigma-Aldrich), 2 mL of hydroiodic acid (57%, Sigma-Aldrich) and 4 mL of acetonitrile. Furthermore, the synthesis was performed at −18 °C, and the black crystallites formed on the glass vial walls were separated from the solution after 20 h.
In the case of AZPbCl3, the small vial contained 0.5 mmol of PbCl2 (98%, Sigma-Aldrich) dissolved in 4 mL of hydrochloric acid (37%, Sigma-Aldrich). Synthesis was performed at RT, and the small crystals, grown at the bottom of the glass vial, were separated after 20 h.
A good match of powder XRD patterns of AZPbBr3 and AZPbI3 with the calculated ones based on the single-crystal data reported by Petrosova et al. [38] confirmed the phase purity of the bulk AZPbBr3 sample and showed some minor impurity phase (peaks at 23.52, 24.82 and 30.63°) in the case of AZPbI3 (see Supplementary Figure S13). However, it should be noticed that the peak calculated at 45.1° and observed by Petrosova et al. as a very weak peak [38] is not observed in our experimental pattern (Supplementary Figure S13). The absence of this peak in our pattern is probably due to a worse signal-to-noise ratio compared to the data reported previously, thus making it difficult to observe very weak peaks. Due to the small number of AZPbCl3 crystals, we do not present their powder XRD pattern, but Raman spectrum confirmed the purity of this phase (Figure 1).
3.2. Materials and Methods
Raman spectra of powdered samples were measured by using a Bruker FT100/S spectrometer (Billerica, MA, USA) with YAG:Nd laser excitation (1064 nm). The spectral resolution was set to be 2 cm−1.
Powder XRD patterns were obtained by using an X’Pert PRO X-ray diffraction system (Malvern Panalytical, Malvern, UK) equipped with a PIXcel ultrafast line detector and Soller slits for CuKα1 radiation (λ = 1.54056 Å). The powders were measured in the reflection mode, and the X-ray tube settings were 30 mA and 40 kV.
The RT absorption spectra of the powdered samples were measured by using a Varian Cary 5E UV–Vis–NIR spectrophotometer (Varian, Palo Alto, CA, USA).
Emission spectra at various temperatures under 266 or 450 nm excitation from a laser diode were measured with the Hamamatsu photonic multichannel analyzer PMA-12, equipped with a BT-CCD linear image sensor (Hamamatsu Photonics, Iwata, Japan). The temperature of the samples was controlled by using a Linkam THMS 600 Heating/Freezing Stage (Linkam, Tadworth, UK).
4. Conclusions
AZPbX3 perovskites were synthesized by using a novel method and characterized by using Raman and optical spectroscopes. The Raman data showed that all bands above 300 cm−1 could be attributed to vibrations of AZ+ cation. Interestingly, similar to other 3D perovskites, one of the bands exhibits very strong dependence on the type of halogen ion, indicating its strong coupling with the inorganic lattice. We assigned this band to the AZ-cage mode. The Raman data also revealed that AZ+ cations form weaker hydrogen bonds with the halide anions than MA+ cations.
The Diffuse reflectance data revealed that the excitonic absorption and PL bands, as well as band gaps of AZPbX3 perovskites, are red shifted compared to their MA and FA counterparts. This behavior is correlated with the size of the organic cation, which increases when going from MA+ to FA+ and then AZ+. The PL intensity of the studied compound strongly decreases upon heating, and the analysis of this behavior allowed us to estimate the energy activation for thermal quenching as 29, 40 and 56 meV for the Br, I and Cl analogue, respectively. These values are comparable to the exciton binding energies reported for the MA and FA lead halide perovskites. Similar to the MA and FA perovskites, the PL of AZPbX3 compounds also exhibited a blue shift upon heating. In conclusion, our studies show that AZPbX3 compounds exhibit very similar properties to those of their MA and FA counterparts. Therefore, the aziridinium analogues and AZ/MA and AZ/FA mixed-cation systems seem to be very attractive materials for light-emitting and solar cell applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27227949/s1, Figure S1: Raman spectra of new and old samples. Figures S2–S4: Energy band gaps. Figure S5: Diffuse reflection spectra of MA, FA and AZ lead halides. Figures S6 and S7: Power dependence of PL intensity for AZPbCl3 and AZPbBr3. Figures S8 and S9: Logarithm of I0/I-1 as a function of 1/kBT for AZPbCl3 and AZPbBr3. Figure S10: Temperature dependence of band center positions for AZPbBr3. Figure S11: Logarithm of I0/I-1 as a function of 1/kBT for AZPbI3. Figure S12: Temperature dependence of band center positions for AZPbI3. Figure S13: XRD patterns. Table S1: Raman wavenumbers (in cm−1) of AZPbX3 (X = Cl, Br, I), together with the proposed assignment.
