Hypsizygus marmoreus as a Source of Indole Compounds and Other Bioactive Substances with Health-Promoting Activities
Abstract
1. Introduction
2. Results
2.1. Analysis of Bioelements
2.2. Analysis of Indole Compounds
2.3. Analysis of Sterol Compounds
2.4. Analysis of Lovastatin Content
2.5. Analysis of Ergothioneine Content
2.6. Analysis of Phenolic Compounds and Phenylalanine Content
2.7. Analysis of Glucan Content
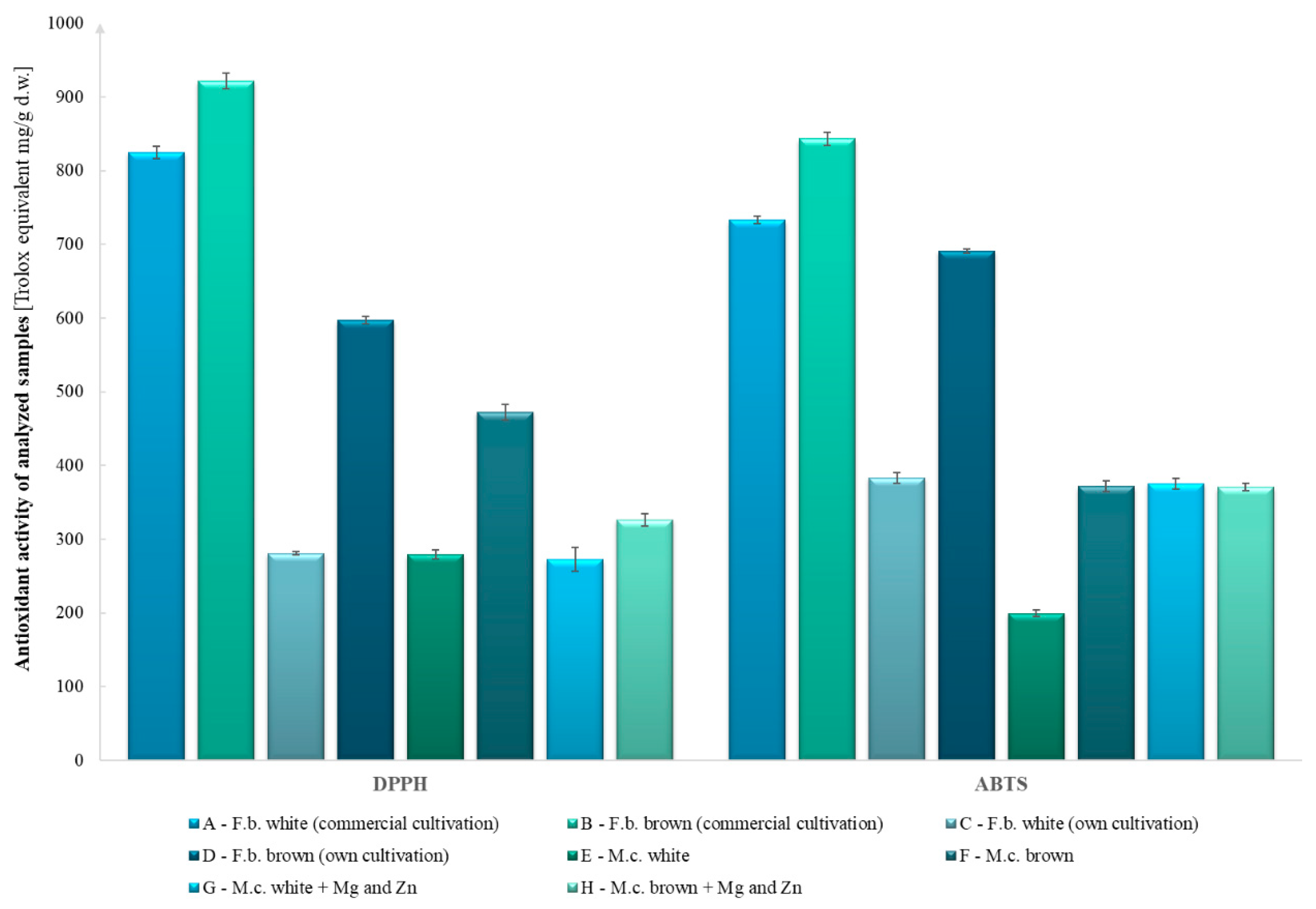
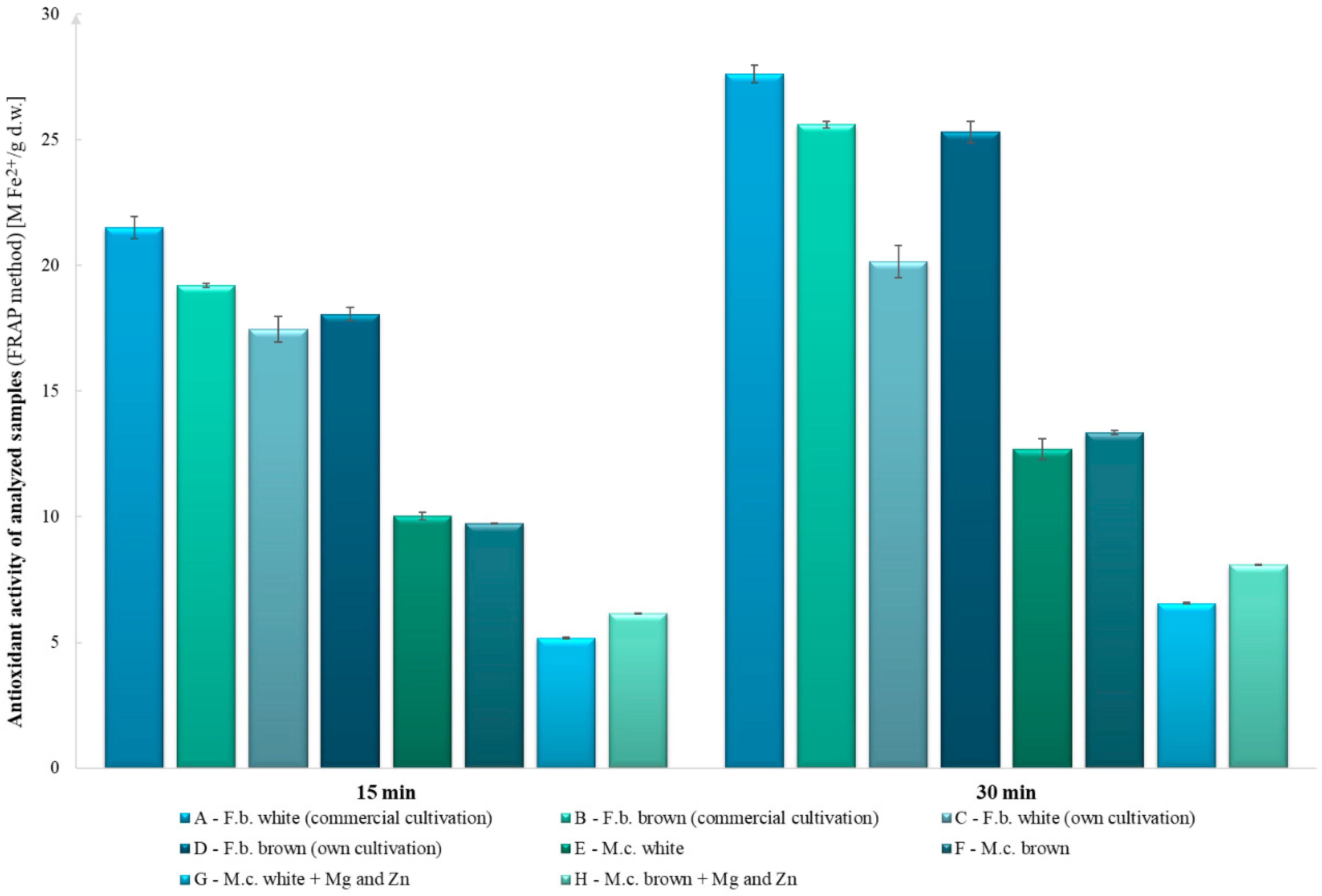
2.8. Analysis of Antioxidant Potential
3. Discussion
4. Materials and Methods
4.1. Mushroom Material and Biotechnological Methods
4.1.1. Mycelial Cultures on a Solid Medium
4.1.2. Shaken Cultures
4.1.3. Aerated Cultures in a Bioreactor
4.1.4. Obtaining Fruiting Bodies of H. marmoreus in Self-Cultivation
4.2. Bioelement Analysis
4.2.1. Mineralization of Samples
4.2.2. Quantitative Analysis
4.3. Analysis of the Content of Organic Compounds
4.3.1. Preparation of Methanolic Extracts
4.3.2. Analysis of Indole Compounds
4.3.3. Analysis of Sterols
4.3.4. Analysis of Lovastatin
4.3.5. Analysis of Ergothioneine Content
4.3.6. Analysis of L-Phenylalanine and Phenolic Compounds
4.3.7. Analysis of Glucan Content
4.4. Determination of Antioxidant Activity
4.4.1. Determination of Antioxidant Activity Using the Dpph• Method
4.4.2. Determination of Antioxidant Activity Using the FRAP Method
4.4.3. Determination of Antioxidant Activity Using the ABTS Method
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lee, K.H.; Morris-Natschke, S.L.; Yang, X.; Huang, R.; Zhou, T.; Wu, S.F.; Shi, Q.; Itokawa, H. Recent progress of research on medicinal mushrooms, foods, and other herbal products used in traditional Chinese medicine. J. Tradit. Complement. Med. 2012, 2, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Avila, S.; Haminiuk, C.W.I. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT Food Sci. Technol. 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Hilszczańska, D. Medicinal properties of macrofungi. For. Res. Pap. 2013, 73, 347–353. