Preparation and Optimization of O/W Emulsions Stabilized by Triglycerol Monolaurate for Curcumin Encapsulation
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of O/W Emulsions
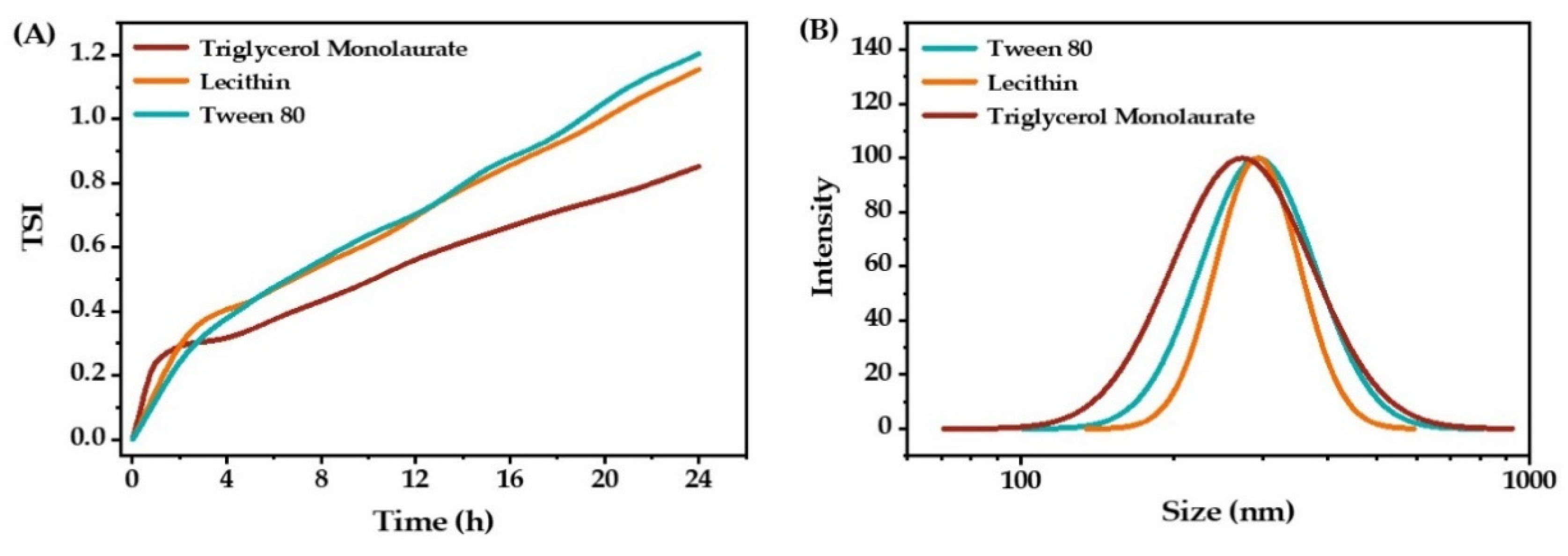
2.1.1. The Type of Emulsifiers
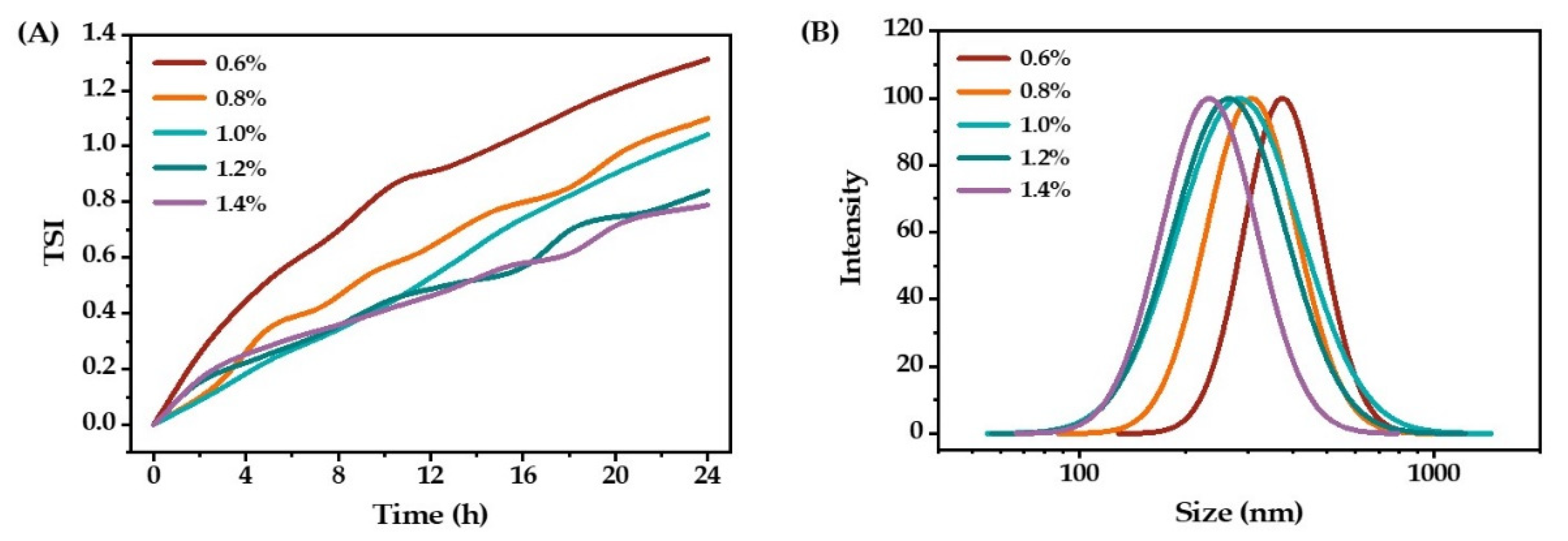
2.1.2. Concentration of Triglycerol Monolaurate
