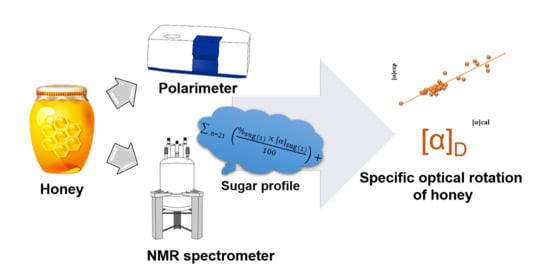
Optical Rotation—A Reliable Parameter for Authentication of Honey?
Abstract
1. Introduction
2. Results
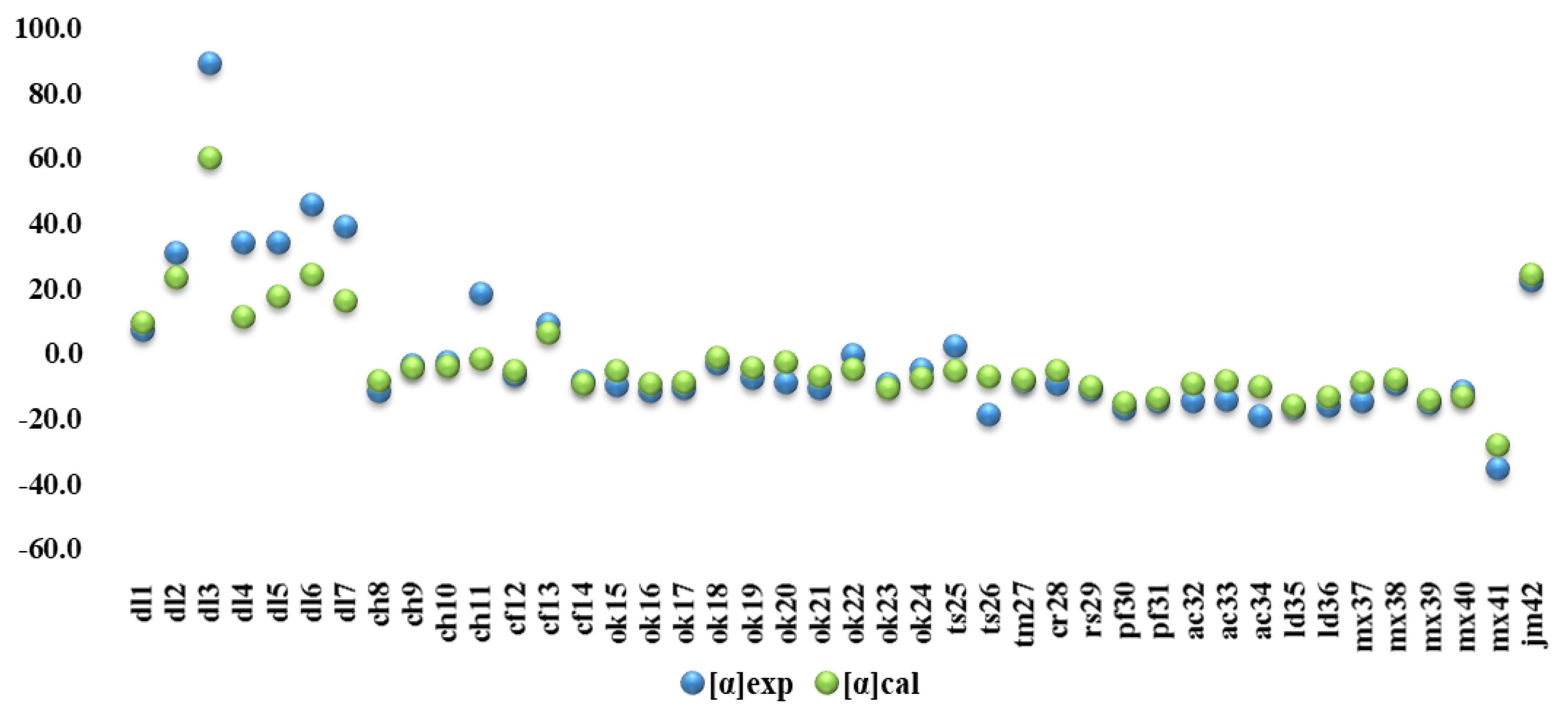
2.1. Measured Specific Optical Angles
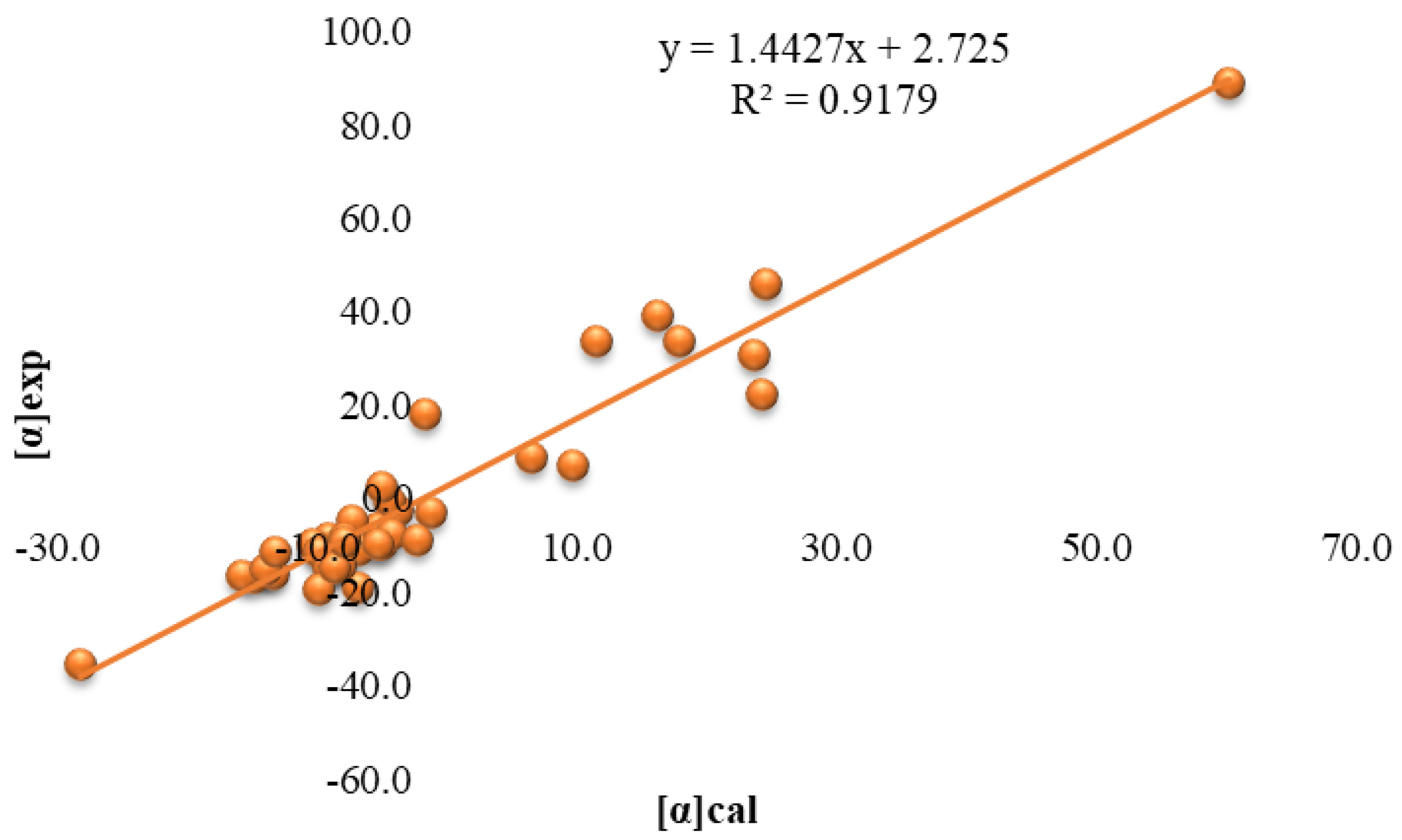
2.2. Calculation of Specific Optical Rotation of Honey/Jam Based on Its Sugar Profile
3. Discussion
4. Materials and Methods
4.1. Honey Samples
4.2. Sample Preparation
4.3. Determination of the Specific Optical Rotation (SOR)
4.4. NMR Spectroscopy
4.5. Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roffet-Salque, M.; Regert, M.; Evershed, R.P.; Outram, A.K.; Cramp, L.J.; Decavallas, O.; Dunne, J.; Gerbault, P.; Mileto, S.; Mirabaud, S.; et al. Widespread exploitation of the honeybee by early Neolithic farmers. Nature 2015, 527, 226–230. [Google Scholar] [CrossRef]
- European Commission. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, 10, 47–52. [Google Scholar]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Boukraâ, L. Honey in Traditional and Modern Medicine, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Dilokthornsakul, W.; Kosiyaporn, R.; Wuttipongwaragon, R.; Dilokthornsakul, P. Potential effects of propolis and honey in COVID-19 prevention and treatment: A systematic review of in silico and clinical studies. J. Integr. Med. 2022, 20, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Bornet, M.; Grosso, C.; Ramalhosa, M.J.; Gouvinhas, I.; Garcia, J.; Rodrigues, F.; Delerue-Matos, C. Honey as an Adjuvant in the Treatment of COVID-19 Infection: A Review. Appl. Sci. 2022, 12, 7800. [Google Scholar] [CrossRef]
- Johnson, R. Food Fraud and Economically Motivated Adulteration of Food and Food Ingredients; CRS: Washington, DC, USA, 2014. [Google Scholar]
- Operation OPSON. Available online: https://www.europol.europa.eu/activities-services/europol-in-action/operations/operation-opson (accessed on 5 December 2022).
