Impact of N-Linked Glycosylation on Therapeutic Proteins
Abstract
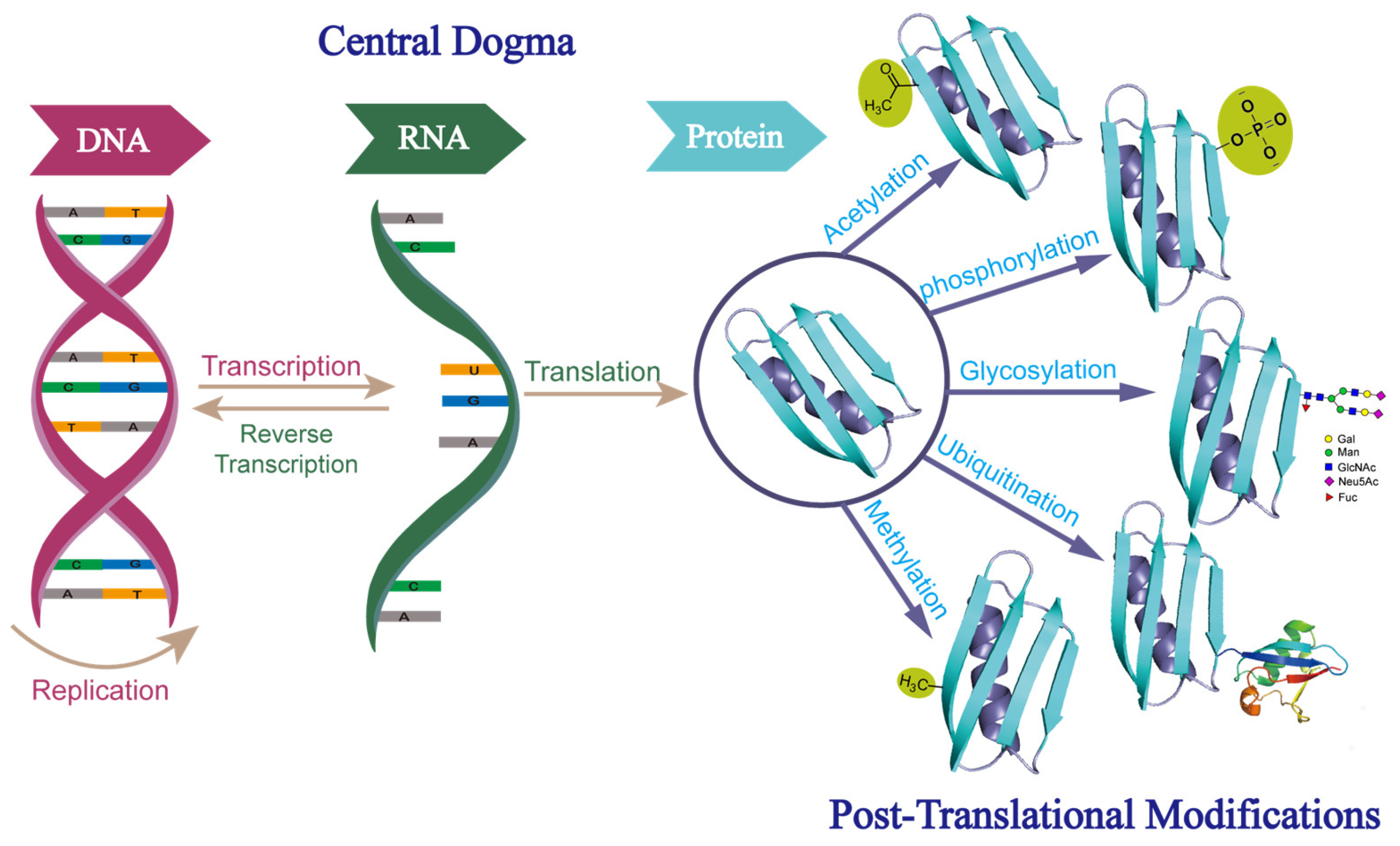
1. Introduction
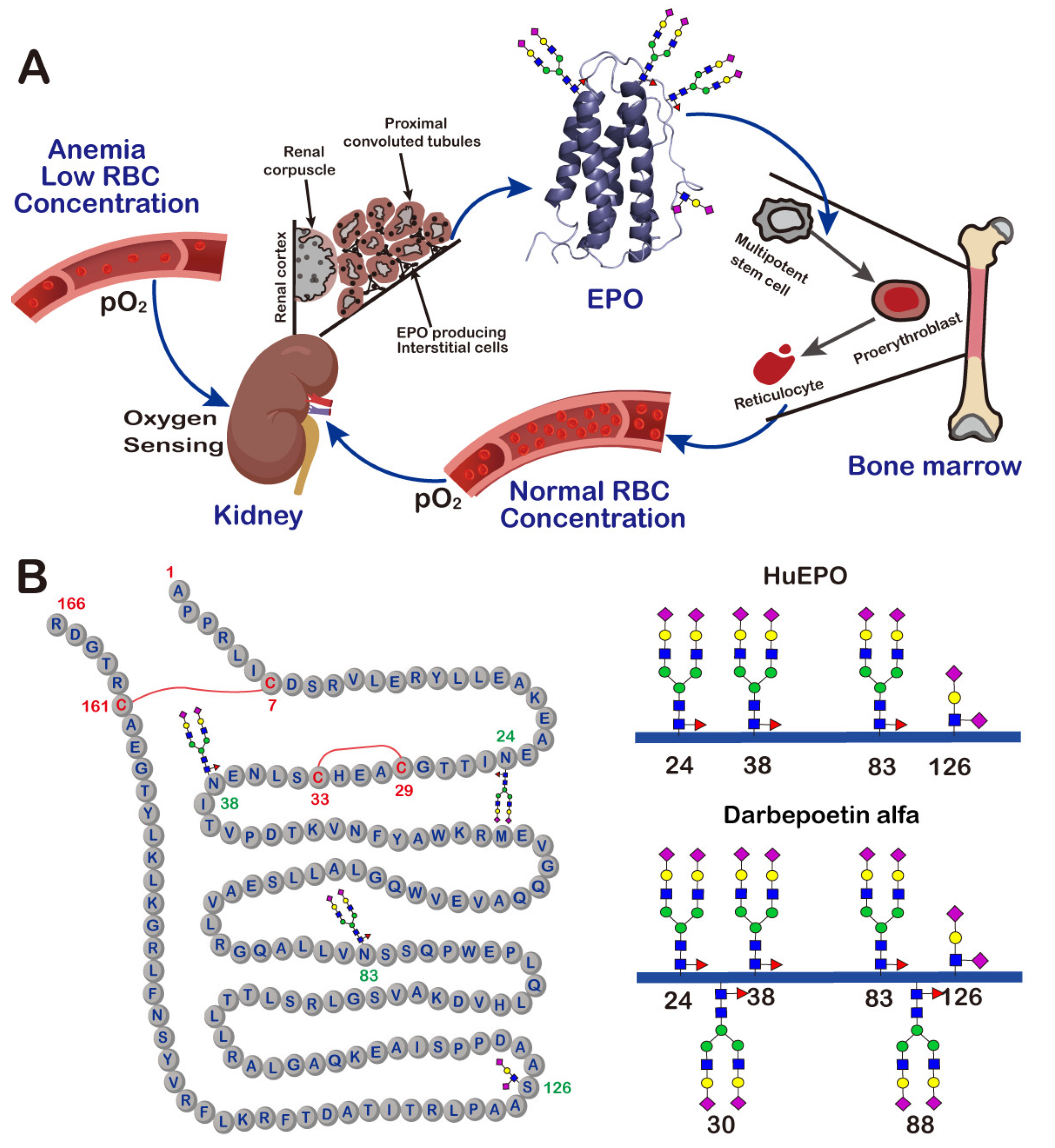
2. Erythropoietin
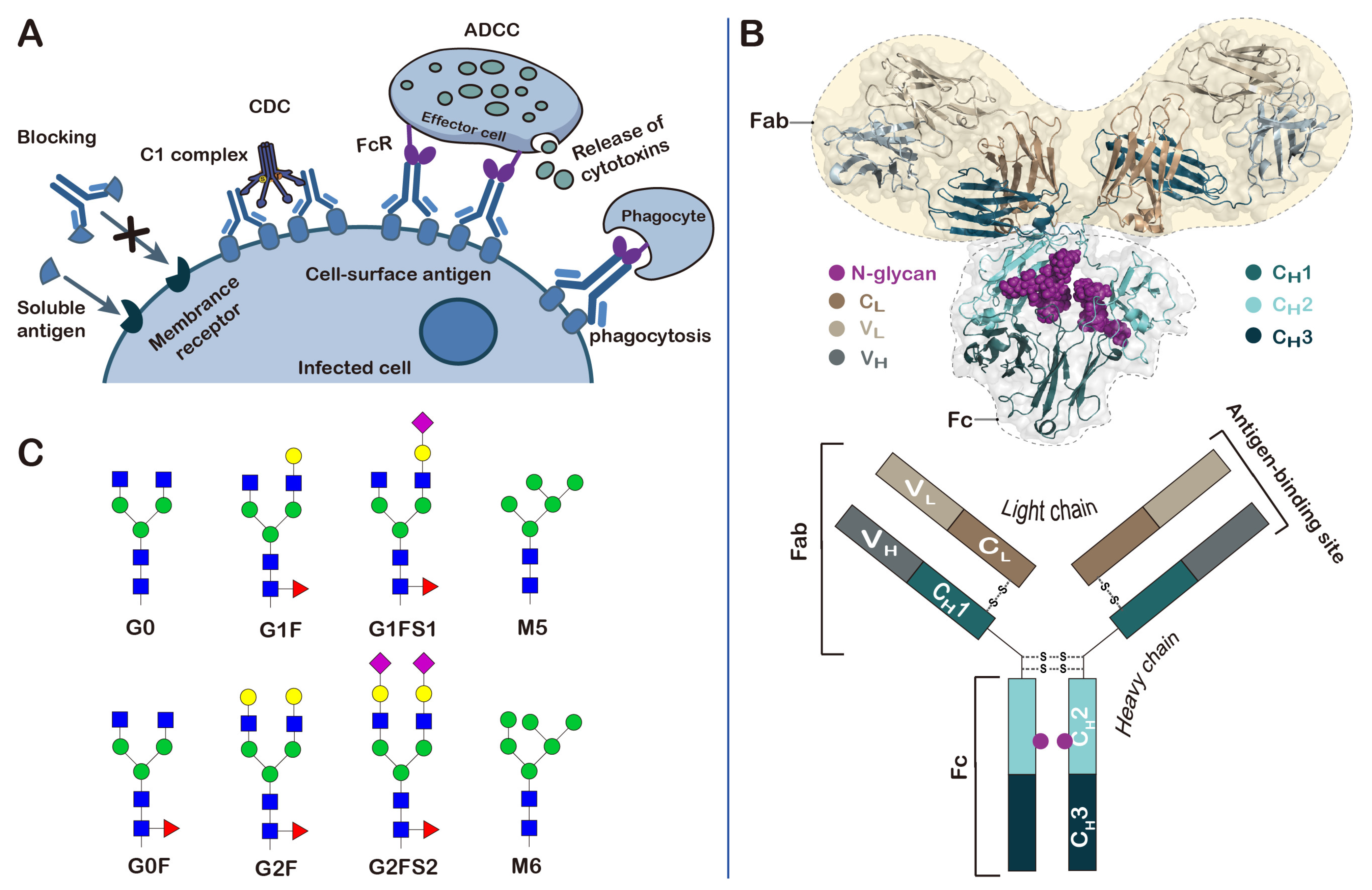
3. Monoclonal Antibodies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ramazi, S.; Zahiri, J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.G. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R. [Google Scholar] [CrossRef] [PubMed]
- Aebi, M. N-linked protein glycosylation in the ER. Biochim. Biophys. Acta 2013, 1833, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yagi, H.; Kuo, C.W.; Khoo, K.H.; Kato, K. An embeddable molecular code for Lewis X modification through interaction with fucosyltransferase 9. Commun. Biol. 2022, 5, 676. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.M.; Dwek, R.A. Glycosylation: Heterogeneity and the 3D structure of proteins. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.B.; Ng, S.K. Impact of host cell line choice on glycan profile. Crit. Rev. Biotechnol. 2018, 38, 851–867. [Google Scholar] [CrossRef]
- Guan, X.; Chaffey, P.K.; Zeng, C.; Greene, E.R.; Chen, L.; Drake, M.R.; Chen, C.; Groobman, A.; Resch, M.G.; Himmel, M.E.; et al. Molecular-scale features that govern the effects of O-glycosylation on a carbohydrate-binding module. Chem. Sci. 2015, 6, 7185–7189. [Google Scholar] [CrossRef]
- Wada, R.; Matsui, M.; Kawasaki, N. Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms. MAbs 2019, 11, 350–372. [Google Scholar] [CrossRef]
- Owczarek, B.; Gerszberg, A.; Hnatuszko-Konka, K. A brief reminder of systems of production and chromatography-based recovery of recombinant protein biopharmaceuticals. Biomed. Res. Int. 2019, 2019, 4216060. [Google Scholar] [CrossRef]
