Can We Form Mesoporous Zeolites by Steam Assisted Crystallization of MCM-41?
Abstract
1. Introduction
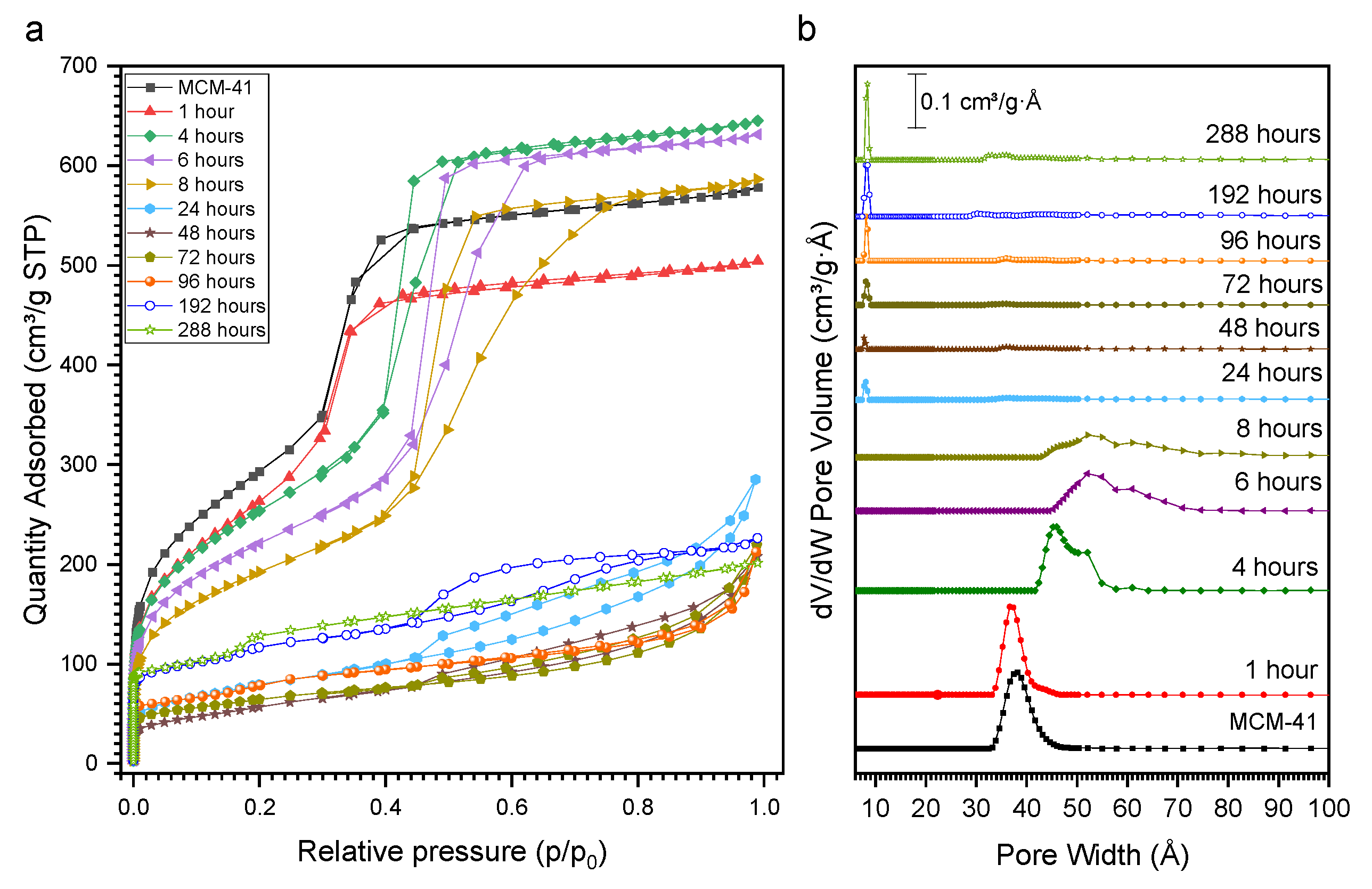
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical Zeolites: Enhanced Utilisation of Microporous Crystals in Catalysis by Advances in Materials Design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Sot. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Navarro, M.T.; Perezpariente, J. Acidity and stability of MCM-41 crystalline aluminosilicates. J. Catal. 1994, 48, 569–574. [Google Scholar] [CrossRef]
- Corma, A.; Grande, M.S.; Gonzalez-Alfaro, V.; Orchilles, A.V. Cracking activity and hydrothermal stability of MCM-41 and its comparison with amorphous silica-alumina and a USY zeolite. J. Catal. 1996, 159, 375–382. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Cejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Opanasenko, M.V.; Roth, W.J.; Čejka, J. Two-Dimensional Zeolites in Catalysis: Current Status and Perspectives. Catal. Sci. Technol. 2016, 6, 2467–2484. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Mesoporosity—A New Dimension for Zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis Strategies in the Search for Hierarchical Zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef]
- Lopez-Orozco, S.; Inayat, A.; Schwab, A.; Selvam, T.; Schwieger, W. Zeolitic Materials with Hierarchical Porous Structures. Adv. Mater. 2011, 23, 2602–2615. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Madsen, C.; Houzvicka, J.; Schmidt, I.; Carlsson, A. Mesoporous Zeolite Single Crystals. J. Am. Chem. Soc. 2000, 122, 7116–7117. [Google Scholar] [CrossRef]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and Industrial Assessment of Synthesis Strategies towards Zeolites with Mesopores. Chem. Cat. Chem. 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Silaghi, M.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Lutz, W.; Shutilov, R.A.; Gavrilov, V.Y. Pore structure of USY zeolites in dependence on steaming condition. Z. Anorg. Und Allg. Chem. 2014, 640, 577–581. [Google Scholar] [CrossRef]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Lakiss, L.; Gilson, J.P.; Thomas, K.; Goupil, J.M.; Fernandez, C.; Valtchev, V. Chemical Equilibrium Controlled Etching of MFI-Type Zeolite and Its Influence on Zeolite Structure, Acidity, and Catalytic Activity. Chem. Mater. 2013, 25, 2759–2766. [Google Scholar] [CrossRef]
- Tanabe, K.; Hölderich, W.F. Industrial application of solid acid–base catalysts. Appl. Catal. A Gen. 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Sachse, A.; Garcia-Martinez, J. Surfactant-templating of zeolites: From design to application. Chem. Mater. 2017, 2, 3827–3853. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Artemova, M.; Demikhova, N.; Smirnova, E.; Roldugina, E.; Stavitskaya, A.; Ivanov, E.; Egazaryants, S.; Vinokurov, V. Micro-mesoporous MCM-41/ZSM-5 Supported Pt and Pd Catalysts for Hydroisomerization of C-8 Aromatic Fraction. Appl. Catal. A Gen. 2020, 603, 117764. [Google Scholar] [CrossRef]