Author Contributions
Conceptualization, D.S. and M.M.; methodology, D.S. and M.P.; validation, D.S. and M.M.; formal analysis, D.S. and M.P.; investigation, D.S. and M.P.; resources, M.M.; data curation, D.S. and M.P.; writing—original draft preparation, D.S., M.M. and M.P.; writing—review and editing, D.S., M.M. and M.P.; visualization, D.S. and M.P.; supervision, M.M.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Li, X.; Hoffman, J.M.; Kanatzidis, M.G. The 2D Halide Perovskite Rulebook: How the Spacer Influences Everything from the Structure to Optoelectronic Device Efficiency. Chem. Rev. 2021, 121, 2230–2291. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically Diverse and Multifunctional Hybrid Organic–Inorganic Perovskites. Nat. Rev. Mater. 2017, 2, 16099. [Google Scholar] [CrossRef]
- Mączka, M.; Zarȩba, J.K.; Gągor, A.; Stefańska, D.; Ptak, M.; Roleder, K.; Kajewski, D.; Soszyński, A.; Fedoruk, K.; Sieradzki, A. [Methylhydrazinium]2PbBr4, a Ferroelectric Hybrid Organic-Inorganic Perovskite with Multiple Nonlinear Optical Outputs. Chem. Mater. 2021, 33, 2331–2342. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wu, J.; Xu, G.; Yang, X.; Cai, R.; Gong, Q.; Zhu, R.; Huang, W. Perovskite Solar Cells for Space Applications: Progress and Challenges. Adv. Mater. 2021, 33, 2006545. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yuan, Z.; Bai, S.; Gao, F.; Sun, B. Recent Progress toward Perovskite Light-Emitting Diodes with Enhanced Spectral and Operational Stability. Mater. Today Nano 2019, 5, 100028. [Google Scholar] [CrossRef]
- Moseley, O.D.I.; Doherty, T.A.S.; Parmee, R.; Anaya, M.; Stranks, S.D. Halide Perovskites Scintillators: Unique Promise and Current Limitations. J. Mater. Chem. C 2021, 9, 11588–11604. [Google Scholar] [CrossRef]
- Mączka, M.M.; Ptak, M.; Gągor, A.; Stefańska, D.; Zaręba, J.K.; Sieradzki, A. Methylhydrazinium Lead Bromide: Noncentrosymmetric Three-Dimensional Perovskite with Exceptionally Large Framework Distortion and Green Photoluminescence. Chem. Mater. 2020, 32, 1667–1673. [Google Scholar] [CrossRef]
- Maçzka, M.; Gagor, A.; Zareba, J.K.; Stefanska, D.; Drozd, M.; Balciunas, S.; Šimenas, M.; Banys, J.; Sieradzki, A. Three-Dimensional Perovskite Methylhydrazinium Lead Chloride with Two Polar Phases and Unusual Second-Harmonic Generation Bistability above Room Temperature. Chem. Mater. 2020, 32, 4072–4082. [Google Scholar] [CrossRef]
- Drozdowski, D.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Fedoruk, K.; Mączka, M.; Sieradzki, A. Three-Dimensional Methylhydrazinium Lead Halide Perovskites: Structural Changes and Effects on Dielectric, Linear, and Nonlinear Optical Properties Entailed by the Halide Tuning. J. Phys. Chem. C 2022, 126, 1600–1610. [Google Scholar] [CrossRef]
- Ferrando, A.; Martínez Pastor, J.P.; Suárez, I. Toward Metal Halide Perovskite Nonlinear Photonics. J. Phys. Chem. Lett. 2018, 9, 5612–5623. [Google Scholar] [CrossRef] [PubMed]
- Buizza, L.R.V.; Crothers, T.W.; Wang, Z.; Patel, J.B.; Milot, R.L.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Charge-Carrier Dynamics, Mobilities, and Diffusion Lengths of 2D-3D Hybrid Butylammonium-Cesium-Formamidinium Lead Halide Perovskites. Adv. Funct. Mater. 2019, 29, 1902656. [Google Scholar] [CrossRef]
