Enzymatic Synthesis of Vancomycin-Modified DNA
Abstract
1. Introduction
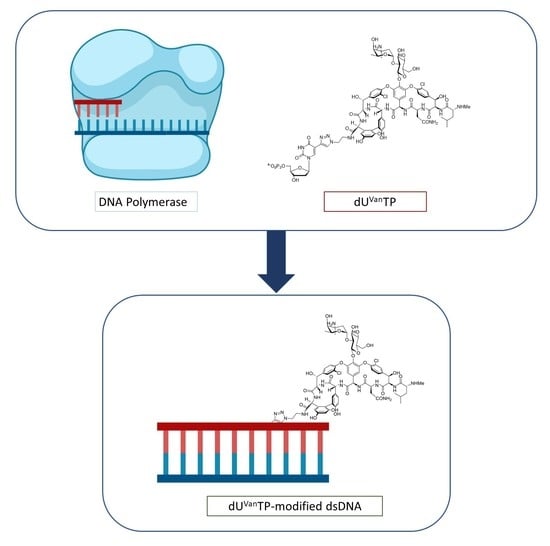
2. Results and Discussion
2.1. Design and Chemical Synthesis of Vancomycin-Modified Nucleoside Triphosphate (dUVanTP)
2.2. Biochemical Characterization of the Modified Nucleotide dUVanTP
2.3. Compatibility of Vancomycin-Modified Nucleotide with SELEX
3. Conclusions
4. Materials and Methods
4.1. Chemical Synthesis of dUVanTP
4.2. Preparation of dUVan Nucleoside Standard
4.3. Digestion and LC-MS Analysis of PEX Reaction Product
4.4. General Protocol for PEX Reactions
4.5. Thermal Denaturation Experiments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Saidjalolov, S.; Braud, E.; Edoo, Z.; Iannazzo, L.; Rusconi, F.; Riomet, M.; Sallustrau, A.; Taran, F.; Arthur, M.; Fonvielle, M.; et al. Click and Release Chemistry for Activity-Based Purification of β-Lactam Targets. Chem. Eur. J. 2021, 27, 7687–7695. [Google Scholar] [CrossRef] [PubMed]
- Shinu, P.; Mouslem, A.K.A.; Nair, A.B.; Venugopala, K.N.; Attimarad, M.; Singh, V.A.; Nagaraja, S.; Alotaibi, G.; Deb, P.K. Progress Report: Antimicrobial Drug Discovery in the Resistance Era. Pharmaceuticals 2022, 15, 413. [Google Scholar] [CrossRef] [PubMed]
- Namivandi-Zangeneh, R.; Sadrearhami, Z.; Dutta, D.; Willcox, M.; Wong, E.H.H.; Boyer, C. Synergy between Synthetic Antimicrobial Polymer and Antibiotics: A Promising Platform To Combat Multidrug-Resistant Bacteria. ACS Infect. Dis. 2019, 5, 1357–1365. [Google Scholar] [CrossRef]
- Bouchet, F.; Atze, H.; Fonvielle, M.; Edoo, Z.; Arthur, M.; Ethève-Quelquejeu, M.; Iannazzo, L. Diazabicyclooctane Functionalization for Inhibition of β-Lactamases from Enterobacteria. J. Med. Chem. 2020, 63, 5257–5273. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sun, J.; Yang, H.; Ji, J.; He, L.; Zhai, L.; Ji, J.; Zhou, P.; Tang, D.; Mu, Y.; et al. Recent Developments to Cope the Antibacterial Resistance via β-Lactamase Inhibition. Molecules 2022, 27, 3832. [Google Scholar] [CrossRef]
- Romero, E.; Oueslati, S.; Benchekroun, M.; D’Hollander, A.C.A.; Ventre, S.; Vijayakumar, K.; Minard, C.; Exilie, C.; Tlili, L.; Retailleau, P.; et al. Azetidinimines as a novel series of non-covalent broad-spectrum inhibitors of β-lactamases with submicromolar activities against carbapenemases KPC-2 (class A), NDM-1 (class B) and OXA-48 (class D). Eur. J. Med. Chem. 2021, 219, 113418. [Google Scholar] [CrossRef]
- Fu, X.; Yu, J.; Dai, N.; Huang, Y.; Lv, F.; Liu, L.; Wang, S. Optical Tuning of Antibacterial Activity of Photoresponsive Antibiotics. ACS Appl. Bio Mater. 2020, 3, 4751–4755. [Google Scholar] [CrossRef]
- Okano, A.; Isley, N.A.; Boger, D.L. Total Syntheses of Vancomycin-Related Glycopeptide Antibiotics and Key Analogues. Chem. Rev. 2017, 117, 11952–11993. [Google Scholar] [CrossRef]
- Acharya, Y.; Dhanda, G.; Sarkar, P.; Haldar, J. Pursuit of next-generation glycopeptides: A journey with vancomycin. Chem. Commun. 2022, 58, 1881–1897. [Google Scholar] [CrossRef]
