An Iron-NDC Framework with a Cage Structure and an Optothermal Conversion in NIR Window
Abstract
1. Introduction
2. Results and Discussions
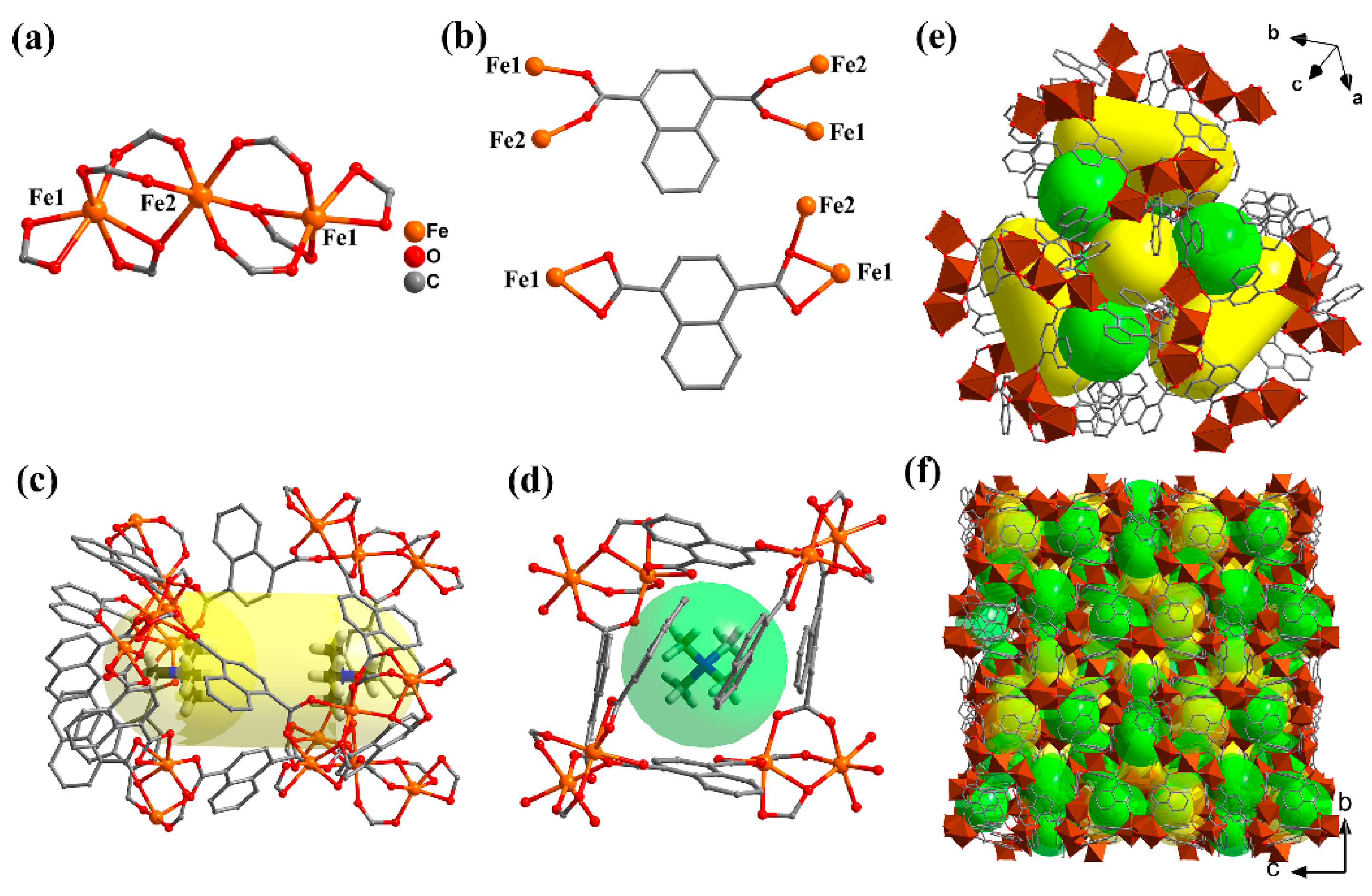
2.1. Crystal Structure Description
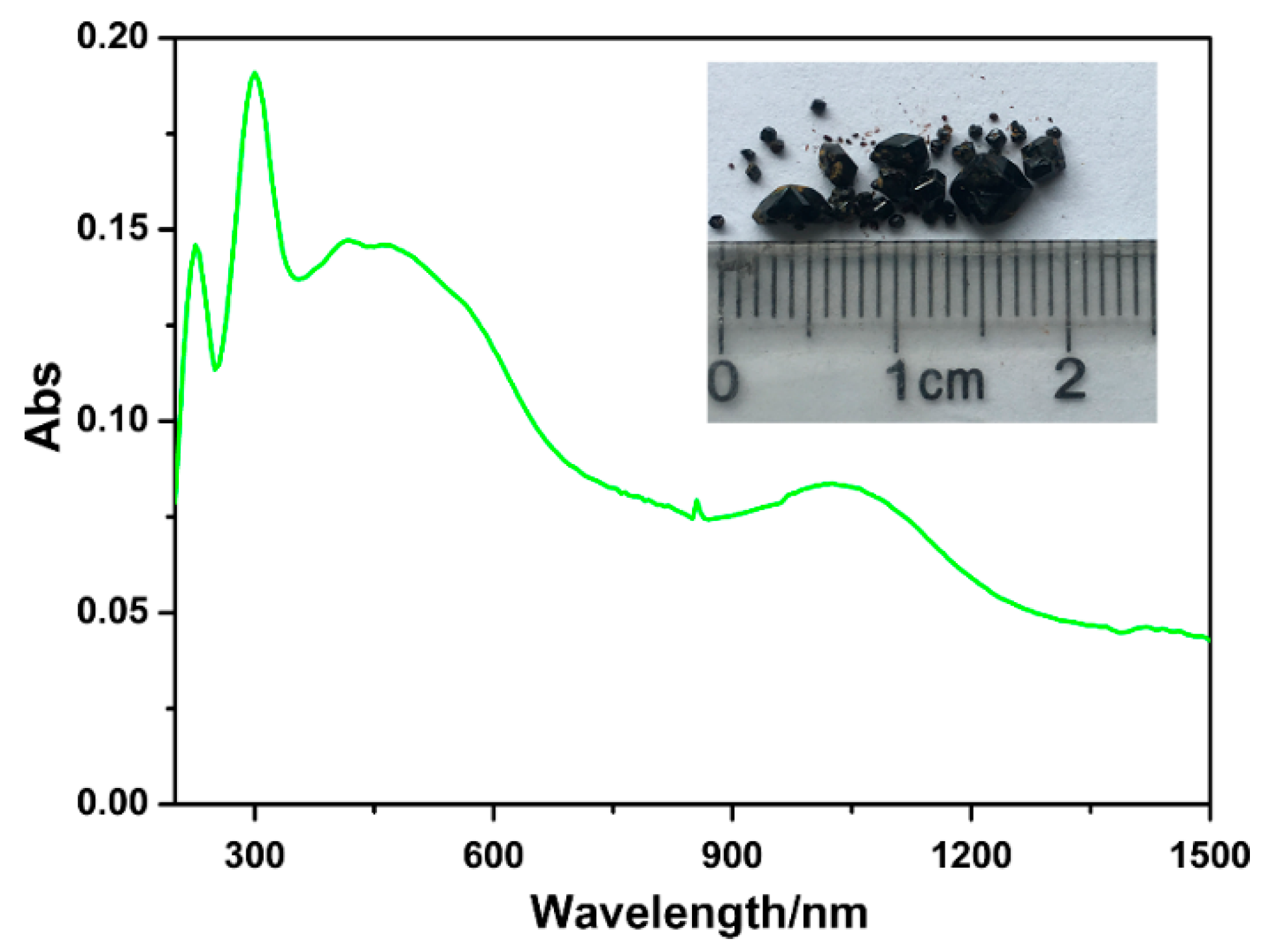
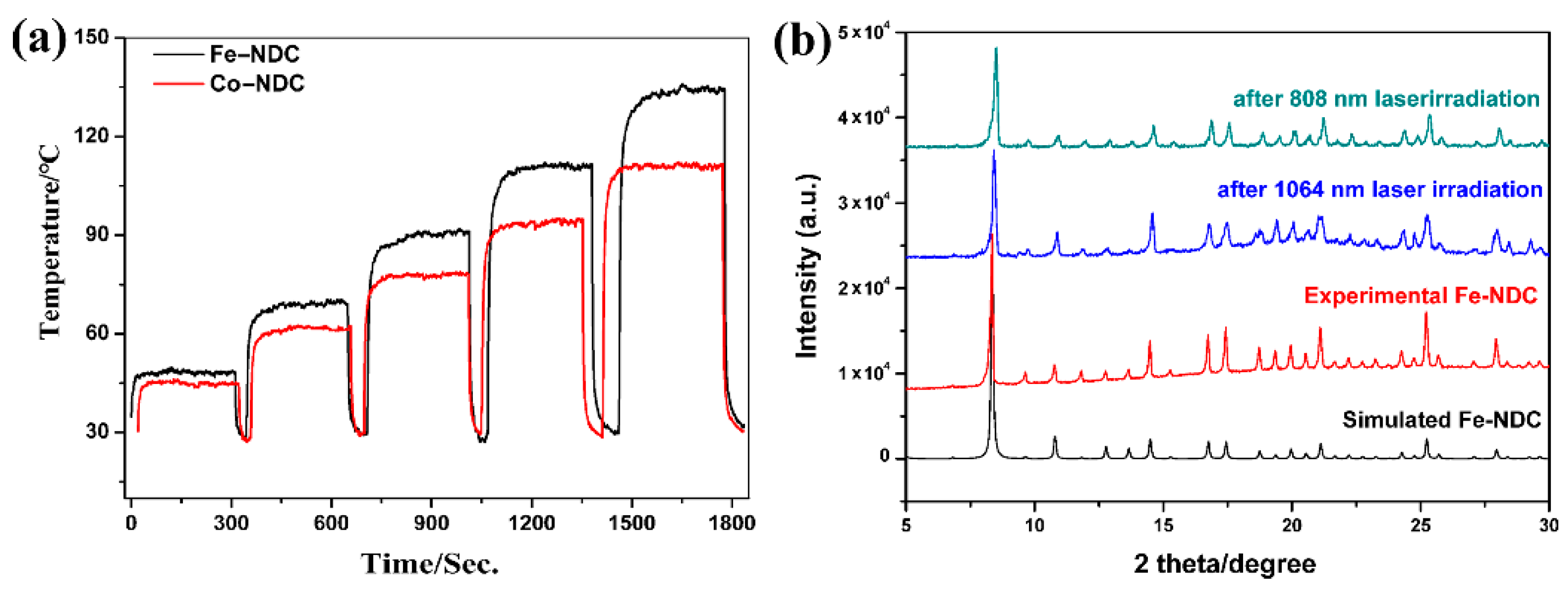
2.2. Basic Physical Measurements
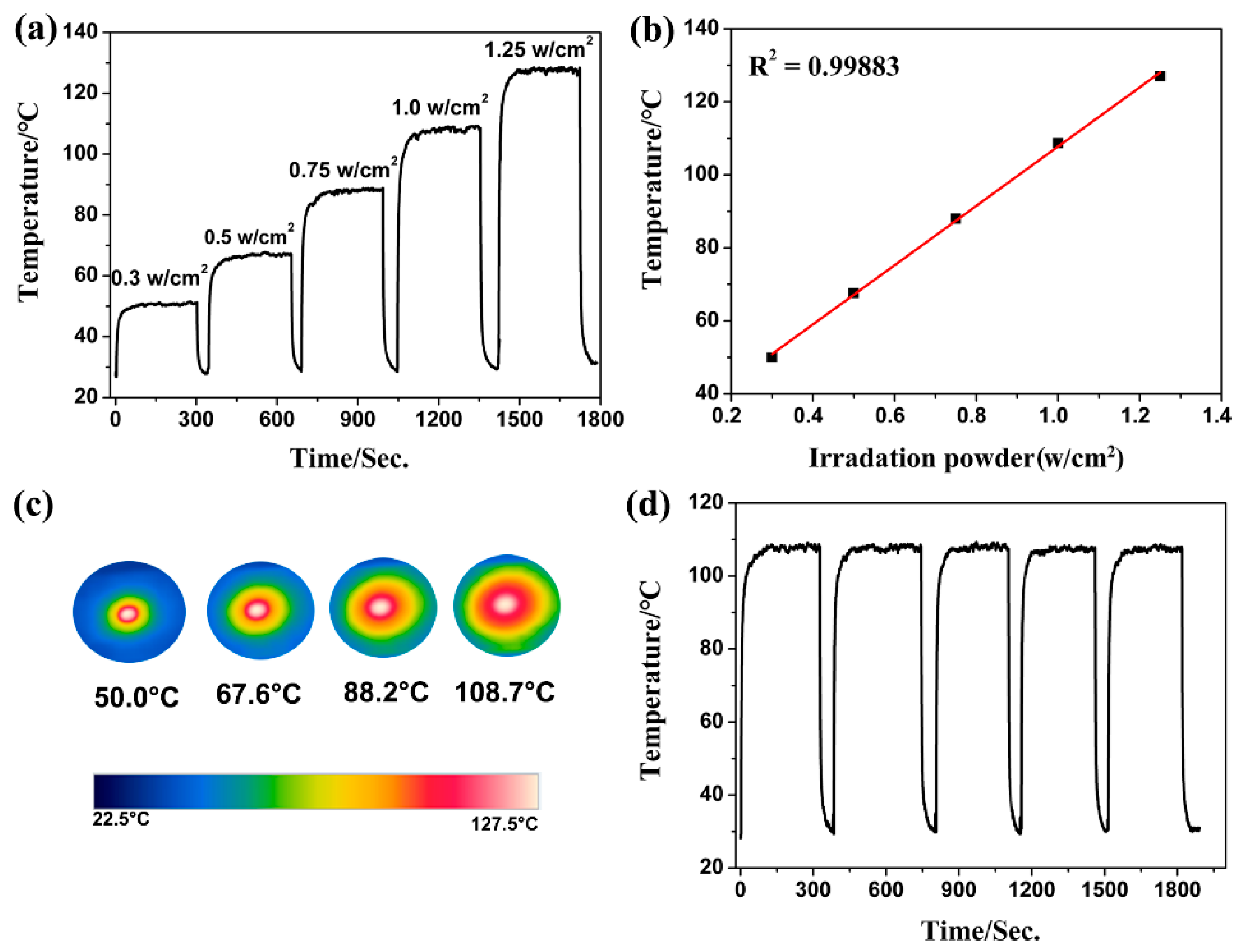
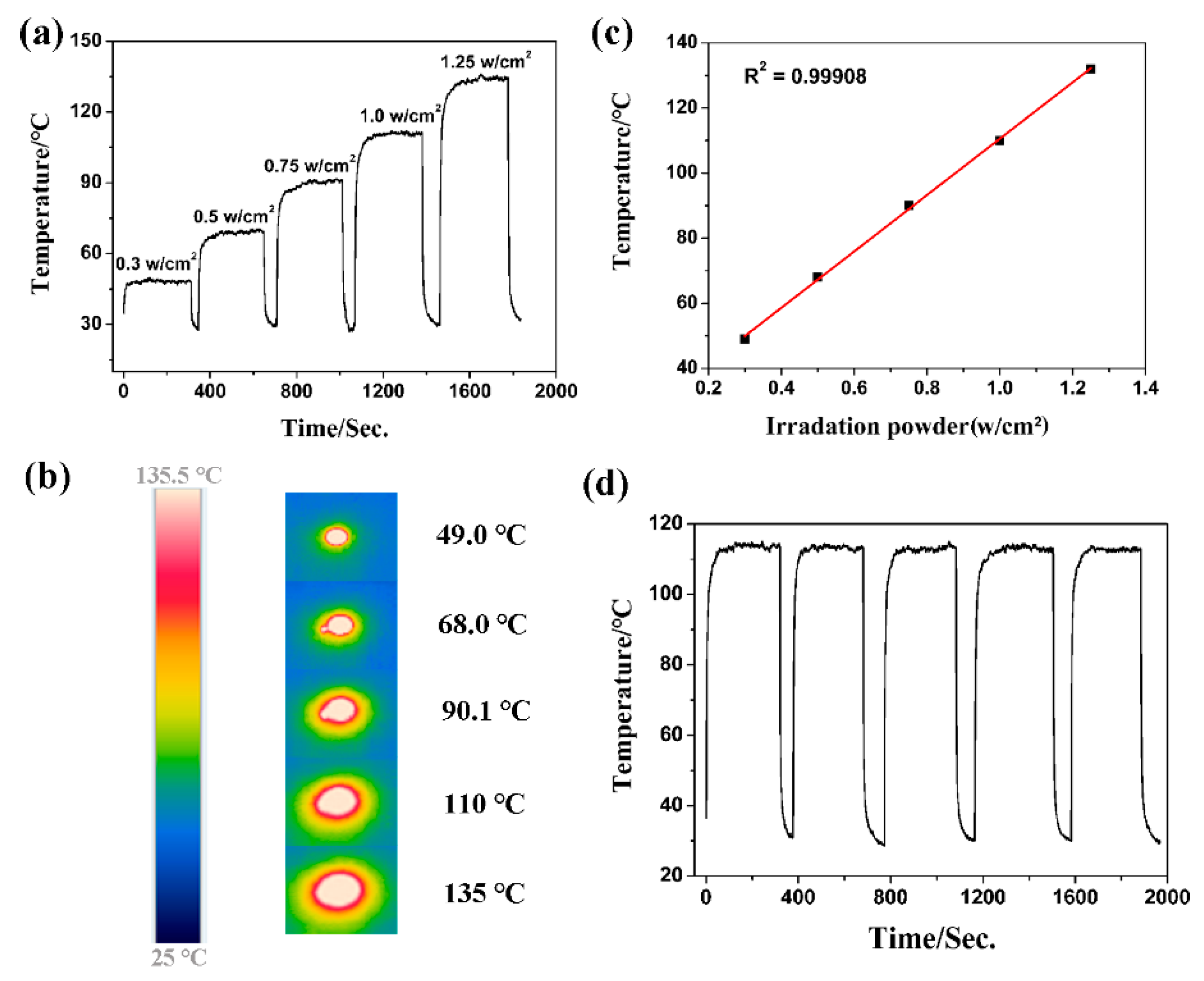
2.3. Photothermal Conversion Characterizations
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, G.Y.; Owens, G.; Chu, D.; Xu, H. Photothermal materials: A key platform enabling highly efficient water evaporation driven by solar energy. Mater. Today Energy 2019, 12, 277–296. [Google Scholar] [CrossRef]
- Xu, J.-W.; Yao, K.; Xu, Z.-K. Nanomaterials with a photothermal effect for antibacterial activities: An overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dunn, A.; Lin, J.; Shi, D. Chapter 13—Photothermal effect of nanomaterials for efficient energy applications, In Novel Nanomaterials for Biomedical, Environmental and Energy Applications, Micro and Nano Technologies; Wang, X., Chen, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 415–434. [Google Scholar]