- D’Annibale, A.; Panetta, R.; Tarquini, O.; Colapietro, M.; Quaranta, S.; Cassetta, A.; Barba, L.; Chita, G.; Latini, A. Synthesis, Physico-Chemical Characterization and Structure of the Elusive Hydroxylammonium Lead Iodide Perovskite NH3OHPbI3. Dalt. Trans. 2019, 48, 5397–5407. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.V.; Dick, B.; Rheingold, A.L.; Zhang, C.; Liu, X.; Vardeny, Z.V.; Miller, J.S. Structures of a Complex Hydrazinium Lead Iodide, (N2H5)15Pb3I21, Possessing [Pb2I9]5−, [PbI6]4−, and I − Ions and α- and β-(N2H5)PbI3. Chem.-Eur. J. 2018, 24, 222–229. [Google Scholar] [CrossRef]
- Tian, J.; Cordes, D.B.; Quarti, C.; Beljonne, D.; Slawin, A.M.Z.; Zysman-Colman, E.; Morrison, F.D. Stable 6H Organic−Inorganic Hybrid Lead Perovskite and Competitive Formation of 6H and 3C Perovskite Structure with Mixed A Cations. ACS Appl. Energy Mater. 2019, 2, 5427–5437. [Google Scholar] [CrossRef]
- Smółka, S.; Mączka, M.; Drozdowski, D.; Stefan, D.; Gągor, A.; Sieradzki, A.; Zaręba, J.K.; Ptak, M. Effect of Dimensionality on Photoluminescence and Dielectric Properties of Imidazolium Lead Bromides. Inorg. Chem. 2022, 61, 15225–15238. [Google Scholar] [CrossRef]
- Oku, T. Crystal Structures of Perovskite Halide Compounds Used for Solar Cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Jang, D.M.; Kim, D.H.; Park, K.; Park, J.; Lee, J.W.; Song, J.K. Ultrasound Synthesis of Lead Halide Perovskite Nanocrystals. J. Mater. Chem. C 2016, 4, 10625–10629. [Google Scholar] [CrossRef]
- Nandi, P.; Giri, C.; Swain, D.; Manju, U.; Topwal, D. Room Temperature Growth of CH3NH3PbCl3 Single Crystals by Solvent Evaporation Method. CrystEngComm 2019, 21, 656–661. [Google Scholar] [CrossRef]
- Hsu, H.-P.; Li, L.-C.; Shellaiah, M.; Sun, K.W. Structural, Photophysical, and Electronic Properties of CH3NH3PbCl3 Single Crystals. Sci. Rep. 2019, 9, 13311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, B.; Zheng, K.; Yang, S.; Li, Y.; Deng, W.; He, R. Formamidinium Lead Bromide (FAPbBr3) Perovskite Microcrystals for Sensitive and Fast Photodetectors. Nano-Micro Lett. 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Bartesaghi, D.; Wei, H.; Hutter, E.M.; Huang, J.; Savenije, T.J. Photoluminescence from Radiative Surface States and Excitons in Methylammonium Lead Bromide Perovskites. J. Phys. Chem. Lett. 2017, 8, 4258–4263. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.C.A.; Tiwari, D.; Duchi, M.; Donaldson, P.M.; Clark, I.P.; Fermin, D.J.; Oliver, T.A.A. Investigating the Role of the Organic Cation in Formamidinium Lead Iodide Perovskite Using Ultrafast Spectroscopy. J. Phys. Chem. Lett 2018, 9, 35. [Google Scholar] [CrossRef]
- Chen, C.-X.; Wang, J.; Gao, M.; Shi, D. Distinctive Bulk-and Surface-Specific Photoluminescence and Photocarrier Dynamics in CH3NH3PbI3 Perovskite. Cryst. Growth Des. 2020, 17, 51. [Google Scholar] [CrossRef]
- Schilcher, M.J.; Robinson, P.J.; Abramovitch, D.J.; Tan, L.Z.; Rappe, A.M.; Reichman, D.R.; Egger, D.A. The Significance of Polarons and Dynamic Disorder in Halide Perovskites. ACS Energy Lett. 2021, 6, 2162–2173. [Google Scholar] [CrossRef]