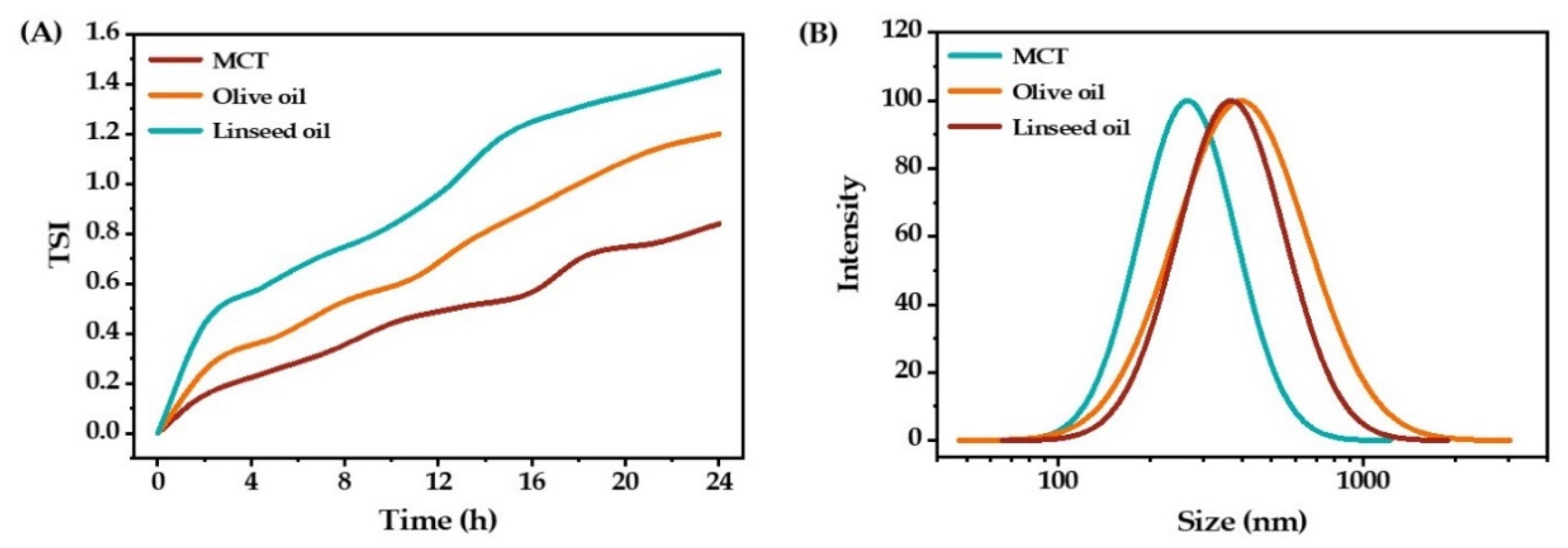
2.1.3. Oil Type
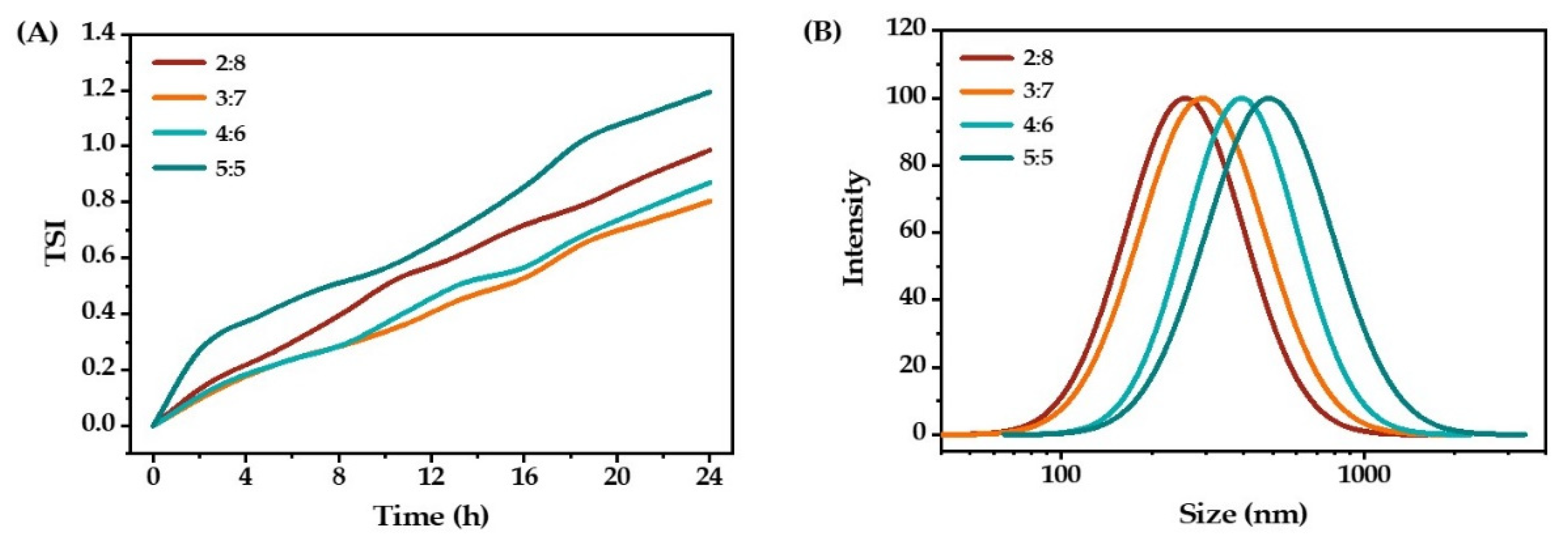
2.1.4. Oil-to-Water Ratio
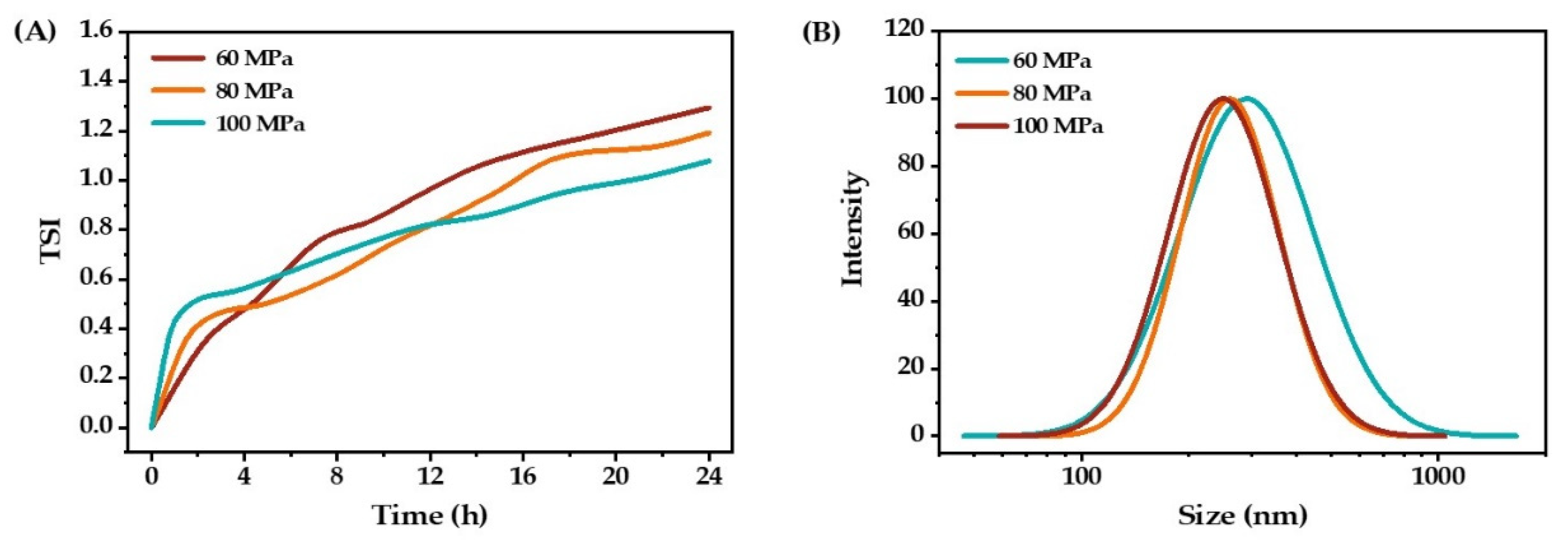
