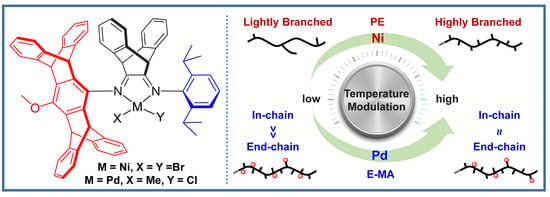
Unsymmetrical Strategy on α-Diimine Nickel and Palladium Mediated Ethylene (Co)Polymerizations
Abstract
1. Introduction
2. Results
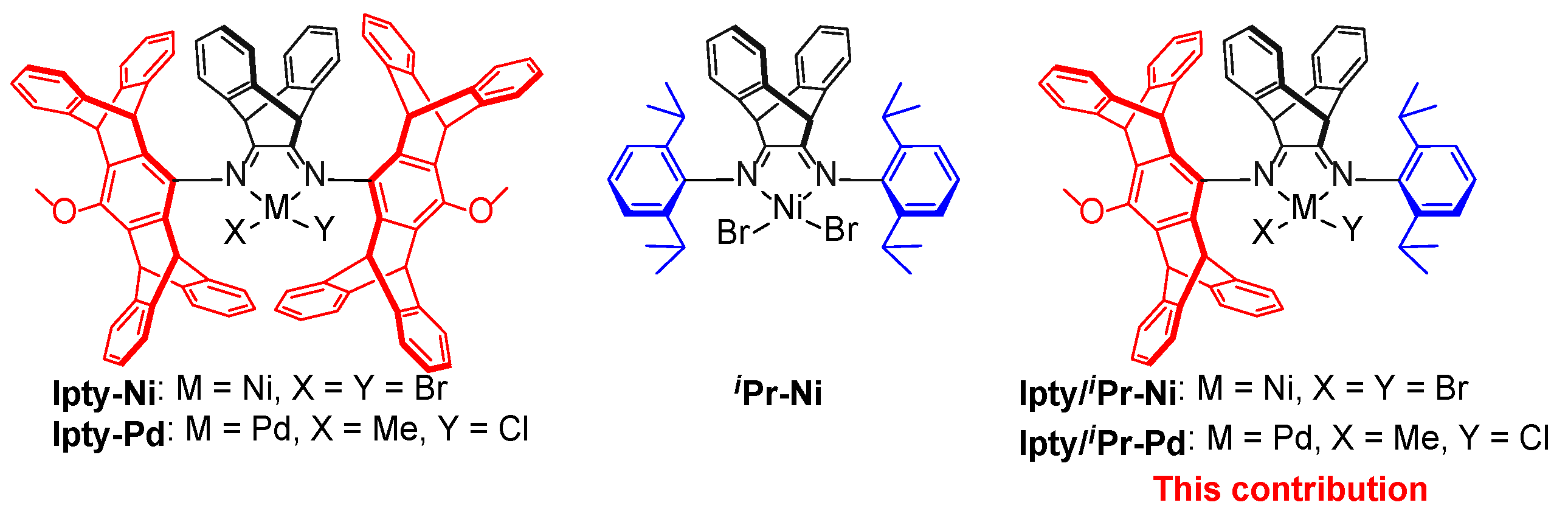
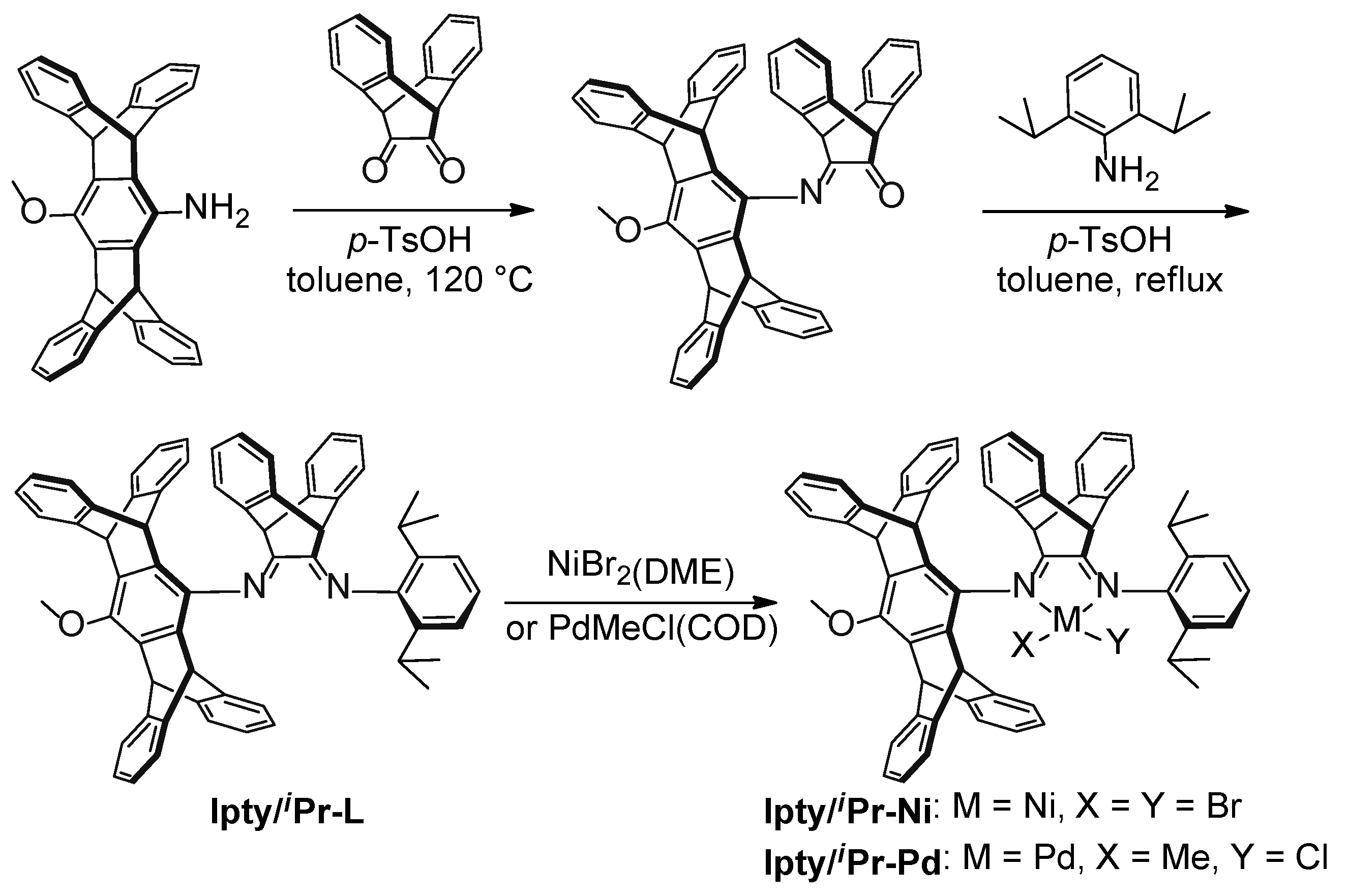
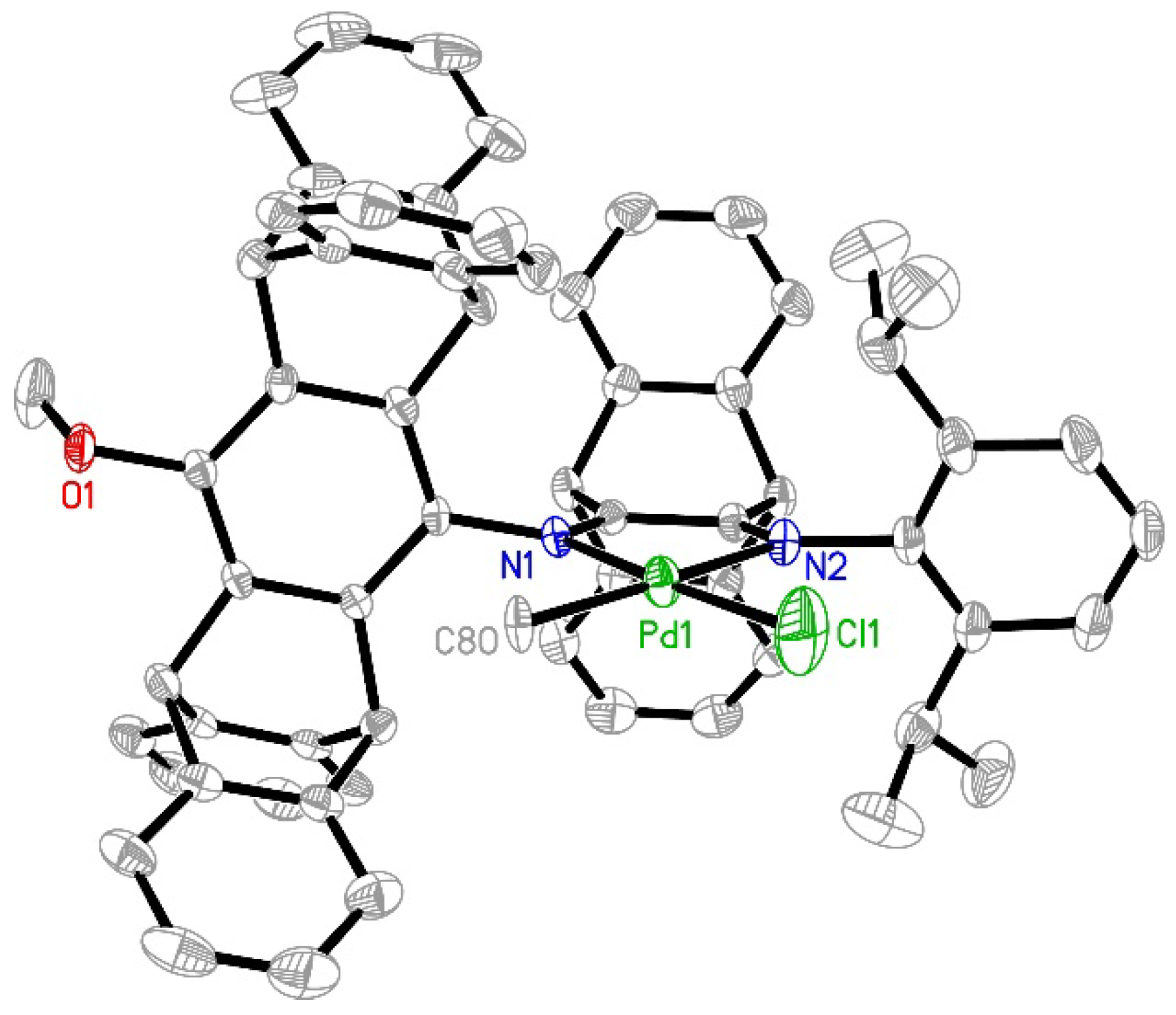
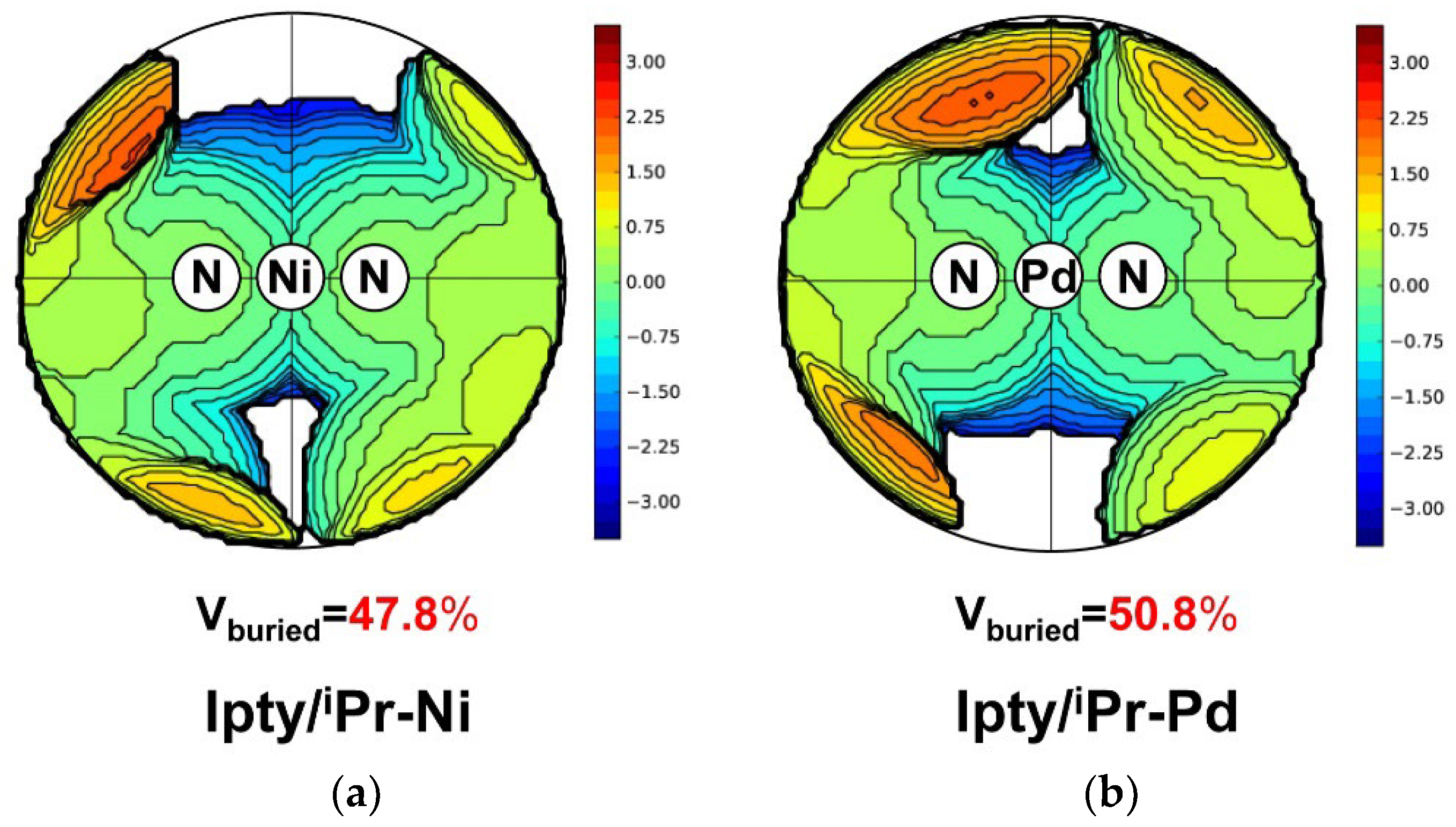
2.1. Synthesis and Characterization of Ipty/iPr-Ni and Ipty/iPr-Pd
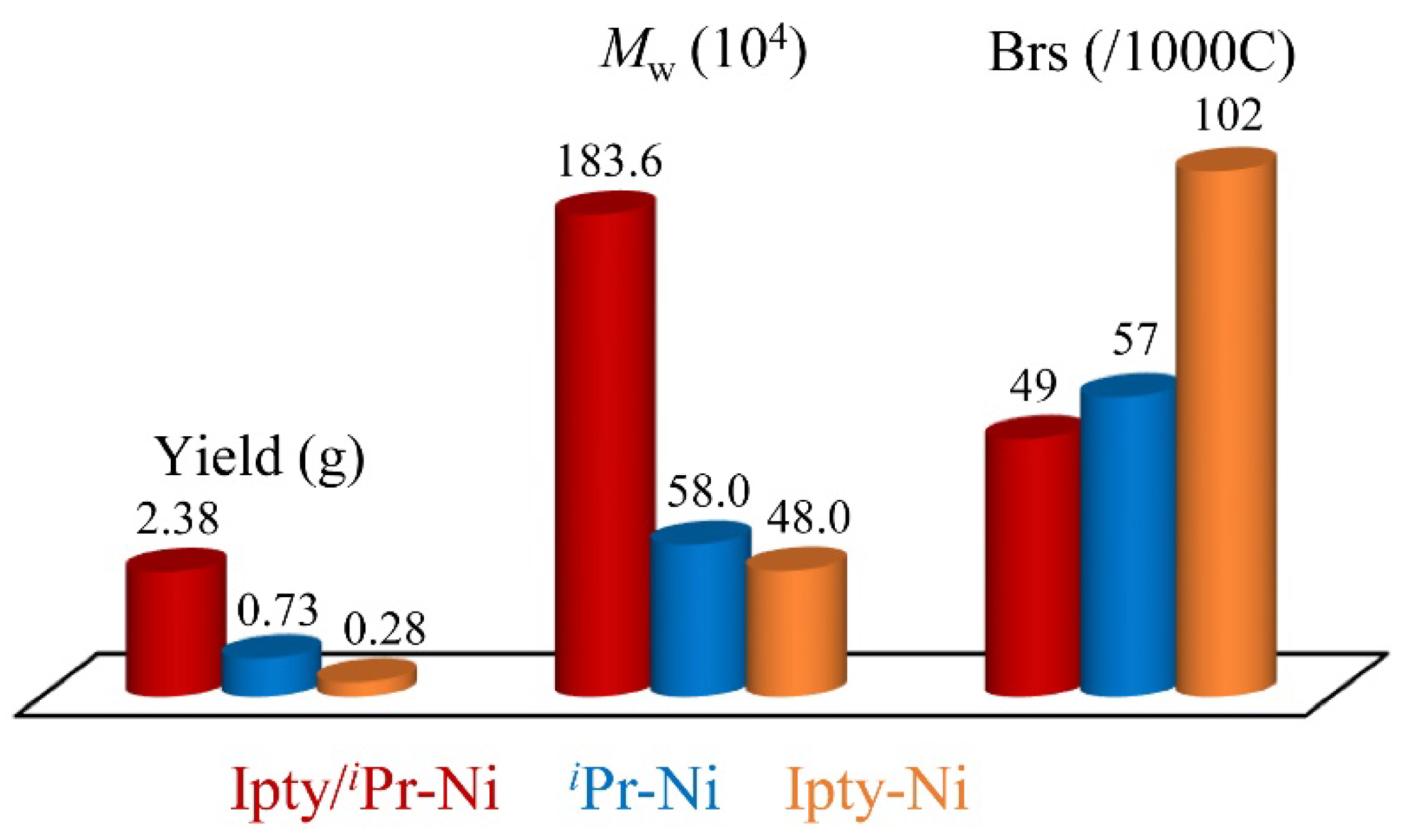
2.2. Ethylene Polymerizaiton by Ipty/iPr-Ni
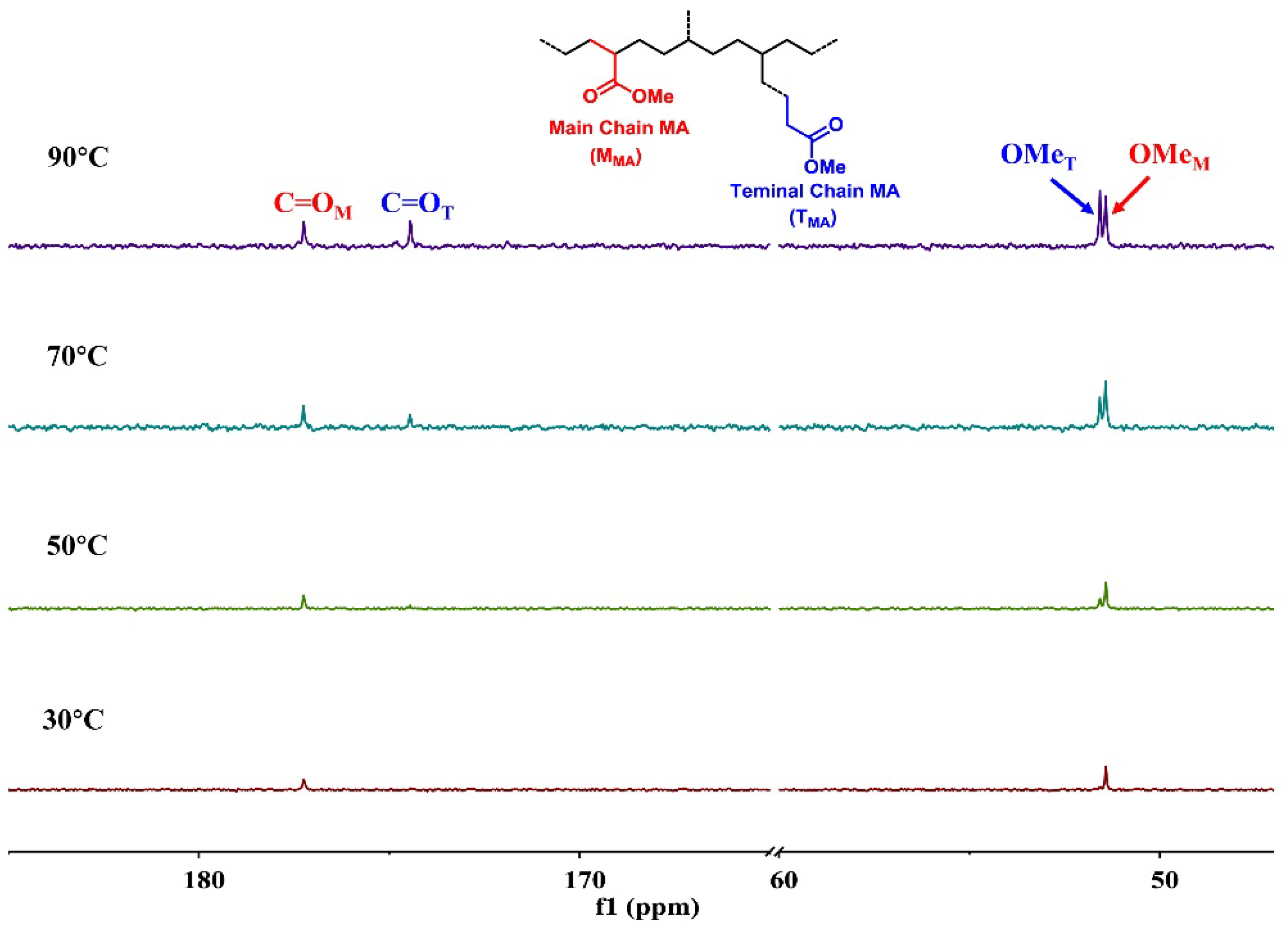
2.3. Copolymerizaiton of Ethylene and Polar Monomer by Ipty/iPr-Ni
2.4. Mechanical Properties of (Co)Polymers Generatedr by Ipty/iPr-Ni
2.5. Ethylene Polymerizaiton by Ipty/iPr-Pd
2.6. Copolymerizaiton of Ethylene and Polar Monomer by Ipty/iPr-Pd
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Ligands and Catalysts
3.2.1. Synthesis of Pentiptycene Aminoanisole
3.2.2. Synthesis of Ligand Ipty/iPr-L
3.2.3. Synthesis of the Nickel Catalyst Ipty/iPr-Ni
3.2.4. Synthesis of the Nickel Catalyst Ipty/iPr-Pd
3.3. X-ray Diffraction
3.4. General Procedures for the Polymerizations
3.4.1. A General Procedure for Ethylene Polymerization
3.4.2. A General Procedure for the Copolymerization of Ethylene with Polar Monomer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mecking, S.; Johnson, L.K.; Wang, L.; Brookhart, M. Mechanistic Studies of the Palladium-Catalyzed Copolymerization of Ethylene and α-Olefins with Methyl Acrylate. J. Am. Chem. Soc. 1998, 120, 888–899. [Google Scholar] [CrossRef]
- Johnson, L.K.; Mecking, S.; Brookhart, M. Copolymerization of Ethylene and Propylene with Functionalized Vinyl Monomers by Palladium(II) Catalysts. J. Am. Chem. Soc. 1996, 118, 267–268. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and α-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Wu, R.; Wu, W.K.; Stieglitz, L.; Gaan, S.; Rieger, B.; Heuberger, M. Recent Advances on α-Diimine Ni and Pd Complexes for Catalyzed Ethylene (Co)Polymerization: A Comprehensive Review. Coord. Chem. Rev. 2023, 474, 214844. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Hu, X.; Wang, C.; Jian, Z. Advances on Controlled Chain Walking and Suppression of Chain Transfer in Catalytic Olefin Polymerization. ACS Catal. 2022, 12, 14304–14320. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring Ethylene/Polar Vinyl Monomer Copolymerizations Using Ni and Pd α-Diimine Catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Hu, X.; Kang, X.; Jian, Z. Suppression of Chain Transfer at High Temperature in Catalytic Olefin Polymerization. Angew. Chem. Int. Ed. 2022, 61, e202207363. [Google Scholar] [CrossRef]
