Isolation, Purification, and Antioxidant Activities of Polysaccharides from Choerospondias axillaris Leaves
Abstract
1. Introduction
2. Results and Discussion
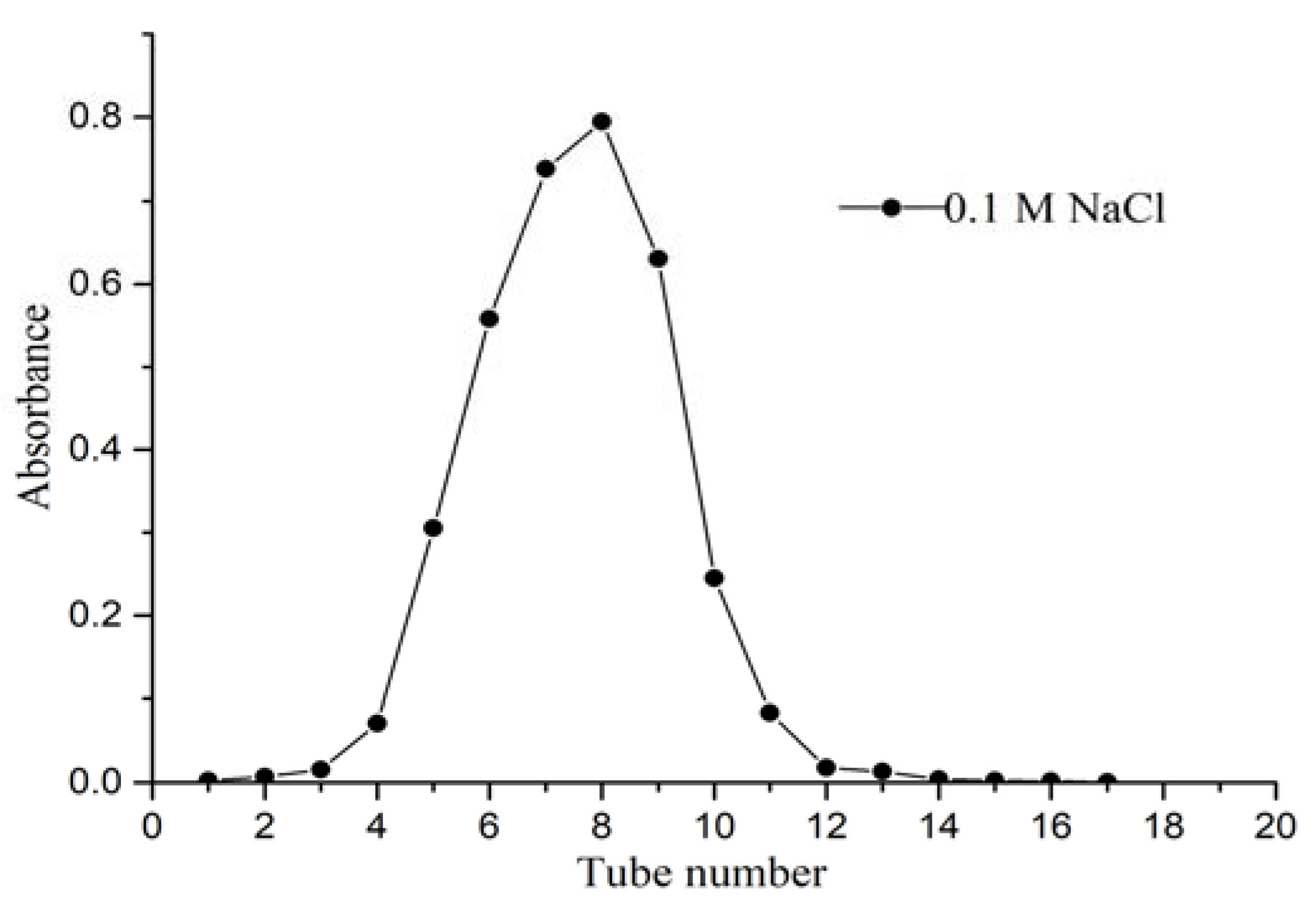
