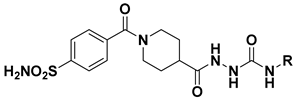
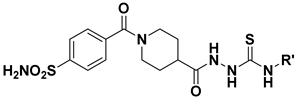
Abstract
Here we report a small library of hydrazinocarbonyl-ureido and thioureido benzenesulfonamide derivatives, designed and synthesized as potent and selective human carbonic anhydrase inhibitors (hCAIs). The synthesized compounds were evaluated against isoforms hCA I, II, IX and XII using acetazolamide (AAZ) as standard inhibitor. Several urea and thiourea derivatives showed inhibitory activity at low nanomolar levels with selectivity against the cytosolic hCA II isoform, as well as the transmembrane, tumor-associated enzymes hCA IX and XII. The thiourea derivatives showed enhanced potency as compared to urea analogues. Additionally, eight compounds 5g, 5m, 5o, 5q, 6l, 6j, 6o and 6u were selected for docking analysis on isoform I, II, IX, XII to illustrate the potential interaction with the enzyme to better understand the activity against the different isoforms.
1. Introduction
Carbonic Anhydrase (CA) is a well-known family of metalloenzymes which is involved in the reversible conversion of CO2 into hydrogen carbonate ions and protons, water-soluble products that regulate the physiological pH. In the past years, CAs have been extensively studied, identifying eight different families: α, β, γ, δ, ζ, η-, θ- and ι-, of which αCAs are present in humans [1,2]. At the moment, 15 different αCAs isoforms have been distinguished, of which 12 are catalytically active: CAs I-IV, CA VA-VB, CA VI, CA VII, CA IX and CAs XII-XIV, distinguished by their different catalytic efficiencies and cellular localization [2,3]. Three of them, CA VIII, X and XI are called CA-related proteins CARPs. The active isoforms have been further clustered in four different classes differing on localization: hCAs I, II, III, VII, and XIII are the cytosolic isoforms, hCAs IV, IX, XII, and XIV are membrane-associated isoforms, hCAs VA and VB are predominantly expressed in mitochondria, whereas hCA VI is present in saliva and milk. hCAs are spread in several tissues and organs which several implications in physiological processes. Therefore, dysregulation of hCAs is related with several pathological processes such as glaucoma, epilepsy, edema, obesity and tumors [3,4,5]. All hCAs have in common a highly conserved active site where an Zn2+, is coordinated by His94, His96, His119 and by a water molecule, which is crucial for the catalytic activity [6]. Among all hCA, two isoforms, hCA IX and hCA, have been intensively studied as targets for the development of antiproliferative compounds, due to their role in survival of hypoxic tumors [6,7,8,9,10,11]. Cancer is generally characterized by an abnormal cell growth and spreading into neighboring tissues, but typically this overgrowing is not followed by correct vascularization with a poor oxygen and nourishment delivery as a consequence. Inevitably, this condition is related with the presence of multiple hypoxic regions which might limit the tumor progression. The hypoxia environment leads to important changes in gene expressions as an adaptive process, necessary for continual progression and metastasis, mediated by hypoxia-inducible transcription factor (HIF-1α), ref. [12], which promotes anaerobic glycolysis, a metabolic modifications crucial for cell survival [13]. As a result of anaerobic metabolism, a massive amount of lactic acid is present into the cytosol, with a consequent reduction in intracellular pH, which is incompatible with biochemical reaction of the cell [14]. In this context, hCA IX and hCA XII are overexpressed in cancer cells as an important tool for the control of intracellular pH, which allows tumor cells to become highly proliferative, aggressive, and resistant to numerous pharmacological therapies [15]. Therefore, the development of selective hCA IX and hCA XII inhibitors represents an appealing approach for the development of potential antiproliferative compounds. The majority of hCA inhibitors have been defined as zinc-binders [16,17] and among them, primary sulfonamides are the most common hCAi due to their unique interaction, not only with Zn2+, but also with the nearby residues [18,19]. As a continuation of our efforts in the design and synthesis of new potent and selective CAIs [18,19,20,21,22], this study presents a small library of benzenesulfonamide based-compounds, designed to extend the SAR piperidinyl-hydrazidoureides. The new benzenesulfonamides were endowed with a piperidine ring liked to ureido/thioureidoaryl tail and were evaluated against hCA I, hCA II, hCA IX, and hCA XII isoforms. Docking studies were also carried out to better understand the interaction with the different isoforms and confirm the acquired inhibition data.
2. Results
2.1. Chemistry
The target 4-sulfamoylbenzoyl-piperidine derivatives 5a–u and 6a–y were obtained through the synthetic pathway shown in Scheme 1. The key intermediate ethyl 1-(4-sulfamoylbenzoyl)piperidine-4-carboxylate (3) was prepared by amide coupling between 4-sulfamoylbenzoic acid (1) and ethyl piperidine-4-carboxylate (2). The condensation was accomplished in dry acetonitrile solution (CH3CN), using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) as coupling agent, in the presence of 1-hydroxybenzotriazole hydrate (HOBt). The intermediate 3 was converted in the corresponding hydrazide 4 by reaction with hydrazine hydrate in absolute ethanol (EtOH). Finally, hydrazinecarbonyl(piperidine-1-carbonyl)benzenesulfonamide (4) was reacted with substituted isocyanates or isothiocyanates to obtain the corresponding ureas 5a–u and thioureas 6a–y, respectively. The structure of the new compounds was confirmed by analytical data and is consistent with reported studies [18].
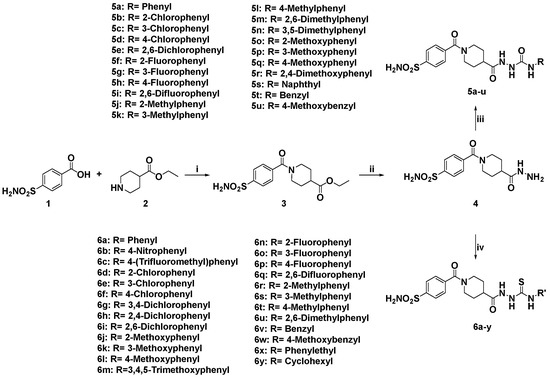
Scheme 1.
General synthetic procedure for sulfonamides subsets 5a–u and 6a–y. Reagents and conditions: (i) EDCI, HOBt, dry CH3CN r.t. 12 h, yield 77%; (ii) NH2NH2·H2O, absolute EtOH, reflux 3 h, yield 78%; (iii) substituted isocyanates, absolute EtOH, reflux 6 h, yield 34–91%; (iv) substituted isothiocyanates, absolute EtOH, reflux 6 h, yield 44–98%.
2.2. Carbonic Anhydrase Inhibition
The hCA I, hCA II, hCA IX and hCA XII inhibitory activity of sulfonamide derivatives 5a–u (Table 1) and 6a–y (Table 2) was assayed by a stopped flow CO2 hydrase assay using the standard inhibitor acetazolamide as positive control [23].

Table 1.
Inhibition data of human CA isoforms CA I, II, IX and XII with sulfonamides 5a–u reported here and the standard sulfonamide inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay [23].

Table 2.
Inhibition data of human CA isoforms CA I, II, IX and XII with sulfonamides 6a–y reported here and the standard sulfonamide inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay [23].
All urea derivatives 5a–u demonstrated active on the tested hCA isoforms with Ki values ranging from low to high nanomolar concentration. The presence of a 2-chlorine on the aryl ring (compound 5b) resulted in high activity on hCA-II, IX and XII while hCA-I was inhibited at higher concentrations. Moving the chlorine atom into 3-position (compound 5c) hCA II activity was retained, while the activity on hCA XII and hCA I was worsened. Interestingly, the shift on the chlorine atom into 4-position (compound 5d) reduced the activity against the four isoforms and hCA I particularly. The presence of a second chlorine atom (compound 5e) caused a slight reduction in activity on hCA II and improvement in selectivity on hCA IX and hCA XII/hCA I when compared with the 2-Cl derivative 5b.
The replacement of 2-chlorine with a methoxy group providing compound 5o, improved the activity on hCA IX and hCA XII as well as the selectivity toward the target isoforms as compared to hCA I and hCA II. Similar results were obtained by the 4-methoxyphenylurea 5q. Shifting the methoxy group into 3-position (compound 5p) retained the activity on hCA IX and hCA XII and increased about 2-fold the activity on hCA II. The introduction of a second methoxy group (compound 5r) produced a further increase in hCA II activity as well as reduction in hCA IX and hCA XII/hCA I selectivity as compared to the 2-methoxy 5o and 4-methoxy 5q analogs.
