General Procedure for Steglich Coupling
To a stirred solution of the corresponding carboxylic acid (1.0–1.2 eq) in dry dichloromethane (CH2Cl2) (0.02–0.05 M), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC∙HCl) (1.5 eq), and N,N-dimethyl-4-aminopyridine (DMAP) (0.5 eq) are added under nitrogen atmosphere and at r.t. The reaction is stirred for 15 min, and the corresponding alcohol (1.0–1.2 eq) is added. The reaction mixture is then left stirring overnight at r.t. After reaction completion (TLC monitoring), 1M HCl solution is added, and the mixture is extracted with CH2Cl2. The collected organic phases are then dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude reaction mixtures are eventually purified by flash column chromatography or Biotage®, when needed.
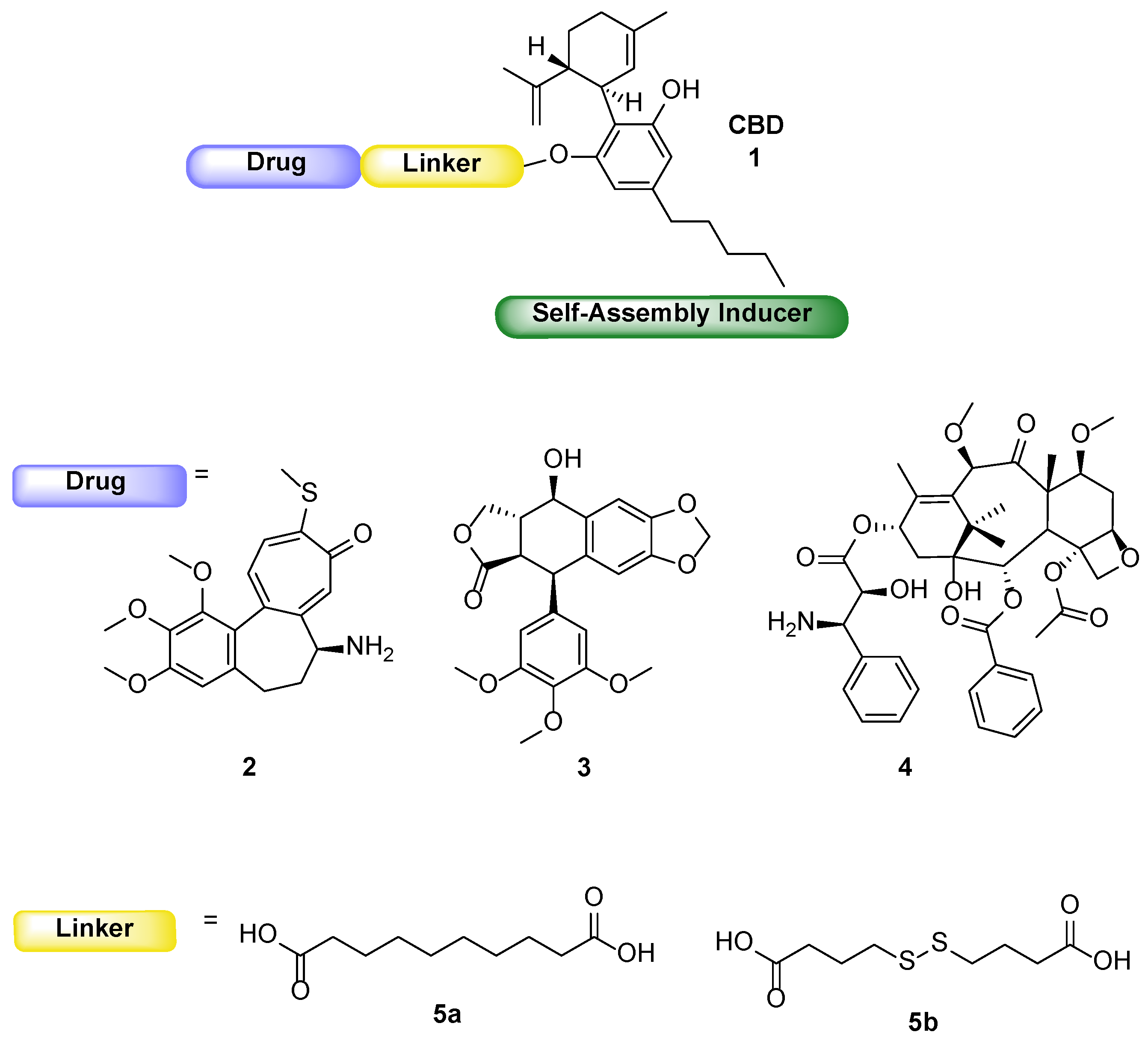
Following the general procedure for the Steglich coupling, to a solution of 4,4′-dithiodibutyric acid 5b (1000 mg, 4.20 mmol), EDC∙HCl (472 mg, 2.46 mmol), DMAP (75 mg, 0.62 mmol) in dry CH2Cl2 (25 mL, 0.1 M) and pyridine (2.5 mL), trimethylsilyl ethanol (0.26 mL, 1.84 mmol) is added to obtain 503 mg of product 16b with 81% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.17 (m, 2H), 2.73 (m, 4H), 2.52 (t, J = 7.3 Hz, 2H), 2.42 (t, J = 7.3 Hz, 2H), 2.03 (m, 4H), 0.98 (m, 2H), 0.04 (s, 9H).
HRMS (ESI+): m/z [M + Na]+ calcd. for C13H26O4S2SiNa, 361.0939; found, 361.0940.
Spectroscopic data are consistent with those described in the literature [
9].
Following the general procedure for the Steglich coupling, to a solution of sebacic acid 5a (1000 mg, 4.94 mmol), EDC∙HCl (559 mg, 2.92 mmol), DMAP (89 mg, 0.73 mmol) in dry CH2Cl2 (25 mL, 0.1 M) and pyridine (2.5 mL), trimethylsilyl ethanol (0.31 mL, 2.18 mmol) were added to obtain 560 mg of product 16a with 85% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.20–4.11 (m, 2H), 2.34 (t, J = 7.5 Hz, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.69–1.55 (m, 4H), 1.37–1.25 (m, 8H), 1.03–0.93 (m, 2H), 0.04 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 180.2, 174.2, 62.5, 34.6, 34.2, 29.2, 29.1, 29.1, 25.0, 24.7, 17.4, −1.4.
HRMS (ESI+): m/z [M + Na]+ calcd. for C15H30O4SiNa, 325.1811; found, 325.1815.
Following the general procedure for the Steglich coupling, to a solution of 4,4′-dithiodibutyric acid 5b (1.56 g, 6.54 mmol), EDC∙HCl (751 mg, 3.92 mmol), DMAP (478 mg, 3.91 mmol) in dry CH2Cl2 (52 mL, 0,05 M), 2,2,2-trichloro ethanol (0.25 mL, 2.61 mmol) is added to obtain 567 mg of product 18b with 59% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.75 (s, 2H), 2.74 (q, J = 7.2 Hz, 4H), 2.61 (t, J = 7.2 Hz, 2H), 2.50 (t, J = 7.2 Hz, 2H), 2.16–1.98 (m, 4H).
13C NMR (100 MHz, CDCl3) δ 179.4, 171.4, 95.0, 74.0, 37.6, 37.6, 32.4, 32.3, 24.0, 23.9.
HRMS (ESI+): m/z [M + Na]+ calcd. for C10H15Cl3O4S2Na, 390.9375; found, 390.9377.
Following the general procedure for the Steglich coupling, to a solution of sebacic acid 5a (1.32 g, 6.53 mmol), EDC∙HCl (750 mg, 3.91 mmol), DMAP (480 mg, 3.93 mmol) in dry CH2Cl2 (52 mL, 0.05 M), 2,2,2-trichloro ethanol (0.25 mL, 2.61 mmol) is added to obtain 472 mg of product 18a with 52% yield. Reaction is monitored by TLC (7:3 n-hex/EtOAc + 1% HCOOH) and purified by flash chromatography (8:2 n-hex/EtOAc + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 4.74 (s, 2H), 2.46 (t, J = 7.5 Hz, 2H), 2.35 (t, J = 7.5 Hz, 2H), 1.66 (m, 4H), 1.40–1.26 (m, 8H).