2.1. Isolation and Purification of CALP-1 and CALP-2
2.2. Characteristic of CALP-1 and CALP-2
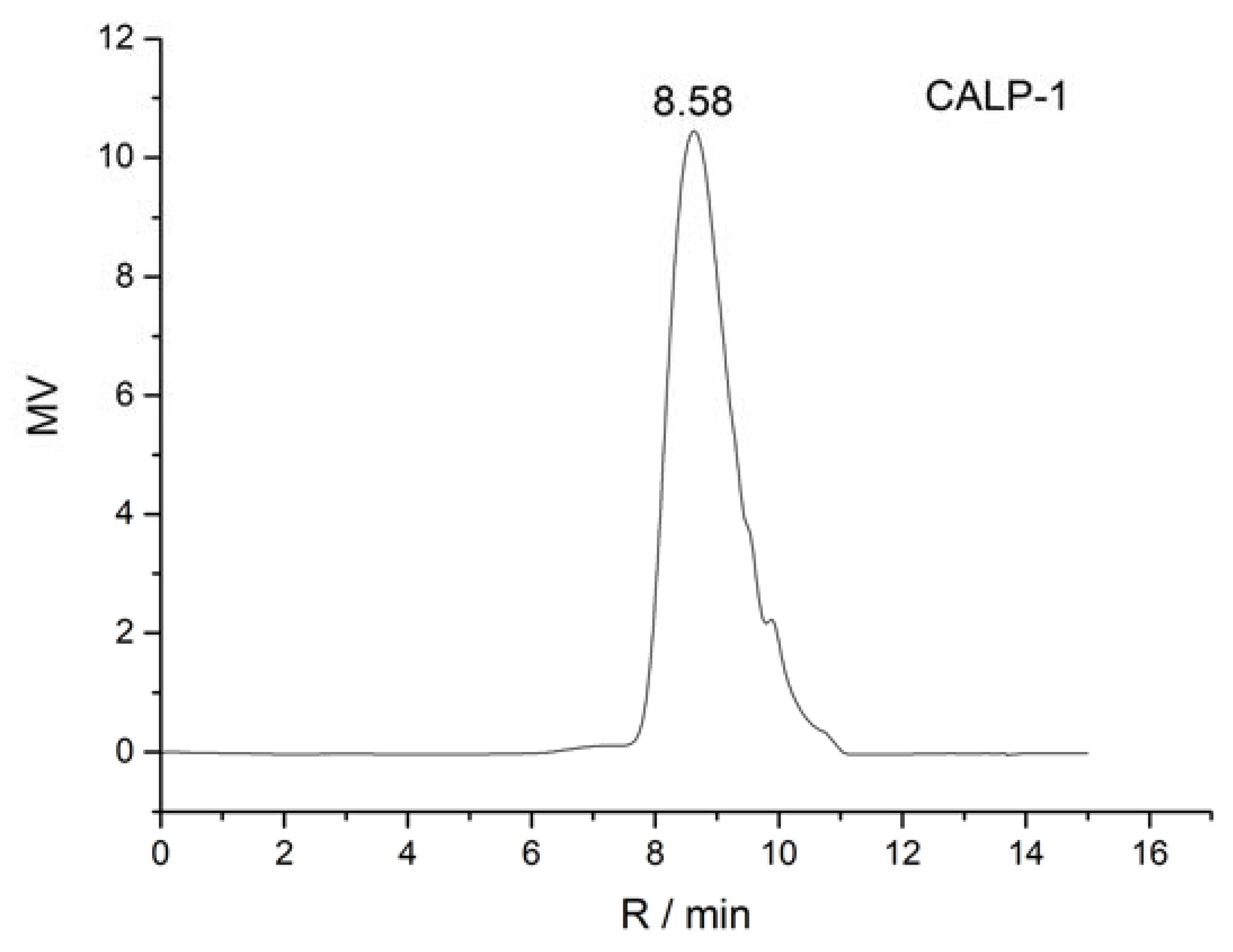
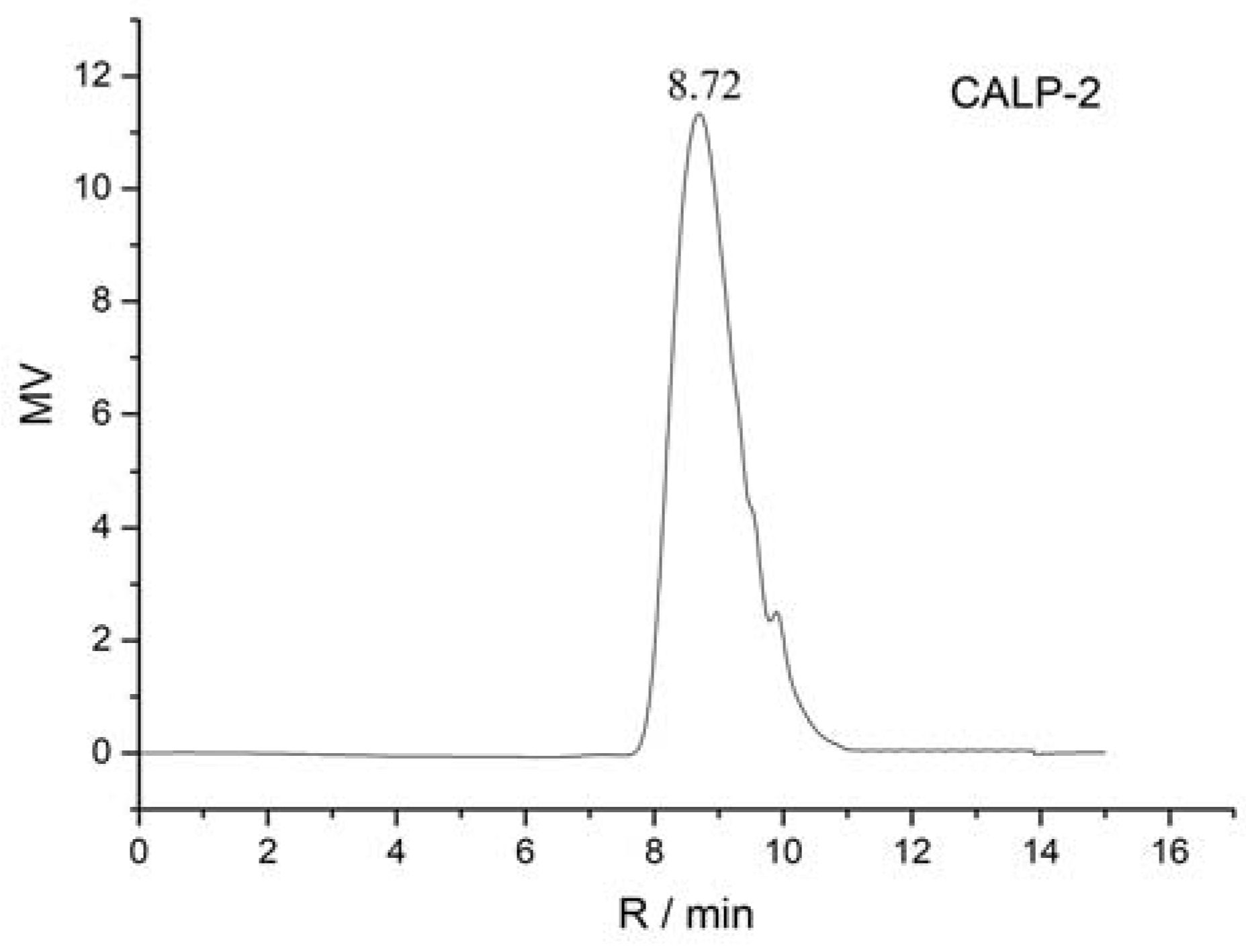
2.2.1. Homogeneity and Molecular Weight of CALP-1 and CALP-2