- Silverman, S.M.; Moses, J.E.; Sharpless, K.B. Reengineering Antibiotics to Combat Bacterial Resistance: Click Chemistry [1,2,3]-Triazole Vancomycin Dimers with Potent Activity against MRSA and VRE. Chem. Eur. J. 2017, 23, 79–83. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Wright, G.D.; Dutka-Malen, S.; Arthur, M.; Courvalin, P.; Walsh, C.T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: Biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991, 30, 10408–10415. [Google Scholar] [CrossRef]
- Walsh; Wright, Introduction: Antibiotic Resistance. Chem. Rev. 2005, 105, 391–394. [CrossRef]
- Petchiappan, A.; Chatterji, D. Antibiotic Resistance: Current Perspectives. ACS Omega 2017, 2, 7400–7409. [Google Scholar] [CrossRef]
- Müller, A.; Klöckner, A.; Schneider, T. Targeting a cell wall biosynthesis hot spot. Nat. Prod. Rep. 2017, 34, 909–932. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Yang, K.; Li, R.; Xiao, X.; Wang, Z. Antagonizing Vancomycin Resistance in Enterococcus by Surface Localized Antimicrobial Display-Derived Peptides. ACS Infect. Dis. 2020, 6, 761–767. [Google Scholar] [CrossRef]
- Melander, R.J.; Melander, C. The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef]
- Chang, M.; Mahasenan, K.V.; Hermoso, J.A.; Mobashery, S. Unconventional Antibacterials and Adjuvants. Acc. Chem. Res. 2021, 54, 917–929. [Google Scholar] [CrossRef]
- Chiosis, G.; Boneca, I.G. Selective Cleavage of D-Ala-D-Lac by Small Molecules: Re-Sensitizing Resistant Bacteria to Vancomycin. Science 2001, 293, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.; Sarkar, P.; Samaddar, S.; Haldar, J. A Vancomycin Derivative with a Pyrophosphate-Binding Group: A Strategy to Combat Vancomycin-Resistant Bacteria. Angew. Chem. Int. Ed. 2016, 55, 7836–7840. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; He, N.; Ou, Y.; Feng, W. Design and Synthesis of New Vancomycin Derivatives. ChemistrySelect 2020, 5, 6670–6673. [Google Scholar] [CrossRef]
- Okano, A.; Isley, N.A.; Boger, D.L. Peripheral modifications of [Ψ[CH2NH]Tpg4]vancomycin with added synergistic mechanisms of action provide durable and potent antibiotics. Proc. Natl. Acad. Sci. USA 2017, 114, E5052–E5061. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Ho, P.L.; Yu, C.-W.; Chow, K.-H.; Gu, H.; Xu, B. Self-assembled multivalent vancomycin on cell surfaces against vancomycin-resistant enterococci (VRE). Chem. Commun. 2003, 2224–2225. [Google Scholar] [CrossRef]
- Yang, C.; Ren, C.; Zhou, J.; Liu, J.; Zhang, Y.; Huang, F.; Ding, D.; Xu, B.; Liu, J. Dual Fluorescent- and Isotopic-Labelled Self-Assembling Vancomycin for in vivo Imaging of Bacterial Infections. Angew. Chem. Int. Ed. 2017, 56, 2356–2360. [Google Scholar] [CrossRef]
- Griffin, J.H.; Linsell, M.S.; Nodwell, M.B.; Chen, Q.; Pace, J.L.; Quast, K.L.; Krause, K.M.; Farrington, L.; Wu, T.X.; Higgins, D.L.; et al. Multivalent Drug Design. Synthesis and In Vitro Analysis of an Array of Vancomycin Dimers. J. Am. Chem. Soc. 2003, 125, 6517–6531. [Google Scholar] [CrossRef]
- Sarkar, P.; Basak, D.; Mukherjee, R.; Bandow, J.E.; Haldar, J. Alkyl-Aryl-Vancomycins: Multimodal Glycopeptides with Weak Dependence on the Bacterial Metabolic State. J. Med. Chem. 2021, 64, 10185–10202. [Google Scholar] [CrossRef]