13C NMR (100 MHz, CDCl3) δ 180.4, 172.2, 95.2, 73.9, 34.1, 34.0, 29.1, 29.0, 29.0, 29.0, 24.8, 24.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C12H19Cl3O4Na, 355.0247; found, 355.0248
(S)-10-oxo-10-((1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)amino)decanoic acid (9a)
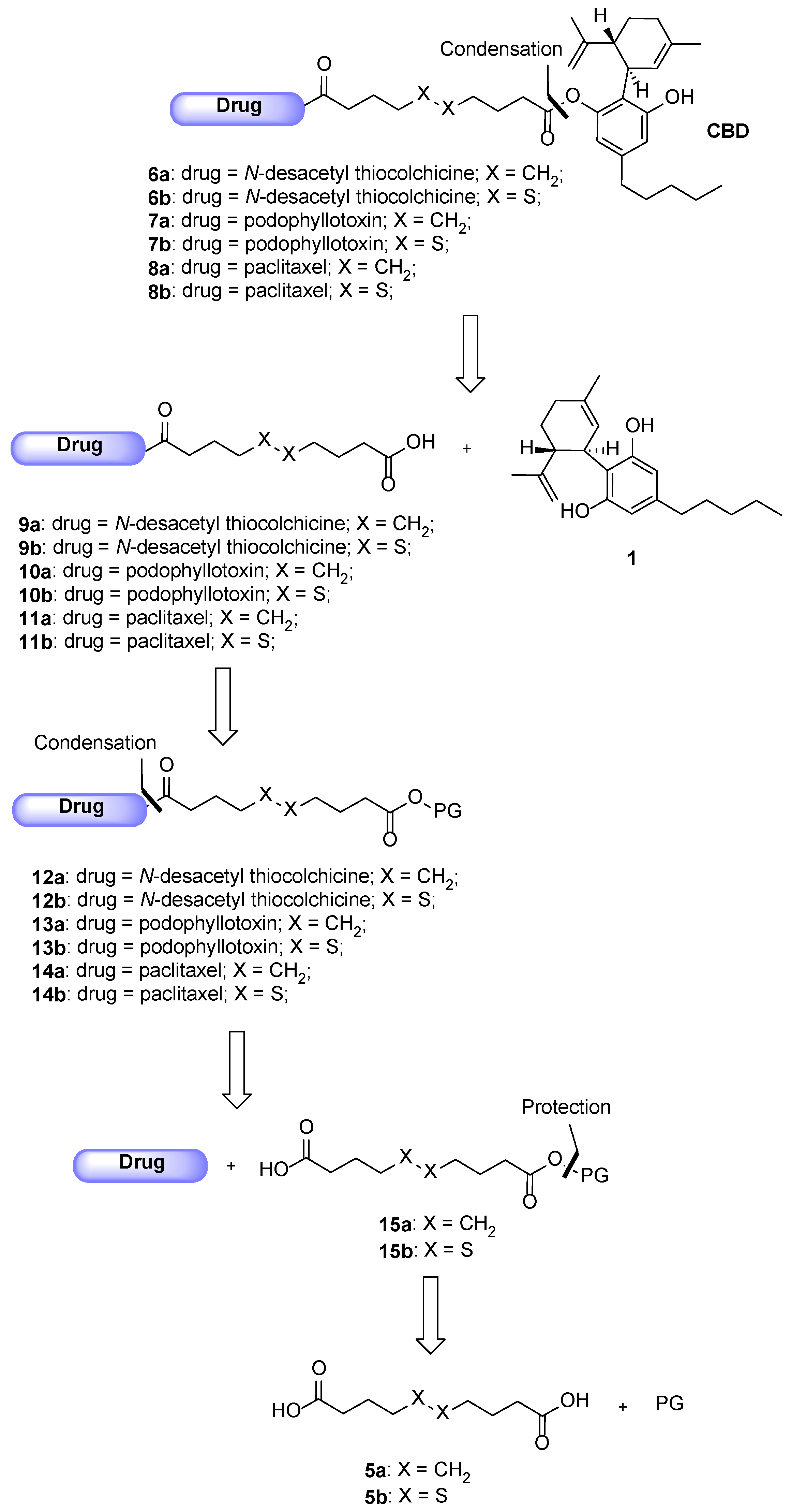
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 5a (135 mg, 0.67 mmol), EDC∙HCl (56 mg, 0.29 mmol), DMAP (4 mg, 0.03 mmol), TEA (0.30 mL, 2.14 mmol) in dry CH2Cl2 (3.0 mL, 0.09 M), 2 (100 mg, 0.27 mmol) is added to obtain 93 mg of product 9a with 62% yield. Reaction is monitored by TLC (CH2Cl2/MeOH 95:5) and purified by flash chromatography (CH2Cl2/MeOH 95:5 eluent mixture).
1H NMR (400 MHz, CDCl3) δ 7.60 (s, 1H), 7.44 (s, 1H), 7.34 (d, J = 10.5 Hz, 1H), 7.11 (d, J = 10.5 Hz, 1H), 6.53 (s, 1H), 4.71 (dt, J = 12.8, 6.7 Hz, 1H), 3.93 (s, 3H), 3.89 (s, 3H), 3.65 (s, 3H), 2.51 (dd, J = 13.2, 5.9 Hz, 1H), 2.43 (s, 3H), 2.41–2.25 (m, 3H), 2.26–2.17 (m, 3H), 1.95–1.84 (m, 1H), 1.67–1.49 (m, 4H), 1.34–1.21 (m, 8H).
HRMS (ESI+): m/z [M + Na]+ calcd. for C30H39NO7SNa, 580.2345; found, 580.2348.
Spectroscopic data are consistent to the ones reported in the literature [
30].
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 10-oxo-10-(((S)-1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)amino)decanoate (6a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 9a (50 mg, 0.09 mmol), EDC∙HCl (19 mg, 0.98 mmol), DMAP (1 mg, 0.01 mmol), TEA (0.01 mL, 0.98 mmol) in dry CH2Cl2 (2 mL, 0.04 M), 1 (28 mg, 0.09 mmol) are added to obtain 36 mg of product 6a with 45% yield. Reaction is monitored by TLC (n-hex/EtOAc 1:1) and purified by flash chromatography (n-hex/EtOAc 1:1).
1H NMR (400 MHz, CDCl3) δ 7.36–7.27 (m, 2H), 7.08 (d, J = 10.5 Hz, 1H), 6.53 (s, 2H), 6.45 (d, J = 7.4 Hz, 1H), 6.37 (s, 1H), 5.95 (bs, 1H), 5.51 (bs, 1H), 4.67 (dt, J = 13.2, 6.8 Hz, 1H), 4.62–4.57 (m, 1H), 4.44 (bs, 1H), 3.94 (s, 3H), 3.90 (s, 3H), 3.66 (s, 3H), 3.49 (bs, 1H), 2.59–2.32 (m, 9H), 2.31–2.13 (m, 4H), 2.11–1.64 (m, 12H), 1.65–1.50 (m, 3H), 1.39–1.22 (m, 14H), 0.88 (t, J = 7.7 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 182.2, 173.0, 172.2, 158.4, 153.8, 152.0, 151.3, 141.8, 138.8, 135.8, 135.1, 134.5, 129.9, 128.6, 127.8, 127.0, 125.8, 123.5, 114.5, 111.4, 107.5, 61.8, 61.5, 56.2, 52.0, 45.7, 37.9, 37.0, 36.5, 35.5, 34.4, 31.6, 30.6, 30.3, 30.1, 29.8, 29.4, 29.2, 28.1, 27.0, 25.6, 24.9, 23.7, 22.6, 19.9, 15.3, 14.1.
HRMS (ESI+):m/z [M + Na]+ calcd. for C51H67NO8SNa, 876.4485; found, 876.4491.
: -72.8 (c 1 in CHCl3).
(S)-4-((4-oxo-4-((1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a] heptalen-7-yl)amino)butyl)disulfaneyl)butanoic acid (9b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 5b (238 mg, 1.0 mmol), EDC∙HCl (84 mg, 0.44 mmol), DMAP (5 mg, 0.04 mmol), TEA (0.45 mL, 3.2 mmol) in dry CH2Cl2 (4.5 mL, 0.01 M), 2 (150 mg, 0.40 mmol) is added to obtain 117 mg of product 9b with 49% yield. Reaction is monitored by TLC (CH2Cl2/MeOH 98:2 + 1% HCOOH) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H), 7.56 (d, J = 10.7 Hz, 1H), 7.33 (d, J = 10.7 Hz, 1H), 6.56 (s, 1H), 4.85–4.72 (m, 1H), 3.97–3.89 (m, 6H), 3.76–3.59 (m, 3H), 2.88–2.67 (m, 5H), 2.63–2.41 (m, 9H), 2.43–2.26 (m, 2H), 2.13–1.88 (m, 4H).
HRMS (ESI+): m/z [M + Na]+ calcd. for C28H35NO7S3Na, 616.1473; found, 616.1478.