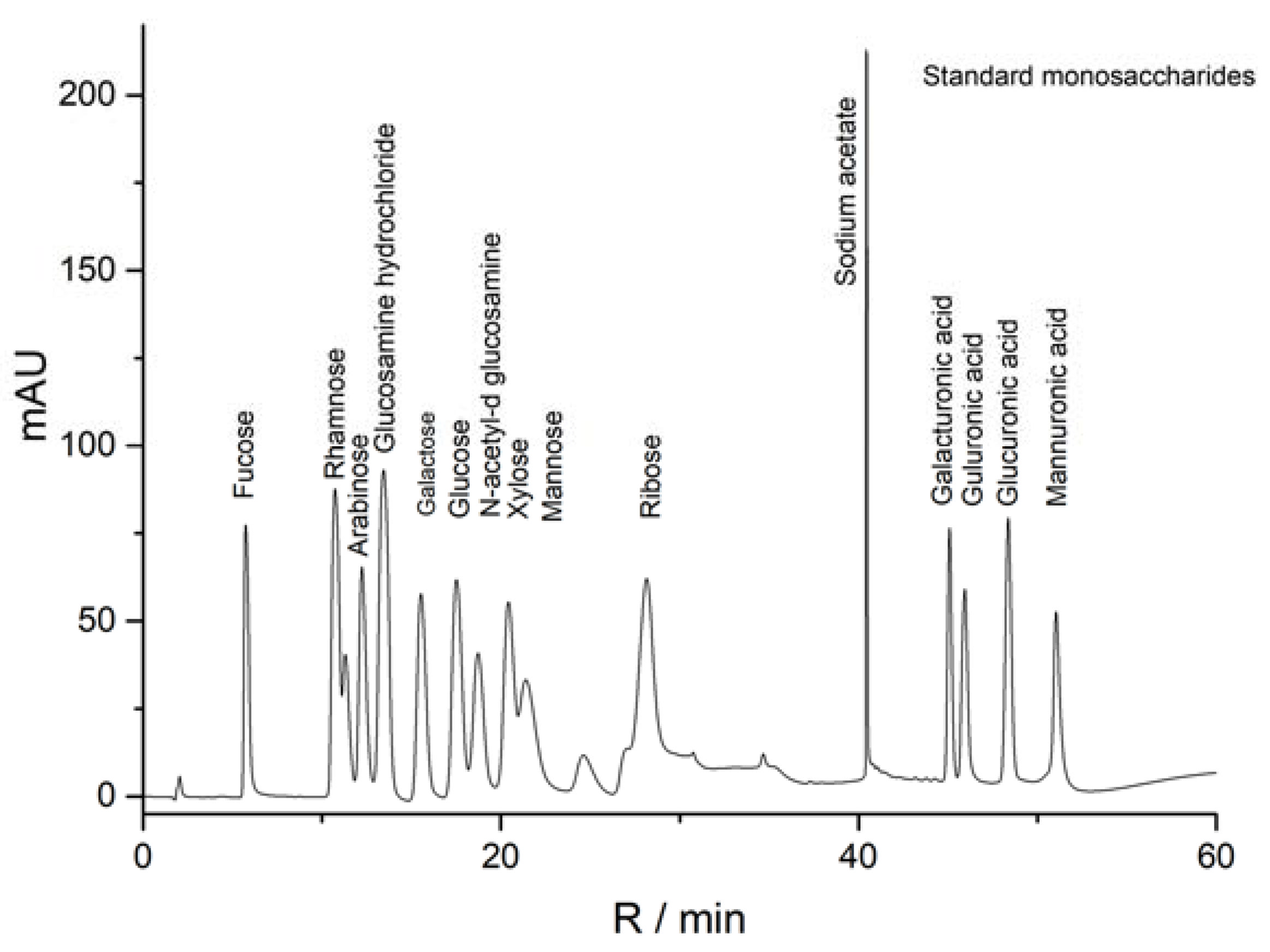
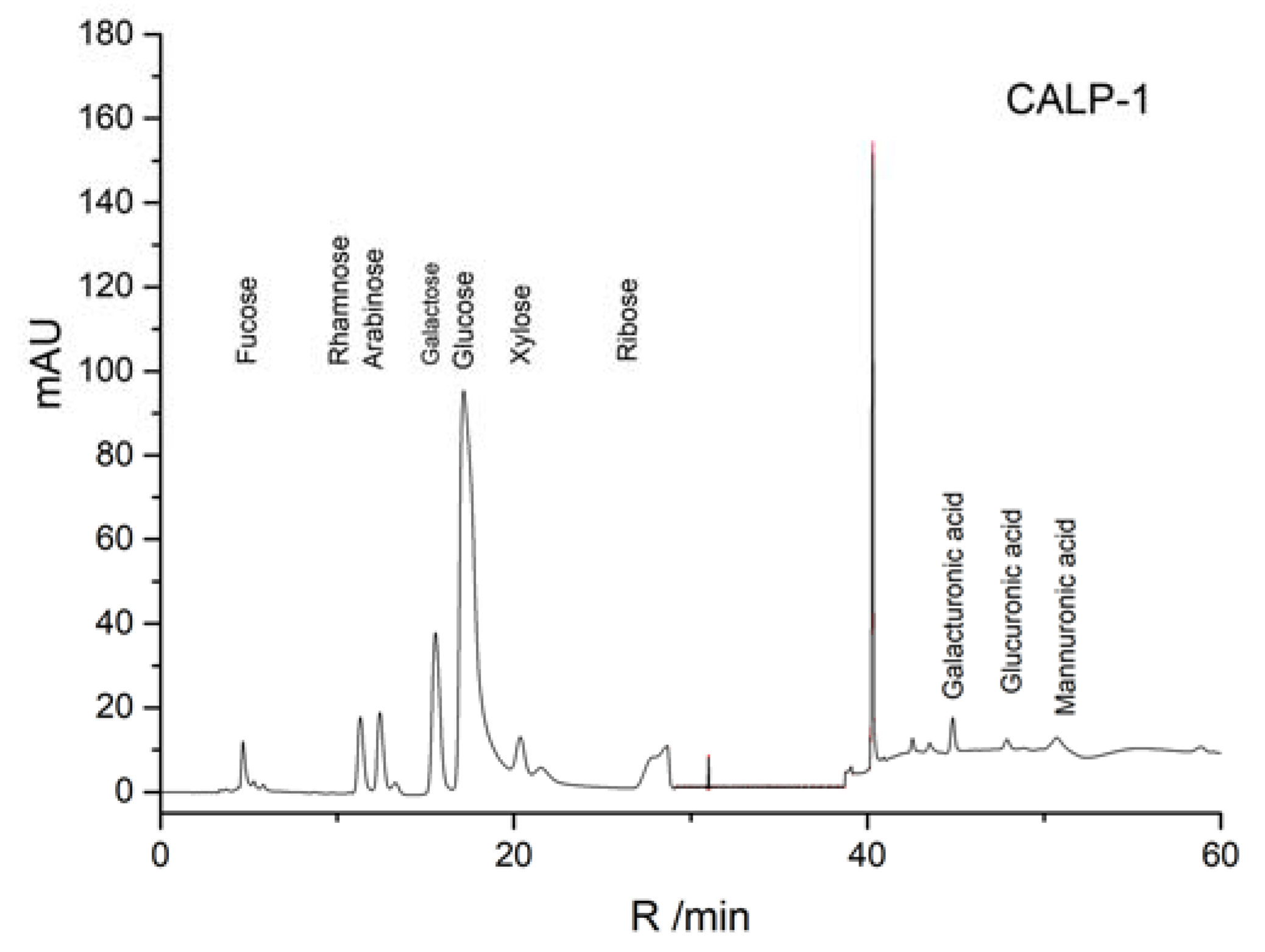
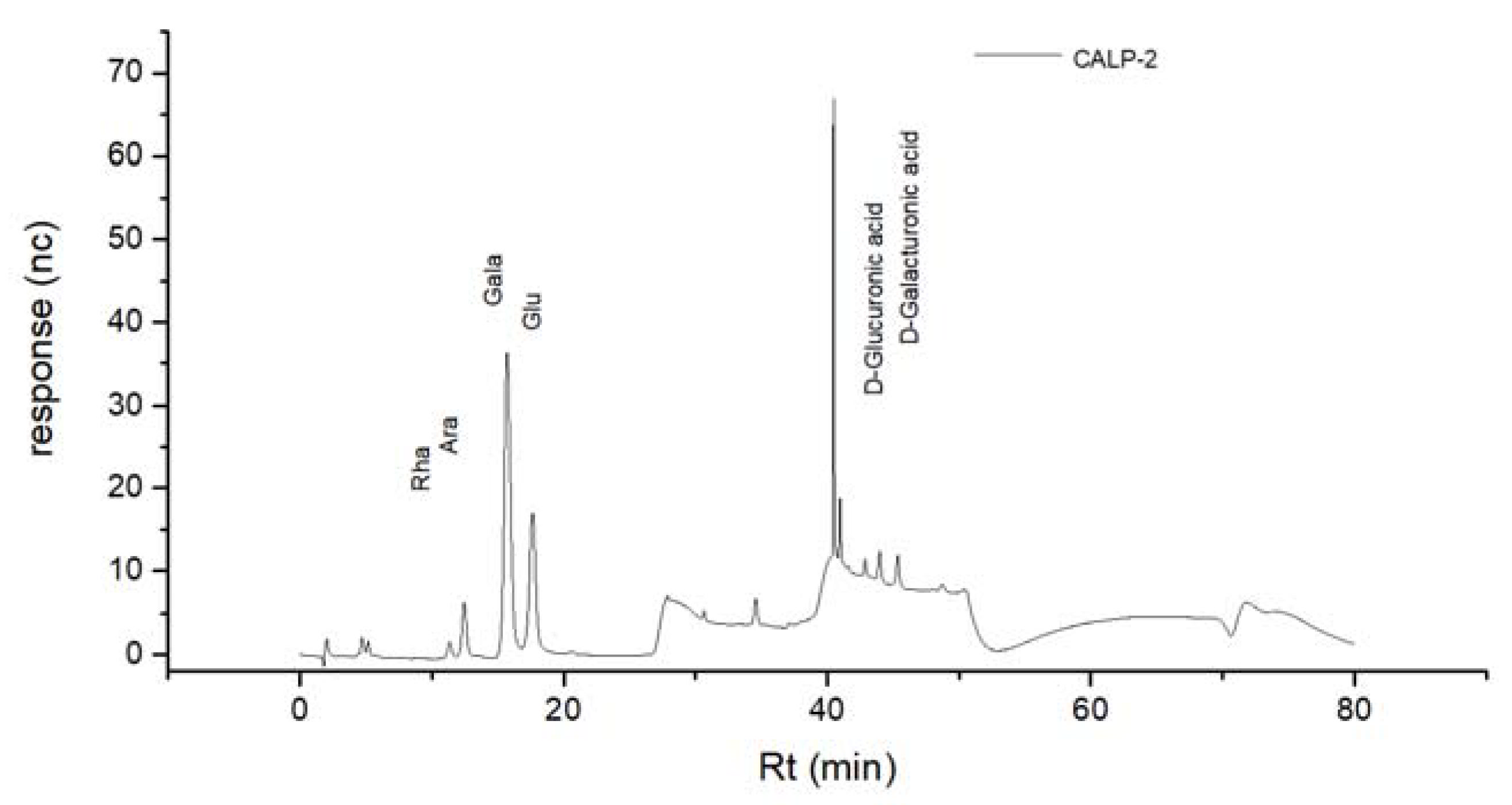
2.2.2. Monosaccharide Compositions of Polysaccharide Fractions