- Han, B.; Zhang, Y.-L.; Chen, Q.-D.; Sun, H.-B. Carbon-Based Photothermal Actuators. Adv. Funct. Mater. 2018, 28, 1802235. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Chang, R.; Xing, R.; Yan, X. Supramolecular Photothermal Nanomaterials as an Emerging Paradigm toward Precision Cancer Therapy. Sci. Bull. 2019, 29, 1806877. [Google Scholar] [CrossRef]
- He, W.; Zhou, L.; Wang, M.; Cao, Y.; Chen, X.; Hou, X. Structure development of carbon-based solar-driven water evaporation systems. Sci. Bull. 2021, 66, 1472–1483. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef]
- Nawaz, F.; Yang, Y.; Zhao, S.; Sheng, M.; Pan, C.; Que, W. Innovative salt-blocking technologies of photothermal materials in solar-driven interfacial desalination. J. Mater. Chem. A 2021, 9, 16233–16254. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Y.; Lin, J.; Huang, P. Nanomaterials for photoacoustic imaging in the second near-infrared window. Biomate. Sci. 2019, 7, 472–479. [Google Scholar] [CrossRef]
- Zhang, X.; An, L.; Tian, Q.; Lin, J.; Yang, S. Tumor microenvironment-activated NIR-II reagents for tumor imaging and therapy. J. Mater. Chem. B 2020, 8, 4738–4747. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, B.; Jiang, X.; Yuan, Z.; Chen, S.; Sun, P.; Fan, Q.; Huang, W. Double-acceptor conjugated polymers for NIR-II fluorescence imaging and NIR-II photothermal therapy applications. J. Mater. Chem. B 2021, 9, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Jeffrey, R.L.