Spectroscopic data are consistent to the ones reported in literature [
31].
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 4-((4-oxo-4-(((S)-1,2,3-trimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7 yl)amino)butyl)disulfaneyl)butanoate (6b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 9b (70 mg, 0.18 mmol), EDC∙HCl (38 mg, 0.20 mmol), DMAP (3 mg, 0.02 mmol), TEA (0.2 mL, 1.44 mmol) in dry CH2Cl2 (4 mL, 0.01 M), 1 (57 mg, 0.18 mmol) is added to obtain 32 mg of product 6b with 20% yield. Reaction is monitored by TLC (n-hex/EtOAc 3:7) and purified by flash chromatography (n-hex/EtOAc 3:7 + 1% HCOOH).
1H NMR (400 MHz, CDCl3) δ 7.37–7.27 (m, 2H), 7.07 (d, J = 10.5 Hz, 1H), 6.99–6.83 (m, 1H), 6.76–6.65 (m, 1H), 6.53 (bs, 2H), 6.37 (s, 1H), 5.97 (bs, 1H), 5.47 (bs, 1H), 4.64 (dt, J = 11.9, 6.6 Hz, 1H), 4.61–4.56 (m, 1H), 4.44 (s, 1H), 3.93 (s, 3H), 3.89 (s, 3H), 3.66 (s, 3H), 2.83–2.72 (m, 3H), 2.72–2.60 (m, 4H), 2.55–2.41 (m, 7H), 2.42–2.29 (m, 2H), 2.27–2.05 (m, 4H), 1.99 (q, J = 7.3 Hz, 2H), 1.85–1.68 (m, 5H), 1.62–1.47 (m, 3H), 1.36–1.18 (m, 8H), 0.97–0.77 (m, 3H).
13C NMR (100 MHz, CDCl3) δ 182.4, 171.8, 171.7, 158.5, 155.7, 153.8, 151.4, 142.9, 141.8, 138.5, 134.9, 134.5, 128.6, 126.8, 125.8, 124.5, 114.7, 111.5, 107.6, 61.8, 61.5, 56.2, 52.1, 45.8, 38.4, 38.0, 37.6, 36.9, 35.5, 35.3, 34.6, 32.5, 31.6, 30.6, 30.5, 30.3, 30.1, 29.8, 24.7, 24.7, 24.3, 23.8, 22.6, 20.0, 15.3, 14.2.
HRMS (ESI+):m/z [M + Na]+ calcd. for C49H63NO8S3Na, 912.3613; found, 912.3619.
: -117.1 (c 0.96 in CHCl3).
(5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7] naphtho[2,3-d][1,3]dioxol-5-yl 4-((4-oxo-4-(2-(trimethylsilyl)ethoxy)butyl)disulfaneyl) butanoate (17b)
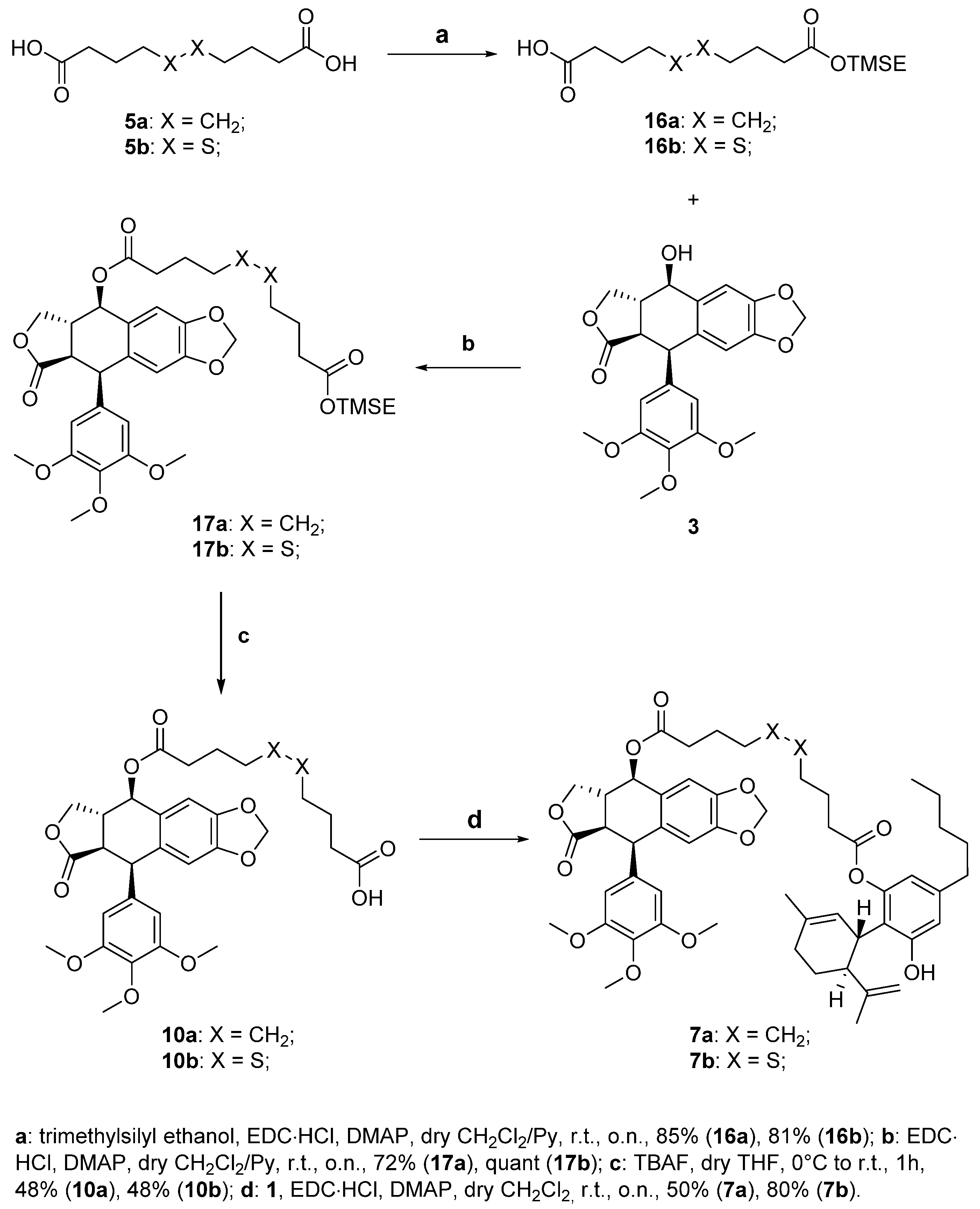
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16b (74.0 mg, 0.22 mmol), EDC∙HCl (41.7 mg, 0.22 mmol), DMAP (11.0 mg, 0.09 mmol) in dry CH2Cl2 (9 mL, 0.02 M), 3 (75 mg, 0.18 mmol) is added to obtain 1 mg of product 17b quantitatively. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.74 (s, 1H), 6.51 (s, 1H), 6.36 (s, 2H), 5.95 (d, J = 5.4 Hz, 2H), 5.86 (d, J = 9.0 Hz, 1H), 4.57 (d, J = 3.9 Hz, 1H), 4.39–4.30 (m, 1H), 4.22–4.09 (m, 3H), 3.77 (s, 3H), 3.73 (s, 6H), 2.90 (dd, J = 14.5, 4.5 Hz, 1H), 2.87–2.75 (m, 1H), 2.77–2.65 (m, 4H), 2.65–2.45 (m, 2H), 2.38 (t, J = 7.4 Hz, 2H), 2.12–1.93 (m, 4H), 1.00–0.91 (m, 2H), 0.01 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 173.6, 173.3, 172.9, 152.5 (2C), 148.1, 147.5, 137.0, 134.8, 132.3, 128.2, 109.6, 108.0 (2C), 106.9, 101.6, 73.6, 71.3, 62.6, 60.6, 56.1 (2C), 45.4, 43.6, 38.6, 37.7, 37.6, 32.7, 32.5, 24.2, 24.0, 17.3, −1.5 (3C).
HRMS (ESI+):m/z [M + Na]+ calcd. for C35H46O11S2SiNa, 757.2148; found, 757.2150.