- Schuster, J.; Koulov, A.; Mahler, H.C.; Detampel, P.; Huwyler, J.; Singh, S.; Mathaes, R. In vivo stability of therapeutic proteins. Pharm. Res. 2020, 37, 23. [Google Scholar] [CrossRef]
- Marshall, S.A.; Lazar, G.A.; Chirino, A.J.; Desjarlais, J.R. Rational design and engineering of therapeutic proteins. Drug Discov. Today 2003, 8, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Sola, R.J.; Griebenow, K. Glycosylation of therapeutic proteins: An effective strategy to optimize efficacy. BioDrugs 2010, 24, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.M.; Elliott, S. Glycoengineering: The effect of glycosylation on the properties of therapeutic proteins. J. Pharm. Sci. 2005, 94, 1626–1635. [Google Scholar] [CrossRef]
- Dammen-Brower, K.; Epler, P.; Zhu, S.; Bernstein, Z.J.; Stabach, P.R.; Braddock, D.T.; Spangler, J.B.; Yarema, K.J. Strategies for glycoengineering therapeutic proteins. Front. Chem. 2022, 10, 863118. [Google Scholar] [CrossRef] [PubMed]
- Majewska, N.I.; Tejada, M.L.; Betenbaugh, M.J.; Agarwal, N. N-Glycosylation of IgG and IgG-like recombinant therapeutic proteins: Why is it important and how can we control it? Annu. Rev. Chem. Biomol. Eng. 2020, 11, 311–338. [Google Scholar] [CrossRef]
- Thompson, N.; Wakarchuk, W. O-glycosylation and its role in therapeutic proteins. Biosci. Rep. 2022, 42, BSR20220094. [Google Scholar] [CrossRef]
- Delobel, A. Glycosylation of therapeutic proteins: A critical quality attribute. Methods Mol. Biol. 2021, 2271, 1–21. [Google Scholar] [CrossRef]