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Telfer, S.; Xu, Q. Special issue for The 6th International Conference on Metal-Organic Frameworks & Open Framework Compounds (MOF2018). Coord. Chem. Rev. 2019, 398, 112982. [Google Scholar]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Re. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Jiang, H.-L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Espín, J.; Garzón-Tovar, L.; Carné-Sánchez, A.; Imaz, I.; Maspoch, D. Photothermal Activation of Metal-Organic Frameworks Using a UV-Vis Light Source. ACS Appl. Mater. Interfaces 2018, 10, 9555–9562. [Google Scholar] [CrossRef]
- Lü, B.; Chen, Y.; Li, P.; Wang, B.; Müllen, K.; Yin, M. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat. Commun. 2019, 10, 767. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Xiong, J.; Lin, Z.; Wei, W.; Xu, Y. Near-infrared photothermal conversion of stable radicals photoinduced from a viologen-based coordination polymer. Chem. Commun. 2020, 56, 7399–7402. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Xu, N.; Murase, R.; Yang, Z.-M.; DQAlessandro, D.M.; Zuo, J.-L.; Zhu, J. Persistent Radical Tetrathiafulvalene-Based 2D Metal-Organic Frameworks and Their Application in Efficient Photothermal Conversion. Angew. Chem. Int. Ed. 2021, 60, 4789–4795. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, Y.-Y.; Su, J.; Wang, H.-Y.; Zuo, J.-L. Charge Transfer Metal-Organic Framework Containing Redox-Active TTF/NDI Units for Highly Efficient Near-Infrared Photothermal Conversion. Chem. Eur. J. 2021, 27, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.W.; Bjorgaard, J.A.; Brown, K.E.; Chavez, D.E.; Hanson, S.K.; Scharff, R.J.; Tretiak, S.; Veauthier, J.M. Energetic Chromophores: Low-Energy Laser Initiation in Explosive Fe(II) Tetrazine Complexes. J. Am. Chem. Soc. 2016, 138, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Szimhardt, N.; Wurzenberger, M.H.H.; Klapötke, T.M.; Lechner, J.T.; Reichherzer, H.; Unger, C.C.; Stierstorfer, J. Highly functional energetic complexes: Stability tuning through coordination diversity of isomeric propyl-linked ditetrazoles. J. Mater. Chem. A 2018, 6, 6565–6577. [Google Scholar] [CrossRef]
- Wurzenberger, M.H.H.; Szimhardt, N.; Stierstorfer, J. Copper(II) Chlorate Complexes: The Renaissance of a Forgotten and Misjudged Energetic Anion. J. Am. Chem. Soc. 2018, 140, 3206–3209. [Google Scholar] [CrossRef]
- Tan, B.; Chen, C.; Chen, Y.-R.; Zhang, J.; Yang, G.-Y. NIR light-driven deflagration of energetic copper complexes through photothermal effect. CrystEngComm 2022, 24, 7493–7499. [Google Scholar] [CrossRef]