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Kapri, M.; Tiwari, A.; Sharma, S. Medicinal importance of mushroom mycelium: Mechanisms and applications. J. Funct. Foods 2019, 56, 182–193. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.T.; Yang, Y.C.; Li, Y.H.; Mau, J.L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Sułkowska-Ziaja, K. Mushrooms as a source of biological active indole compounds. In Indole: Synthesis, Functions and Reactions; Pratt, B., Ed.; Nova Science Publishers: New York, NY, USA, 2019; pp. 61–119. [Google Scholar]
- Liu, M.; Meng, G.; Zhang, J.; Zhao, H.; Jia, L. Antioxidant and hepatoprotective activities of mycelia selenium polysaccharide by Hypsizigus marmoreus SK-02. Biol. Trace Elem. Res. 2016, 172, 437–448. [Google Scholar] [CrossRef]
- Akavia, E.; Beharav, A.; Wasser, S.P.; Nevo, E. Disposal of agro-industrial by-products by organic cultivation of the culinary and medicinal mushroom Hypsizygus marmoreus. Waste Manag. 2009, 29, 1622–1627. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Wang, X.; Zhu, Y.; Zhang, J.; Liu, H.; Jia, L. Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int. J. Biol. Macromol. 2018, 111, 121–128. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Comparison of phenolics, antioxidant, and antiproliferative activities of two Hypsizygus marmoreus varieties. J. Food Sci. 2020, 85, 2227–2235. [Google Scholar] [CrossRef]
- Kała, K.; Krakowska, A.; Szewczyk, A.; Ostachowicz, B.; Szczurek, K.; Fijałkowska, A.; Muszyńska, B. Determining the amount of potentially bioavailable phenolic compounds and bioelements in edible mushroom mycelia of Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes. Food Chem. 2021, 352, 129456. [Google Scholar] [CrossRef]
- Jędrejko, K.; Kała, K.; Sułkowska-Ziaja, K.; Krakowska, A.; Zięba, P.; Marzec, K.; Szewczyk, A.; Sękara, A.; Pytko-Polończyk, J.; Muszyńska, B. Cordyceps militaris–fruiting bodies, mycelium, and supplements: Valuable component of daily diet. Antioxidants 2022, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sękara, A.; Muszyńska, B. Selenium and zinc biofortification of Pleurotus eryngii mycelium and fruiting bodies as a tool for controlling their biological activity. Molecules 2020, 25, 889. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites–strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Komendacki, P.; Kała, K.; Opoka, W.; Rojowski, J. L-Tryptophan and its derivatives in edible mushrooms species. Med. Int. Revuo 2014, 26, 82–88. [Google Scholar]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural occurrence, analysis, biosynthesis, biotechnology, physiology and toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Delgado-Andrade, C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- Davarynejad, D.G.; Hamed, K.; Nyéki, J.; Szabó, Z.; Nagy, P.T. Levels of some micronutrient in dried and fresh fruit samples of apricot cultivars. Int. J. Hortic. Sci. 2012, 18, 25–30. [Google Scholar] [CrossRef]
- Karmańska, A. Skład chemiczny i wartość odżywcza—Buna shimeji—Hypsizygus tessalatus i soplówki jeżowatej Hericium erinaceus. Bromat. Chem. Toksykol. 2012, 45, 1271–1275. (In Polish) [Google Scholar]
- Włodarczyk, A.; Krakowska, A.; Sułkowska-Ziaja, K.; Suchanek, M.; Zięba, P.; Opoka, W.; Muszyńska, B. Pleurotus spp. mycelia enriched in magnesium and zinc salts as a potential functional food. Molecules 2020, 26, 162. [Google Scholar] [CrossRef] [PubMed]
- Kała, K.; Krakowska, A.; Sułkowska-Ziaja, K.; Szewczyk, A.; Reczyński, W.; Opoka, W.; Muszyńska, B. Kinetics of extracted bioactive components from mushrooms in artificial digestive juices. Int. J. Food Prop. 2017, 20, 1796–1817. [Google Scholar] [CrossRef]
- Kała, K.; Krakowska, A.; Gdula-Argasińska, J.; Opoka, W.; Muszyńska, B. Assessing the bioavailability of zinc and indole compounds from mycelial cultures of the bay mushroom Imleria badia (Agaricomycetes) using in vitro models. Int. J. Med. Mushrooms 2019, 21, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska-Ziaja, K.; Hałaszuk, P.; Mastej, M.; Piechaczek, M.; Muszyńska, B. Mycosteroles—Characteristics and biological importance. Med. Int. Revuo 2016, 27, 26–34. [Google Scholar]