4-((4-oxo-4-(((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl)oxy)butyl)disulfaneyl)butanoic acid (10b)
To a well-stirred solution of compound 17b (141 mg, 0.192 mmol) in dry THF (3.9 mL, 0.05M), 1M TBAF in THF (1.92 mL, 1.92 mmol) is added at 0 °C under nitrogen atmosphere. The reaction is stirred at r.t. and monitored by TLC (n-hex/EtOAc 1:1 + 1% HCOOH). After 1 h, the reaction is quenched with saturated NH4Cl aqueous solution and extracted with abundant CH2Cl2. The organic phase is then dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude mixture is then purified by Biotage® (gradient with n-hex/EtOAc + 1% HCOOH eluent mixture) to obtain 59 mg of the desired product 10b (48% yield).
1H NMR (400 MHz, CDCl3) δ 6.74 (d, J = 5.0 Hz, 1H), 6.54 (s, 1H), 6.38 (s, 2H), 5.99–5.92 (m, 2H), 5.73 (d, J = 4.9 Hz, 1H), 4.47–4.33 (m, 2H), 4.28 (dd, J = 9.6, 3.0 Hz, 1H), 3.82 (s, 3H), 3.80 (s, 6H), 3.29 (dd, J = 9.2, 3.6 Hz, 1H), 3.03–2.92 (m, 1H), 2.79–2.62 (m, 4H), 2.52–2.45 (m, 2H), 2.43–2.25 (m, 2H), 2.08–1.90 (m, 4H).
13C NMR (100 MHz, CDCl3) δ 178.5, 177.5, 172.7, 153.3 (2C), 148.5, 147.3, 139.0, 136.9, 131.3, 126.2, 109.9, 108.4, 105.5 (2C), 101.5, 72.6, 70.9, 61.0, 56.3 (2C), 45.5, 44.3, 39.8, 37.8, 37.6, 32.5, 32.3, 24.0, 24.0.
HRMS (ESI+): m/z [M + Na]+ calcd. for C30H34O11S2Na, 657.1440; found, 657.1443.
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 4-((4-oxo-4-(((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6, 8,8a,9-hexahydrofuro[3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl)oxy)butyl)disulfaneyl)butanoate (7b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 10b (59 mg, 0.093 mmol), EDC∙HCl (22 mg, 0.112 mmol), DMAP (6 mg, 0.047 mmol) in dry CH2Cl2 (2 mL, 0.05 M), 1 (44 mg, 0.140 mmol) is added to obtain 69 mg of product 7b with 80% yield. Reaction is monitored by TLC (1:1 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.73 (s, 1), 6.54 (s, 2H), 6.40–6.37 (m, 3H), 5.95 (dd, J = 10.4, 1.4 Hz, 2H), 5.74 (d, J = 5.0 Hz, 1H), 5.52 (s, 1H), 4.63–4.57 (m, 1H), 4.48–4.35 (m, 3H), 4.28 (dd, J = 9.6, 2.9 Hz, 1H), 3.83 (s, 3H), 3.80 (s, 6H), 3.47 (s, 1H), 3.28 (dd, J = 9.2, 3.6 Hz, 1H), 3.02–2.92 (m, 1H), 2.83–2.72 (m, 2H), 2.68 (t, J = 7.0 Hz, 2H), 2.64 (s, 2H), 2.58–2.44 (m, 3H), 2.44–2.27 (m, 2H), 2.28–2.16 (m, 1H), 2.17–2.05 (m, 3H), 1.98 (p, J = 7.1 Hz, 2H), 1.87–1.69 (m, 5H), 1.63–1.50 (m, 5H), 1.36–1.24 (m, 4H), 0.87 (t, J = 6.8 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 177.3, 172.7, 171.4, 153.4 (2C), 148.6, 147.4, 143.0, 142.2, 139.0, 133.0, 131.4, 126.3, 123.3, 114.8, 114.1, 111.5, 111.2, 110.0, 108.3, 105.6 (2C), 101.6, 72.6, 70.9, 61.0, 56.4 (2C), 53.6, 45.7, 45.6, 44.3, 39.9, 38.1, 37.6 (2C), 35.5, 32.5 (2C), 31.6, 30.6, 30.3, 29.8, 28.0, 24.1, 24.0, 23.7, 22.6, 14.1.
HRMS (ESI+): m/z [M + Na]+ calcd. for C51H62O12S2Na, 953.3580; found, 953.3582.
: -39.5 (c 0.32 in CHCl3).
1-((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro [3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl) 10-(2-(trimethylsilyl)ethyl) decanedioate (17a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16a (67 mg, 0.22 mmol), EDC∙HCl (42 mg, 0.22 mmol), DMAP (11 mg, 0.09 mmol) in dry CH2Cl2 (9 mL, 0.02 M), 3 (75 mg, 0.18 mmol) is added to obtain 91 mg of product 17a with 72% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.74 (s, 1H), 6.53 (s, 1H), 6.39 (s, 2H5.98 (dd, J = 6.5, 1.3 Hz, 2H), 5.88 (d, J = 9.1 Hz, 1H), 4.60 (d, J = 4.4 Hz, 1H), 4.36 (dd, J = 9.3, 7.0 Hz, 1H), 4.24–4.11 (m, 3H), 3.81 (s, 3H), 3.75 (s, 6H), 2.92 (dd, J = 14.5, 4.4 Hz, 1H), 2.88–2.74 (m, 1H), 2.49–2.33 (m, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.73–1.54 (m, 4H), 1.36–1.26 (m, 8H), 1.03–0.92 (m, 2H), 0.03 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 174.2, 174.0, 173.7, 152.7 (2C), 148.1, 147.6, 137.2, 134.9, 132.4, 128.5, 109.7, 108.1 (2C), 107.0, 101.6, 73.4, 71.4, 62.4, 60.8, 56.2 (2C), 45.6, 43.8, 38.8, 34.5, 34.4, 29.1, 29.1, 29.1, 29.1, 25.0, 24.9, 17.3, −1.5 (3C).
HRMS (ESI+):m/z [M + Na]+ calcd. for C37H50O11SiNa, 721.3020; found, 721.3023.
10-oxo-10-(((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7]naphtho[2,3-d][1,3]dioxol-5-yl)oxy)decanoic acid (10a)
To a well-stirred solution of compound 17a (139 mg, 0.199 mmol) in dry THF (4.0 mL, 0.05M), 1M TBAF in THF (2.00 mL, 2.00 mmol) is added at 0 °C under nitrogen atmosphere. The reaction is stirred at r.t. and monitored by TLC (n-hex/EtOAc 1:1 + 1% HCOOH). After 1 h, the reaction is quenched with saturated NH4Cl aqueous solution and extracted with abundant CH2Cl2. The organic phase is then dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude mixture is then purified by Biotage® (gradient with n-hex/EtOAc + 1% HCOOH eluent mixture) to obtain 57 mg of the desired product 10a (48% yield).
1H NMR (400 MHz, CDCl3) δ 6.71 (s, 1H), 6.52 (s, 1H), 6.39 (s, 2H), 5.94 (dd, J = 10.8, 1.4 Hz, 2H), 5.72 (d, J = 5.0 Hz, 1H), 4.41 (dd, J = 9.6, 6.8 Hz, 1H), 4.36 (d, J = 3.7 Hz, 1H), 4.29 (dd, J = 9.7, 2.9 Hz, 1H), 3.81 (s, 2H), 3.78 (s, 6H), 3.26 (dd, J = 9.2, 3.7 Hz, 1H), 2.99–2.88 (m, 1H), 2.33 (t, J = 7.5 Hz, 2H), 2.21 (td, J = 7.5, 4.6 Hz, 2H), 1.66–1.49 (m, 4H), 1.35–1.22 (m, 9H).
13C NMR (100 MHz, CDCl3) δ 179.6, 177.4, 173.5, 153.3 (2C), 148.4, 147.3, 138.9, 136.9, 131.3, 126.4, 109.8, 108.2, 105.5 (2C), 101.5, 72.3, 71.0, 60.9, 56.2 (2C), 45.6, 44.2, 39.9, 34.3, 34.0, 29.0 (2C), 29.0, 29.0, 24.8, 24.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C32H38O11Na, 621.2312; found, 621.2313.