- Hu, X.; Kang, X.; Zhang, Y.; Jian, Z. Facile Access to Polar Functionalized Ultrahigh Molecular Weight Polyethylene at Ambient Conditions. CCS Chem. 2022, 4, 1680–1694. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Mecking, S.; Jian, Z. Ultrahigh Branching of Main-Chain-Functionalized Polyethylenes by Inverted Insertion Selectivity. Angew. Chem. Int. Ed. 2020, 59, 14296–14302. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, Y.; Kou, S.; Jian, Z. A Concerted Double-Layer Steric Strategy Enables an Ultra-Highly Active Nickel Catalyst to Access Ultrahigh Molecular Weight Polyethylenes. J. Catal. 2020, 390, 30–36. [Google Scholar] [CrossRef]
- Guo, L.; Sun, W.; Li, S.; Xu, G.; Dai, S. Bulky yet Flexible Substituents in Insertion Polymerization with α-Diimine Nickel and Palladium Systems. Polym. Chem. 2019, 10, 4866–4871. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Luo, Y.; Chen, C. A Second-Coordination-Sphere Strategy to Modulate Nickel- and Palladium-Catalyzed Olefin Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 11604–11609. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C. Direct Synthesis of Functionalized High-Molecular-Weight Polyethylene by Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2016, 55, 13281–13285. [Google Scholar] [CrossRef]
- Takano, S.; Takeuchi, D.; Osakada, K.; Akamatsu, N.; Shishido, A. Dipalladium Catalyst for Olefin Polymerization: Introduction of Acrylate Units into the Main Chain of Branched Polyethylene. Angew. Chem. Int. Ed. 2014, 53, 9246–9250. [Google Scholar] [CrossRef]
- Allen, K.E.; Campos, J.; Daugulis, O.; Brookhart, M. Living Polymerization of Ethylene and Copolymerization of Ethylene/Methyl Acrylate Using “Sandwich” Diimine Palladium Catalysts. ACS Catal. 2015, 5, 456–464. [Google Scholar] [CrossRef]
- Vaidya, T.; Klimovica, K.; LaPointe, A.M.; Keresztes, I.; Lobkovsky, E.B.; Daugulis, O.; Coates, G.W. Secondary Alkene Insertion and Precision Chain-Walking: A New Route to Semicrystalline “Polyethylene” from α-Olefins by Combining Two Rare Catalytic Events. J. Am. Chem. Soc. 2014, 136, 7213–7216. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A Robust Ni(II) α-Diimine Catalyst for High Temperature Ethylene Polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef]
- Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti, O.; Rieger, B. New Nickel(II) Diimine Complexes and the Control of Polyethylene Microstructure by Catalyst Design. J. Am. Chem. Soc. 2007, 129, 9182–9191. [Google Scholar] [CrossRef]
- Camacho, D.H.; Salo, E.V.; Ziller, J.W.; Guan, Z. Cyclophane-Based Highly Active Late-Transition-Metal Catalysts for Ethylene Polymerization. Angew. Chem. Int. Ed. 2004, 43, 1821–1825. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Jian, Z. Comprehensive Studies of Ligand Electronic Effect on Unsymmetrical α-Diimine Nickel(II) Promoted Ethylene (Co)Polymerizations. Polym. Chem. 2020, 11, 4005–4012. [Google Scholar] [CrossRef]
- Li, S.; Xu, G.; Dai, S. A Remote Nonconjugated Electron Effect in Insertion Polymerization with α-Diimine Nickel and Palladium Species. Polym. Chem. 2020, 11, 2692–2699. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Chen, C. Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization. Polymers 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Popeney, C.S.; Guan, Z. Effect of Ligand Electronics on the Stability and Chain Transfer Rates of Substituted Pd(II) α-Diimine Catalysts. Macromolecules 2010, 43, 4091–4097. [Google Scholar] [CrossRef]
- Popeney, C.; Guan, Z. Ligand Electronic Effects on Late Transition Metal Polymerization Catalysts. Organometallics 2005, 24, 1145–1155. [Google Scholar] [CrossRef]
- Zhong, L.; Zheng, H.; Du, C.; Du, W.; Liao, G.; Cheung, C.S.; Gao, H. Thermally Robust α-Diimine Nickel and Palladium Catalysts with Constrained Space for Ethylene (Co)Polymerizations. J. Catal. 2020, 384, 208–217. [Google Scholar] [CrossRef]
- Dall’Anese, A.; Rosar, V.; Cusin, L.; Montini, T.; Balducci, G.; D’Auria, I.; Pellecchia, C.; Fornasiero, P.; Felluga, F.; Milani, B. Palladium-Catalyzed Ethylene/Methyl Acrylate Copolymerization: Moving from the Acenaphthene to the Phenanthrene Skeleton of α-Diimine Ligands. Organometallics 2019, 38, 3498–3511. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.; Liang, G.; Gao, H.; Wu, Q. Enhancing Thermal Stability and Living Fashion in α-Diimine-Nickel-Catalyzed (Co)Polymerization of Ethylene and Polar Monomer by Increasing the Steric Bulk of Ligand Backbone. Macromolecules 2017, 50, 2675–2682. [Google Scholar] [CrossRef]