The replacement of 2-chlorine with a fluorine atom (compound 5f) maintained the good activity on hCA II, hCA IX and hCA XII increasing the hCA IX, hCA XII/hCA I selectivity. The shift of fluorine atom into 3-position (compound 5g) produced reduction in activity on hCA IX while the Ki values on hCA II and hCA XII are similar. Shifting the fluorine into 4-position (compound 5h) improved hCA IX inhibitory activity with high hCA IX/hCA I and hCA IX/hCA II selectivity. The introduction of a second fluorine atom (compound 5i) slight improved the activity on all hCA tested isoforms as compared to 2-fluorine analog 5f, while the selectivity on hCA IX and hCA XII was worsened.
The introduction at 2-position of a methyl group (compound 5j) gave good activity on all CA isoforms. The shift of the methyl group into 3-position (compound 5k) produced change in the selectivity, the tumor-associated isoforms hCA IX and hCA XII being the best inhibited. Shifting the methyl into 4-position (compound 5l) produced a further increase in hCA IX selectivity. The introduction of a second methyl group (compound 5m) produced an increase in hCA IX, hCA XII/hCA I selectivity as compared to the 2-methyl analog 5j.
The unsubstituted thiourea 6a showed high inhibitory activity on hCA XII as well as about 8-fold selectivity on hCA I and hCA II. The introduction of a nitro group into 4-position (compound 6b) gave selectivity on hCA IX if compared to hCA I (about 22-fold) and at minor extent if compared to hCA II and hCA XII (about 4- and 3-fold, respectively).
The replacement of the nitro group with a trifluoromethyl one (compound 6c) produced the best activity on hCA II in thiourea series, also displaying high activity on hCA IX and hCA XII.
The introduction of a 2-chlorine atom (compound 6d) produced reversal of hCA IX and hCA XII inhibition potency as compared to the unsubstituted analog 6a while the selectivity towards these isoforms/hCA I was preserved. The shift of the chlorine into 3-position (compound 6e) did not affect activity as well as selectivity. The shift of the chlorine into 4-position (compound 6f) produced reduction in hCA II activity as compared to the 2- and 3-chlorine analogs. Interestingly, thiourea 6f showed improvement of inhibitory activity on all the tested isoforms as compared to the urea analog 5d. The introduction of a second chlorine atom (compound 6g) produced increase in hCA IX activity as compared to the 3- and 4-chlorine derivatives followed by a high hCA IX/hCA I, hCA II selectivity. The shift of the second chlorine in 2-position (compound 6h) caused about a 10-fold increase in hCA XII activity as compared to the 3,4-dichloro analog. Moving the second chlorine in 6-position (compound 6i) produced an increase in hCA XII inhibitory activity as compared to 2-chlorine analog as well as a high increase in hCA XII/hCA I and hCA II selectivity.
The replacement of the 2-chlorine with a methoxy group (compound 6j) slightly improved the activity on all hCA isoforms attended by about 35-fold hCA IX, hCA XII/hCA I selectivity. Interestingly, the shift of the methoxy group into 4-position (compound 6l) or in 3-position (compound 6k) reduced hCA IX and hCA XII activity and selectivity. The presence of a 3,4,5-trimethoxy group (compound 6m) produced reduction in activity on hCA II as compared to 4-methoxy and 3-methoxy analogs but also a better hCA XII selectivity if compared to the 4-methoxy analog.
The introduction of a fluorine atom at 2-position (compound 6n) produced high activity on hCA II, hCA IX and hCA XII. On shifting the fluorine atom on 3-position (compound 6o) the low nanomolar hCA IX and hCA XII activity was maintained, with about 100- and 20-fold selectivity as compared to hCA I and hCA II, respectively. The 2,6-difluoro derivative 6q displayed the same activity profile of 6o with a reduction in selectivity. The 4-fluorine derivative 6p showed reduction in activity and selectivity as compared to all fluorine-substituted thiourea derivatives. Furthermore, fluorine-substituted thioureas showed better hCA XII inhibitory activity than the urea analogs.
The 2-methylsubstitued thiourea 6r showed the best activity on hCA IX. The shift of methyl group into 3-position (compound 6s) caused about 8-fold increase in hCA II and hCA XII while the activity on hCA IX is almost unchanged as compared to the 2-methyl analog. The shift on the methyl group into 4-position (compound 6t) caused a decrease in activity on all isoforms as compared to 2- and 3-methyl analogs, but the selectivity of hCA IX and hCA XII versus hCA I was maintained. The introduction of a second methyl group (compound 6u) produced the best hCA IX and hCA XII selective compound of the methyl-substituted thioureas.
2.3. Molecular Docking
Eight selected compounds, four ureas and four thioureas (5g, 5m, 5o, 5q, 6l, 6j, 6o and 6u), were docked in the four CA isoforms analyzed in this work to explore their interactions with CA active sites. The docking poses (Figures S1 and S2) are in agreement with what was already described in our previous study [20]. As expected, in this case the leading interaction is also represented by the coordination between the negatively charged nitrogen and the Zinc ion with the sulfonamide group deeply fitted into the active site. Moreover, as seen in other papers, the hydrogen of the sulfonamide establishes an H-Bond with the T199 which helps stabilize the system.
Despite the greater flexibility of this series of compounds which is responsible for the high variability of the observed binding pose, is it possible to retrieve some interaction that characterized the binding poses on the different isoforms. Concerning hCA II, the interactions with ASN62 and/or ASN67 are quite frequent as already observed. GLN92 is the recurrent interaction with hCA IX, especially for the thioureas series. Last but not least, the recurrent interaction with the hCA XII isoform is represented by the H-Bond between the ureidic/thioureidic -NH and SER132.
3. Materials and Methods
3.1. Chemistry
All commercially available solvents and reagents were used without further purification. 1H NMR spectra were recorded on an Inova 500 spectrometer (Varian, Palo Alto, CA, USA). 1H and 13C NMR spectra for compounds 5m, 5o, 6j and 6u were recorded on Bruker Avance III HD 600 spectrometer. The chemical shifts (δ) are reported in part per million downfield from tetramethylsilane (TMS), which was used as internal standard. The spectra were recorded in hexadeuteriodimethylsulphoxide (DMSO-d6). Infrared spectra were recorded on a Vector 22 spectrometer (Bruker, Bremen, Germany) in Nujol mulls. The main bands are given in cm−1. Positive-ion electrospray ionization (ESI) mass spectra were recorded on a double-focusing MAT 95 instrument (Finnigan, Waltham, MA, USA) with BE geometry. Melting points (mp) were determined with a SMP1 Melting Point apparatus (Stuart Scientific, Stone, UK) and are uncorrected. All products reported showed 1H NMR spectra in agreement with the assigned structures. The purity of the tested compounds was determined by combustion elemental analyses conducted by the Microanalytical Laboratory of the Chemistry Department of the University of Ferrara with a MT-5 CHN recorder elemental analyser (Yanagimoto, Kyoto, Japan) and the values found were within 0.4% of theoretical values. As previously reported, 4-(4-(Hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (4), ureas 5a, 5c, 5e, 5h, 5l, 5m, and 5r–u were synthesized [18]. Briefly, the key intermediate 4 was prepared by this procedure
A 4-(Aminosulfonyl)benzoic acid (1) (4.2 g, 20 mmol), EDCI (3.9 g, 22 mmol) and HOBt (2.7 g, 20 mmol) were dissolved in anhydrous CH3CN (100 mL). The resulting mixture was stirred at rt for 30 min, then ethyl isonipecotate (2) (3.1 g, 20 mmol) was added. The mixture was stirred at rt for 12 h. The solvent was removed in vacuo and the residue was dissolved in ethyl acetate (30 mL) and washed sequentially with water (2 × 10 mL), saturated NaHCO3 aqueous solution (2 × 10 mL), 10% aqueous citric acid (2 × 10 mL) and brine (2 × 10 mL). The organic layer was dried over anhydrous sodium sulfate (Na2SO4), filtered and evaporated under reduced pressure. The residue was tritured with isopropyl ether (iPr2O) and the formed solid was filtered off, dried to obtain ethyl 1-(4-sulfamoylbenzoyl)piperidine-4-carboxylate (3) in 77% yield [18]. A mixture of crude ester 3 (4.9 g, 15 mmol) and hydrazine monohydrate (2.5 mL, 45 mmol) in EtOH was refluxed overnight. After cooling, the formed precipitate was filtered off, washed with water (3 × 10 mL), dried and used in the next step without further purification. Yield 78% [18].