- Singh, B.; Hlavac, A.G. Novel Conversion of N, N-Dimethylformamide Dimethyl Acetal to Tetramethylammonium Salts by Its Reaction with 5-Methyl-4-Isoxazolecarboxylic Acid Derivatives. Chem. Lett. 1991, 20, 41–42. [Google Scholar] [CrossRef]
- Neumeyer, J.L.; Cannon, J.G. Reaction of Methyl Bromide with Dimethylformamide. J. Org. Chem. 1961, 26, 4681–4682. [Google Scholar] [CrossRef]
- Cai, X.-H.; Guo, H. N,N-Dimethylformamide (DMF): An Inexpensive and Attractive Reactant. Curr. Org. Chem. 2021, 25, 1977–2004. [Google Scholar] [CrossRef]
- Espín, J.; Garzón-Tovar, L.; Boix, G.; Imaz, I.; Maspoch, D. The photothermal effect in MOFs: Covalent post-synthetic modification of MOFs mediated by UV-Vis light under solvent-free conditions. Chem. Commun. 2018, 54, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Q.; Zhao, M.; Bi, L.-Y.; Hu, Y.-Q.; Gou, G.; Li, J.; Zheng, Y.-Z. Two-Dimensional Silver(I)-Dithiocarboxylate Coordination Polymer Exhibiting Strong Near-Infrared Photothermal Effect. Inorg. Chem. 2019, 58, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.; Lin, J. Yolk-Shell Structured Au Nanostar@Metal-Organic Framework for Synergistic Chemo-photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2019, 19, 6772–6780. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Cai, P.; Yan, T.; Yang, Z.-M.; Yuan, S.; Zuo, J.-L.; Zhou, H.-C. Enhancing the photothermal conversion of tetrathiafulvalene-based MOFs by redox doping and plasmon resonance. Chem. Sci. 2022, 13, 1657–1664. [Google Scholar] [CrossRef]
- Yan, T.; Li, Y.-Y.; Gu, Q.-Y.; Li, J.; Su, J.; Wang, H.-Y.; Zuo, J.-L. A Tetrathiafulvalene/Naphthalene Diimide-Containing Metal-Organic Framework with fsc Topology for Highly Efficient Near-Infrared Photothermal Conversion. Inorg. Chem. 2022, 61, 3078–3085. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, J.-W.; Jiang, X.; Chen, J.; Wang, T.; Chen, K.-J. Band Gap Modulation Enabled by TCNQ Loading in a Ru-Based Metal-Organic Framework for Enhanced Near-Infrared Absorption and Photothermal Conversion. Cryst. Growth Des. 2021, 21, 729–734. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C. Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
| Empirical Formula | C59H61Fe3N3O20 | C59H61Co3N3O20 |
|---|---|---|
| Formula weight | 1299.65 | 1308.89 |
| Crystal system | Cubic | Cubic |
| Space group | I213 | I213 |
| T/K | 100(2) | 295(2) K |
| λ/Å | 0.71073 | 0.71073 |
| a/Å | 25.80060(10) | 25.6346(3) |
| b/Å | 25.80060(10) | 25.6346(3) |
| c/Å | 25.80060(10) | 25.6346(3) |
| α/º | 90 | 90 |
| β/º | 90 | 90 |