- Manning, L.; Smith, R.; Soon, J.M. Developing an organizational typology of criminals in the meat supply chain. Food Policy 2016, 59, 44–54. [Google Scholar] [CrossRef]
- García, N.L. The current situation on the international honey market. Bee World 2018, 95, 89–94. [Google Scholar] [CrossRef]
- El-Sofany, A.; Naggar, Y.A.; Naiem, E.; Giesy, J.P.; Seif, A. Authentication of the botanical and geographic origin of Egyptian honey using pollen analysis methods. J. Apic. Res. 2020, 59, 946–955. [Google Scholar] [CrossRef]
- Mesele, T.L. Review on physico-chemical properties of honey in Eastern Africa. J. Apic Res. 2021, 60, 33–45. [Google Scholar] [CrossRef]
- Sotiropoulou, N.S.; Xagoraris, M.; Revelou, P.K.; Kaparakou, E.; Kanakis, C.; Pappas, C.; Tarantilis, P. The use of SPME-GC-MS IR and Raman techniques for botanical and geographical authentication and detection of adulteration of honey. Foods 2021, 10, 1671. [Google Scholar] [CrossRef]
- Zielińska, S.; Wesołowska, M.; Bilek, M.; Kaniuczak, J.; Dżugan, M. The saccharide profile of Polish honeys depending on their botanical origin. J. Microbiol. Biotechnol. Food Sci. 2021, 3, 387–390. [Google Scholar]
- Piazza, M.; Oddo, L. Bibliographical review of the main European unifloral honeys. Apidologie 2004, 35, 94–111. [Google Scholar] [CrossRef]
- Tarapatskyy, M.; Sowa, P.; Zaguła, G.; Dżugan, M.; Puchalski, C. Assessment of the Botanical Origin of Polish Honeys Based on Physicochemical Properties and Bioactive Components with Chemometric Analysis. Molecules 2021, 26, 4801. [Google Scholar] [CrossRef] [PubMed]
- Dinkov, D. A scientific note on the specific optical rotation of three honey types from Bulgaria. Apidologie 2003, 34, 319–320. [Google Scholar] [CrossRef][Green Version]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.G.; Marcazzan, G.L.; Piro, R.; et al. Honey quality and international regulatory standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- IHC-International Honey Commission. Available online: https://www.ihc-platform.net/ (accessed on 5 December 2022).
- Primorac, L.; Angelkov, B.; Mandić, M.L.; Kenjerić, D.; Nedeljko, M.; Flanjak, I.; Perl Pirički, A.; Arapceska, M. Comparison of the Croatian and Macedonian honeydew honey. J. Cent. Eur. Agric. 2009, 10, 263–264. [Google Scholar]
- Serrano, S.; Rodríguez, I.; Moreno, R.; Rincón, F. Detection of key factors affecting specific optical rotation determination in honey. CYTA–J. Food 2019, 17, 574–580. [Google Scholar] [CrossRef]
- Gerginova, D.; Dimova, D.; Simova, S. Preliminary NMR and chemometric study of pine jams used as medicinal remedies. Bulg. Chem. Commun. 2017, 49, 215–220. [Google Scholar]
- Gerginova, D.; Simova, S.; Popova, M.; Stefova, M.; Stanoeva, J.P.; Bankova, V. NMR Profiling of North Macedonian and Bulgarian Honeys for Detection of Botanical and Geographical Origin. Molecules 2020, 25, 4687. [Google Scholar] [CrossRef]
- Popova, M.; Gerginova, D.; Trusheva, B.; Simova, S.; Tamfu, A.N.; Ceylan, O.; Clark, K.; Bankova, V. A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula ferruginea. Foods 2021, 10, 997. [Google Scholar] [CrossRef]
- Primorac, L.; Flanjak, I.; Kenjerić, D.; Bubalo, D.; Topolnjak, Z. Specific rotation and carbohydrate profile of Croatian unifloral honeys. Czech J. Food Sci. 2011, 29, 515–519. [Google Scholar] [CrossRef]
- Kolayli, S.; Can, Z.; Çakir, H.; Okan, O.; Yildiz, O. An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: Their physico-chemical, antioxidant and phenolic compounds properties. Turkish J. Biochem. 2018, 43, 362–374. [Google Scholar] [CrossRef]
- Dimins, F.; Kuka, P.; Cakste, I. Content of carbohydrates and specific rotation angle of honey. Foodbalt 2008, 121–125. [Google Scholar]
- Bogdanov, S. Harmonised Methods of the International Honey Commission. 2009, 1–63. Available online: http://www.ihc–platform.net/ihcmethods2009.pdf (accessed on 5 December 2022).