- Long, B.K.; Eagan, J.M.; Mulzer, M.; Coates, G.W. Semi-Crystalline Polar Polyethylene: Ester-Functionalized Linear Polyolefins Enabled by a Functional-Group-Tolerant, Cationic Nickel Catalyst. Angew. Chem. Int. Ed. 2016, 55, 7106–7110. [Google Scholar] [CrossRef]
- Liu, J.; Chen, D.; Wu, H.; Xiao, Z.; Gao, H.; Zhu, F.; Wu, Q. Polymerization of α-Olefins Using a Camphyl α-Diimine Nickel Catalyst at Elevated Temperature. Macromolecules 2014, 47, 3325–3331. [Google Scholar] [CrossRef]
- Peng, D.; Chen, C. Photoresponsive Palladium and Nickel Catalysts for Ethylene Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2021, 60, 22195–22200. [Google Scholar] [CrossRef]
- Wang, G.; Peng, D.; Chen, C. Interplay of Supramolecular Chemistry and Photochemistry with Palladium-Catalyzed Ethylene Polymerization. CCS Chem. 2020, 2, 2025–2034. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, C. Accessing Multiple Catalytically Active States in Redox-Controlled Olefin Polymerization. ACS Catal. 2017, 7, 7490–7494. [Google Scholar] [CrossRef]
- Anderson, W.C., Jr.; Rhinehart, J.L.; Tennyson, A.G.; Long, B.K. Redox-Active Ligands: An Advanced Tool to Modulate Polyethylene Microstructure. J. Am. Chem. Soc. 2016, 138, 774–777. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, S.; Zhang, W.; Chen, C. Systematic Investigations of Ligand Steric Effects on α-Diimine Palladium Catalyzed Olefin Polymerization and Copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Ma, Y.; Han, M.; Solan, G.A.; Yang, W.; Liang, T.; Sun, W.-H. Trifluoromethoxy-Substituted Nickel Catalysts for Producing Highly Branched Polyethylenes: Impact of Solvent, Activator and N,N′-Ligand on Polymer Properties. Polym. Chem. 2022, 13, 1040–1058. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. Plastomeric-Like Polyethylenes Achievable Using Thermally Robust N,N′-Nickel Catalysts Appended with Electron Withdrawing Difluorobenzhydryl and Nitro Groups. Dalton Trans. 2019, 48, 1878–1891. [Google Scholar] [CrossRef]