| γ/º | 90 | 90 |
| V/Å3 | 17,174.7(2) | 16,845.3(6) |
| Z | 12 | 12 |
| Dc/Mg·m−3 | 1.508 | 1.548 |
| μ/mm−1 | 0.831 | 0.959 |
| F(000) | 8088 | 8124 |
| Measured refls. | 89,269 | 12,550 |
| Independent refls. | 7772 | 6278 |
| Rint | 0.0398 | 0.0249 |
| No. of parameters | 362 | 383 |
| GOF | 1.048 | 1.067 |
| a R1, b wR2 [I > 2σ(I)] | 0.0517, 0.1410 | 0.0441, 0.1255 |
| a R1, b wR2 (all data) | 0.0613, 0.1525 | 0.0596, 0.1390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, B.; Wu, Z.-F.; Huang, X.-Y. An Iron-NDC Framework with a Cage Structure and an Optothermal Conversion in NIR Window. Molecules 2022, 27, 8789. https://doi.org/10.3390/molecules27248789
Tan B, Wu Z-F, Huang X-Y. An Iron-NDC Framework with a Cage Structure and an Optothermal Conversion in NIR Window. Molecules. 2022; 27(24):8789. https://doi.org/10.3390/molecules27248789
Chicago/Turabian StyleTan, Bin, Zhao-Feng Wu, and Xiao-Ying Huang. 2022. "An Iron-NDC Framework with a Cage Structure and an Optothermal Conversion in NIR Window" Molecules 27, no. 24: 8789. https://doi.org/10.3390/molecules27248789
APA StyleTan, B., Wu, Z.-F., & Huang, X.-Y. (2022). An Iron-NDC Framework with a Cage Structure and an Optothermal Conversion in NIR Window. Molecules, 27(24), 8789. https://doi.org/10.3390/molecules27248789