- Jang, M.J.; Lee, Y.H.; Ju, Y.C.; Kim, S.M.; Koo, H.M. Effect of color of light emitting diode on development of fruit body in Hypsizygus marmoreus. Mycobiology 2013, 41, 63–66. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide—UHPLC-MS/MS analysis. Food Chem. 2022, 369, 130927. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell Signal. 2021, 87, 110122. [Google Scholar] [CrossRef]
- Lo, Y.C.; Lin, S.Y.; Ulziijargal, E.; Chen, S.Y.; Chien, R.C.; Tzou, Y.J.; Mau, J.L. Comparative study of contents of several bioactive components in fruiting bodies and mycelia of culinary-medicinal mushrooms. Int. J. Med. Mushrooms 2012, 14, 357–363. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Vudhya Gowrisankar, Y.; Chen, X.Z.; Yang, Y.C.; Yang, H.L. The antiaging activity of ergothioneine in UVA-irradiated human dermal fibroblasts via the inhibition of the AP-1 pathway and the activation of Nrf2-mediated antioxidant genes. Oxid. Med. Cell. Longev. 2020, 2020, 2576823. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.D.S.; Corte, L.E.D.; Bueno, J.C.M.; Spinosa, W.A.; Resende, J.T.V.; Hata, F.T.; Cabrera, L.C.; Zeffa, D.M.; Gonçalves, L.S.A.; Constantino, L.V. Firmness and biochemical composition of Shitake and Shimeji commercialized in natura and consumers’ opinion survey. Hortic. Bras. 2021, 39, 425–431. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jian, S.Y.; Lian, P.Y.; Mau, J.L. Antioxidant properties of extracts from a white mutant of the mushroom Hypsizigus marmoreus. J. Food Compos. Anal. 2008, 21, 116–124. [Google Scholar] [CrossRef]
- Shah, S.R.; Ukaegbu, C.I.; Hamid, H.A.; Alara, O.R. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var.) extracted with different solvents. J. Food Meas. Charact. 2018, 12, 1947–1961. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Díaz, T.; López, Ó.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- Savini, S.; Loizzo, M.R.; Tundis, R.; Mozzon, M.; Foligni, R.; Longo, E.; Morozova, K.; Scampicchio, M.; Martin-Vertedor, D.; Boselli, E. Fresh refrigerated Tuber melanosporum truffle: Effect of the storage conditions on the antioxidant profile, antioxidant activity and volatile profile. Eur. Food Res. Technol. 2017, 243, 2255–2263. [Google Scholar] [CrossRef]