- McComas, C.C.; Crowley, B.M.; Boger, D.L. Partitioning the Loss in Vancomycin Binding Affinity for d-Ala-d-Lac into Lost H-Bond and Repulsive Lone Pair Contributions. J. Am. Chem. Soc. 2003, 125, 9314–9315. [Google Scholar] [CrossRef]
- Flint, A.J.; Davis, A.P. Vancomycin mimicry: Towards new supramolecular antibiotics. Org. Biomol. Chem. 2022, 20, 7694–7712. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Pourhajibagher, M.; Raoofian, R.; Tabarzad, M.; Bahador, A. Therapeutic applications of nucleic acid aptamers in microbial infections. J. Biomed. Sci. 2020, 27, 6. [Google Scholar] [CrossRef]
- Zhang, X.; Soontornworajit, B.; Zhang, Z.; Chen, N.; Wang, Y. Enhanced Loading and Controlled Release of Antibiotics Using Nucleic Acids As an Antibiotic-Binding Effector in Hydrogels. Biomacromolecules 2012, 13, 2202–2210. [Google Scholar] [CrossRef]
- Catuogno, S.; Esposito, C.L.; De Franciscis, V. Aptamer-Mediated Targeted Delivery of Therapeutics: An Update. Pharmaceuticals 2016, 9, 69. [Google Scholar] [CrossRef]
- Lijuan, C.; Xing, Y.; Minxi, W.; Wenkai, L.; Le, D. Development of an aptamer-ampicillin conjugate for treating biofilms. Biochem. Biophys. Res. Commun. 2017, 483, 847–854. [Google Scholar] [CrossRef]
- Chen, X.-F.; Zhao, X.; Yang, Z. Aptamer-Based Antibacterial and Antiviral Therapy against Infectious Diseases. J. Med. Chem. 2021, 64, 17601–17626. [Google Scholar] [CrossRef]
- Ocsoy, I.; Yusufbeyoglu, S.; Yılmaz, V.; McLamore, E.S.; Ildız, N.; Ülgen, A. DNA aptamer functionalized gold nanostructures for molecular recognition and photothermal inactivation of methicillin-Resistant Staphylococcus aureus. Colloids Surf. B Biointerfaces 2017, 159, 16–22. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Flamme, M.; Felder, P.S.; Karges, J.; Bonhomme, F.; Gandioso, A.; Malosse, C.; Gasser, G.; Hollenstein, M. A ruthenium–oligonucleotide bioconjugated photosensitizing aptamer for cancer cell specific photodynamic therapy. RSC Chem. Biol. 2022, 3, 85–95. [Google Scholar] [CrossRef]
- Mallikaratchy, P.; Tang, Z.; Tan, W. Cell Specific Aptamer–Photosensitizer Conjugates as a Molecular Tool in Photodynamic Therapy. ChemMedChem 2008, 3, 425–428. [Google Scholar] [CrossRef]
- Röthlisberger, P.; Hollenstein, M. Aptamer chemistry. Adv. Drug Deliv. Rev. 2018, 134, 3–21. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.K.; El-Khoury, R.; Thorpe, J.D.; Damha, M.J.; Hollenstein, M. Recent progress in non-native nucleic acid modifications. Chem. Soc. Rev. 2021, 50, 5126–5164. [Google Scholar] [CrossRef] [PubMed]
- Figazzolo, C.; Ma, Y.; Tucker, J.H.R.; Hollenstein, M. Ferrocene as a potential electrochemical reporting surrogate of abasic sites in DNA. Org. Biomol. Chem. 2022, 20, 8125–8135. [Google Scholar] [CrossRef] [PubMed]