- Du, S.; Kong, S.; Shi, Q.; Mao, J.; Guo, C.; Yi, J.; Liang, T.; Sun, W.-H. Enhancing the Activity and Thermal Stability of Nickel Complex Precatalysts Using 1-[2,6-Bis(bis(4-fluorophenyl)methyl)-4-methyl phenylimino]-2-aryliminoacenaphthylene Derivatives. Organometallics 2015, 34, 582–590. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, G.; Wang, H.; Jing, K.; Dai, S. A Dual Steric Enhancement Strategy in α-Diimine Nickel and Palladium Catalysts for Ethylene Polymerization and Copolymerization. Organometallics 2022, 41, 124–132. [Google Scholar] [CrossRef]
- Hai, Z.; Lu, Z.; Li, S.; Cao, Z.-Y.; Dai, S. The Synergistic Effect of Rigid and Flexible Substituents on Insertion Polymerization with α-Diimine Nickel and Palladium Catalysts. Polym. Chem. 2021, 12, 4643–4653. [Google Scholar] [CrossRef]
- Guo, L.; Liu, W.; Li, K.; Sun, M.; Sun, W.; Zhao, L.; Jiang, G.; Peng, H.; Liu, Z.; Dai, S. Synthesis of Functional and Hyperbranched Ethylene Oligomers Using Unsymmetrical α-Diimine Palladium Catalysts. Eur. Polym. J. 2019, 115, 185–192. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Zhang, Y.; Jian, Z. Unsymmetrical Strategy Makes Significant Differences in α-Diimine Nickel and Palladium Catalyzed Ethylene (Co)Polymerizations. ChemCatChem 2020, 12, 2497–2505. [Google Scholar] [CrossRef]
- Lu, Z.; Liao, Y.; Fan, W.; Dai, S. Efficient Suppression of the Chain Transfer Reaction in Ethylene Coordination Polymerization with Dibenzosuberyl Substituents. Polym. Chem. 2022, 13, 4090–4099. [Google Scholar] [CrossRef]
- Lu, W.; Liao, Y.; Dai, S. Facile Access to Ultra-Highly Branched Polyethylenes Using Hybrid “Sandwich” Ni(II) and Pd(II) Catalysts. J. Catal. 2022, 411, 54–61. [Google Scholar] [CrossRef]
- Wang, X.; Dong, B.; Yang, Q.; Liu, H.; Zhang, C.; Zhang, X. α-Diimine Nickel Complexes Bearing Axially Bulky Terphenyl and Equatorially Bulky Dibenzobarrelene Groups: Synthesis, Characterization and Olefin Polymerization Studies. Polym. Chem. 2020, 11, 6783–6793. [Google Scholar] [CrossRef]
- Rosar, V.; Montini, T.; Balducci, G.; Zangrando, E.; Fornasiero, P.; Milani, B. Palladium-Catalyzed Ethylene/Methyl Acrylate Co-Oligomerization: The Effect of a New Nonsymmetrical α-Diimine with the 1,4-Diazabutadiene Skeleton. ChemCatChem 2017, 9, 3402–3411. [Google Scholar] [CrossRef]
- Yuan, S.; Yue, E.; Wen, C.; Sun, W.-H. Synthesis, Characterization, and Ethylene Polymerization of 1-[2,4-Bis(bis(4-fluorophenyl)methyl)naphthylimino]-2-aryliminoacenaphthylnickel Bromides: Influences of Polymerization Parameters on Polyethylenes. RSC Adv. 2016, 6, 7431–7438. [Google Scholar] [CrossRef]