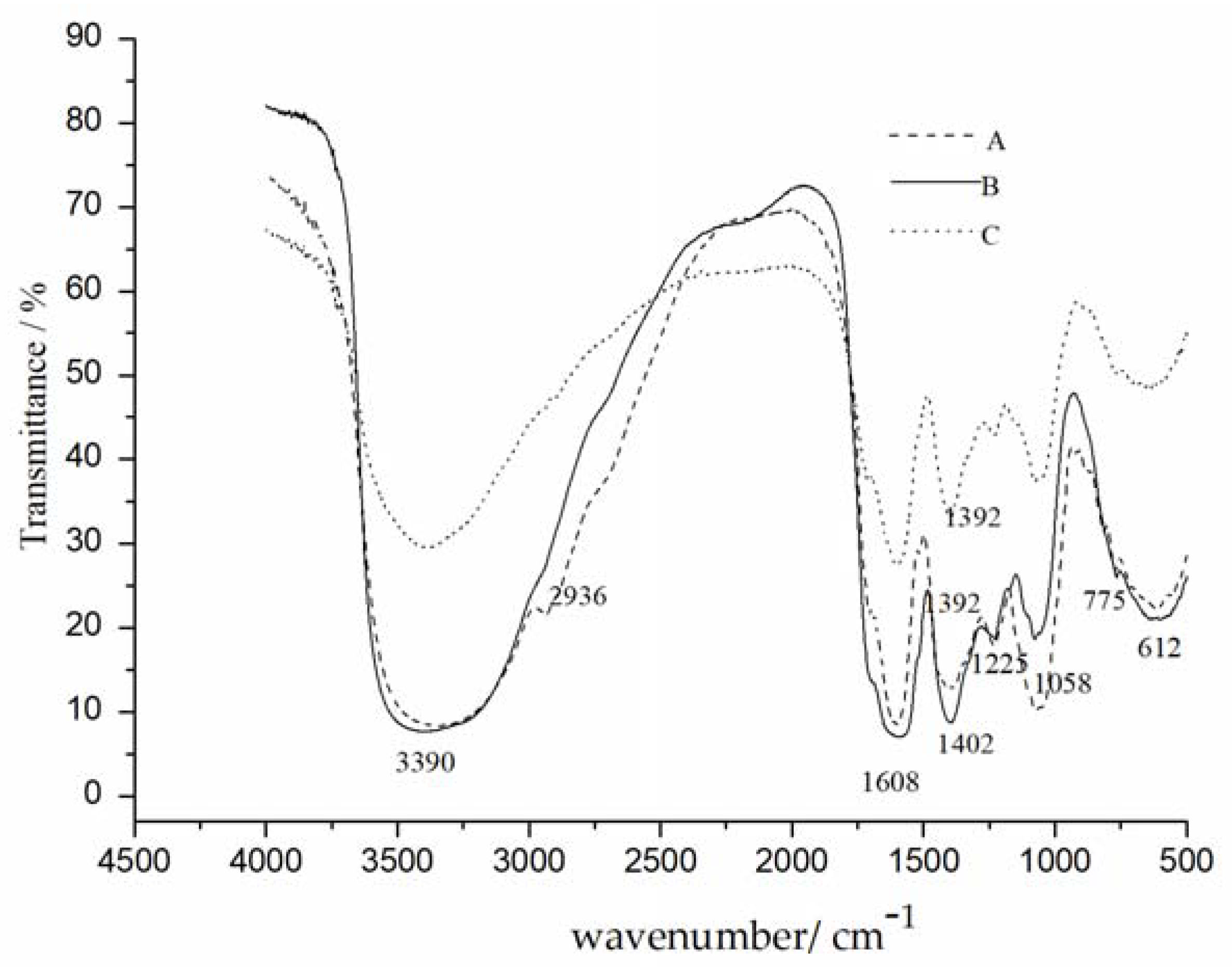
2.2.3. FTIR Analysis
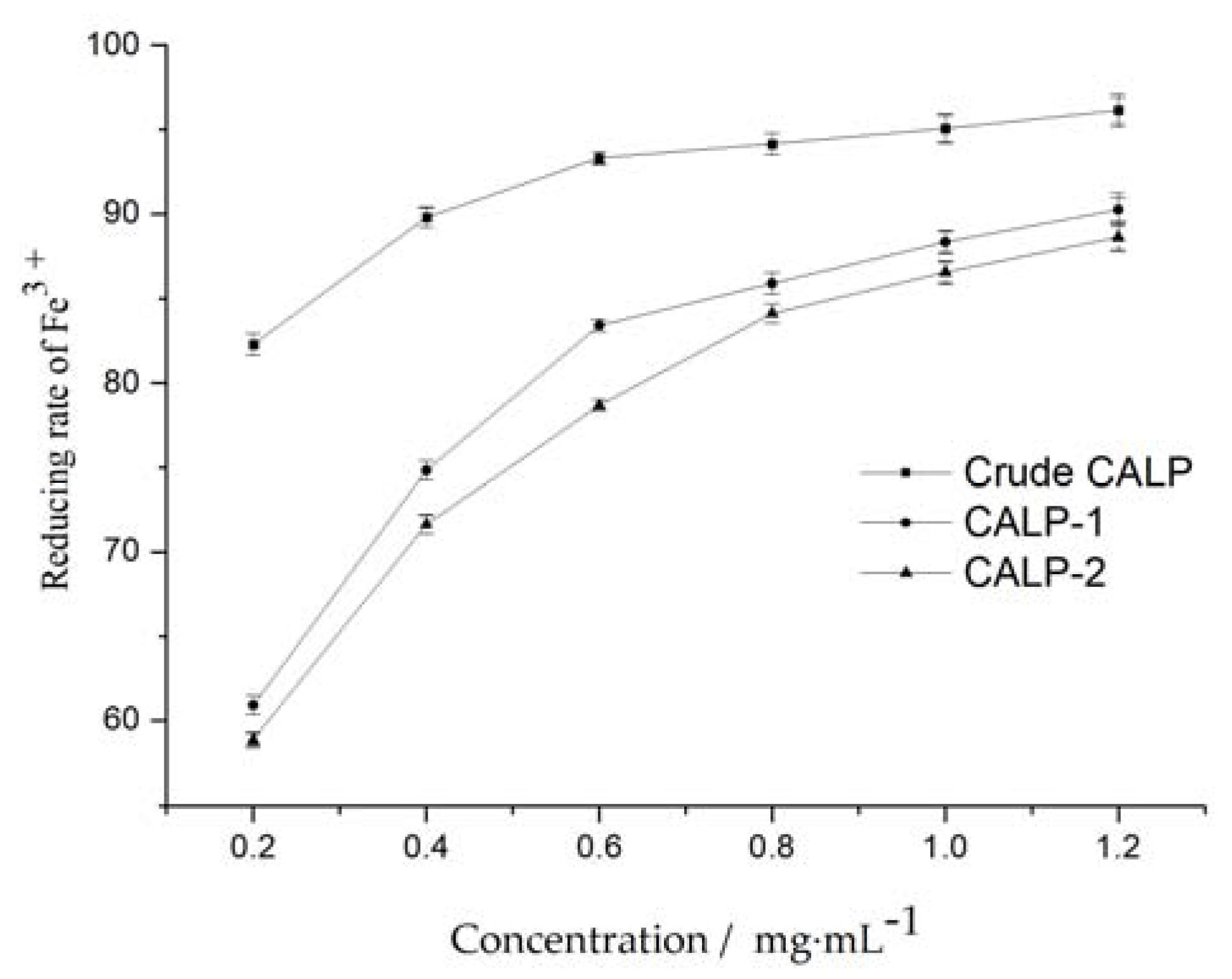
2.3. Antioxidant Activity Analysis of Polysaccharide In Vitro
2.3.1. Determination of Reducing Power