2.1.5. Homogenization Pressure and Processing Cycles
2.1.6. Multivariate Optimization by Response Surface Methodology (RSM)
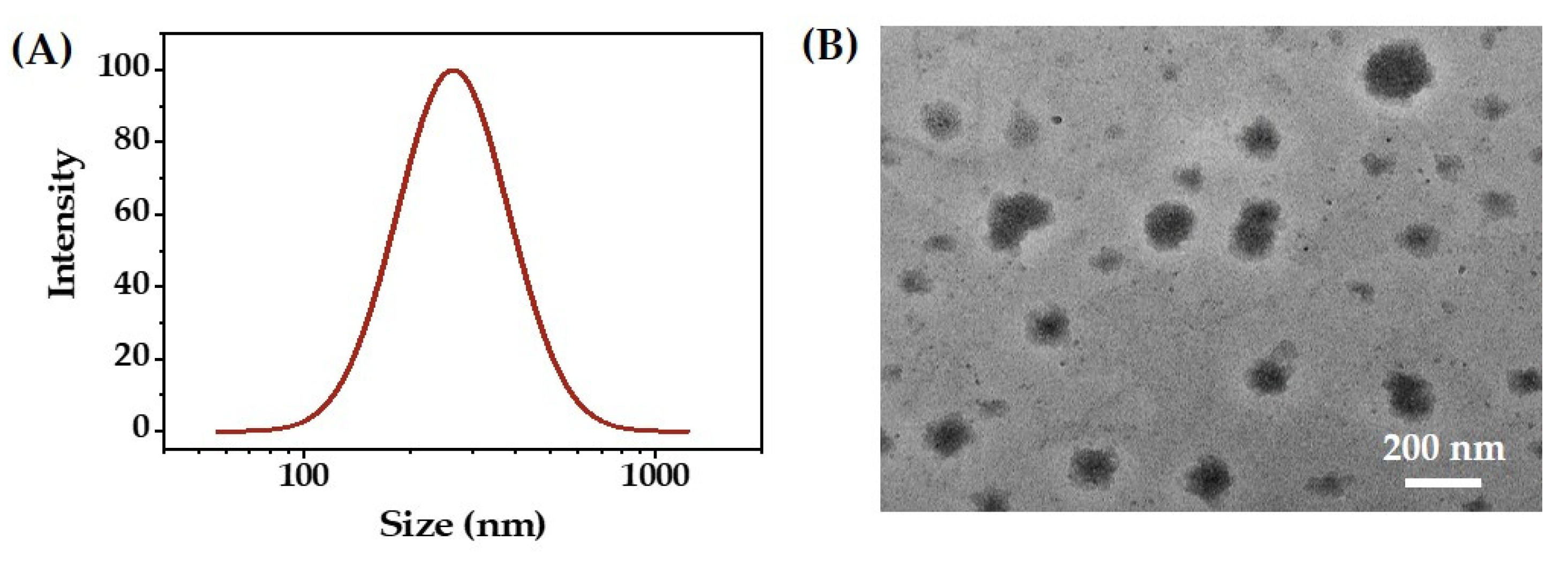