- Oddoux, L. Recherches sur les Mycéliums Secondaires des Homobasidiés en Culture Pure; Imprimerie de Trevoux: Lyon, France, 1957. [Google Scholar]
- Sułkowska-Ziaja, K.; Szewczyk, A.; Galanty, A.; Gdula-Argasińska, J.; Muszyńska, B. Chemical composition and biological activity of extracts from fruiting bodies and mycelial cultures of Fomitopsis betulina. Mol. Biol. Rep. 2018, 45, 2535–2544. [Google Scholar] [CrossRef]
- Yuan, J.P.; Kuang, H.C.; Wang, J.H.; Liu, X. Evaluation of ergosterol and its esters in the pileus, gill, and stipe tissues of agaric fungi and their relative changes in the comminuted fungal tissues. Appl. Microbiol. Biotechnol. 2008, 80, 459–465. [Google Scholar] [CrossRef]
- Pansuriya, R.C.; Singhal, R.S. Supercritical fluid extraction of lovastatin from the wheat bran obtained after solid-state fermentation. Food Technol. Biotechnol. 2009, 47, 159–165. [Google Scholar]
- Zhou, T.; Liu, Q.; Jiang, W.; Chen, N. A new strategy for quantitative analysis of ergothioneine in fermentation broth by RP–HPLC. Lect. Notes Electr. Eng. 2014, 249, 313–321. [Google Scholar]
- Ellnain-Wojtaszek, M.; Zgórka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]

| Mushroom Material | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analyzed Bioelements | F.b. White (Commercial Cultivation) | F.b. Brown (Commercial Cultivation) | F.b. White (Own Cultivation) | F.b. Brown (Own Cultivation) | M.c. White | M.c. Brown | M.c. White + Mg and Zn | M.c. Brown + Mg and Zn |
| Na | 14.5 ± 0.7 A | 27.6 ± 0.7 B | 25.1 ± 1.4 B | 37.1 ± 1.0 C | 4.17 ± 0.20 D | 6.76 ± 0.31 D | 28.5 ± 1.7 E | 30.3 ± 0.4 E |
| K | 1211 ± 33 A | 1610 ± 61 B | 2159 ± 111 C | 2656 ± 64 D | 686 ± 24 E | 788 ± 50 E,F | 564 ± 28 E,G | 607 ± 41 E,G |
| Mg | 197 ± 9 A | 238 ± 4 B | 172 ± 9 A | 218 ± 5 B | 285 ± 8 C | 325 ± 16 D | 303 ± 11 C,D | 330 ± 20 D |
| Ca | 4.10 ± 0.30 A | 5.87 ± 0.18 B | 8.37 ± 0.31 C | 9.51 ± 0.24 C | 5.23 ± 0.21 B | 6.60 ± 0.37 B | 25.8 ± 1.3 D | 25.7 ± 0.2 D |
| Zn | 11.2 ± 0.5 A,C | 13.4 ± 0.2 A,B | 6.47 ± 0.22 D | 9.52 ± 0.18 C | 12.8 ± 0.3 B | 17.4 ± 0.3 E | 29.1 ± 1.7 F | 30.2 ± 2.2 F |
| Cu | 1.73 ± 0.13 A | 1.84 ± 0.09 A | 1.14 ± 0.08 B | 1.42 ± 0.07 C | 2.71 ± 0.10 D | 3.53 ± 0.11 E | 0.321 ± 0.025 F | 0.285 ± 0.045 F |