2.3.2. DPPH Radical Scavenging Activity
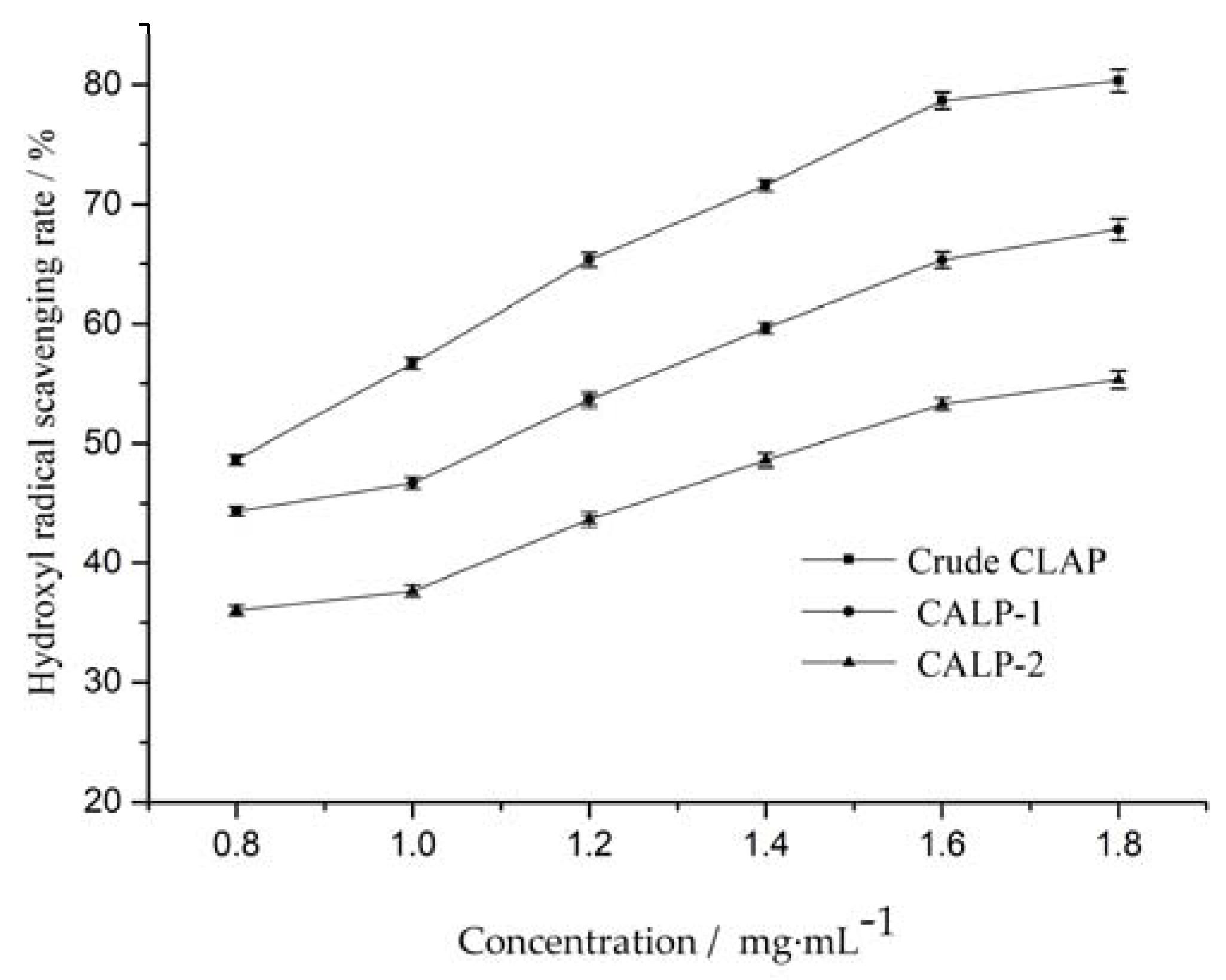
2.3.3. Hydroxyl Radical Scavenging Activity
3. Materials and Methods
3.1. Chemicals and Sample Material
3.2. Extraction and Purification of Polysaccharides
3.3. Characterization of CALP-1 and CALP-2
3.3.1. Determination of Molecular Weight
3.3.2. Analysis of Monosaccharide Composition
- (1)
- Preparation of standard solution and samples.