- Li, S.; Dai, S. 8-Arylnaphthyl Substituent Retarding Chain Transfer in Insertion Polymerization with Unsymmetrical α-Diimine Systems. Polym. Chem. 2020, 11, 7199–7206. [Google Scholar] [CrossRef]
- Xia, J.; Kou, S.; Mu, H.; Jian, Z. Slow-Chain-Walking Polymerization of Ethylene and Highly Chain-Straightening Polymerization of 1-Hexene to Access Semicrystalline Polyolefins. Eur. Polym. J. 2022, 166, 111022. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Zhang, C.; Tang, T.; Zhang, X. Propylene Homopolymerization and Copolymerization with Ethylene by Acenaphthene-Based α-Diimine Nickel Complexes to Access EPR-Like Elastomers. Polym. Chem. 2021, 12, 6307–6318. [Google Scholar] [CrossRef]
- Ma, X.; Hu, X.; Zhang, Y.; Mu, H.; Cui, L.; Jian, Z. Preparation and in situ Chain-End-Functionalization of Branched Ethylene Oligomers by Monosubstituted α-Diimine Nickel Catalysts. Polym. Chem. 2019, 10, 2596–2607. [Google Scholar] [CrossRef]
- Fang, J.; Sui, X.; Li, Y.; Chen, C. Synthesis of Polyolefin Elastomers from Unsymmetrical α-Diimine Nickel Catalyzed Olefin Polymerization. Polym. Chem. 2018, 9, 4143–4149. [Google Scholar] [CrossRef]
- Sui, X.; Hong, C.; Pang, W.; Chen, C. Unsymmetrical α-Diimine Palladium Catalysts and Their Properties in Olefin (Co)Polymerization. Mater. Chem. Front. 2017, 1, 967–972. [Google Scholar] [CrossRef]
- Cherian, A.E.; Rose, J.M.; Lobkovsky, E.B.; Coates, G.W. A C2-Symmetric, Living α-Diimine Ni(II) Catalyst: Regioblock Copolymers from Propylene. J. Am. Chem. Soc. 2005, 127, 13770–13771. [Google Scholar] [CrossRef]
- Dai, S.; Li, G.; Lu, W.; Liao, Y.; Fan, W. Suppression of Chain Transfer via a Restricted Rotation Effect of Dibenzosuberyl Substituents in Polymerization Catalysis. Polym. Chem. 2021, 12, 3240–3249. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, C.-Q.; Hu, X.-Q.; Xia, Y.; Chi, Y.; Zhang, Y.-X.; Jian, Z.-B. Benzosuberyl Substituents as a “Sandwich-Like” Function in Olefin Polymerization Catalysis. Chin. J. Polym. Sci. 2021, 39, 984–993. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, Z. Polar Additive Triggered Chain Walking Copolymerization of Ethylene and Fundamental Polar Monomers. Polym. Chem. 2022, 13, 4966–4972. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, X.; Jian, Z. Selective Branch Formation in Ethylene Polymerization to Access Precise Ethylene-Propylene Copolymers. Nat. Commun. 2022, 13, 725. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, Z. Polar Additive Triggered Branching Switch and Block Polyolefin Topology in Living Ethylene Polymerization. Macromolecules 2021, 54, 3191–3196. [Google Scholar] [CrossRef]
- Huo, P.; Liu, W.; He, X.; Wang, H.; Chen, Y. Nickel (II) Complexes with Three-Dimensional Geometry α-Diimine Ligands: Synthesis and Catalytic Activity toward Copolymerization of Norbornene. Organometallics 2013, 32, 2291–2299. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Wang, Y.; Pickens, D.B.; Motta, A.; Wang, Q.J.; Chung, Y.-W.; Lohr, T.L.; Marks, T.J. Highly Branched Polyethylene Oligomers via Group IV-Catalysed Polymerization in very Nonpolar Media. Nat. Catal. 2019, 2, 236–242. [Google Scholar] [CrossRef]
- Johnson, L.; Wang, L.; McLain, S.; Bennett, A.; Dobbs, K.; Hauptman, E.; Ionkin, A.; Ittel, S.; Kunitsky, K.; Marshall, W.; et al. Copolymerization of Ethylene and Acrylates by Nickel Catalysts, Beyond Metallocenes; American Chemical Society: Washington, DC, USA, 2003; pp. 131–142. [Google Scholar]
- Lebarbé, T.; Maisonneuve, L.; Nga Nguyen, T.H.; Gadenne, B.; Alfos, C.; Cramail, H. Methyl 10-Undecenoate as a Raw Material for the Synthesis of Renewable Semi-Crystalline Polyesters and Poly(Ester-Amide)s. Polym. Chem. 2012, 3, 2842–2851. [Google Scholar] [CrossRef]
- Zhong, S.; Tan, Y.; Zhong, L.; Gao, J.; Liao, H.; Jiang, L.; Gao, H.; Wu, Q. Precision Synthesis of Ethylene and Polar Monomer Copolymers by Palladium-Catalyzed Living Coordination Copolymerization. Macromolecules 2017, 50, 5661–5669. [Google Scholar] [CrossRef]
- Bézier, D.; Daugulis, O.; Brookhart, M. Oligomerization of Ethylene Using a Diphosphine Palladium Catalyst. Organometallics 2017, 36, 443–447. [Google Scholar] [CrossRef]
- Mondal, R.; Shah, B.K.; Neckers, D.C. Photogeneration of Heptacene in a Polymer Matrix. J. Am. Chem. Soc. 2006, 128, 9612–9613. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, Y.; Cui, L.; Mu, H.; Jian, Z. Pentiptycenyl Substituents in Insertion Polymerization with α-Diimine Nickel and Palladium Species. Organometallics 2019, 38, 2075–2083. [Google Scholar] [CrossRef]
- SMART, Version 5.054; Bruker AXS Inc.: Madison, WI, USA, 2000.