3.1.1. General Procedure for the Preparation of Benzenesulfonamidohydrazido Ureas (5a–u)
The appropriate isocyanate (1 mmol) was added to a solution of 4-(4-(hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide 4 (0.33 g, 1 mmol) in dry EtOH (5 mL). The reaction mixture was refluxed overnight and then stirred until room temperature was reached. The formed precipitate was separated by suction, washed with Et2O (2 × 5 mL) and recrystalized from EtOH.
- N-(2-Chlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5b). Yield 67% m.p. 214–215 °C. ESIMS (m/z): 480, 482 (M+H)+.1H NMR (DMSO-d6): δ 1.58 (m, 2H, CH2), 1.70, 1.94 (m, 2H, CH2), 2.53, 2.90 (m, 2H, CH2), 3.10, 3.51 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.03 (m, 1H, Ar), 7.28 (m, 1H, Ar), 7.43 (m, 1H, Ar), 7.46 (s, 2H, NH2), 7.57 (d, J = 7.5 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 8.07 (m, 1H, Ar), 8.17 (s, 1H, NH), 8.74 (s, 1H, NH), 9.83 (s, 1H, NH). IR (Nujol) 3334, 1608 cm−1. Elemental analysis: calculated for C20H22ClN5O5S (479.94) %C 50.05, %H 4.62, %N 14.59, found %C 50.09, %H 4.60 %N 14.64.
- N-(4-Chlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5d). Yield 72% m.p. 209–210 °C. ESIMS (m/z): 497 (M+H)+. 1H NMR (DMSO-d6): δ 1.55 (m, 2H, CH2), 1.74, 2.32 (m, 2H, CH2), 2.50, 2.88 (m, 2H, CH2), 3.06, 3.49 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.29 (m, 1H, Ar), 7.44 (m, 4H, Ar and NH2), 7.57 (m, 3H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 8.07 (s, 1H, NH), 9.01 (s, 1H, NH), 9.68 (s, 1H, NH). IR (Nujol) 3303, 3161, 1615 cm−1. Elemental analysis: calculated for C20H22ClN5O5S (496.00) %C 48.43, %H 4.47, %N 14.12, found %C 48.48, %H 4.46 %N 14.17.
- N-(2-fluorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5f). Yield 82% m.p. 229–230 °C. ESIMS (m/z): 464 (M+H)+. 1H NMR (DMSO-d6): δ 1.57 (br s, 2H, CH2) 1.69, 1.84 (br s, 2H, CH2), 2.53, 2.90 (br s, 2H, CH2), 3.10, 3.51 (br s, 2H, CH2), 4.44 (br s, 1H, CH), 6.99 (m, 1H, Ar), 7.13 (m, 1H, Ar), 7.22 (m, 1H, Ar), 7.45 (s, 2H, NH2), 7.56 (d, J = 8.5 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 8.30 (m, 1H, Ar), 8.34 (s, 1H, NH), 8.51 (s, 1H, NH), 9.78 (s, 1H, NH). IR (Nujol) 3269, 1667, 1614 cm−1. Elemental analysis: calculated for C20H22FN5O5S (463.48) %C 51.83, %H 4.78, %N 15.11, found %C 51.77, %H 4.76, %N 15.15.
- N-(3-fluorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5g). Yield 40% m.p. 214–215 °C. ESIMS (m/z): 464 (M+H)+. 1H NMR (DMSO-d6): δ 1.57 (br s, 2H, CH2) 1.71, 1.86 (br s, 2H, CH2), 2.54, 2.89 (br s, 2H, CH2), 3.08, 3.44 (br s, 2H, CH2), 4.45 (br s, 1H, CH), 6.76 (m, 1H, Ar), 7.15 (m, 1H, Ar), 7.27 (m, 1H, Ar), 7.45 (s, 3H, Ar and NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 8.13 (s, 1H, NH), 8.96 (s, 1H, NH), 9.70 (s, 1H, NH). IR (Nujol) 3355, 3264, 1673, 1615 cm−1. Elemental analysis: calculated for C20H22FN5O5S (463.48) %C 51.83, %H 4.78, %N 15.11, found %C 51.88, %H 4.77, %N 15.07.
- N-(2,6-difluorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5i). Yield 34% m.p. 194–195 °C. ESIMS (m/z): 482 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (br s, 2H, CH2) 1.70, 1.85 (br s, 2H, CH2), 2.47, 2.87 (br s, 2H, CH2), 3.08, 3.50 (br s, 2H, CH2), 4.44 (br s, 1H, CH), 7.11 (m, 2H, Ar), 7.28 (m, 1H, Ar), 7.45 (s, 2H, NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 8.22 (s, 1H, NH), 8.31 (s, 1H, NH), 9.74 (s, 1H, NH). IR (Nujol) 3321, 3220, 1645, 1613 cm−1. Elemental analysis: calculated for C20H21F2N5O5S (481,47) %C 49.89, %H 4.40, %N 14.55, found %C 49.83, %H 4.41, %N 14.59.
- 2-(1-(4-Sulfamoylbenzoyl)piperidine-4-carbonyl)-N-(o-tolyl)hydrazinecarboxamide (5j). Yield 87% m.p. 238–240 °C. ESIMS (m/z): 460 (M+H)+. 1H NMR (DMSO-d6): δ 1.57 (br s, 2H, CH2), 1.60, 1.85 (br s, 2H, CH2), 2.25 (s, 3H, CH3), 2.53, 2.89 (br s, 2H, CH2), 3.10, 3.51 (br s, 2H, CH2), 4.45 (br s, 1H, CH), 6.76 (d, J = 7.5 Hz, 1H, Ar), 7.12 (m, 1H, Ar), 7.23 (m, 2H, Ar), 7.45 (s, 2H, NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 7.95 (s, 1H, NH), 8.61 (s, 1H, NH), 9.66 (s, 1H, NH). IR (Nujol) 3358, 3243, 1639, 1615 cm−1. Elemental analysis: calculated for C21H25N5O5S (459.52) %C 54.89, %H 5.48, %N 15.24, found %C 54.94, %H 5.46, %N 15.18.
- 2-(1-(4-Sulfamoylbenzoyl)piperidine-4-carbonyl)-N-(m-tolyl)hydrazinecarboxamide (5k). Yield 91% m.p. 222–225 °C. ESIMS (m/z): 460 (M+H)+. 1H NMR (DMSO-d6): δ 1.58 (br s, 2H, CH2), 1.74, 1.86 (br s, 2H, CH2), 2.26 (s, 3H, CH3), 2.53, 2.90 (br s, 2H, CH2), 3.10, 3.51 (br s, 2H, CH2), 4.46 (br s, 1H, CH), 6.77 (d, J = 7.5 Hz, 1H, Ar), 7.13 (m, 1H, Ar), 7.23 (m, 2H, Ar), 7.46 (s, 2H, NH2), 7.58 (d, J = 8.5 Hz, 2H, Ar), 7.89 (d, J = 8.5 Hz, 2H, Ar), 8.63 (s, 1H, NH), 9.03 (s, 1H, NH), 9.68 (s, 1H, NH). IR (Nujol) 3288, 3054, 1639, 1615 cm−1. Elemental analysis: calculated for C21H25N5O5S (459.52) %C 54.89, %H 5.48, %N 15.24, found %C 54.94, %H 5.46, %N 15.18.
- N-(2,6-Dimethylphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxanide (5m). Yield 85% m.p. 212–213 °C. ESIMS (m/z): 474 (M+H)+. 1H NMR (DMSO-d6): δ 1.57 (m, 2H, CH2), 1.71, 1.83 (m, 2H, CH2), 2.15 (s, 6H, 2CH3), 2.51, 2.87 (m, 2H, CH2), 3.08, 3.51 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.03 (m, 3H Ar) 7.45 (s, 2H, NH2), 7.58 (d, J = 8.0 Hz, 2H, Ar), 7.63 (s, 1H, NH), 7.83 (s, 1H, NH), 7.88 (m, 3H, Ar), 9.68 (s, 1H, NH). 13C NMR (DMSO-d6): δ 173.8, 167.7, 156.3, 144.6, 139.4, 136.0, 135.3, 127.5 (2C), 127.2 (2C), 126.0, 125.8 (2C), 64.9, 46.5, 40.9, 28.3, 27.8, 18.1 (2C), 15.1. IR (Nujol) 3323, 3224, 1611 cm−1. Anal. Calcd for C22H27N5O5S (473.55) %C 55.80, %H 5.75, %N 14.79, found %C 55.85, %H 5.76, %N 14.75
- N-(3,5-Dimethylphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5n). Yield 90%. m.p. 216–217 °C. ESIMS (m/z): 474 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.70, 1.84 (m, 2H, CH2), 2.19 (s, 6H, CH3), 2.53, 2.89 (m, 2H, CH2), 3.09, 3.50 (m, 2H, CH2), 4.45 (m, 1H, CH), 6.58 (s, 1H, Ar), 7.04 (s, 2H, Ar), 7.43 (s, 2H, NH2), 7.56 (d, J = 8.0 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 7.91 (s, 1H, NH), 8.51 (s, 1H, NH), 9.65 (s, 1H, NH). IR (Nujol) 3289, 3068, 1644, 1556 cm−1. Elemental analysis: calculated for C22H27N5O5S (473.55) %C 55.80, %H 5.75, %N 14.79, found %C 55.85, %H 5.76, %N 14.72.