- Leone, D.L.; Hubalek, M.; Pohl, R.; Sykorova, V.; Hocek, M. 1,3-Diketone-Modified Nucleotides and DNA for Cross-Linking with Arginine-Containing Peptides and Proteins. Angew. Chem. Int. Ed. 2021, 60, 17383–17387. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.W.; Röthlisberger, P.; Mechaly, A.E.; Weber, P.; Levi-Acobas, F.; Lo, Y.; Wong, A.W.C.; Kinghorn, A.B.; Haouz, A.; Savage, G.P.; et al. Evolution of abiotic cubane chemistries in a nucleic acid aptamer allows selective recognition of a malaria biomarker. Proc. Natl. Acad. Sci. USA 2020, 117, 16790–16798. [Google Scholar] [CrossRef]
- Siegl, J.; Plückthun, O.; Mayer, G. Dependence of click-SELEX performance on the nature and average number of modified nucleotides. RSC Chem. Biol. 2022, 3, 288–294. [Google Scholar] [CrossRef]
- Siegl, J.; Nikolin, C.; Phung, N.L.; Thoms, S.; Blume, C.; Mayer, G. Split–Combine Click-SELEX Reveals Ligands Recognizing the Transplant Rejection Biomarker CXCL9. ACS Chem. Biol. 2022, 17, 129–137. [Google Scholar] [CrossRef]
- Röthlisberger, P.; Levi-Acobas, F.; Hollenstein, M. New synthetic route to ethynyl-dUTP: A means to avoid formation of acetyl and chloro vinyl base-modified triphosphates that could poison SELEX experiments. Bioorg. Med. Chem. Lett. 2017, 27, 897–900. [Google Scholar] [CrossRef]
- Kucharczyk, M.; Brzezowska, M.; Maciąg, A.; Lis, T.; Jeżowska-Bojczuk, M. Structural features of the Cu2+–vancomycin complex. J. Inorg. Biochem. 2008, 102, 936–942. [Google Scholar] [CrossRef]
- Świątek, M.; Valensin, D.; Migliorini, C.; Gaggelli, E.; Valensin, G.; Jeżowska-Bojczuk, M. Unusual binding ability of vancomycin towards Cu2+ ions. Dalton Trans. 2005, 3808–3813. [Google Scholar] [CrossRef]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef]
- Nguyen, H.; Abramov, M.; Rozenski, J.; Eremeeva, E.; Herdewijn, P. In Vivo Assembly and Expression of DNA Containing Non-canonical Bases in the Yeast Saccharomyces cerevisiae. ChemBioChem 2022, 23, e202200060. [Google Scholar] [CrossRef]
- Cahová, H.; Pohl, R.; Bednárová, L.; Nováková, K.; Cvačka, J.; Hocek, M. Synthesis of 8-bromo-, 8-methyl- and 8-phenyl-dATP and their polymerase incorporation into DNA. Org. Biomol. Chem. 2008, 6, 3657–3660. [Google Scholar] [CrossRef]
- Hollenstein, M. Synthesis of Deoxynucleoside Triphosphates that Include Proline, Urea, or Sulfonamide Groups and Their Polymerase Incorporation into DNA. Chem. Eur. J. 2012, 18, 13320–13330. [Google Scholar] [CrossRef]
- Zaboikin, M.; Freter, C.; Srinivasakumar, N. Gaussian decomposition of high-resolution melt curve derivatives for measuring genome-editing efficiency. PLoS ONE 2018, 13, e0190192. [Google Scholar]
- Baccaro, A.; Marx, A. Enzymatic Synthesis of Organic-Polymer-Grafted DNA. Chem. Eur. J. 2010, 16, 218–226. [Google Scholar] [CrossRef]
- Kočalka, P.; Andersen, N.K.; Jensen, F.; Nielsen, P. Synthesis of 5-(1,2,3-Triazol-4-yl)-2′-deoxyuridines by a Click Chemistry Approach: Stacking of Triazoles in the Major Groove Gives Increased Nucleic Acid Duplex Stability. ChemBioChem 2007, 8, 2106–2116. [Google Scholar] [CrossRef]
- Gimenez Molina, A.; Raguraman, P.; Delcomyn, L.; Veedu, R.N.; Nielsen, P. Oligonucleotides containing 2′-O-methyl-5-(1-phenyl-1,2,3-triazol-4-yl)uridines demonstrate increased affinity for RNA and induce exon-skipping in vitro. Bioorg. Med. Chem. 2022, 55, 116559. [Google Scholar] [CrossRef]