- Zhong, X.; D’Antona, A.M.; Scarcelli, J.J.; Rouse, J.C. New opportunities in glycan engineering for therapeutic proteins. Antibodies 2022, 11, 5. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef]
- Lee, J.; Vernet, A.; Gruber, N.G.; Kready, K.M.; Burrill, D.R.; Way, J.C.; Silver, P.A. Rational engineering of an erythropoietin fusion protein to treat hypoxia. Protein Eng. Des. Sel. 2021, 34, gzab025. [Google Scholar] [CrossRef]
- Miyake, T.; Kung, C.K.; Goldwasser, E. Purification of human erythropoietin. J. Biol. Chem. 1977, 252, 5558–5564. [Google Scholar] [CrossRef] [PubMed]
- Recny, M.A.; Scoble, H.A.; Kim, Y. Structural characterization of natural human urinary and recombinant DNA-derived erythropoietin. Identification of des-arginine 166 erythropoietin. J. Biol. Chem. 1987, 262, 17156–17163. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.H.; Everett, R.; Wang, F.F.; Arakawa, T.; Goldwasser, E. Structural characterization of human erythropoietin. J. Biol. Chem. 1986, 261, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Lowy, P.H.; Keighley, G.; Borsook, H. Inactivation of erythropoietin by neuraminidase and by mild substitution reactions. Nature 1960, 185, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Shoemaker, C.; Rudersdorf, R.; Neill, S.D.; Kaufman, R.J.; Mufson, A.; Seehra, J.; Jones, S.S.; Hewick, R.; Fritsch, E.F.; et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 1985, 313, 806–810. [Google Scholar] [CrossRef]
- Lin, F.K.; Suggs, S.; Lin, C.H.; Browne, J.K.; Smalling, R.; Egrie, J.C.; Chen, K.K.; Fox, G.M.; Martin, F.; Stabinsky, Z.; et al. Cloning and expression of the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1985, 82, 7580–7584. [Google Scholar] [CrossRef]