- (2-Methoxyphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5o). Yield 84% m.p. 219–220 °C. ESIMS (m/z): 476 (M+H)+. 1H NMR (DMSO-d6): δ 1.60 (br s, 2H, CH2), 1.70, 1.86 (br s, 2H, CH2), 2.53-2.91 (br s, 2H, CH2), 3.11, 3.52 (br s, 2H, CH2), 3.84 (s, 3H, OCH3), 4.46 (br s, 1H, CH), 6.87 (m, 1H, Ar), 6.93 (m, 1H, Ar), 7.00 (m, 1H, Ar), 7.46 (s, 2H, NH2), 7.59 (d, J = 8.5 Hz, 2H, Ar), 7.90 (d, J = 8.5 Hz, 2H, Ar), 8.04 (d, J = 8.0 Hz, 1H, Ar), 8.09 (s, 1H, NH), 8.59 (s, 1H, NH), 9.78 (s, 1H, NH). 13C NMR (DMSO-d6): δ 173.8, 167.8, 155.1, 147.5, 144.7, 139.5, 128.4, 127.4 (2C), 125.9 (2C), 121.9, 120.6, 118.0, 110.7, 55.7, 46.2, 40.9, 28.5, 27.9, 18.6. IR (Nujol) 3298, 3100, 1663, 1606 cm−1. Elemental analysis: calculated for C21H25N5O6S (475,52) %C 53.04, %H 5.30, %N 14.73, found %C 53.09, %H 5.31, %N 14.69. m/z 476.
- (3-Methoxyphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5p). Yield 62% m.p. 218–220 °C. ESIMS (m/z): 476 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (br s, 2H, CH2), 1.70, 1.85 (br s, 2H, CH2), 2.54, 2.87 (br s, 2H, CH2), 3.09, 3.51 (br s, 2H, CH2), 3.71 (s, 3H, OCH3), 4.45 (br s, 1H, CH), 6.54 (m, 1H, Ar), 6.94 (m, 1H, Ar), 7.14 (m, 2H, Ar), 7.44 (s, 2H, NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 7.98 (s, 1H, NH), 8.70 (s, 1H, NH), 9.67 (s, 1H, NH). IR (Nujol) 3317, 1651, 1614 cm−1. Elemental analysis: calculated for C21H25N5O6S (475,52) %C 53.04, %H 5.30, %N 14.73, found %C 52.99, %H 5.31, %N 14.77.
- (4-Methoxyphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarboxamide (5q). Yield 78% m.p. 228–230 °C. ESIMS (m/z): 476 (M+H)+. 1H NMR (DMSO-d6): δ 1.58 (br s, 2H, CH2), 1.74, 1.86 (br s, 2H, CH2), 2.54, 2.86 (br s, 2H, CH2), 3.08, 3.51 (br s, 2H, CH2), 3.70 (s, 3H, OCH3), 4.45 (br s, 1H, CH), 6.54 (d, J = 9.5 Hz, 1H, Ar), 7.33 (d, J = 8.5 Hz, 1H, Ar), 7.46 (s, 2H, NH2), 7.58 (m, 3H, Ar), 7.88 (m, 3H, Ar), 8.53 (s, 1H, NH), 9.02 (s, 1H, NH), 9.65 (s, 1H, NH). IR (Nujol) 3315, 3216, 1680, 1617 cm−1. Elemental analysis: calculated for C21H25N5O6S (475,52) %C 53.04, %H 5.30, %N 14.73, found %C 52.98, %H 5.32, %N 14.76.
3.1.2. General Procedure for the Preparation of N-aryl-4-Sulfamoylbenzoyl-piperidine-4-carbonyl-hydrazincarbothioamides (6a–y)
A mixture of 4-(4-(hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide 4 (0.33 g, 1 mmol) and the appropriate isothiocyanate (1 mmol) in absolute EtOH (5 mL) was refluxed overnight. After cooling, the formed precipitate was filtered off, washed with Et2O (2 × 5 mL) and recrystalized from EtOH.
- N-phenyl-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazine-1-carbothioamide (6a). Yield 98% m.p. 210–211 °C. ESIMS (m/z): 462 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.76, 1.92 (m, 2H, CH2), 2.54, 2.87 (m, 2H, CH2), 3.08, 3.51 (m, 2H, CH2), 4.44 (m, 1H, CH), 7.15 (m, 1H, Ar), 7.32 (m, 3H, Ar), 7.44 (s, 3H, Ar and NH2), 7.56 (d, J = 7.5 Hz, 2H, Ar), 7.87 (m, 3H, Ar and NH), 9.51 (s, 1H, NH), 9.92 (s, 1H, NH). IR (Nujol) 3334, 3242, 3050, 1687, 1613 cm−1. Elemental analysis: calculated for C20H23N5O4S2 (461.56) %C 52.05, %H 5.02, %N 15.17, found %C 52.11, %H 5.02, %N 15.14.
- N-(4-Nitrophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6b). Yield 85% m.p. 236–237 °C. ESIMS (m/z): 507 (M+H)+. 1H NMR (DMSO-d6) δ 1.56 (m, 2H, CH2), 1.76, 1.91 (m, 2H, CH2), 2.56, 2.89 (m, 2H, CH2), 3.09, 3.52 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.43 (s, 2H, NH2), 7.56 (d, J = 8.0 Hz, 2H, Ar), 7.87 (m, 4H, Ar), 8.19 (d, J = 9.0 Hz, 2H, Ar), 9.86 (s, 1H, NH), 9.93 (s, 1H, NH), 9.97 (s, 1H, NH). IR (Nujol) 3317, 3220, 3137, 1678, 1598 cm−1. Elemental analysis: calculated for C20H22N6O6S2 (506.46) %C 47.42, %H 4.38, %N 16.59, found %C 47.47, %H 4.39, %N 16.63.
- 4-(4-(4-(4-(Trifluoromethyl)phenyl)piperazine-1-carbonyl)piperidine-1-carbonyl)benzenesulfonamide (6c). Yield 47% m.p. 201–203 °C. ESIMS (m/z): 530 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.76, 1.91 (m, 2H, CH2), 2.54, 2.88 (m, 2H, CH2), 3.08, 3.51 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.42 (s, 2H, NH2), 7.56 (d, J = 8.0 Hz, 2H, Ar), 7.66 (d, J = 8.5 Hz, 2H, Ar), 7.73 (d, J = 8.5 Hz, 2H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 9.75 (s, 2H, NH), 9.96 (s, 1H, NH). IR (Nujol) 3300, 1686, 1619 cm−1. Elemental analysis: calculated for C21H22F3N5O4S2 (529.55) %C 47.63, %H 4.19, %N 13.23, found %C 47.58, %H 4.20, %N 13.27.
- N-(2-Chlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6d). Yield 98% m.p. 209–210 °C. ESIMS (m/z): 497 (M+H)+. 1H NMR (DMSO-d6): δ 1.57 (m, 2H, CH2), 1.77, 1.93 (m, 2H, CH2), 2.63, 2.88 (m, 2H, CH2), 3.08, 3.53 (m, 2H, CH2), 4.46 (m, 1H, CH), 7.27 (m, 1H, Ar), 7.34 (m, 1H, Ar), 7.46 (s, 2H, NH2), 7.49 (m, 2H, Ar), 7.57 (d, J = 8.0 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 9.34 (s, 1H, NH), 9.69 (s, 1H, NH), 10.01 (s, 1H, NH). IR (Nujol) 3362, 3254, 1682, 1619 cm−1. Elemental analysis: calculated for C20H22ClN5O4S2 (496.00) %C 48.43, %H 4.47, %N 14.12, found %C 48.38, %H 4.45, %N 14.17.