| Fe | 5.66 ± 0.18 A | 7.79 ± 0.55 B | 4.48 ± 0.19 C | 6.46 ± 0.17 A,D | 6.49 ± 0.31 A,E | 9.08 ± 0.41 F | 8.49 ± 0.10 B,F | 7.17 ± 0.25 B,D,F |
| Mushroom Material | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analyzed Compounds | F.b. White (Commercial Cultivation) | F.b. Brown (Commercial Cultivation) | F.b. White (Own Cultivation) | F.b. Brown (Own Cultivation) | M.c. White | M.c. Brown | M.c. White + Mg and Zn | M.c. Brown + Mg and Zn |
| Indole compounds [mg 100 g−1 d.w. ± SD] | ||||||||
| L-Tryptophan | 59.9 ± 0.5 A | 72.1 ± 0.4 B | 12.5 ± 0.2 C | 8.97 ± 0.38 D | 18.8 ± 0.8 E | 25.6 ± 1.1 F | 17.3 ± 0.4 E,G | 18.7 ± 0.2 E,G |
| 5-Hydroxy-L-tryptophan | nd | nd | nd | nd | 3.07 ± 0.09 A | 11.4 ± 0.8 B | nd | 0.319 ± 0.008 C |
| Serotonin | nd | * | nd | nd | nd | nd | * | nd |
| Tryptamine | 14.2 ± 0.7 A | 29.3 ± 0.6 B | * | nd | 0.153 ± 0.009 C | nd | * | 0.131 ± 0.095 C |
| 5-Methyltryptamine | nd | 27.2 ± 0.3 A | nd | nd | 3.39 ± 0.02 B | 4.71 ± 0.03 C | 9.42 ± 0.44 D | 3.19 ± 0.01 B |
| Melatonin | * | nd | * | * | nd | * | 4.37 ± 0.15 A | 4.99 ± 0.24 B |
| Sterols [mg 100 g−1 d.w. ± SD] | ||||||||
| Ergosterol | 74.8 ± 1.0 A | 116 ± 1 B | 45.3 ± 0.1 C | 69.3 ± 0.2 D | 24.9 ± 0.1 E | 58.8 ± 0.7 F | 142 ± 4 G | 166 ± 1 H |
| Ergosterol peroxide | 2.60 ± 0.01 A | 9.74 ± 0.04 B | nd | * | * | * | 6.01 ± 0.21 C | 15.8 ± 0.3 D |
| Phenolic compounds [mg 100 g−1 d.w. ± SD] | ||||||||
| p-Hydroxybenzoic acid | 23.5 ± 0.1 A | 10.7 ± 0.1 B | 0.300 ± 0.037 C | 0.309 ± 0.005 C | nd | 1.45 ± 0.03 D | nd | nd |
| Protocatechuic acid | 16.6 ± 0.1 A | 11.0 ± 0.1 B | 1.65 ± 0.03 C | 1.22 ± 0.04 D | 3.70 ± 0.11 E | nd | 4.14 ± 0.05 F | 4.39 ± 0.05 G |
| Vanillic acid | 4.21 ± 0.10 A | nd | nd | nd | nd | nd | nd | nd |
| Cinnamic acid | 6.50 ± 0.03 A | 8.88 ± 0.01 B | nd | nd | nd | 1.26 ± 0.08 C | nd | nd |
| Other bioactive compounds [mg 100 g−1 d.w. ± SD] | ||||||||
| Lovastatin | 74.5 ± 0.6 A | 62.4 ± 0.5 B | 43.9 ± 0.6 C | 66.7 ± 2.6 D | 29.2 ± 0.6 E | 19.2 ± 0.1 F | 15.3 ± 0.1 G | 15.0 ± 0.3 G |