- (2)
- HPAEC conditions.
3.3.3. FTIR Analysis
3.3.4. Assay of Antioxidant Activity In Vitro of CALP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, C.-W.; Cui, C.B.; Cai, B.; Han, B.; Li, M.-M.; Fan, M. Flavanoidal constituents of Choerospondias axillaries and their in vitro antitumor and anti-hypoxia activities. Chin. J. Med. Chem. 2005, 19, 138–141. [Google Scholar]
- Hua, W.; Xiang, D.G.; Gao, C.Z.; Lei, C.; Wen, B.Y. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. [Google Scholar]
- Yang, G.-L.; Zhong, C.-L.; Zhang, Q.; Cheng, S.-X. Optimization of extraction of total flavonoids from Choerospondias axillaris leaves by response surface methodology and analysis of antioxidant activity. Cereals Oils 2022, 35, 133–137. [Google Scholar]
- Li, Q.; Wang, X.; Dai, T.; Liu, C.; Li, T.; Mcclements, D.J.; Chen, J.; Liu, J. Proanthocyanidins, Isolated from Choerospondias axillaris Fruit Peels, Exhibit Potent Antioxidant Activities in Vitro and Novel Anti-angiogenic Property in Vitro and in Vivo. J. Agric. Food Chem. 2016, 64, 3546–3556. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Zhai, Y.; McClements, D.J.; Liu, J. Separation and characterization of polyphenolics from underutilized byproducts of fruit production (Choerospondias axillaris peels): Inhibitory activity of proanthocyanidins against glycolysis enzymes. Food Funct. 2015, 6, 3693–3701. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Liu, C. Anti-tumour effects of polysaccharides isolated from Artemisia annua L by inducing cell apoptosis and immunomodulatory anti-hepatoma effects of polysaccharides. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 15–22. [Google Scholar] [CrossRef]
- Khaled, A.; Babak, G.; Ali, A.; Kambiz, J. Extraction, purification, physicochemical properties and antioxidant activity of a new polysaccharide from Ocimum album L. seed. Int. J. Biol. Macromol. 2021, 180, 643–653. [Google Scholar]
- Su, J.; Sun, J.; Jian, T.; Zhang, G.; Ling, J. Immunomodulatory and Antioxidant Effects of Polysaccharides from the Parasitic Fungus Cordyceps kyushuensis. BioMed Res. Int. 2020, 2020, 8257847. [Google Scholar] [CrossRef]
- Huang, G.; Mei, X.; Hu, J. The Antioxidant Activities of Natural Polysaccharides. Curr. Drug Targets 2017, 18, 1296–1300. [Google Scholar] [CrossRef]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, J.; Ding, Y.; Cheng, J.; Yang, S. In vitro antioxidant and immunostimulating activities of polysaccharides from Ginkgo biloba leaves. Int. J. Biol. Macromol. 2019, 124, 972–980. [Google Scholar] [CrossRef]
- Hwang, K.C.; Shin, H.Y.; Kim, W.J.; Seo, M.S.; Kim, H. Effects of a high-molecular-weight polysaccharides isolated from Korean persimmon on the antioxidant, anti-inflammatory, and antiwrinkle activity. Molecules 2021, 26, 1600. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, J.D.; Ni, D.J.; Hao, Q.Q. Study on extraction conditions and free radical scavenging activity of polysaccharides from persimmon leaves. Acta Hortic. 2018, 219–224. [Google Scholar] [CrossRef]
- Liang, X.; Gao, Y.; Pan, Y.; Zou, Y.; He, M.; He, C.; Li, L.; Yin, Z.; Lv, C. Purification, Chemical Characterization and Antioxidant Activities of Polysaccharides Isolated from Mycena dendrobii. Carbohydr. Polym. 2018, 203, 45–51. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yang, Y.M.; Feng, H.A.N.; Zhang, H.N.; Du, J.X.; Hao, X.M. Immunological Effects of Total Flavones from Leaves of Choerospondias axillaris on Mice. Chin. Herb. Med. 2013, 5, 121–124. [Google Scholar]
- Zhu, L.; Zhang, C.; Li, C.; Zhou, Y. Studies on the Chemical Constituents of Mongolian Medicine Guangzao. Chin. Herb. Med. 2003, 23–24. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Liu, W.; Liu, J. Comparison of bioactivities and phenolic composition of Choerospondias axillaris peels and fleshes. J. Sci. Food Agric. 2016, 96, 2462–2471. [Google Scholar] [CrossRef]
- Demirci, M.A.; Ipek, Y.; Gul, F.; Ozen, T.; Demirtas, I. Extraction, isolation of heat-resistance phenolic compounds, antioxidant properties, characterization and purification of 5-hydroxymaltol from Turkish Apple pulps. Food Chem. 2018, 269, 111–117. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.D.; Li, Y. Antitumor and immunomodulatory activity of polysaccharide isolated from Trametes orientalis. Carbohydr. Polym. 2015, 131, 248–254. [Google Scholar] [CrossRef]
- Liang, Y.R.; Li, W.-Q.; Liu, S.G. Infrared spectrometry of sulfate polysaccharides of Chlorella autotropica. Mar. Sci. 2007, 31, 4–12. [Google Scholar]