1-((1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl) 10-((5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9-hexahydrofuro[3’,4’:6,7] naphtho[2,3-d][1,3]dioxol-5-yl) decanedioate (7a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 10a (59 mg, 0.093 mmol), EDC∙HCl (22 mg, 0.112 mmol), DMAP (6 mg, 0.047 mmol) in dry CH2Cl2 (2 mL, 0.05 M), 1 (44 mg, 0.140 mmol) is added to obtain 69 mg of product 7a with 80% yield. Reaction is monitored by TLC (1:1 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 6.72 (s, 1H), 6.56–6.51 (m, 2H), 6.40 (s, 2H), 6.37 (d, J = 1.8 Hz, 1H), 5.95 (dd, J = 11.0, 1.4 Hz, 2H), 5.74 (d, J = 5.1 Hz, 1H), 5.53 (s, 1H), 4.62–4.57 (m, 1H), 4.47–4.35 (m, 3H), 4.31 (dd, J = 9.6, 2.8 Hz, 1H), 3.83 (s, 3H), 3.80 (s, 6H), 3.47 (s, 1H), 3.26 (dd, J = 9.2, 3.7 Hz, 1H), 2.99–2.89 (m, 1H), 2.59–2.37 (m, 5H), 2.30–2.14 (m, 3H), 2.11–2.01 (m, 1H), 1.86–1.61 (m, 7H), 1.60 (s, 3H), 1.59–1.50 (m, 4H), 1.42–1.21 (m, 12H), 0.87 (t, J = 6.9 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 177.3, 173.5, 153.4, 148.5, 147.3, 139.0, 137.0, 131.3, 126.5, 109.9, 108.2, 105.6 (2C), 101.5, 72.3, 71.0, 68.1, 60.9, 56.3 (2C), 45.7 (2C), 44.3, 39.9, 38.0, 35.5 (2C), 34.4, 34.3, 31.6, 30.6, 30.3, 29.2, 29.2, 29.2, 29.2, 28.2, 25.7, 24.9, 24.9, 23.7, 22.6, 20.0, 14.1.
HRMS (ESI+): m/z [M + Na]+ calcd. for C53H66O12Na, 917.4452; found, 917.4457.
: -24.5 (c 1.07 in CHCl3).
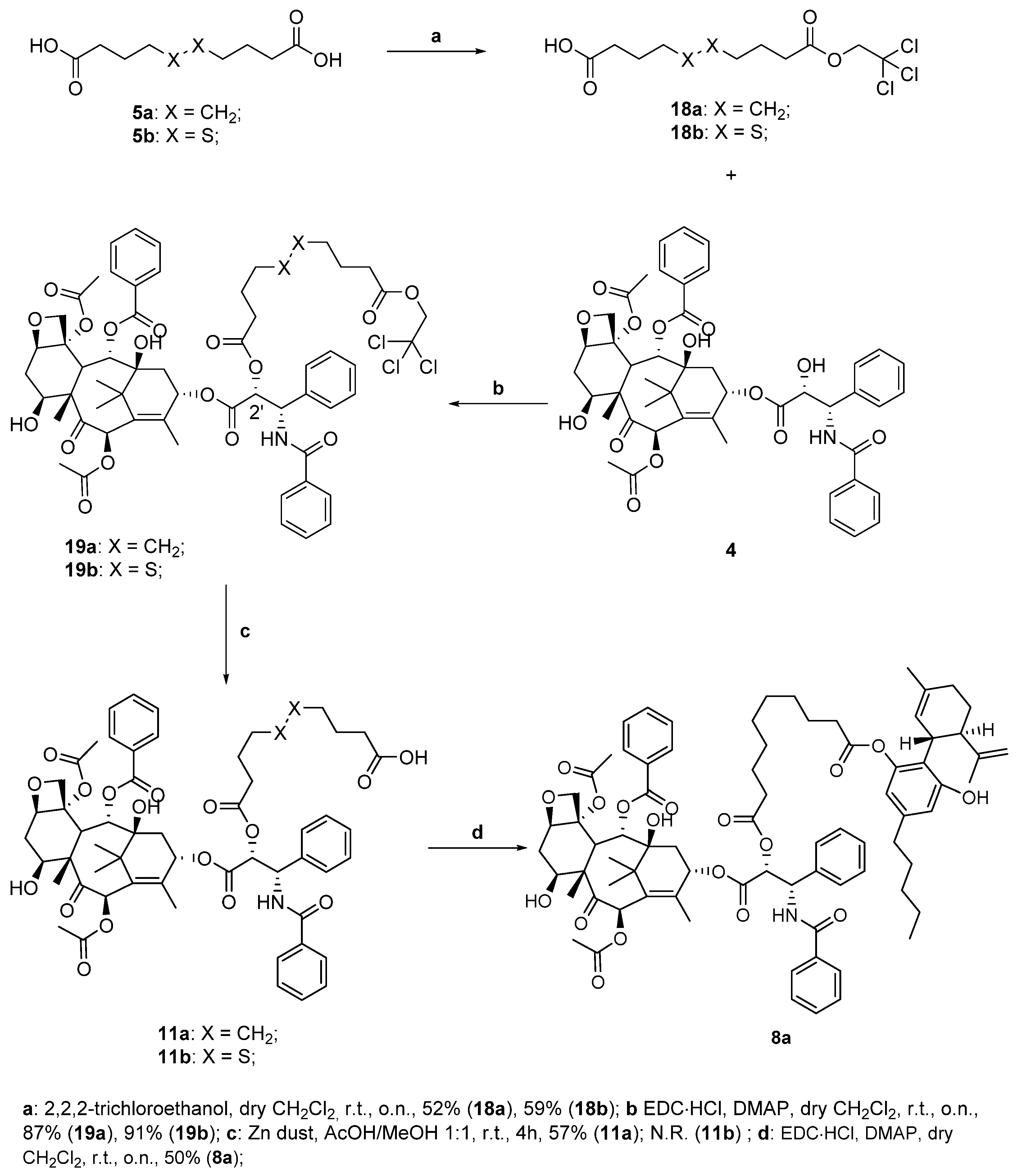
(2aR,4S,4aS,6R,9S,11S,12S,12bS)-9-(((R)-17-((S)-benzamido(phenyl)methyl)-2,2-dimethyl-6,15-dioxo-5,16-dioxa-10,11-dithia-2-silaoctadecan-18-oyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4] benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate (18b)
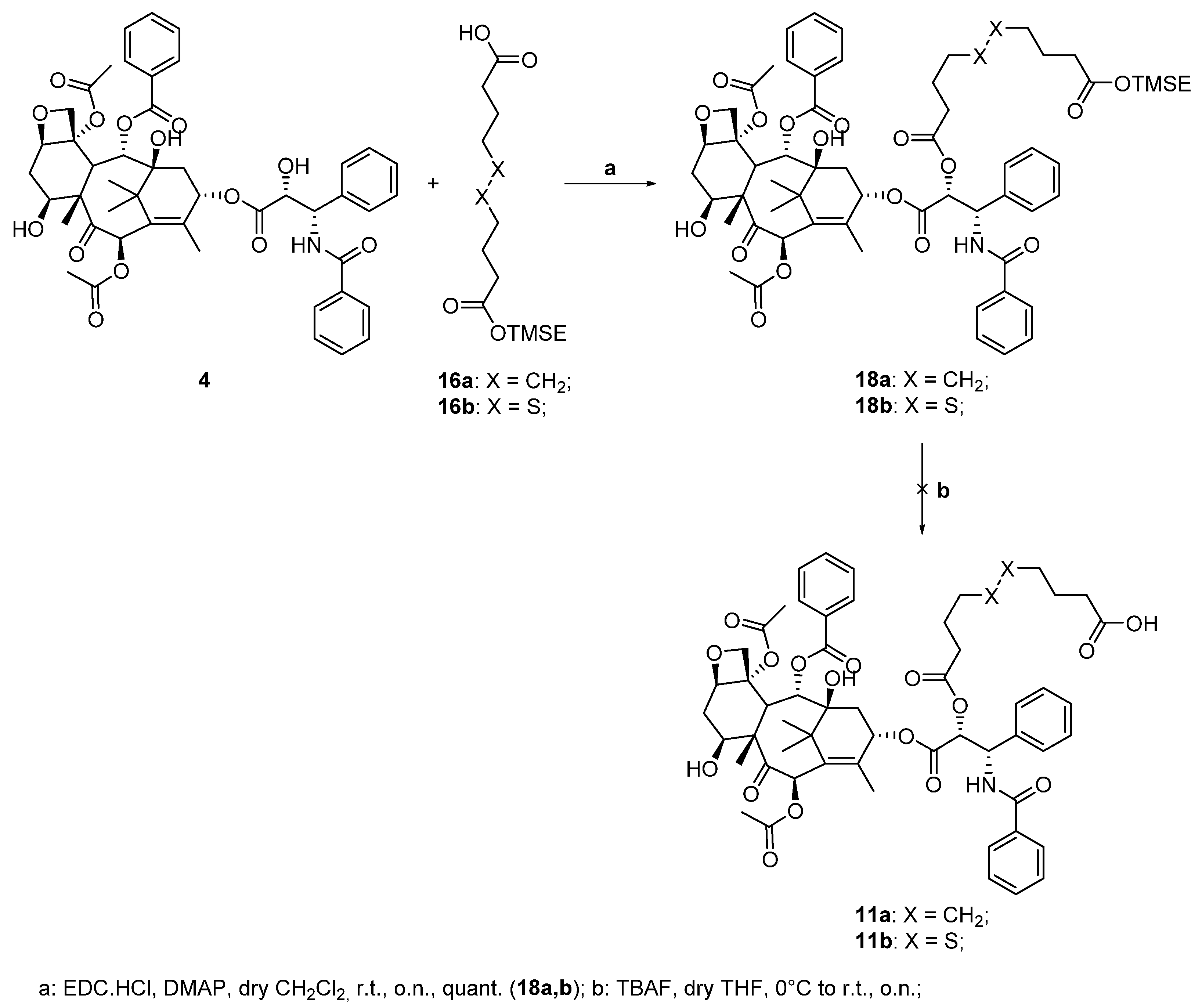
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16b (36 mg, 0.11 mmol), EDC∙HCl (21 mg, 0.11 mmol), DMAP (6 mg, 0.045 mmol) in dry CH2Cl2 (1.5 mL, 0.05 M), 4 (75 mg, 0.09 mmol) is added to obtain 100 mg of product 18b quantitatively. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.17–8.10 (m, 2H), 7.77–7.70 (m, 2H), 7.65–7.56 (m, 1H), 7.55–7.46 (m, 3H), 7.46–7.30 (m, 7H), 6.94 (d, J = 9.2 Hz, 1H), 6.32–6.21 (m, 2H), 5.98 (dd, J = 9.2, 3.1 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.1 Hz, 1H), 4.97 (dd, J = 9.6, 2.3 Hz, 1H), 4.44 (dd, J = 10.9, 6.6 Hz, 1H), 4.31 (d, J = 8.5 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 4.18–4.08 (m, 2H), 3.82 (d, J = 7.0 Hz, 1H), 2.75–2.61 (m, 4H), 2.61–2.48 (m, 4H), 2.46 (s, 3H), 2.42–2.32 (m, 3H), 2.24–2.12 (m, 4H), 2.09–1.96 (m, 4H), 1.94 (s, 3H), 1.92–1.80 (m, 2H), 1.68 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H), 1.03–0.91 (m, 2H), 0.03 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 203.9, 171.2, 169.9, 168.1, 167.3, 167.0, 142.7, 137.0, 133.7, 132.1, 130.3 (2C), 129.2 (2C), 128.8 (2C), 128.6 (2C), 127.2 (2C), 126.6 (2C), 84.5, 76.5, 75.7, 75.2, 74.1, 72.1, 71.9, 62.8, 58.5, 52.8, 45.7, 37.7, 37.1, 35.7, 35.6, 32.8, 32.0, 26.9, 24.3, 24.0, 22.8, 22.2, 17.4, 14.9, 9.7, −1.4 (3C).
HRMS (ESI+): m/z [M + Na]+ calcd. for C60H75NO17S2SiNa, 1196.4143; found, 1196.4149.