- N-(3-Chlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6e). Yield 61% m.p. 226–227 °C. ESIMS (m/z): 497 (M+H)+. 1H NMR (DMSO-d6): δ 1.52 (m, 2H, CH2), 1.72, 1.89 (m, 2H, CH2), 2.53, 2.85 (m, 2H, CH2), 3.05, 3.49 (m, 2H, CH2), 4.43 (m, 1H, CH), 7.19 (d, J = 7.0 Hz, 1H, Ar), 7.25 (m, 3H, Ar), 7.42 (s, 2H, NH2), 7.55 (d, J = 8.0 Hz, 2H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 8.33 (s, 1H, NH), 9.24 (s, 1H, NH), 9.77 (s, 1H, NH). IR (Nujol) 3277, 3175, 3087, 1677, 1544 cm−1. Elemental analysis: calculated for C20H22ClN5O4S2 (496.00) %C 48.43, %H 4.47, %N 14.12, found %C 48.36, %H 4.48, %N 14.09.
- N-(4-Chlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6f). Following the general procedure, the title compound was prepared starting from 4-chlorophenylisothiocyanate. Yield 79% m.p. 222–223 °C. ESIMS (m/z): 497 (M+H)+. 1H NMR (DMSO-d6): δ 1.55 (s, 2H, CH2), 1.74, 1.91 (m, 2H, CH2), 2.63, 2.88 (m, 2H, CH2), 3.08, 3.51 (m, 2H, CH2), 4.46 (m, 1H, CH), 7.36 (d, J = 8.5 Hz, 2H, Ar), 7.42 (s, 2H, NH2), 7.46 (m, 2H, Ar), 7.56 (d, J = 7.5 Hz, 2H, Ar), 7.86 (d, J = 8.0 Hz, 2H, Ar), 9.59 (s, 2H, NH), 9.91 (s, 1H, NH). IR (Nujol) 3322, 3290, 3185, 1686, 1590 cm−1. Elemental analysis: calculated for C20H22ClN5O4S2 (496.00) %C 48.43, %H 4.47, %N 14.12, found %C 48.48, %H 4.48, %N 14.08. m/z 597.
- N-(3,4-Dichlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6g). Yield 97% m.p. 232–233 °C. ESIMS (m/z): 531 (M+H)+. 1H NMR (DMSO-d6): δ 1.55 (m, 2H, CH2), 1.76, 1.91 (m, 2H, CH2), 1.53, 2.88 (m, 2H, CH2), 3.08, 3.52 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.43 (s, 2H, NH2), 7.47 (d, J = 8.0 Hz, 2H, Ar), 7.56 (d, J = 7.0 Hz, 2H, Ar), 7.82 (s, 1H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 9.64 (s, 1H, NH), 9.76 (s, 1H, NH), 9.95 (s, 1H, NH). IR (Nujol) 3343, 3271, 3149, 1683, 1540 cm−1. Elemental analysis: calculated for C20H21Cl2N5O4S2 (530.45) %C 45.29, %H 3.99, %N 13.20, found %C 45.33, %H 3.97, %N 13.25.
- N-(2,4-Dichlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6h). Yield 81% m.p. 220–221 °C. ESIMS (m/z): 531 (M+H)+. 1H NMR (DMSO-d6): δ 1.55 (m, 2H, CH2), 1.74, 1.89 (m, 2H, CH2), 2.53, 2.87 (m, 2H, CH2), 3.07, 3.50 (m, 2H, CH2), 4.44 (m, 1H, CH), 7.39 (m, 2H, Ar), 7.42 (s, 2H, NH2), 7.54 (s, 1H, Ar), 7.55 (d, J = 8.0 Hz, 2H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 9.35 (s, 1H, NH), 9.74 (s, 1H, NH), 9.99 (s, 1H, NH). IR (Nujol) 3243, 1681 cm−1. Elemental analysis: calculated for C21H21N5O5S2 (530.45) %C 45.29, %H 3.99, %N 13.20, found %C 45.23, %H 3.98, %N 13.23.
- N-(2,6-Dichlorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6i). Yield 90% m.p. 179–180 °C. ESIMS (m/z): 531 (M+H)+. 1H NMR (DMSO-d6): δ 1.57 (m, 2H, CH2), 1.79, 1.95 (m, 2H, CH2), 2.53, 2.87 (m, 2H, CH2), 3.08, 3.53 (m, 2H, CH2), 4.46 (m, 1H, CH), 7.33 (m, 1H, Ar), 7.44 (s, 2H, NH2), 7.49 (m, 2H, Ar), 7.57 (d, J = 8.0 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 9.42 (s, 1H, NH), 9.71 (s, 1H, NH), 10.00 (s, 1H, NH). IR (Nujol) 3451, 3228, 1691, 1619 cm−1. Elemental analysis: calculated for C21H21N5O5S2 (530.45) %C 45.29, %H 3.99, %N 13.20, found %C 45.24, %H 4.02, %N 13.17.
- N-(2-methoxyphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazine-1-carbothioamide (6j). Yield 98% m.p. 214–215 °C. ESIMS (m/z): 492 (M+H)+. 1H NMR (DMSO-d6): δ 1.61 (m, 2H, CH2), 1.76, 1.93 (m, 2H, CH2), 2.59, 2.91 (m, 2H, CH2), 3.12, 3.54 (m, 2H, CH2), 3.80 (s, 3H, OCH3), 4.48 (m, 1H, CH), 6.93 (t, J = 8.27, 1H, Ar), 7.04 (d, J = 8.26, 1H, Ar), 7.15 (t, J = 8.50, 1H, Ar), 7.47 (s, 2H, NH2), 7.59 (d, J = 8.5 Hz, 2H, Ar), 7.90 (d, J = 8.5 Hz, 2H, Ar), 8.09 (m, 1H, Ar), 8.91 (s, 1H, NH), 9.65 (s, 1H, NH), 10.08 (s, 1H, NH). 13C NMR (DMSO-d6): δ 167.8, 151.3, 144.7, 139.4, 127.6, 127.3 (2C), 125.9 (2C), 124.6, 119.8 (2C), 111.3, 56.1, 55.8 (2C), 46.5, 40.9, 28.3, 27.8, 18.6. IR (Nujol) 3305, 3272, 3223, 1683, 1590 cm−1. Elemental analysis: calculated for C21H25N5O5S2 (491.58) %C 51.31, %H 5.13, %N 13.04, found %C 51.37, %H 5.11, %N 13.07.
- N-(3-methoxyphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazine-1-carbothioamide (6k). Yield 80% m.p. 214–215 °C. ESIMS (m/z): 492 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.76, 1.92 (m, 2H, CH2), 2.52, 2.89 (m, 2H, CH2), 3.09, 3.52 (m, 2H, CH2), 3.74 (s, 2H, OCH3), 4.46 (m, 1H, CH), 6.73 (m, 1H, Ar), 7.00 (m, 1H, Ar), 7.23 (m, 2H, Ar), 7.45 (s, 2H, NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 9.53 (s, 2H, NH), 9.91 (s, 1H, NH). IR (Nujol) 3312, 3277, 3205, 1684, 1601, 1591 cm−1. Elemental analysis: calculated for C21H25N5O5S2 (491.58) %C 51.31, %H 5.13, %N 13.04, found %C 51.38, %H 5.11, %N 13.06.
- N-(4-Methoxyphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6l). Yield 84% m.p. >250 °C. ESIMS (m/z): 492 (M+H)+. 1H NMR (DMSO-d6) δ 1.54 (m, 2H, CH2), 1.75, 1.91 (m, 2H, CH2), 1.54, 1.87 (m, 2H, CH2), 3.07, 3.51 (m, 2H, CH2), 3.73 (s, 3H, OCH3), 4.44 (m, 1H, CH), 6.87 (d, J = 8.0 Hz, 2H, Ar), 7.24 (d, J = 7.5 Hz, 2H, Ar), 7.43 (s, 2H, NH2), 7.56 (d, J = 8.0 Hz, 2H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 9.38 (s, 1H, NH), 9.43 (s, 1H, NH), 9.86 (s, 1H, NH). IR (Nujol) 3335, 323, 1680, 1543 cm−1. Elemental analysis: calculated for C21H25N5O5S2 (491.58) %C 51.31, %H 5.13, %N 13.05, found %C 51.36, %H 5.11, %N 13.01. m/z 492.
- 2-(1-(4-Sulfamoylbenzoyl)piperidine-4-carbonyl)-N-(3,4,5-trimethoxyphenyl)hydrazinecarbothioamide (6m). Yield 44% m.p. >250 °C. ESIMS (m/z): 552 (M+H)+. 1H NMR (DMSO-d6): δ 1.55 (m, 2H, CH2), 1.75, 1.90 (m, 2H, CH2), 2.53, 2.87 (m, 2H, CH2), 3.08, 3.52 (m, 2H, CH2), 3.63 (s, 3H, OCH3), 3.73 (s, 6H, OCH3), 4.44 (m, 1H, CH), 6.82 (s, 2H, Ar), 7.43 (s, 2H, NH2), 7.56 (d, J = 8 Hz, 2H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 9.48 (s, 2H, NH), 9.88 (s, 1H, NH). IR (Nujol) 3533, 3284, 3168, 1692, 1565 cm−1. Elemental analysis: calculated for C23H29N5O7S2 (551.64) %C 50.08, %H 5.30, %N 12.70, found %C 50.01, %H 5.32, %N 12.66.