- SAINT and SADABS, Version 6.22; Bruker AXS Inc.: Madison, WI, USA, 2000.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]


| Entry | Co-Cat. | Yield (g) | Act. (106) b | Mn (104) c | Mw (104) c | Mw/Mn c | Brs d | Tm (°C) e |
|---|---|---|---|---|---|---|---|---|
| 1 | MMAO | 2.32 | 6.96 | 81.2 | 141.7 | 1.7 | 65 | 54.6 |
| 2 | MAO | 1.62 | 4.86 | 92.0 | 150.3 | 1.6 | 64 | 60.2 |
| 3 | Et2AlCl | 1.87 | 5.61 | 48.1 | 88.3 | 1.8 | 86 | 35.3 |
| Entry | T (°C) | Yield (g) | Act. (106) b | Mn (104) c | Mw (104) c | Mw/Mn c | Brs d | Tm (°C) e |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 2.32 | 13.92 | 166.3 | 231.4 | 1.4 | 16 | 111.9 |
| 2 | 30 | 2.38 | 14.28 | 111.3 | 183.6 | 1.6 | 49 | 78.0 |
| 3 | 50 | 1.96 | 11.76 | 65.6 | 120.3 | 1.8 | 71 | 58.0 |
| 4 | 70 | 1.87 | 11.22 | 40.4 | 74.0 | 1.8 | 81 | 45.9 |
| 5 | 90 | 1.18 | 7.08 | 36.8 | 69.6 | 1.9 | 94 | - |
| 6 f | 120 | 0.57 | 1.37 | 10.4 | 20.6 | 2.0 | 105 | - |
| 7 g | 50 | 3.20 | 19.20 | 96.2 | 173.1 | 1.8 | 62 | 71.7 |
| 8 h | 30 | 0.73 | 4.38 | 33.5 | 58.0 | 1.7 | 57 | 39.3 |
| 9 i | 30 | 0.28 | 1.68 | 29.8 | 48.0 | 1.6 | 102 | 118.7 |
| Entry | p (bar) | Yield (g) | Act. (106) b | X (mol%) c | Mn (104) d | Mw (104) d | Mw/Mn d | Brs c | Tm (°C) e |
|---|---|---|---|---|---|---|---|---|---|
| 1 f | 8 | 1.57 | 0.63 | 0.12 | 25.7 | 44.3 | 1.7 | 85 | - |
| 2 | 8 | 1.55 | 0.62 | 0.18 | 22.0 | 40.4 | 1.8 | 68 | 55.2 |
| 3 | 6 | 1.16 | 0.46 | 0.21 | 22.2 | 40.2 | 1.8 | 71 | 54.2 |
| 4 | 2 | 0.19 | 0.08 | 0.41 | 7.6 | 15.0 | 2.0 | 81 | - |
| Entry | T (°C) | P (bar) | Yield (g) | Act. (106) b | Mn (104) c | Mw (104) c | Mw/Mn c | Brs d |
|---|---|---|---|---|---|---|---|---|
| 1 e | 0 | 8 | 1.38 | 0.06 | 10.5 | 15.5 | 1.5 | 164 |
| 2 | 30 | 8 | 0.55 | 0.37 | 9.7 | 15.2 | 1.6 | 156 |
| 3 | 50 | 8 | 1.86 | 1.24 | 7.1 | 16.2 | 2.3 | 147 |
| 4 | 70 | 8 | 1.15 | 0.77 | 3.7 | 7.7 | 2.1 | 139 |
| 5 | 90 | 8 | 0.28 | 0.19 | 2.2 | 4.6 | 2.1 | 133 |
| 6 | 120 | 8 | 0.10 | 0.07 | 1.1 | 2.2 | 2.0 | 124 |
| 7 | 50 | 4 | 1.63 | 1.08 | 8.0 | 16.4 | 2.0 | 150 |
| 8 | 50 | 2 | 1.01 | 0.67 | 5.2 | 11.1 | 2.1 | 149 |
| Entry | T (°C) | Yield (g) | Act. (104) b | X (mol%) c | Mn (104) d | Mw (104) d | Mw/Mn d | Brs c | MMA:TMA e |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 0.43 | 0.54 | 0.50 | 1.6 | 3.6 | 2.3 | 154 | 92:8 |
| 2 | 50 | 0.71 | 0.89 | 0.77 | 4.0 | 6.6 | 1.6 | 145 | 73:27 |
| 3 | 70 | 0.87 | 1.09 | 1.25 | 3.9 | 7.4 | 1.9 | 138 | 62:38 |
| 4 | 90 | 0.33 | 0.42 | 1.98 | 1.8 | 3.8 | 2.1 | 128 | 53:47 |
| 5 f | 70 | 0.90 | 1.12 | 0.71 | 4.7 | 9.3 | 2.0 | 143 | 64:36 |
| 6 g | 70 | 0.38 | 0.48 | 2.21 | 2.5 | 4.6 | 1.9 | 137 | 60:40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, Y.; Jian, Z. Unsymmetrical Strategy on α-Diimine Nickel and Palladium Mediated Ethylene (Co)Polymerizations. Molecules 2022, 27, 8942. https://doi.org/10.3390/molecules27248942
Ma X, Zhang Y, Jian Z. Unsymmetrical Strategy on α-Diimine Nickel and Palladium Mediated Ethylene (Co)Polymerizations. Molecules. 2022; 27(24):8942. https://doi.org/10.3390/molecules27248942
Chicago/Turabian StyleMa, Xin, Yixin Zhang, and Zhongbao Jian. 2022. "Unsymmetrical Strategy on α-Diimine Nickel and Palladium Mediated Ethylene (Co)Polymerizations" Molecules 27, no. 24: 8942. https://doi.org/10.3390/molecules27248942
APA StyleMa, X., Zhang, Y., & Jian, Z. (2022). Unsymmetrical Strategy on α-Diimine Nickel and Palladium Mediated Ethylene (Co)Polymerizations. Molecules, 27(24), 8942. https://doi.org/10.3390/molecules27248942