- Kontos, A.G.; Manolis, G.K.; Kaltzoglou, A.; Palles, D.; Kamitsos, E.I.; Kanatzidis, M.G.; Falaras, P. Halogen–NH2+ Interaction, Temperature-Induced Phase Transition, and Ordering in (NH2CHNH2)PbX3 (X = Cl, Br, I) Hybrid Perovskites. J. Phys. Chem. C 2020, 124, 8479–8487. [Google Scholar] [CrossRef]
- Herz, L.M. How Lattice Dynamics Moderate the Electronic Properties of Metal-Halide Perovskites. J. Phys. Chem. Lett. 2018, 9, 6853–6863. [Google Scholar] [CrossRef]
- Nakada, K.; Matsumoto, Y.; Shimoi, Y.; Yamada, K.; Furukawa, Y. Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I, Br). Molecules 2019, 24, 626. [Google Scholar] [CrossRef]
- Ibaceta-Jaña, J.; Muydinov, R.; Rosado, P.; Mirhosseini, H.; Chugh, M.; Nazarenko, O.; Dirin, D.N.; Heinrich, D.; Wagner, M.R.; Kühne, T.D.; et al. Vibrational Dynamics in Lead Halide Hybrid Perovskites Investigated by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 5604–5614. [Google Scholar] [CrossRef]
- Ruan, S.; McMeekin, D.P.; Fan, R.; Webster, N.A.S.; Ebendorff-Heidepriem, H.; Cheng, Y.B.; Lu, J.; Ruan, Y.; McNeill, C.R. Raman Spectroscopy of Formamidinium-Based Lead Halide Perovskite Single Crystals. J. Phys. Chem. C 2020, 124, 2265–2272. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Goñi, A.R.; Frost, J.M.; Skelton, J.; Brivio, F.; Rodríguez-Martínez, X.; Weber, O.J.; Pallipurath, A.; Alonso, M.I.; Campoy-Quiles, M.; et al. Dynamic Disorder, Phonon Lifetimes, and the Assignment of Modes to the Vibrational Spectra of Methylammonium Lead Halide Perovskites. Phys. Chem. Chem. Phys. 2016, 18, 27051–27066. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Ptak, M. Temperature-Dependent Raman Studies of FAPbBr3 and MAPbBr3 Perovskites: Effect of Phase Transitions on Molecular Dynamics and Lattice Distortion. Solids 2022, 3, 111–121. [Google Scholar] [CrossRef]
- Mączka, M.; Zienkiewicz, J.A.; Ptak, M. Comparative Studies of Phonon Properties of Three-Dimensional Hybrid Organic−Inorganic Perovskites Comprising Methylhydrazinium, Methylammonium, and Formamidinium Cations. J. Phys. Chem. C 2022, 2022, 4056. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Vasconcelos, D.L.M.; Giriunas, L.; Freire, P.T.C.; Bertmer, M.; Banys, J.; Simenas, M. NMR and Raman Scattering Studies of Temperature-and Pressure-Driven Phase Transitions in CH3NH2NH2PbCl3 Perovskite. J. Phys. Chem. C 2020, 124, 26999–27008. [Google Scholar] [CrossRef]
- Sendner, M.; Nayak, P.K.; Egger, D.A.; Beck, S.; Müller, C.; Epding, B.; Kowalsky, W.; Kronik, L.; Snaith, H.J.; Pucci, A.; et al. Optical Phonons in Methylammonium Lead Halide Perovskites and Implications for Charge Transport. Mater. Horiz. 2016, 3, 613–620. [Google Scholar] [CrossRef]
- Schuck, G.; Többens, D.M.; Koch-Müller, M.; Efthimiopoulos, I.; Schorr, S. Infrared Spectroscopic Study of Vibrational Modes across the Orthorhombic–Tetragonal Phase Transition in Methylammonium Lead Halide Single Crystals. J. Phys. Chem. C 2018, 122, 5227–5237. [Google Scholar] [CrossRef]
- Petrosova, H.R.; Kucheriv, O.I.; Shova, S.; Gural’skiy, I.A. Aziridinium Cation Templating 3D Lead Halide Hybrid Perovskites. Chem. Commun. 2022, 58, 5745–5748. [Google Scholar] [CrossRef]