| Ergothioneine | 23.4 ± 2.5 A | 19.9 ± 1.4 A | 17.1 ± 1.4 A | 14.7 ± 1.5 A | 25.6 ± 1.6 B | 73.0 ± 7.4 C | 80.4 ± 4.3 C | 24.4 ± 1.3 A |
| L-Phenylalanine | 292 ± 16 A | 422 ± 12 B | 97 ± 2 C | 109 ± 2 C, D | 132 ± 2 D | 228 ± 12 E | 111 ± 6 D | 103 ± 4 C,D |
| Glucans [g 100−1 g d.w. ± SD] | ||||||||
| Total glucans | 41.1 ± 1.0 A | 32.0 ± 1.0 B | 61.4 ± 1.0 C | 58.0 ± 1.5 D | 36.0 ± 1.2 E | 35.0 ± 0.5 E | 41.4 ± 0.8 C,F | 42.3 ± 1.2 A,F |
| α-Glucans ** | 1.93 ± 0.16 A | 3.80 ± 0.11 B | 13.2 ± 0.2 C | 11.3 ± 0.2 D | 10.4 ± 0.4 E | 11.6 ± 0.3 D | 9.50 ± 0.11 F | 9.57 ± 0.37 F |
| β-Glucans *** | 39.1 ± 1.0 A | 28.2 ± 1.1 B | 48.2 ± 0.8 C | 46.8 ± 1.4 C | 25.4 ± 1.1 B,D | 23.4 ± 0.7 D | 31.9 ± 0.8 E | 32.7 ± 1.6 E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kała, K.; Pająk, W.; Sułkowska-Ziaja, K.; Krakowska, A.; Lazur, J.; Fidurski, M.; Marzec, K.; Zięba, P.; Fijałkowska, A.; Szewczyk, A.; et al. Hypsizygus marmoreus as a Source of Indole Compounds and Other Bioactive Substances with Health-Promoting Activities. Molecules 2022, 27, 8917. https://doi.org/10.3390/molecules27248917
Kała K, Pająk W, Sułkowska-Ziaja K, Krakowska A, Lazur J, Fidurski M, Marzec K, Zięba P, Fijałkowska A, Szewczyk A, et al. Hypsizygus marmoreus as a Source of Indole Compounds and Other Bioactive Substances with Health-Promoting Activities. Molecules. 2022; 27(24):8917. https://doi.org/10.3390/molecules27248917
Chicago/Turabian StyleKała, Katarzyna, Wojciech Pająk, Katarzyna Sułkowska-Ziaja, Agata Krakowska, Jan Lazur, Maciej Fidurski, Krystian Marzec, Piotr Zięba, Agata Fijałkowska, Agnieszka Szewczyk, and et al. 2022. "Hypsizygus marmoreus as a Source of Indole Compounds and Other Bioactive Substances with Health-Promoting Activities" Molecules 27, no. 24: 8917. https://doi.org/10.3390/molecules27248917
APA StyleKała, K., Pająk, W., Sułkowska-Ziaja, K., Krakowska, A., Lazur, J., Fidurski, M., Marzec, K., Zięba, P., Fijałkowska, A., Szewczyk, A., & Muszyńska, B. (2022). Hypsizygus marmoreus as a Source of Indole Compounds and Other Bioactive Substances with Health-Promoting Activities. Molecules, 27(24), 8917. https://doi.org/10.3390/molecules27248917