- Lombardero, M.; Kovacs, K.; Scheithauer, B.W. Erythropoietin: A hormone with multiple functions. Pathobiology 2011, 78, 41–53. [Google Scholar] [CrossRef]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef]
- Nalbant, D.; Saleh, M.; Goldman, F.D.; Widness, J.A.; Veng-Pedersen, P. Evidence of receptor-mediated elimination of erythropoietin by analysis of erythropoietin receptor mRNA expression in bone marrow and erythropoietin clearance during anemia. J. Pharmacol. Exp. Ther. 2010, 333, 528–532. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting-strategies and applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef]
- Glanz, V.Y.; Kashirskikh, D.A.; Grechko, A.V.; Yet, S.F.; Sobenin, I.A.; Orekhov, A.N. Sialidase activity in human blood serum has a distinct seasonal pattern: A pilot study. Biology 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Padhi, D.; Jang, G. Pharmacology of darbepoetin alfa. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S4), iv2–iv9. [Google Scholar] [CrossRef] [PubMed]
- Egrie, J.C.; Dwyer, E.; Browne, J.K.; Hitz, A.; Lykos, M.A. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp. Hematol. 2003, 31, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Chairman and CEO Letter and Amgen Inc. 2021 Annual Report. Available online: https://investors.amgen.com/static-files/1789c7c6-0a07-49d4-bd81-e1afc7cb1f6d (accessed on 29 March 2022).
- Darling, R.J.; Kuchibhotla, U.; Glaesner, W.; Micanovic, R.; Witcher, D.R.; Beals, J.M. Glycosylation of erythropoietin affects receptor binding kinetics: Role of electrostatic interactions. Biochemistry 2002, 41, 14524–14531. [Google Scholar] [CrossRef]