- Pérez-Osorio, M.A.; Milot, R.L.; Filip, M.R.; Patel, J.B.; Herz, L.M.; Johnston, M.B.; Giustino, F. Vibrational Properties of the Organic-Inorganic Halide Perovskite CH3NH3PbI3 from Theory and Experiment: Factor Group Analysis, First-Principles Calculations, and Low-Temperature Infrared Spectra. J. Phys. Chem. C 2015, 119, 25703–25718. [Google Scholar] [CrossRef]
- Ledinský, M.; Löper, P.; Niesen, B.; Holovský, J.; Moon, S.J.; Yum, J.H.; De Wolf, S.; Fejfar, A.; Ballif, C. Raman Spectroscopy of Organic-Inorganic Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 401–406. [Google Scholar] [CrossRef]
- Quarti, C.; Grancini, G.; Mosconi, E.; Bruno, P.; Ball, J.M.; Lee, M.M.; Snaith, H.J.; Petrozza, A.; Angelis, F. De The Raman Spectrum of the CH3NH3PbI3 Hybrid Perovskite: Interplay of Theory and Experiment. J. Phys. Chem. Lett. 2014, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Mattoni, A.; Filippetti, A.; Saba, M.I.; Caddeo, C.; Delugas, P. Temperature Evolution of Methylammonium Trihalide Vibrations at the Atomic Scale. J. Phys. Chem. Lett. 2016, 7, 529–535. [Google Scholar] [CrossRef]
- Ciupa-Litwa, A.; Ptak, M.; Kucharska, E.; Hanuza, J.; Mączka, M. Vibrational Properties and DFT Calculations of Perovskite-Type Methylhydrazinium Manganese Hypophosphite. Molecules 2020, 25, 5215. [Google Scholar] [CrossRef] [PubMed]
- Potts, W.J. The Fundamental Vibration Frequencies of Ethylene Oxide and Ethylene Imine. Spectrochim. Acta 1965, 21, 511–527. [Google Scholar] [CrossRef]
- Mitchell, R.W.; Burr, J.C.; Merritt, J.A. Vibrational Spectra of Normal, Imine-Deuterated and 15N Ethyleneimine. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 195–207. [Google Scholar] [CrossRef]
- Ngom, M.; Kwabia-Tchana, F.; Perrin, A.; Guillemin, J.-C.; Flaud, J.-M.; Demaison, J.; Ngom, E.A. New Vibrational Assignments for the ν 1 to ν 17 Vibrational Modes of Aziridine and First Analysis of the High-Resolution Infrared Spectrum of Aziridine between 720 and 1050 cm−1. Mol. Phys. 2011, 109, 2153–2161. [Google Scholar] [CrossRef][Green Version]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Techn. Phys. 1931, 12, 593–601. [Google Scholar]
- Ptak, M.; Sieradzki, A.; Šimėnas, M.; Maczka, M. Molecular Spectroscopy of Hybrid Organic–Inorganic Perovskites and Related Compounds. Coord. Chem. Rev. 2021, 448, 214180. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, S.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. Development of Formamidinium Lead Iodide-Based Perovskite Solar Cells: Efficiency and Stability. Chem. Sci. 2022, 13, 2167–2183. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Correa-Baena, J.-P.; Pazoki, M.; Saliba, M.; Schenk, K.; Grä, M.; Hagfeldt, A. Exploration of the Compositional Space for Mixed Lead Halogen Perovskites for High Efficiency Solar Cells. Energy Environ. Sci 2016, 9, 1706–1724. [Google Scholar] [CrossRef]
- Simenas, M.; Balciunas, S.; Wilson, J.N.; Svirskas, S.; Kinka, M.; Garbaras, A.; Kalendra, V.; Gagor, A.; Szewczyk, D.; Sieradzki, A.; et al. Suppression of Phase Transitions and Glass Phase Signatures in Mixed Cation Halide Perovskites. Nat. Commun. 2020, 11, 5103. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y. Luminescence Spectroscopy of Lead-Halide Perovskites: Materials Properties and Application as Photovoltaic Devices. J. Mater. Chem. C 2017, 5, 3427–3437. [Google Scholar] [CrossRef]