- N-(2-Fluorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6n). Yield 98% m.p. 219–220 °C. ESIMS (m/z): 480 (M+H)+.1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.77, 1.93 (m, 2H, CH2), 2.63, 2.88 (m, 2H, CH2), 3.09, 3.52 (m, 2H, CH2), 4.46 (m, 1H, CH), 7.17 (m, 1H, Ar), 7.21 (m, 1H, Ar), 7.44 (s, 2H, NH2), 7.25 (m, 2H, Ar), 7.57 (d, J = 8.0 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 9.32 (s, 1H, NH), 9.70 (s, 1H, NH), 9.96 (s, 1H, NH). IR (Nujol) 3305, 3199, 1683, 1592 cm−1. Elemental analysis: calculated for C20H22FN5O4S2 (479.55) %C 50.09, %H 4.62, %N 14.60, found %C 50.17, %H 4.60, %N 14.63.
- N-(3-Fluorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6o). Yield 46% m.p. 214–215 °C. ESIMS (m/z): 480 (M+H)+.1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.76, 1.92 (m, 2H, CH2), 2.55, 2.89 (m, 2H, CH2), 3.10, 3.53 (m, 2H, CH2), 4.46 (m, 1H, CH), 6.97 (s, 1H, Ar), 7.26 (m, 1H, Ar), 7.35 (m, 2H, Ar), 7.45 (s, 2H, NH2), 7.57 (d, J = 8.0 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 9.68 (s, 2H, NH), 9.96 (s, 1H, NH). IR (Nujol) 3333, 3266, 1686, 1620 cm−1. Elemental analysis: calculated for C20H22FN5O4S2 (479.55) %C 50.09, %H 4.62, %N 14.60, found %C 50.02, %H 4.64, %N 14.64. m/z 480.
- N-(4-Fluorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6p). Yield 85% m.p. 215–216 °C. ESIMS (m/z): 480 (M+H)+. 1H NMR (DMSO-d6) δ 1.55 (m, 2H, CH2), 1.76, 1.91 (m, 2H, CH2), 2.53, 2.87 (m, 2H, CH2), 3.08, 3.51 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.14 (d, J = 8.5 Hz, 2H, Ar), 7.39 (m, 2H, Ar), 7.45 (s, 2H, NH2), 7.56 (d, J = 8.0 Hz, 2H, Ar), 7.87 (d, J = 8.0 Hz, 2H, Ar), 9.54 (s, 2H, NH), 9.90 (s, 1H, NH). IR (Nujol) 3320, 3175, 1685, 1563 cm−1. Elemental analysis: calculated for C20H22FN5O4S2 (479.55) %C 50.09, %H 4.62, %N 14.60, found %C 50.03, %H 4.60, %N 14.64.
- N-(2,6-Difluorophenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6q). Yield 77% m.p. 242–243 °C. ESIMS (m/z): 498 (M+H)+. 1H NMR (DMSO-d6) δ 1.55 (m, 2H, CH2), 1.76, 1.92 (m, 2H, CH2), 2.55, 2.86 (m, 2H, CH2), 3.07, 3.38 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.11 (d, J = 8.0 Hz, 2H, Ar), 7.33 (d, J = 7.0 Hz, 1H, Ar), 7.43 (s, 2H, NH2), 7.56 (d, J = 8.0 Hz, 2H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 9.15 (s, 1H, NH), 9.83 (s, 1H, NH), 10.02 (s, 1H, NH). IR (Nujol) 3314, 3279, 3201, 1658, 1566 cm−1. Elemental analysis: calculated for C20H21F2N5O4S2 (497.54) %C 48.28, %H 4.25, %N 14.08, found %C 48.33, %H 4.26, %N 14.05.
- 2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)-N-(o-tolyl)hydrazine-1-carbothioamide (6r). Yield 98% m.p. 209–210 °C. ESIMS (m/z): 476 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.77, 1.92 (m, 2H, CH2), 2.14 (s, 2H, CH3), 2.52, 2.87 (m, 2H, CH2), 3.08, 3.52 (m, 2H, CH2), 4.46 (m, 1H, CH), 7.16 (m, 4H, Ar), 7.45 (s, 2H, NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 9.29 (s, 1H, NH), 9.42 (s, 1H, NH), 9.92 (s, 1H, NH). IR (Nujol) 3300, 3192, 1685, 1591 cm−1. Elemental analysis: calculated for C21H25N5O4S2 (475.58) %C 53.04, %H 5.30, %N 14.73, found %C 52.98, %H 5.32, %N 14.77.
- 2-(1-(3-sulfamoylbenzoyl)piperidine-4-carbonyl)-N-(p-tolyl)hydrazine-1-carbothioamide (6s). Yield 72% m.p. 214–215 °C. ESIMS (m/z): 476 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.77, 1.92 (m, 2H, CH2), 2.29 (s, 2H, CH3), 2.52, 2.87 (m, 2H, CH2), 3.08, 3.52 (m, 2H, CH2), 4.46 (m, 1H, CH), 6.99 (m, 1H, Ar), 7.19 (m, 3H Ar), 7.46 (s, 2H, NH2), 7.59 (d, J = 8.5 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 9.49 (s, 2H, NH), 9.91 (s, 1H, NH). IR (Nujol) 3316, 3291, 3197, 1684, 1591 cm−1. Elemental analysis: calculated for C21H25N5O4S2 (475.58) %C 53.04, %H 5.30, %N 14.73, found %C 52.98, %H 5.32, %N 14.76.
- 2-(1-(3-sulfamoylbenzoyl)piperidine-4-carbonyl)-N-(p-tolyl)hydrazine-1-carbothioamide (6t). Yield 80% m.p. 234–235 °C. ESIMS (m/z): 476 (M+H)+. 1H NMR (DMSO-d6): δ 1.56 (m, 2H, CH2), 1.76, 1.92 (m, 2H, CH2), 2.28 (s, 2H, CH3), 2.53, 2.88 (m, 2H, CH2), 3.09, 3.52 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.13 (d, J = 7.5 Hz, 2H, Ar), 7.28 (d, J = 7.5 Hz, 2H, Ar), 7.45 (s, 2H, NH2), 7.57 (d, J = 8.5 Hz, 2H, Ar), 7.87 (d, J = 8.5 Hz, 2H, Ar), 9.44 (s, 2H, NH), 9.89 (s, 1H, NH). IR (Nujol) 3304, 3272, 3151, 1686, 1621 cm−1. Elemental analysis: calculated for C21H25N5O4S2 (475.58) %C 53.04, %H 5.30, %N 14.73, found %C 53.09, %H 5.28, %N 14.21.
- N-(2,6-Dimethylphenyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6u). Yield 64% m.p. 233–234 °C. ESIMS (m/z): 490 (M+H)+. 1H NMR (DMSO-d6): δ 1.57 (m, 2H, CH2), 1.71, 1.83 (m, 2H, CH2), 2.15 (s, 6H, 2CH3), 2.51, 2.87 (m, 2H, CH2), 3.08, 3.51 (m, 2H, CH2), 4.45 (m, 1H, CH), 7.03 (m, 3H Ar) 7.45 (s, 2H, NH2), 7.58 (d, J = 8.0 Hz, 2H, Ar), 7.83 (s, 1H, NH), 7.88 (m, 3H, Ar and NH), 9.68 (s, 1H, NH). 13C NMR (DMSO-d6): δ 173.22, 167.8, 167.7, 144.6, 139.5, 136.9, 136.6, 127.5, 127.3 (2C), 127.2 (2C), 126.8, 125.9 (2C), 46.5, 41.0, 40.0, 28.6, 28.0, 17.9 (2C) IR (Nujol) 3330, 3238, 1689 cm−1. Elemental analysis: calculated for C22H27N5O4S2 (489.61) %C 53.97, %H 5.56, %N 14.30, found %C 54.03, %H 5.55, %N 14.26.