- Koeppen, B.M.; Stanton, B.A. Glomerular filtration and renal blood flow. In Renal Physiology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 27–43. [Google Scholar]
- Macdougall, I.C.; Gray, S.J.; Elston, O.; Breen, C.; Jenkins, B.; Browne, J.; Egrie, J. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J. Am. Soc. Nephrol. 1999, 10, 2392–2395. [Google Scholar] [CrossRef]
- Kwak, C.Y.; Park, S.Y.; Lee, C.G.; Okino, N.; Ito, M.; Kim, J.H. Enhancing the sialylation of recombinant EPO produced in CHO cells via the inhibition of glycosphingolipid biosynthesis. Sci. Rep. 2017, 7, 13059. [Google Scholar] [CrossRef]
- Lee, S.J.; Evers, S.; Roeder, D.; Parlow, A.F.; Risteli, J.; Risteli, L.; Lee, Y.C.; Feizi, T.; Langen, H.; Nussenzweig, M.C. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 2002, 295, 1898–1901. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Palinsky, W.; Bierau, H. Glycoengineered antibodies: Towards the next-generation of immunotherapeutics. Glycobiology 2019, 29, 199–210. [Google Scholar] [CrossRef]
- Bournazos, S.; Wang, T.T.; Dahan, R.; Maamary, J.; Ravetch, J.V. Signaling by antibodies: Recent progress. Annu. Rev. Immunol. 2017, 35, 285–311. [Google Scholar] [CrossRef]
- Wang, X.; Mathieu, M.; Brezski, R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell 2018, 9, 63–73. [Google Scholar] [CrossRef]
- AbbVie Reports Full-Year and Fourth-Quarter 2021 Financial Results. Available online: https://news.abbvie.com/news/press-releases/abbvie-reports-full-year-and-fourth-quarter-2021-financial-results.htm (accessed on 2 February 2022).