- Fan, H.; Sun, H.; Haque, M.M.; Peng, X. Effect of Triazole-Modified Thymidines on DNA and RNA Duplex Stability. ACS Omega 2019, 4, 5107–5116. [Google Scholar] [CrossRef]
- Balintová, J.; Welter, M.; Marx, A. Antibody–nucleotide conjugate as a substrate for DNA polymerases. Chem. Sci. 2018, 9, 7122–7125. [Google Scholar] [CrossRef]
- Hollenstein, M. Deoxynucleoside triphosphates bearing histamine, carboxylic acid, and hydroxyl residues—Synthesis and biochemical characterization. Org. Biomol. Chem. 2013, 11, 5162–5172. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.F.; Jackson, K.M.; Boyle, M.M.; Buss, J.A.; Potapov, V.; Gehring, A.M.; Zatopek, K.M.; Corrêa, I.R., Jr.; Ong, J.L.; Jack, W.E. Therminator DNA Polymerase: Modified Nucleotides and Unnatural Substrates. Front. Mol. Biosci. 2019, 6, 28. [Google Scholar] [CrossRef]
- Dunn, M.R.; Larsen, A.C.; Zahurancik, W.J.; Fahmi, N.E.; Meyers, M.; Suo, Z.; Chaput, J.C. DNA Polymerase-Mediated Synthesis of Unbiased Threose Nucleic Acid (TNA) Polymers Requires 7-Deazaguanine To Suppress G:G Mispairing during TNA Transcription. J. Am. Chem. Soc. 2015, 137, 4014–4017. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.F.; Wang, J.; Wu, W.; Karouby, J.; Li, H.; Stupi, B.P.; Jack, W.E.; Hersh, M.N.; Metzker, M.L. Rapid incorporation kinetics and improved fidelity of a novel class of 3′-OH unblocked reversible terminators. Nucleic Acids Res. 2012, 40, 7404–7415. [Google Scholar] [CrossRef] [PubMed]
- Diafa, S.; Evequoz, D.; Leumann, C.J.; Hollenstein, M. Enzymatic Synthesis of 7,5-Bicyclo-DNA Oligonucleotides. Chem. Asian J. 2017, 12, 1347–1352. [Google Scholar] [CrossRef]
- Jang, M.-Y.; Song, X.-P.; Froeyen, M.; Marlière, P.; Lescrinier, E.; Rozenski, J.; Herdewijn, P. A Synthetic Substrate of DNA Polymerase Deviating from the Bases, Sugar, and Leaving Group of Canonical Deoxynucleoside Triphosphates. Chem. Biol. 2013, 20, 416–423. [Google Scholar] [CrossRef][Green Version]
- Renders, M.; Miller, E.; Lam, C.H.; Perrin, D.M. Whole cell-SELEX of aptamers with a tyrosine-like side chain against live bacteria. Org. Biomol. Chem. 2017, 15, 1980–1989. [Google Scholar] [CrossRef]
- Chan, K.Y.; Kinghorn, A.B.; Hollenstein, M.; Tanner, J.A. Chemical Modifications for a Next Generation of Nucleic Acid Aptamers. ChemBioChem 2022, 23, e202200006. [Google Scholar] [CrossRef]
- Gawande, B.N.; Rohloff, J.C.; Carter, J.D.; von Carlowitz, I.; Zhang, C.; Schneider, D.J.; Janjic, N. Selection of DNA aptamers with two modified bases. Proc. Natl. Acad. Sci. USA 2017, 114, 2898–2903. [Google Scholar] [CrossRef]
- Minagawa, H.; Sawa, H.; Fujita, T.; Kato, S.; Inaguma, A.; Hirose, M.; Orba, Y.; Sasaki, M.; Tabata, K.; Nomura, N.; et al. A high-affinity aptamer with base-appended base-modified DNA bound to isolated authentic SARS-CoV-2 strains wild-type and B.1.617.2 (delta variant). Biochem. Biophys. Res. Commun. 2022, 614, 207–212. [Google Scholar] [CrossRef]