- N-Benzyl-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6v). Following the general procedure, the title compound was prepared starting from benzylisothiocyanate. Yield 83% m.p. >250 °C. ESIMS (m/z): 476 (M+H)+. 1H NMR (DMSO-d6): δ 1.52 (m, 2H, CH2), 1.73, 1.88 (m, 2H, CH2), 2.45, 2.84 (m, 2H, CH2), 3.10, 3.49 (s, 2H, CH2), 4.42 (m, 1H, CH), 4.70 (s, 2H, CH2), 7.19 (m, 1H, Ar), 7.24 (m, 4H, Ar), 7.46 (s, 2H, NH2), 7.59 (d, J = 8.0 Hz, 2H, Ar), 7.88 (d, J = 8.5 Hz, 2H, Ar), 8.33 (s, 1H, NH), 9.24 (s, 1H, NH), 9.77 (s, 1H, NH). IR (Nujol) 3333, 3244, 1688, 1560 cm−1. Elemental analysis: calculated for C21H25N5O4S2 (475.58) %C 53.03, %H 5.30, %N 14.73, found %C 52.96, %H 5.29, %N 14.76.
- N-(4-Methoxybenzyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6w). Yield 64% m.p. 240–241 °C. ESIMS (m/z): 506 (M+H)+. 1H NMR (DMSO-d6): δ 1.51 (m, 2H, CH2), 1.72, 1.88 (m, 2H, CH2), 2.54, 2.85 (m, 2H, CH2), 3.04, 3.49 (m, 2H, CH2), 3.71 (s, 3H, OCH3), 4.42 (m, 1H, CH), 4.62 (s, 2H, CH2), 6.84 (d, J = 8.5 Hz, 2H, Ar), 7.19 (d, J = 8.5 Hz, 2H, Ar), 7.42 (s, 2H, NH2), 7.54 (d, J = 8.5 Hz, 2H, Ar), 7.85 (d, J = 8 Hz, 2H, Ar), 8.23 (s, 1H, NH), 9.18 (s, 1H, NH), 9.73 (s, 1H, NH). IR (Nujol) 3347, 3249, 3150, 1669, 1548 cm−1. Elemental analysis: calculated for C22H27N5O5S2 (505.61) %C 52.26, %H 5.38, %N 13.85, found %C 52.31, %H 5.36, %N 13.82.
- N-(1-Phenylethyl)-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6x). Yield 49% m.p. 196–197 °C. ESIMS (m/z): 490 (M+H)+. 1H NMR (DMSO-d6): δ 1.41 (d, J = 7.0, 3H, CH3), 1.53 (m, 2H, CH2), 1.72, 1.88 (m, 2H, CH2), 2.62, 2.86 (m, 2H, CH2), 3.06, 3.49 (m, 2H, CH2), 4.43 (m, 1H, CH), 5.57 (m, 1H, CH), 7.20 (m, 1H, Ar), 7.29 (m, 4H, Ar), 7.42 (s, 2H, NH2), 7.55 (d, J = 8.5 Hz, 2H, Ar), 7.86 (d, J = 8.5 Hz, 2H, Ar), 7.99 (s, 1H, NH), 9.14 (s, 1H, NH), 9.71 (s, 1H, NH). IR (Nujol) 3335, 3230, 3087, 1688, 1597 cm−1. Elemental analysis: calculated for C22H27N5O4S2 (489.61) %C 53.97, %H 5.56, %N 14.30, found %C 53.92, %H 5.58, %N 14.32.
- N-Cyclohexyl-2-(1-(4-sulfamoylbenzoyl)piperidine-4-carbonyl)hydrazinecarbothioamide (6y). Yield 67% m.p. 183–184 °C. ESIMS (m/z): 468 (M+H)+. 1H NMR (DMSO-d6) δ 1.06 (m, 2H, CH2), 1.22 (m, 4H, CH2), 1.54 (m, 4H, CH2), 1.56 (m, 2H, CH2), 1.77, 1.86 (m, 2H, CH2), 2.53, 2.86 (m, 2H, CH2), 3.06, 3.50 (m, 2H, CH2), 4.03 (m, 1H, CH), 4.43 (m, 1H, CH), 7.36 (s, 1H, NH), 7.43 (s, 2H, NH2), 7.55 (d, J = 7.5 Hz, 2H, Ar), 7.86 (d, J = 8.0 Hz, 2H, Ar), 8.99 (s, 1H, NH), 9.64 (s, 1H, NH). IR (Nujol) 3324, 3177, 1672, 1555 cm−1. Elemental analysis: calculated for C20H29N5O4S2 (467.61) %C 51.37, %H 6.25, %N 14.98, found %C 51.42, %H 6.26, %N 14.94.
3.2. Carbonic Anhydrase Inhibition
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity using the Khalifah procedure [23]. The used indicator was phenol red (0.2 mM), the absorbance maximum was of 557 nm, and the buffer was 20 mM Hepes (pH 7.5), whereas 20 mM Na2SO4 were employed for maintaining the ionic strength constant. Initial rates of the CA-catalyzed CO2 hydration reaction were followed for a 10–100 s, working at CO2 concentrations from 1.7 to 17 mM. Six traces of the initial 5–10% of the reaction have been used for each inhibitor for the assessment of the initial velocity. Uncatalyzed rates were subtracted from the observed total rates. Standard acetazolamide and tested compounds stock solutions (0.1 mM) were prepared in 10% DMSO aqueous solution and were diluted up to 0.01 nM with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min, for assuring the formation of the E–I complex. The inhibition constants were obtained by non-linear least squares using the Cheng–Prusoff equation, as reported earlier [24,25,26] and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlier. Their concentrations in the assay system were of 5.7–11.9 nM [21,27,28].
3.3. Molecular Docking
Molecular Docking simulations were carried out using Glide, the docking package of the Schrödinger suite [29]. The crystal structures of CAI (pdb 3w6h), CAII (pdb 3hs4), CAIX (pdb 3aia) and CAXII (pdb 1jd0) were retrieved from RCSB Protein Data Bank web server (http://www.rcsb.org/ (accessed on 1 March 2022)). All pdb files were pre-processed using the Protein Preparation workflow available on Maestro [30]. 3D ligands were prepared using an in-house python script developed using RDKit toolkit [31] and minimized using MMFF94 forcefield. The receptor grid was centred on the co-crystallized ligand, grid size was defined as 20 × 20 × 20 Å, and prepared following the standard protocol. Molecular docking was performed using the XP available method and the top scored pose was selected for the analysis.
4. Conclusions
In the present study, we described a small library of benzenesulfonamides as potential hCAIs, endowed with a piperidine ring, using hydrazinocarbonyl ureido/thioureido moiety as tail of inhibitors. Ureido derivatives 5a–u and thioureido derivatives 6a–y displayed different activities and a broad spectrum of selectivity against hCAI, hCAII, hCAIX and hCAXII. Overall, the presence of one or two halogens in different positions on the aromatic ring, strongly influence the activity and selectivity towards the CA isoforms, observing also significant differences moving from chlorine to fluorine and maintaining the same position in the ring. Regarding ureido derivatives, the 4-fluorophenyl derivative 5h displayed the best activity against the cancer-related isoform hCAIX, with Ki 2.1 nM while the analogue 4-chlorophenyl 5d resulted as about 18-fold less active against the same isoform. The introduction of methoxy group in ortho and para-position (compounds 5o–5q) resulted in a high potency and selectivity against both hCAIX and hCAXII whereas the shifting of methoxy group in meta-position (compound 5p) resulted in a decrease in selectivity. Compound 5u, endowed with g-methoxybenzyl group, resulted as the best compound of the series against hCAXII, with Ki 6.4 nM and about 6-fold more selective if compared with hCAII, and hCAIX. Moving on thioureido derivatives, the 3,4-dichlorophenyl derivative 6g inhibited hCAIX at low nanomolar levels, with a Ki 4.7 nM, also displaying good selectivity if compared with other isoforms. One of the most interesting compounds of the series resulted as the 3-fluorophenyl derivative 6o, with excellent potency and selectivity against both hCAIX and hCAXII. A similar trend was also observed for compound 6u, provided with 2,6-dimethylphenyl group. Finally, molecular docking analysis revealed the plausible key interactions that might explain the high activity and selectivity of these compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27175370/s1, Figures S1 and S2 best docking pose in CAI, CAII, CAIX and CAXII for 5g, 5m, 5o, 5q, 6l, 6j, 6o, 6u; NMR spectra of the new ureas 5 and thioureas 6, CAI, CAII, CAIX and CAXII inhibition curves for 5g, 5m, 5o, 5q, 6l, 6j, 6o, 6u.