1-((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl) 10-(2-(trimethylsilyl)ethyl) decanedioate (18a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16a (71 mg, 0.071 mmol), EDC∙HCl (14.0 mg, 0.071 mmol), DMAP (4.0 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0,05 M), 4 (50 mg, 0.059 mmol) is added to obtain 59.9 mg of product 18a with 87% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 7.4 Hz, 2H), 7.73 (d, J = 7.4 Hz, 2H), 7.58 (t, J = 7.4 Hz, 1H), 7.53–7.44 (m, 3H), 7.44–7.34 (m, 6H), 7.34–7.27 (m, 1H), 7.00 (d, J = 9.1 Hz, 1H), 6.28 (s, 1H), 6.27–6.16 (m, 1H), 5.93 (dd, J = 9.2, 3.5 Hz, 1H), 5.65 (d, J = 7.1 Hz, 1H), 5.50 (d, J = 3.5 Hz, 1H), 4.94 (dd, J = 9.6, 2.3 Hz, 1H), 4.42 (dd, J = 10.9, 6.6 Hz, 1H), 4.28 (d, J = 8.5 Hz, 1H), 4.21–4.09 (m, 3H), 3.79 (d, J = 7.0 Hz, 1H), 2.60–2.46 (m, 1H), 2.43 (s, 3H), 2.42–2.28 (m, 3H), 2.24 (t, J = 7.5 Hz, 3H), 2.19 (s, 3H), 2.17–2.07 (m, 1H), 1.92 (s, 3H), 1.90–1.76 (m, 1H), 1.65 (s, 3H), 1.63–1.50 (m, 4H), 1.32–1.18 (m, 11H), 1.11 (s, 3H), 1.01–0.91 (m, 2H), 0.02 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 203.9, 174.0, 172.8, 171.2, 169.8, 168.2, 167.2, 167.0, 142.8, 137.1, 133.8, 133.7, 132.8, 132.0, 130.2 (2C), 129.3, 129.1 (2C), 128.8 (2C), 128.7 (2C), 128.5, 127.2 (2C), 126.7 (2C), 84.5, 81.1, 79.1, 76.5, 75.6, 75.2, 73.9, 72.1, 71.8, 62.4, 58.5, 53.0, 45.7, 43.2, 35.6, 34.5 (2C), 33.8, 29.1, 29.1, 29.0, 28.9, 26.8, 24.9, 24.7, 22.7, 22.2, 20.9, 17.3, 14.8, 9.7, −1.4 (3C).
HRMS (ESI+): m/z [M + Na]+ calcd. for C62H79NO17SiNa, 1160.5015; found, 1160.5019.
1-((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl) 10-(2,2,2-trichloroethyl) decanedioate (19a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 18a (24 mg, 0.071 mmol), EDC∙HCl (14 mg, 0.071 mmol), DMAP (4 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0.05 M), 4 (50 mg, 0.059 mmol) is added to obtain 60 mg of product 19a with 87% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.18–8.10 (m, 2H), 7.78–7.70 (m, 2), 7.65–7.57 (m, 1H), 7.56–7.47 (m, 3H), 7.46–7.30 (m, 7H), 6.87 (d, J = 9.2 Hz, 1H), 6.32–6.21 (m, 2H), 5.95 (dd, J = 9.2, 3.2 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.50 (d, J = 3.2 Hz, 1H), 4.98 (dd, J = 9.8, 2.3 Hz, 1H), 4.74 (s, 2H), 4.45 (dd, J = 10.9, 6.6 Hz, 1H), 4.32 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.82 (d, J = 7.0 Hz, 1H), 2.56 (ddd, J = 14.7, 9.8, 6.6 Hz, 1H), 2.49–2.28 (m, 8H), 2.23 (s, 3H), 2.21–2.10 (m, 1H), 1.94 (d, J = 1.4 Hz, 3H), 1.89 (ddd, J = 14.6, 11.0, 2.4 Hz, 1H), 1.79–1.51 (m, 7H), 1.35–1.21 (m, 11H), 1.13 (s, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 172.8 (2C), 171.3, 169.9, 168.2, 167.2, 167.1, 142.9, 137.1, 133.8, 133.8, 132.9, 132.1, 130.3, 129.3 (4C), 129.1 (4C), 128.8, 128.6, 127.2 (2C), 126.6 (2C), 95.2, 84.6, 81.2, 79.2, 76.5, 75.7, 75.2, 74.0, 73.9, 72.2, 71.9, 58.6, 52.9, 45.7, 43.3, 35.7, 35.6, 34.0, 33.8, 29.1, 29.0 (2C), 28.9, 26.9, 24.8, 22.8, 22.2, 20.9, 14.9, 14.3, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C59H68Cl3NO17Na, 1190.3451; found, 1190.3454.