- Gerginova, D. NMR Metabolomics of Honey and Wine. Ph.D. Thesis, IOCCP-BAS, Sofia, Bulgaria, 2022. [Google Scholar]


| Diluted (n = 7) * | Chestnut (n = 4) | Coniferous (n = 3) | Oak (n = 10) | Thistle (n = 2) | Thyme (n = 1) | |
|---|---|---|---|---|---|---|
| min max | +7.2 +89.2 | −11.5 +18.4 | −8.1 +9.0 | −11.3 −0.2 | −18.8 +2.5 | |
| avg | +40.2 | +0.3 | −1.8 | −7.5 | −8.3 | −8.3 |
| Coriander (n = 1) | Rapeseed (n = 1) | Polyfloral (n = 2) | Acacia (n = 3) | Linden (n = 2) | Mixed (n = 5) | |
| min max | −16.9 −14.8 | −19.2 −14.1 | −16.3 −15.8 | −35.2 −9.3 | ||
| avg | −9.4 | −11.2 | −15.9 | −16.0 | −16.1 | −17.0 |
| Saccharides in Honey | Individual Angle [α]sug | Sugar Quantity (%) g/100 g | Contribution to the OR |
|---|---|---|---|
| Glucose (G) | 52.70 | 28.92 | 15.20 |
| Fructose (F) | −92.40 | 37.48 | −34.60 |
| Gentiobiose (Gb) | 10.00 | 0.00 | 0.00 |
| Sugar (Su) | 66.50 | 0.25 | 0.20 |
| Isomaltose (IMa) | 120.00 | 1.65 | 2.00 |
| Isomaltulose (IMu) | 97.20 | 0.00 | 0.00 |
| Kojibiose (Kb) | 133.00 | 0.54 | 0.70 |
| Leucrose (Lu) | −6.80 | 0.15 | 0.00 |
| Maltose (Ma) | 130.40 | 1.87 | 2.40 |
| Maltulose (Mu) | 64.00 | 1.79 | 1.10 |
| Nigerose (Ng) | 120.00 | 0.59 | 0.70 |
| ααTrehalose (Tr) | 199.00 | 0.00 | 0.00 |
| αβTrehalose | 82.00 | 0.31 | 0.30 |
| Trehalulose (Tru) | 50.00 | 0.61 | 0.30 |
| Turanose (Nu) | 75.00 | 2.09 | 1.60 |
| Erlose (Er) | 121.80 | 0.71 | 0.90 |
| 1−Kestose (1−Ks) | 28.00 | 0.18 | 0.10 |
| Maltotriose (Mr) | 160.00 | 0.00 | 0.00 |
| Melezitose (Mz) | 88.20 | 0.00 | 0.00 |
| Panose (Pa) | 154.00 | 0.31 | 0.50 |
| Raffinose (Rf) | 101.00 | 0.39 | 0.40 |
| Specific optical rotation angle of honey: | −8.3 | ||
| G | F | Gb | IMa | IMu | Kb | |
| 0.09 * | −0.89 ** | −0.22 | −0.20 | −0.19 | −0.54 | |
| Ma | Mu | Ng | Su | ααTr | αβTr | |
| 0.88 | −0.44 | −0.50 | 0.15 | −0.16 | −0.58 | |
| Tu | Er | 1-Ks | Mr | Mz | Pa | |
| −0.62 | −0.35 | −0.21 | 0.89 | −0.07 | −0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerginova, D.; Kurteva, V.; Simova, S. Optical Rotation—A Reliable Parameter for Authentication of Honey? Molecules 2022, 27, 8916. https://doi.org/10.3390/molecules27248916
Gerginova D, Kurteva V, Simova S. Optical Rotation—A Reliable Parameter for Authentication of Honey? Molecules. 2022; 27(24):8916. https://doi.org/10.3390/molecules27248916
Chicago/Turabian StyleGerginova, Dessislava, Vanya Kurteva, and Svetlana Simova. 2022. "Optical Rotation—A Reliable Parameter for Authentication of Honey?" Molecules 27, no. 24: 8916. https://doi.org/10.3390/molecules27248916
APA StyleGerginova, D., Kurteva, V., & Simova, S. (2022). Optical Rotation—A Reliable Parameter for Authentication of Honey? Molecules, 27(24), 8916. https://doi.org/10.3390/molecules27248916