- Reusch, D.; Tejada, M.L. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015, 25, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Wagner-Rousset, E.; Bussat, M.C.; Lokteff, M.; Klinguer-Hamour, C.; Haeuw, J.F.; Goetsch, L.; Wurch, T.; Van Dorsselaer, A.; Corvaia, N. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr. Pharm. Biotechnol. 2008, 9, 482–501. [Google Scholar] [CrossRef]
- Tebbey, P.W.; Varga, A.; Naill, M.; Clewell, J.; Venema, J. Consistency of quality attributes for the glycosylated monoclonal antibody Humira(R) (adalimumab). MAbs 2015, 7, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Nimmerjahn, F. Fc Mediated Activity of Antibodies Structural and Functional Diversity Preface; Thermo Fisher: Waltham, MA, USA, 2019; Volume 423, pp. V–VI. [Google Scholar]
- Anthony, R.M.; Nimmerjahn, F.; Ashline, D.J.; Reinhold, V.N.; Paulson, J.C.; Ravetch, J.V. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008, 320, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Barb, A.W.; Prestegard, J.H. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat. Chem. Biol. 2011, 7, 147–153. [Google Scholar] [CrossRef]
- Subedi, G.P.; Hanson, Q.M.; Barb, A.W. Restricted motion of the conserved immunoglobulin G1 N-glycan is essential for efficient FcgammaRIIIa binding. Structure 2014, 22, 1478–1488. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.; Bae, J.S.; Kim, Y.J.; Kang, H.A.; Kim, S.H.; Lee, S.J.; Lim, K.J.; Lee, J.W.; Jung, S.K.; et al. Analytical similarity assessment of rituximab biosimilar CT-P10 to reference medicinal product. MAbs 2018, 10, 380–396. [Google Scholar] [CrossRef]
- Van de Bovenkamp, F.S.; Hafkenscheid, L.; Rispens, T.; Rombouts, Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J. Immunol. 2016, 196, 1435–1441. [Google Scholar] [CrossRef]
- Corsiero, E.; Carlotti, E.; Jagemann, L.; Perretti, M.; Pitzalis, C.; Bombardieri, M. H and L Chain Affinity Maturation and/or Fab N-Glycosylation Influence Immunoreactivity toward Neutrophil Extracellular Trap Antigens in Rheumatoid Arthritis Synovial B Cell Clones. J. Immunol. 2020, 204, 2374–2379. [Google Scholar] [CrossRef]
- Van de Bovenkamp, F.S.; Derksen, N.I.L.; Ooijevaar-de Heer, P.; van Schie, K.A.; Kruithof, S.; Berkowska, M.A.; van der Schoot, C.E.; H, I.J.; van der Burg, M.; Gils, A.; et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc. Natl. Acad. Sci. USA 2018, 115, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Van de Bovenkamp, F.S.; Derksen, N.I.L.; van Breemen, M.J.; de Taeye, S.W.; Ooijevaar-de Heer, P.; Sanders, R.W.; Rispens, T. Variable Domain N-Linked Glycans Acquired During Antigen-Specific Immune Responses Can Contribute to Immunoglobulin G Antibody Stability. Front. Immunol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kiyoshi, M.; Anraku, M.; Hashii, N.; Oda-Ueda, N.; Ueda, T.; Ohkuri, T. Glycosylation decreases aggregation and immunogenicity of adalimumab Fab secreted from Pichia pastoris. J. Biochem. 2021, 169, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Reslan, M.; Sifniotis, V.; Cruz, E.; Sumer-Bayraktar, Z.; Cordwell, S.P.; Kayser, V. Enhancing the stability of adalimumab by engineering additional glycosylation motifs. Int. J. Biol. Macromol. 2020, 158, 189–196. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmana, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Higel, F.; Seidl, A.; Sorgel, F.; Friess, W. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm. 2016, 100, 94–100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Liu, W.; Li, Y.; Ma, B.; Shang, S.; Tan, Z. Impact of N-Linked Glycosylation on Therapeutic Proteins. Molecules 2022, 27, 8859. https://doi.org/10.3390/molecules27248859
Chen B, Liu W, Li Y, Ma B, Shang S, Tan Z. Impact of N-Linked Glycosylation on Therapeutic Proteins. Molecules. 2022; 27(24):8859. https://doi.org/10.3390/molecules27248859
Chicago/Turabian StyleChen, Baoquan, Wenqiang Liu, Yaohao Li, Bo Ma, Shiying Shang, and Zhongping Tan. 2022. "Impact of N-Linked Glycosylation on Therapeutic Proteins" Molecules 27, no. 24: 8859. https://doi.org/10.3390/molecules27248859
APA StyleChen, B., Liu, W., Li, Y., Ma, B., Shang, S., & Tan, Z. (2022). Impact of N-Linked Glycosylation on Therapeutic Proteins. Molecules, 27(24), 8859. https://doi.org/10.3390/molecules27248859