1-((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl) 10-(2,2,2-trichloroethyl) decanedioate (19b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 18b (26.0 mg, 0.071 mmol), EDC∙HCl (14.0 mg, 0.071 mmol), DMAP (4.0 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0,05 M), 4 (50.0 mg, 0.059 mmol) is added to obtain 77.7 mg of product 19b with 91% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.17–8.10 (m, 2H), 7.78–7.69 (m, 2H), 7.65–7.55 (m, 1H), 7.56–7.46 (m, 3H), 7.47–7.31 (m, 7H), 6.90 (d, J = 9.2 Hz, 1H), 6.32–6.21 (m, 2H), 5.97 (dd, J = 9.2, 3.1 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.1 Hz, 1H), 4.97 (dd, J = 9.7, 2.3 Hz, 1H), 4.74 (s, 2H), 4.45 (dd, J = 10.9, 6.6 Hz, 1H), 4.32 (d, J = 8.4 Hz, 1H), 4.23–4.17 (m, 1H), 3.82 (d, J = 7.0 Hz, 1H), 2.74–2.46 (m, 9H), 2.46 (s, 3H), 2.38 (dd, J = 15.4, 9.3 Hz, 1H), 2.23 (s, 3H), 2.22–2.12 (m, 1H), 2.12–1.95 (m, 4H), 1.95 (s, 3H), 1.94–1.83 (m, 1H), 1.68 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 172.8 (2C), 171.3, 169.9, 168.2, 167.2, 167.1, 142.9, 137.1, 133.8, 133.8, 132.9, 132.1, 130.3, 129.3 (4C), 129.1 (4C), 128.8, 128.6, 127.2 (2C), 126.6 (2C), 95.2, 84.6, 81.2, 79.2, 76.5, 75.7, 75.2, 74.0, 73.9, 72.2, 71.9, 58.6, 52.9, 45.7, 43.3, 35.7, 35.6, 34.0, 33.8, 28.9, 26.9, 24.8, 22.8, 22.2, 20.9, 14.9, 14.3, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C57H64Cl3NO17S2Na, 1226.2579; found, 1226.2583.
10-(((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl)oxy)-10-oxodecanoic acid (11a)
Following the reported procedure described by Negretti et al. [
32], to a solution of the trichloroethyl ester
19a (59.9 mg, 0.051 mmol) in AcOH/MeOH 1:1 (2 mL, 0.03 M), zinc dust (83.4 mg, 1.275 mmol) is added at r.t. under vigorous stirring. The reaction is monitored by TLC (
n-hex/EtOAc 4:6 + 1% HCOOH) and, after 4 h, is filtered over celite washing with MeOH. The organic phase is then washed with H
2O extracting with abundant EtOAc, dried over anhydrous Na
2SO
4, filtered, and evaporated under reduced pressure. The crude mixture is purified by Biotage
® (gradient with
n-hex/EtOAc + 1% HCOOH as eluent mixture), obtaining 30.1 mg of product
11a with 57% yield.
1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 7.6 Hz, 2H), 7.72 (d, J = 7.6 Hz, 2H), 7.60 (t, J = 7.3 Hz, 1H), 7.50 (dd, J = 8.7, 6.6 Hz, 3H), 7.45–7.29 (m, 7H), 6.97 (d, J = 9.2 Hz, 1H), 6.30 (s, 1H), 6.25 (t, J = 9.1 Hz, 1H), 5.96 (dd, J = 9.2, 3.3 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.3 Hz, 1H), 4.97 (dd, J = 9.7, 2.3 Hz, 1H), 4.43 (dd, J = 10.9, 6.7 Hz, 1H), 4.30 (d, J = 8.5 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 3.81 (d, J = 7.0 Hz, 1H), 2.63–2.49 (m, 1H), 2.49–2.30 (m, 6H), 2.30–2.11 (m, 6H), 1.93 (s, 3H), 1.92–1.82 (m, 1H), 1.67 (s, 3H), 1.62–1.49 (m, 4H), 1.33–1.18 (m, 11H), 1.13 (s, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 178.5, 172.9, 171.4, 170.0, 168.3, 167.6, 167.1, 142.8, 137.0, 133.8, 133.7, 133.0, 132.2, 130.3, 129.4 (4C), 129.2 (2C), 128.9, 128.8, 128.6, 127.2 (2C), 126.7 (2C), 84.6, 81.2, 79.1, 76.6, 75.8, 75.3, 73.9, 72.2, 71.9, 58.6, 53.0, 45.7, 43.3, 35.7, 33.9, 33.8, 29.0, 28.8, 26.9, 24.7, 24.7, 22.8, 22.2, 20.9, 14.9, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C57H67NO17Na, 1060.4307; found, 1060.4311.
10-(((1S,2R)-1-benzamido-3-(((2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-diacetoxy-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-9-yl)oxy)-3-oxo-1-phenylpropan-2-yl)oxy)-10-oxodecanoic acid (8a)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 11a (30.1 mg, 0.029 mmol), EDC∙HCl (7.0 mg, 0.044 mmol), DMAP (2.0 mg, 0.015 mmol) in dry CH2Cl2 (1 mL, 0.05 M), 1 (14 mg, 0.044 mmol) is added to obtain 34.1 mg of product 8a with 88% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 7.7 Hz, 2H), 7.73 (d, J = 7.6 Hz, 2H), 7.61 (t, J = 7.4 Hz, 1H), 7.56–7.46 (m, 3H), 7.45–7.30 (m, 7H), 6.88 (d, J = 9.2 Hz, 1H), 6.54 (s, 1H), 6.38 (d, J = 1.7 Hz, 1H), 6.32–6.20 (m, 2H), 5.96 (dd, J = 9.2, 3.3 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.51 (d, J = 3.3 Hz, 2H), 4.97 (dd, J = 9.6, 2.3 Hz, 1H), 4.60 (s, 1H), 4.49–4.40 (m, 2H), 4.31 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 3.82 (d, J = 7.0 Hz, 1H), 3.48 (s, 1H), 2.64–2.31 (m, 11H), 2.22 (s, 3H), 2.20–1.98 (m, 3H), 1.95 (s, 3H), 1.93–1.70 (m, 6H), 1.68 (s, 3H), 1.63–1.52 (m, 7H), 1.42–1.24 (m, 12H), 1.23 (s, 3H), 1.13 (s, 3H), 0.87 (t, J = 6.7 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 204.0, 175.6, 172.8, 171.4, 171.3, 170.9, 169.9, 168.2, 167.2, 167.2, 142.9, 137.1, 135.1, 133.8, 132.9, 132.2, 130.4, 129.3 (4C), 129.2 (4C), 128.9, 128.6, 127.2 (2C), 126.6 (2C), 84.6, 81.2, 79.3, 76.6, 75.7, 75.2, 73.9, 72.3, 71.9, 58.6, 52.9, 45.7, 43.3, 38.0, 35.7, 35.6, 35.5, 34.4, 33.9, 31.6, 30.8, 30.6, 29.3, 29.2, 29.0, 26.9, 24.9, 24.8, 23.7, 22.8, 22.6, 22.3, 20.9, 14.9, 14.1, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C78H95NO18Na, 1356.6447; found, 1356.6452.
: -65.9 (c 0.69 in CHCl3).