- Wu, Y.; Huo, Y.; Xu, L.; Xu, Y.; Wang, X.; Zhou, T. Purification, characterization and antioxidant activity of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 165, 2116–2125. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Zhou, Q.; Hao, S.; Zhou, T.; Xie, H. Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2015, 81, 1026–1030. [Google Scholar] [CrossRef]
- Kim, S.J.; Baek, S.Y.; Kim, M.R. Quality characteristics and antioxidant activities of rice crispy cereal added with dried laver. Korean J. Food Sci. Technol. 2020, 52, 487–494. [Google Scholar]
- Wang, Y.; Guo, M. Purification and structural characterization of polysaccharides isolated from Auricularia cornea var. Li. Carbohydr. Polym. 2020, 230, 1156–1180. [Google Scholar] [CrossRef]
- Li, J.; Gu, F.; Cai, C.; Hu, M.; Fan, L.; He, J.; Yu, G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2020, 143, 806–813. [Google Scholar] [CrossRef]
- Alyassin, M.; Campbell, G.M.; O’Neill, H.M.; Bedford, M.R. Simultaneous determination of cereal monosaccharides, xylo-and arabinoxylo-oligosaccharides and uronic acids using HPAEC-PAD. Food Chem. 2020, 315, 126221. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Qian, L.; Liu, H.; Li, T.; Liu, Y.; Zhang, Z.; Zhang, Y. Purification, characterization and in vitro antioxidant activity of a polysaccharide AAP–3–1 from Auricularia auricula—ScienceDirect. Int. J. Biol. Macromol. 2020, 162, 1453–1464. [Google Scholar] [CrossRef]
- Shang, X.L.; Liu, C.Y.; Dong, H.Y.; Peng, H.H.; Zhu, Z.Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 1300–1316. [Google Scholar] [CrossRef]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Mis, A. FTIR studies of gluten matrix dehydration after fibre polysaccharide addition. Food Chem. 2018, 252, 198–206. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, Q.; Zhang, Y.; Li, J.; Zhu, X.; Zhao, L.; Wen, J.; Liu, J.; Zhao, L.; Zhao, J. Enzyme-Assisted Extraction Optimization, Characterization and Antioxidant Activity of Polysaccharides from Sea Cucumber Phyllophorus proteus. Molecules 2018, 23, 590. [Google Scholar] [CrossRef]



| Standards Products | Regression Equation | Correlation Coefficient (R2) | Linear Range (ug/mL) |
|---|---|---|---|
| Fucose | y = 2.4148 x + 1.1584 | 0.993 | 1–50 |
| Rhamnose | y = 1.2828 x + 0.1854 | 0.995 | 1–50 |
| Arabinose | y = 2.616 x + 0.9552 | 0.993 | 1–50 |
| Glucosamine hydrochloride | y = 3.7482 x + 2.5799 | 0.991 | 1–50 |
| Galactose | y = 3.3186 x + 1.0248 | 0.998 | 1–50 |
| Glucose | y = 3.3404 x + 1.6294 | 0.993 | 1–50 |
| N-acetyl-d glucosamine | y = 2.1423 x + 0.4743 | 0.994 | 1–50 |
| Xylose | y = 3.2735 x + 0.8944 | 0.997 | 1–50 |
| Mannose | y = 2.6926 x − 0.4180 | 0.994 | 1–50 |
| Fructose | y = 1.079 x − 0.2946 | 0.993 | 1–50 |
| Ribose | y = 3.5422 x + 4.9 | 0.999 | 1–50 |
| Galacturonic acid | y = 1.951 x + 0.1441 | 0.999 | 1–50 |
| Glucuronic acid | y = 2.9585 x + 0.0585 | 0.999 | 1–50 |
| Galactosamine hydrochloride | y = 2.821 x + 2.595 | 0.989 | 1–50 |
| Guluronic acid | y = 2.0363 x + 0.0109 | 0.999 | 1–50 |
| Mannuronic acid | y = 2.2052 x − 0.5217 | 0.996 | 1–50 |
| Monosaccharide | Monosaccharide Composition Molar Ratio | |
|---|---|---|
| CALP-1 | CALP-2 | |
| Rhamnose | 5.16 | 1.38 |
| Arabinose | 2.31 | 3.63 |
| Galactose | 5.50 | 18.84 |
| Glucose | 27.18 | 8.28 |
| Xylose | 1.00 | - |
| Mannose | 0.76 | - |
| galacturonic acid | 1.07 | 1.45 |
| Glucuronic acid | 0.22 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Lu, L.; Zheng, Y.; Qin, C.; Chen, Y.; Zhou, Z. Isolation, Purification, and Antioxidant Activities of Polysaccharides from Choerospondias axillaris Leaves. Molecules 2022, 27, 8881. https://doi.org/10.3390/molecules27248881
Zhang Q, Lu L, Zheng Y, Qin C, Chen Y, Zhou Z. Isolation, Purification, and Antioxidant Activities of Polysaccharides from Choerospondias axillaris Leaves. Molecules. 2022; 27(24):8881. https://doi.org/10.3390/molecules27248881
Chicago/Turabian StyleZhang, Qiang, Lianxiang Lu, Yanfei Zheng, Chengrong Qin, Yuexin Chen, and Zhongjie Zhou. 2022. "Isolation, Purification, and Antioxidant Activities of Polysaccharides from Choerospondias axillaris Leaves" Molecules 27, no. 24: 8881. https://doi.org/10.3390/molecules27248881
APA StyleZhang, Q., Lu, L., Zheng, Y., Qin, C., Chen, Y., & Zhou, Z. (2022). Isolation, Purification, and Antioxidant Activities of Polysaccharides from Choerospondias axillaris Leaves. Molecules, 27(24), 8881. https://doi.org/10.3390/molecules27248881