Author Contributions
Conceptualization, V.O., G.B. and C.T.S.; software, A.D.; validation, V.O., G.B. and C.T.S.; formal analysis, A.D.; investigation, D.M. and A.A.; resources, C.T.S. and V.O.; data curation, D.M. and A.A.; writing—original draft preparation, D.M. and A.A.; writing—review and editing, D.M., A.D., A.A., G.B., C.T.S. and V.O.; visualization, D.M., A.A. and A.D.; supervision, V.O. and C.T.S.; funding acquisition, V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Italian Ministero dell’Istruzione, Università e della Ricerca, Italy; grant PRIN 2017, Prot. No. 2010E84AA4_002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 5 and 6 are available from the authors.
References
- Nocentini, A.; Supuran, C.T. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008–2018). Expert Opin. Ther. Pat. 2018, 28, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple Binding Modes of Inhibitors to Carbonic Anhydrases: How to Design Specific Drugs Targeting 15 Different Isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Scozzafava, A.; Conway, J. Carbonic Anhydrase: Its Inhibitors and Activators. J. Am. Chem. Soc. 2005, 127, 3643. [Google Scholar] [CrossRef]
- D’Ambrosio, K.; De Simone, G.; Supuran, C.T. Human Carbonic Anhydrases: Catalytic Properties, Structural Features, and Tissue Distribution. Carbon. Anhydrases Biocatal. 2015, 2, 17–30. [Google Scholar] [CrossRef]
- Stams, T.; Christianson, D.W. X-ray crystallographic studies of mammalian carbonic anhydrase isozymes. In The Carbonic Anhydrases; Birkhäuser: Basel, Switzerland, 2000; Volume 90, pp. 159–174. [Google Scholar] [CrossRef]
- McDonald, P.C.; Winum, J.Y.; Supuran, C.T.; Dedhar, S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012, 3, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, B.; Waheed, A.; Shah, G.N.; Grubb, J.H.; Sly, W.S.; Tu, C.; Silverman, D.N. Purification and kinetic analysis of recombinant CA XII, a membrane carbonic anhydrase overexpressed in certain cancers. Proc. Natl. Acad. Sci. USA 2000, 97, 14212–14217. [Google Scholar] [CrossRef]
- Angeli, A.; Carta, F.; Nocentini, A.; Winum, J.Y.; Zalubovskis, R.; Akdemir, A.; Onnis, V.; Eldehna, W.M.; Capasso, C.; Simone, G.; et al. Carbonic Anhydrase Inhibitors Targeting Metabolism and Tumor Microenvironment. Metabolites 2020, 10, 412. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors: An update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin. Investig. Drugs 2021, 30, 1197–1208. [Google Scholar] [CrossRef]
- Tanini, D.; Carradori, S.; Capperucci, A.; Lupori, L.; Zara, S.; Ferraroni, M.; Ghelardini, C.; Mannelli, L.; Micheli, L.; Lucarini, E.; et al. Chalcogenides-incorporating carbonic anhydrase inhibitors concomitantly reverted oxaliplatin-induced neuropathy and enhanced antiproliferative action. Eur. J. Med. Chem. 2021, 225, 113793. [Google Scholar] [CrossRef]
- D’Ascenzio, M.; Secci, D.; Carradori, S.; Zara, S.; Guglielmi, P.; Cirilli, R.; Pierini, M.; Poli, G.; Tuccinardi, T.; Angeli, A.; et al. 1,3-Dipolar Cycloaddition, HPLC Enantioseparation, and Docking Studies of Saccharin/Isoxazole and Saccharin/Isoxazoline Derivatives as Selective Carbonic Anhydrase IX and XII Inhibitors. J. Med. Chem. 2020, 63, 2470–2488. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 6, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Monti, S.M.; Hilvo, M.; Parkkila, S.; Romano, V.; Scaloni, A.; Pedone, C.; Scozzafava, A.; Supuran, C.T.; De Simone, G. Crystal structure of human carbonic anhydrase XIII and its complex with the inhibitor acetazolamide. Proteins 2009, 74, 164–175. [Google Scholar] [CrossRef]
- Svastová, E.; Hulíková, A.; Rafajová, M.; Zat’ovicová, A.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Petreni, A.; Osman, S.M.; Alasmary, F.A.; Almutairi, T.M.; Nocentini, A.; Supuran, C.T. Binding site comparison for coumarin inhibitors and amine/amino acid activators of human carbonic anhydrases. Eur. J. Med. Chem. 2021, 226, 113875. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Moi, D.; Pinzi, L.; Bonanni, D.; Rastelli, G. Hydroxamic Acid Derivatives: From Synthetic Strategies to Medicinal Chemistry Applications. ACS Omega 2021, 6, 21843–21849. [Google Scholar] [CrossRef]
- Moi, D.; Nocentini, A.; Deplano, A.; Osman, S.M.; AlOthman, Z.A.; Piras, V.; Balboni, G.; Supuran, C.T.; Onnis, V. Appliance of the piperidinyl-hydrazidoureido linker to benzenesulfonamide compounds: Synthesis, in vitro and in silico evaluation of potent carbonic anhydrase II, IX and XII inhibitors. Bioorg. Chem. 2020, 98, 103728. [Google Scholar] [CrossRef]
- Nocentini, A.; Moi, D.; Deplano, A.; Osman, S.M.; AlOthman, Z.A.; Balboni, G.; Supuran, C.T.; Onnis, V. Sulfonamide/sulfamate switch with a series of piperazinylureido derivatives: Synthesis, kinetic and in silico evaluation as carbonic anhydrase isoforms I, II, IV, and IX inhibitors. Eur. J. Med. Chem. 2020, 186, 111896. [Google Scholar] [CrossRef]
- Moi, D.; Nocentini, A.; Deplano, A.; Balboni, G.; Supuran, C.T.; Onnis, V. Structure-activity relationship with pyrazoline-based aromatic sulfamates as carbonic anhydrase isoforms I, II, IX and XII inhibitors: Synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 182, 111638. [Google Scholar] [CrossRef]
- Nocentini, A.; Moi, D.; Balboni, G.; Onnis, V.; Supuran, C.T. Discovery of thiazolin-4-one-based aromatic sulfamates as a new class of carbonic anhydrase isoforms I, II, IV, and IX inhibitors. Bioorg. Chem. 2018, 77, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Moi, D.; Balboni, G.; Salvadori, S.; Onnis, V.; Supuran, C.T. Synthesis and biological evaluation of novel pyrazoline-based aromatic sulfamates with potent carbonic anhydrase isoforms II, IV and IX inhibitory efficacy. Bioorg. Chem. 2018, 77, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef]
- Kiss, L.E.; Ferreira, H.S.; Torrão, L.; Bonifácio, M.J.; Palma, P.N.; Soares-da-Silva, P.; Learmonth, D.A. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J. Med. Chem. 2010, 53, 3396–3411. [Google Scholar] [CrossRef]
- Vullo, D.; Del Prete, S.; Nocentini, A.; Osman, S.M.; AlOthman, Z.A.; Capasso, C.; Bozdag, M.; Carta, F.; Gratteri, P.; Supuran, C.T. Dithiocarbamates effectively inhibit the β-carbonic anhydrase from the dandruff-producing fungus Malassezia globose. Bioorg. Med. Chem. 2017, 25, 1260–1265. [Google Scholar] [CrossRef]
- Del Prete, S.; Angeli, A.; Ghobril, C.; Hitce, J.; Clavaud, C.; Marat, X.; Supuran, C.T.; Capasso, C. Sulfonamide Inhibition Profile of the β-Carbonic Anhydrase from Malassezia restricta, An Opportunistic Pathogen Triggering Scalp Conditions. Metabolites 2020, 10, 39. [Google Scholar] [CrossRef]
- Nocentini, A.; Bonardi, A.; Gratteri, P.; Cerra, B.; Gioiello, A.; Supuran, C.T. Steroids interfere with human carbonic anhydrase activity by using alternative binding mechanisms. J. Enzyme Inhib. Med. Chem. 2018, 33, 1453–1459. [Google Scholar] [CrossRef]
- Bonardi, A.; Vermelho, A.B.; da Silva Cardoso, V.; de Souza Pereira, M.C.; da Silva Lara, L.; Selleri, S.; Gratteri, P.; Supuran, C.T.; Nocentini, A. N-Nitrosulfonamides as Carbonic Anhydrase Inhibitors: A Promising Chemotype for Targeting Chagas Disease and Leishmaniasis. ACS Med. Chem. Lett. 2018, 10, 413–418. [Google Scholar] [CrossRef]
- Glide, version 6.7; Schrödinger LLC: New York, NY, USA, 2015.
- Maestro, version 10.2; Schrödinger LLC: New York, NY, USA, 2015.
- RDKit. Cheminformatics and Machine Learning Software. 2013. Available online: http://www.rdkit.Org (accessed on 1 March 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).