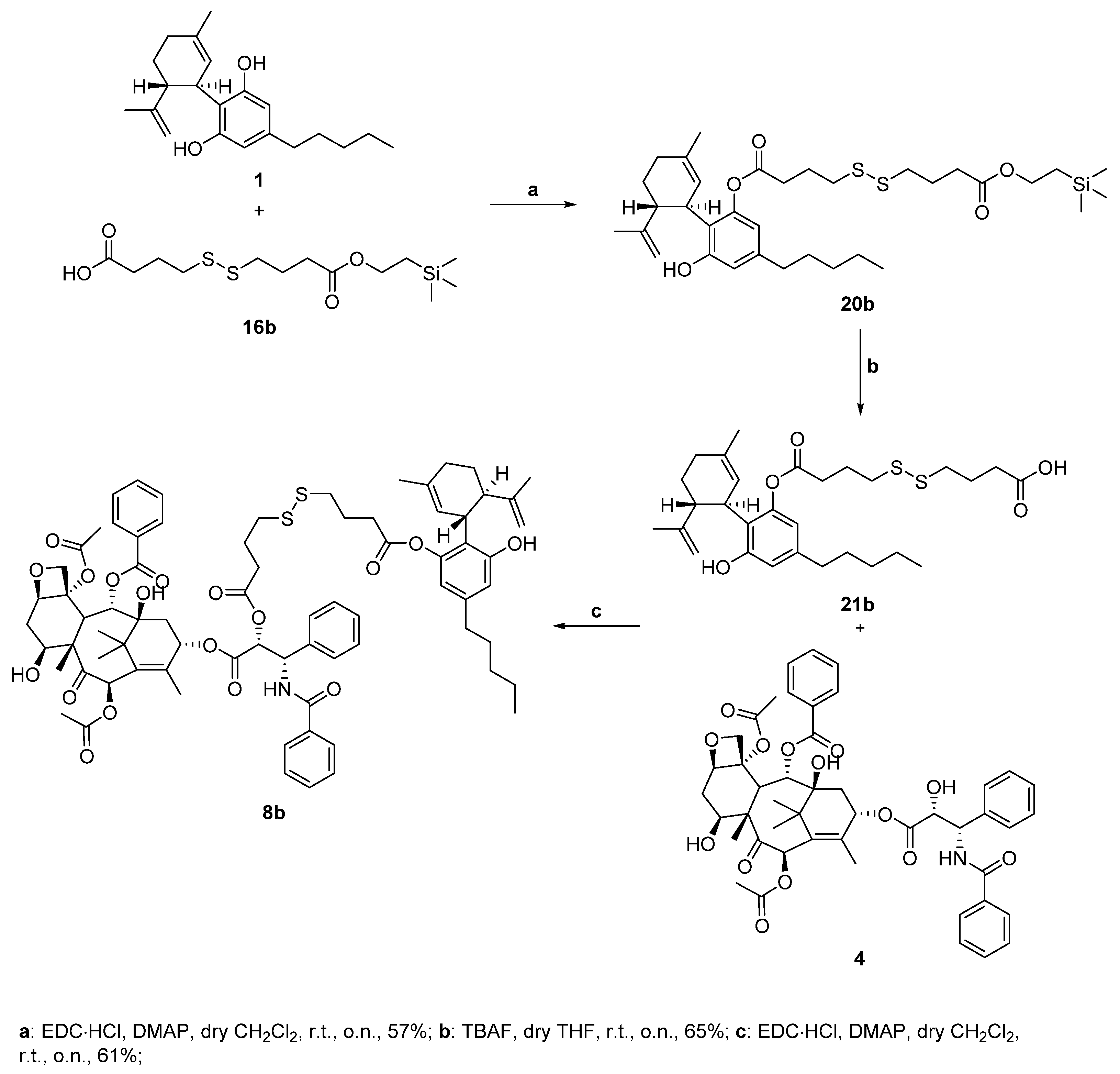
(1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl 4-((4-oxo-4-(2-(trimethylsilyl)ethoxy)butyl)disulfaneyl)butanoate (20b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 16b (150 mg, 0.44 mmol), EDC∙HCl (93 mg, 0.049 mmol), DMAP (71 mg, 0.58 mmol) in dry CH2Cl2 (9 mL, 0.05 M), 1 (279 mg, 0.89 mmol) is added to obtain 159 mg of product 20b with 57% yield. Reaction is monitored by TLC (9:1 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) 6.54 (bs, 1H), 6.38 (s, 1H), 5.98 (bs, 1H), 5.52 (bs, 1H), 4.65–4.55 (m, 1H), 4.44 (bs, 1H), 4.24–4.10 (m, 2H), 3.48 (bs, 1H), 2.78 (t, J = 7.1 Hz, 2H), 2.73 (t, J = 7.1 Hz, 2H), 2.68–2.58 (m, 2H), 2.53–2.38 (m, 5H), 2.29–2.07 (m, 4H), 2.07–1.98 (m, 2H), 1.87–1.67 (m, 5H), 1.61–1.51 (m, 5H), 1.38–1.21 (m, 5H), 1.03–0.95 (m, 2H), 0.87 (t, J = 6.8 Hz, 3H), 0.04 (s, 9H).
13C NMR (100 MHz, CDCl3) δ 172.9, 171.4, 156.8, 149.2, 143.1, 140.7, 139.1, 123.3, 114.8, 114.1, 111.6, 62.6, 45.7, 38.1, 37.6, 35.5, 32.5, 31.6, 30.6, 30.3, 29.8, 28.0, 24.1, 23.8, 22.6, 20.1, 17.3, 14.2, −1.5.
HRMS (ESI+): m/z [M + Na]+ calcd. for C34H54O5S2SiNa, 657.3080; found, 657.3084.
4-((4-(((1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl)oxy)-4-oxobutyl)disulfaneyl)butanoic acid (21b)
To a well-stirred solution of compound 20b (155.0 mg, 0.244 mmol) in dry THF (5.0 mL, 0.05M), 1M TBAF in THF (2.44 mL, 2.44 mmol) is added at 0 °C under nitrogen atmosphere. The reaction is stirred at r.t. and monitored by TLC (n-hex/EtOAc 7:3 + 1% HCOOH). The reaction is quenched with saturated NH4Cl aqueous solution and extracted with abundant EtOAc. The organic phase is then dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude mixture is then purified by flash chromatography (n-hex/EtOAc 8:2 + 1% HCOOH eluent mixture) to obtain 78 mg of the desired product 21b with 65% yield.
1H NMR (400 MHz, CDCl3) δ 6.54 (bs, 1H), 6.38 (s, 1H), 5.98 (bs, 1H), 5.52 (bs, 1H), 4.60 (s, 1H), 4.44 (s, 1H), 3.48 (bs, 1H), 2.78 (t, J = 7.1 Hz, 2H), 2.73 (t, J = 7.1 Hz, 2H), 2.68–2.59 (m, 2H), 2.53–2.38 (m, 4H), 2.23–2.07 (m, 3H), 2.03 (p, J = 7.2 Hz, 2H), 1.86–1.66 (m, 5H), 1.62–1.52 (m, 5H), 1.35–1.24 (m, 5H), 0.93–0.82 (m, 3H).
13C NMR (100 MHz, CDCl3) δ 176.9, 171.4, 155.8, 149.2, 143.1, 140.7, 139.1, 123.3, 114.8, 114.1, 111.6, 45.7, 38.1, 37.6, 35.5, 32.5, 31.6, 30.6, 30.3, 29.8, 28.0, 24.1, 23.8, 22.6, 20.1, 14.2.
HRMS (ESI+): m/z [M + Na]+ calcd. for C29H42O5S2Na, 557.2371; found, 557.2377.
(2aR,4S,4aS,6R,9S,11S,12S,12bS)-9-(((2R,3S)-3-benzamido-2-((4-((4-(((1’R,2’R)-6-hydroxy-5’-methyl-4-pentyl-2’-(prop-1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-2-yl)oxy)-4-oxobutyl)disulfaneyl)butanoyl)oxy)-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca [
3,
4]
benzo [1,2-b]oxete-6,12b(2aH)-diyl diacetate (8b)
Following the general procedure for the Steglich coupling, to a solution of carboxylic acid 21b (32 mg, 0.059 mmol), EDC∙HCl (11 mg, 0.059 mmol), DMAP (4 mg, 0.030 mmol) in dry CH2Cl2 (1.5 mL, 0.05 M), 4 (50 mg, 0.059 mmol) is added to obtain 49 mg of product 8b with 61% yield. Reaction is monitored by TLC (4:6 n-hex/EtOAc) and purified by Biotage® (gradient with n-hex/EtOAc eluent mixture).
1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 7.6 Hz, 2H), 7.76–7.70 (m, 1H), 7.64–7.55 (m, 1H), 7.55–7.45 (m, 3H), 7.45–7.30 (m, 7H), 6.93 (d, J = 9.2 Hz, 1H), 6.54 (s, 1H), 6.37 (d, J = 1.6 Hz, 1H), 6.32–6.20 (m, 2H), 5.97 (dd, J = 9.2, 3.2 Hz, 1H), 5.68 (d, J = 7.0 Hz, 1H), 5.51 (d, J = 3.2 Hz, 1H), 4.97 (d, J = 9.8 Hz, 1H), 4.61–4.56 (m, 1H), 4.48–4.39 (m, 2H), 4.31 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.81 (d, J = 7.0 Hz, 1H), 3.46 (bs, 1H), 2.76–2.43 (m, 15H), 2.37 (dd, J = 15.3, 9.4 Hz, 1H), 2.21 (s, 3H), 2.20–1.95 (m, 8H), 1.96–1.92 (m, 3H), 1.92–1.69 (m, 8H), 1.67 (s, 3H), 1.62–1.50 (m, 6H), 1.35–1.25 (m, 4H), 1.22 (s, 3H), 1.13 (s, 3H), 0.86 (t, J = 6.6 Hz, 3H).
13C NMR (100 MHz, CDCl3) δ 203.9, 172.0, 171.3, 169.9, 168.1, 167.2, 167.1, 142.8, 137.0, 133.8, 132.9, 132.1, 130.3, 129.3, 129.2, 128.8, 128.6, 127.2, 126.6, 117.2, 114.8, 84.6, 81.2, 79.2, 76.5, 75.7, 75.2, 74.2, 72.2, 72.0, 60.5, 58.6, 52.8, 45.7, 43.3, 38.1, 37.5, 37.1, 35.6, 35.5, 32.5, 32.0, 31.6, 30.6, 24.1, 24.0, 23.7, 22.8, 22.6, 22.2, 21.1, 20.9, 20.1, 14.9, 14.3, 14.1, 9.7.
HRMS (ESI+): m/z [M + Na]+ calcd. for C76H91NO18S2Na, 1392.5575; found, 1392.5579.