- Ji, C.; Wang, S.; Li, L.; Sun, Z.; Hong, M.; Luo, J. The First 2D Hybrid Perovskite Ferroelectric Showing Broadband White-Light Emission with High Color Rendering Index. Adv. Funct. Mater. 2019, 29, 1805038. [Google Scholar] [CrossRef]
- Smith, M.D.; Connor, B.A.; Karunadasa, H.I. Tuning the Luminescence of Layered Halide Perovskites. Chem. Rev. 2019, 119, 3104–3139. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kobayashi, T.; Iwanaga, M.; Watanabe, M. Exciton Dynamics Related with Phase Transitions in CsPbCl3 Single Crystals. J. Lumin. 2001, 94–95, 255–259. [Google Scholar] [CrossRef]
- Chen, C.; Hu, X.; Lu, W.; Chang, S.; Shi, L.; Li, L.; Zhong, H.; Han, J.-B. Elucidating the Phase Transitions and Temperature-Dependent Photoluminescence of MAPbBr3 Single Crystal. J. Phys. D Appl. Phys. 2018, 51, 045105. [Google Scholar] [CrossRef]
- Wright, A.D.; Verdi, C.; Milot, R.L.; Eperon, G.E.; Pérez-Osorio, M.A.; Snaith, H.J.; Giustino, F.; Johnston, M.B.; Herz, L.M. Electron–Phonon Coupling in Hybrid Lead Halide Perovskites. Nat. Commun. 2016, 7, 11755. [Google Scholar] [CrossRef]
- Ghosh, S.; Shi, Q.; Pradhan, B.; Kumar, P.; Wang, Z.; Acharya, S.; Pal, S.K.; Pullerits, T.; Karki, K.J. Phonon Coupling with Excitons and Free Carriers in Formamidinium Lead Bromide Perovskite Nanocrystals. J. Phys. Chem. Lett. 2018, 9, 4245–4250. [Google Scholar] [CrossRef]
- Galkowski, K.; Mitioglu, A.A.; Surrente, A.; Yang, Z.; Maude, D.K.; Kossacki, P.; Eperon, G.E.; Wang, J.T.W.; Snaith, H.J.; Plochocka, P.; et al. Spatially Resolved Studies of the Phases and Morphology of Methylammonium and Formamidinium Lead Tri-Halide Perovskites. Nanoscale 2017, 9, 3222–3230. [Google Scholar] [CrossRef]
- Wang, K.H.; Li, L.C.; Shellaiah, M.; Sun, K.W. Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals. Sci. Rep. 2017, 7, 13643. [Google Scholar] [CrossRef]
- Zhumekenov, A.A.; Saidaminov, M.I.; Haque, M.A.; Alarousu, E.; Sarmah, S.P.; Murali, B.; Dursun, I.; Miao, X.H.; Abdelhady, A.L.; Wu, T.; et al. Formamidinium Lead Halide Perovskite Crystals with Unprecedented Long Carrier Dynamics and Diffusion Length. ACS Energy Lett. 2016, 1, 32–37. [Google Scholar] [CrossRef]
- Dai, J.; Zheng, H.; Zhu, C.; Lu, J.; Xu, C. Comparative Investigation on Temperature-Dependent Photoluminescence of CH3NH3PbBr3 and CH(NH2)2PbBr3 Microstructures. J. Mater. Chem. C 2016, 4, 4408–4413. [Google Scholar] [CrossRef]
- Lozhkina, O.A.; Yudin, V.I.; Murashkina, A.A.; Shilovskikh, V.V.; Davydov, V.G.; Kevorkyants, R.; Emeline, A.V.; Kapitonov, Y.V.; Bahnemann, D.W. Low Inhomogeneous Broadening of Excitonic Resonance in MAPbBr3 Single Crystals. J. Phys. Chem. Lett. 2018, 9, 302–305. [Google Scholar] [CrossRef]
- Bokdam, M.; Sander, T.; Stroppa, A.; Picozzi, S.; Sarma, D.D.; Franchini, C.; Kresse, G. Role of Polar Phonons in the Photo Excited State of Metal Halide Perovskites. Sci. Rep. 2016, 6, 28618. [Google Scholar] [CrossRef]
- Jarzyński, S.; Leśniak, S. Recent Progress in the Synthesis of Aziridine Derivatives (Microreview). Chem. Heterocycl. Compd. 2016, 52, 353–355. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).