- Ondruš, M.; Sýkorová, V.; Hocek, M. Traceless enzymatic synthesis of monodispersed hypermodified oligodeoxyribonucleotide polymers from RNA templates. Chem. Commun. 2022, 58, 11248–11251. [Google Scholar] [CrossRef]
- Mei, H.; Liao, J.-Y.; Jimenez, R.M.; Wang, Y.; Bala, S.; McCloskey, C.; Switzer, C.; Chaput, J.C. Synthesis and Evolution of a Threose Nucleic Acid Aptamer Bearing 7-Deaza-7-Substituted Guanosine Residues. J. Am. Chem. Soc. 2018, 140, 5706–5713. [Google Scholar] [CrossRef]
- Freund, N.; Taylor, A.I.; Arangundy-Franklin, S.; Subramanian, N.; Peak-Chew, S.-Y.; Whitaker, A.M.; Freudenthal, B.D.; Abramov, M.; Herdewijn, P.; Holliger, P. A two-residue nascent-strand steric gate controls synthesis of 2′-O-methyl- and 2′-O-(2-methoxyethyl)-RNA. Nat. Chem. 2022. [Google Scholar] [CrossRef]
- Hervey, J.R.D.; Freund, N.; Houlihan, G.; Dhaliwal, G.; Holliger, P.; Taylor, A.I. Efficient synthesis and replication of diverse sequence libraries composed of biostable nucleic acid analogues. RSC Chem. Biol. 2022, 3, 1209–1215. [Google Scholar] [CrossRef]
- Sola, M.; Menon, A.P.; Moreno, B.; Meraviglia-Crivelli, D.; Soldevilla, M.M.; Cartón-García, F.; Pastor, F. Aptamers Against Live Targets: Is In Vivo SELEX Finally Coming to the Edge? Mol. Ther. Nucleic Acids 2020, 21, 192–204. [Google Scholar] [CrossRef]
- Lee, M.; Kang, B.; Lee, J.; Lee, J.; Jung, S.T.; Son, C.Y.; Oh, S.S. De novo selected hACE2 mimics that integrate hotspot peptides with aptameric scaffolds for binding tolerance of SARS-CoV-2 variants. Sci. Adv. 2022, 8, eabq6207. [Google Scholar] [CrossRef]
- Stokes, J.M.; MacNair, C.R.; Ilyas, B.; French, S.; Côté, J.-P.; Bouwman, C.; Farha, M.A.; Sieron, A.O.; Whitfield, C.; Coombes, B.K.; et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2017, 2, 17028. [Google Scholar] [CrossRef]







| P1 | 5′-TAC GAC TCA CTA TAG CCT C’ |
|---|---|
| T1 | 5′-AGA GGC TAT AGT GAG TCG TA |
| T2 | 5′-CAC TCA CGT CAG TGA CAT GCA TGC CGA TGA CTA GTC GTC ACT AGT GCA CGT AAC GTG CTA GTC AGA AAT TTC GCA CCA C |
| T3 | 5′-CAC TCA CGT CAG TGA CAT GC N40 GTC AGA AAT TTC GCA CCA C |
| T4 | 5′-Phos-AGA GGC TAT AGT GAG TCG TA |
| P2 | 5′-FAM-GTG GTG CGA AAT TTC TGA C |
| P3 | 5′-GTG GTG CGA AAT TTC TGA C |
| P4 | 5′-CAC TCA CGT CAG TGA CAT GC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figazzolo, C.; Bonhomme, F.; Saidjalolov, S.; Ethève-Quelquejeu, M.; Hollenstein, M. Enzymatic Synthesis of Vancomycin-Modified DNA. Molecules 2022, 27, 8927. https://doi.org/10.3390/molecules27248927
Figazzolo C, Bonhomme F, Saidjalolov S, Ethève-Quelquejeu M, Hollenstein M. Enzymatic Synthesis of Vancomycin-Modified DNA. Molecules. 2022; 27(24):8927. https://doi.org/10.3390/molecules27248927
Chicago/Turabian StyleFigazzolo, Chiara, Frédéric Bonhomme, Saidbakhrom Saidjalolov, Mélanie Ethève-Quelquejeu, and Marcel Hollenstein. 2022. "Enzymatic Synthesis of Vancomycin-Modified DNA" Molecules 27, no. 24: 8927. https://doi.org/10.3390/molecules27248927
APA StyleFigazzolo, C., Bonhomme, F., Saidjalolov, S., Ethève-Quelquejeu, M., & Hollenstein, M. (2022). Enzymatic Synthesis of Vancomycin-Modified DNA. Molecules, 27(24), 8927. https://doi.org/10.3390/molecules27248927