- Zhou, J.; Hua, Z.; Zhao, J.; Gao, Z.; Zeng, S.; Shi, J. A micro/mesoporous aluminosilicate: Key factors affecting framework crystallization during steam-assisted synthesis and its catalytic property. J. Mater. Chem. 2010, 20, 6764–67710. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhang, K.; Wang, Y.M. Steam-assisted crystallization of TPA+-exchanged MCM-41 type mesoporous materials with thick pore walls. Mater. Res. Bull. 2012, 7, 1774–1782. [Google Scholar] [CrossRef]
- Li, H.; Jin, J.; Wu, W.; Chen, C.; Li, L.; Li, Y.; Zhao, W.; Gu, J.; Chen, G.; Shi, J. Synthesis of a hierarchically macro-/mesoporous zeolite based on a micro-emulsion mechanism. J. Mater. Chem. 2011, 21, 19395. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Shi, J. Competition balance between mesoporous self-assembly and crystallization of zeolite: A key to the formation of mesoporous zeolite. J. Alloys Compd. 2013, 556, 71–78. [Google Scholar] [CrossRef]
- Kamil, M.S.M.; Cheralathan, K.K. Facile synthesis of hydrothermally stable mesoporous ZSM-5 zeolite from Al- SBA-16 via steam assisted crystallization. J. Porous Mater. 2020, 27, 587–601. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Kim, J.M.; Ryoo, R. Characterization of highly ordered MCM-41 silicas using X-ray diffraction and nitrogen adsorption. Langmuir 1999, 15, 5279–5284. [Google Scholar] [CrossRef]
- Mokaya, R. Improving the stability of mesoporous MCM-41 silica via thicker more highly condensed pore walls. J. Phys. Chem. B 1999, 103, 10204–10208. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Palencia-Ruiz, S.; Sachse, A.; Amar, F.; Gucuyener, C.; Bats, N.; Batalha, N.; Pinard, L. Understanding the mechanism of large-scale template elimination during calcination of Mcm-41. Microporous Mesoporous Mater. 2022, 338, 111981. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Batonneau-Gener, I.; Sachse, A. Determination of the Exact Microporous Volume and BET Surface Area in Hierarchical ZSM-5. J. Phys. Chem. C 2019, 123, 4235–4242. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, I.M.S.; Pergher, S.B.C.; Sachse, A. Can We Form Mesoporous Zeolites by Steam Assisted Crystallization of MCM-41? Molecules 2022, 27, 8934. https://doi.org/10.3390/molecules27248934
Souza IMS, Pergher SBC, Sachse A. Can We Form Mesoporous Zeolites by Steam Assisted Crystallization of MCM-41? Molecules. 2022; 27(24):8934. https://doi.org/10.3390/molecules27248934
Chicago/Turabian StyleSouza, Iane M. S., Sibele B. C. Pergher, and Alexander Sachse. 2022. "Can We Form Mesoporous Zeolites by Steam Assisted Crystallization of MCM-41?" Molecules 27, no. 24: 8934. https://doi.org/10.3390/molecules27248934
APA StyleSouza, I. M. S., Pergher, S. B. C., & Sachse, A. (2022). Can We Form Mesoporous Zeolites by Steam Assisted Crystallization of MCM-41? Molecules, 27(24), 8934. https://doi.org/10.3390/molecules27248934