2.2. Characterization of O/W Emulsions
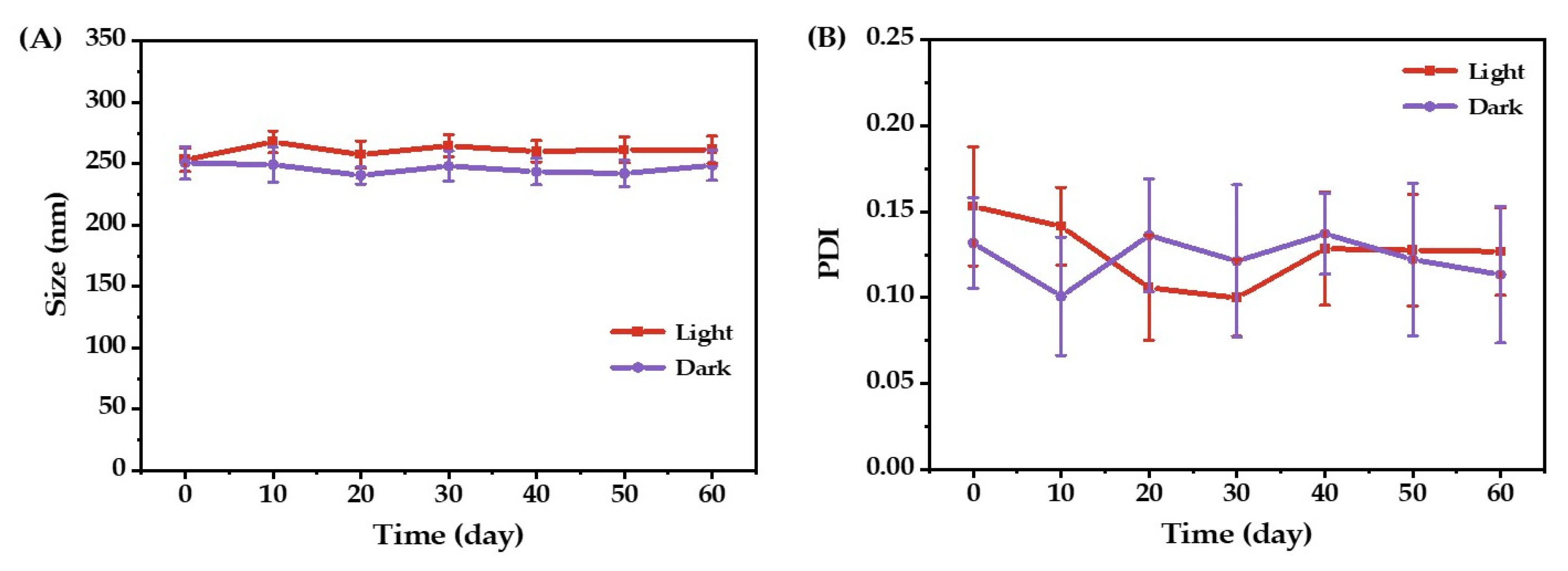
2.3. Storage Stability of O/W Emulsions
2.4. Storage Stability of Curcumin Encapsulated in the O/W Emulsions
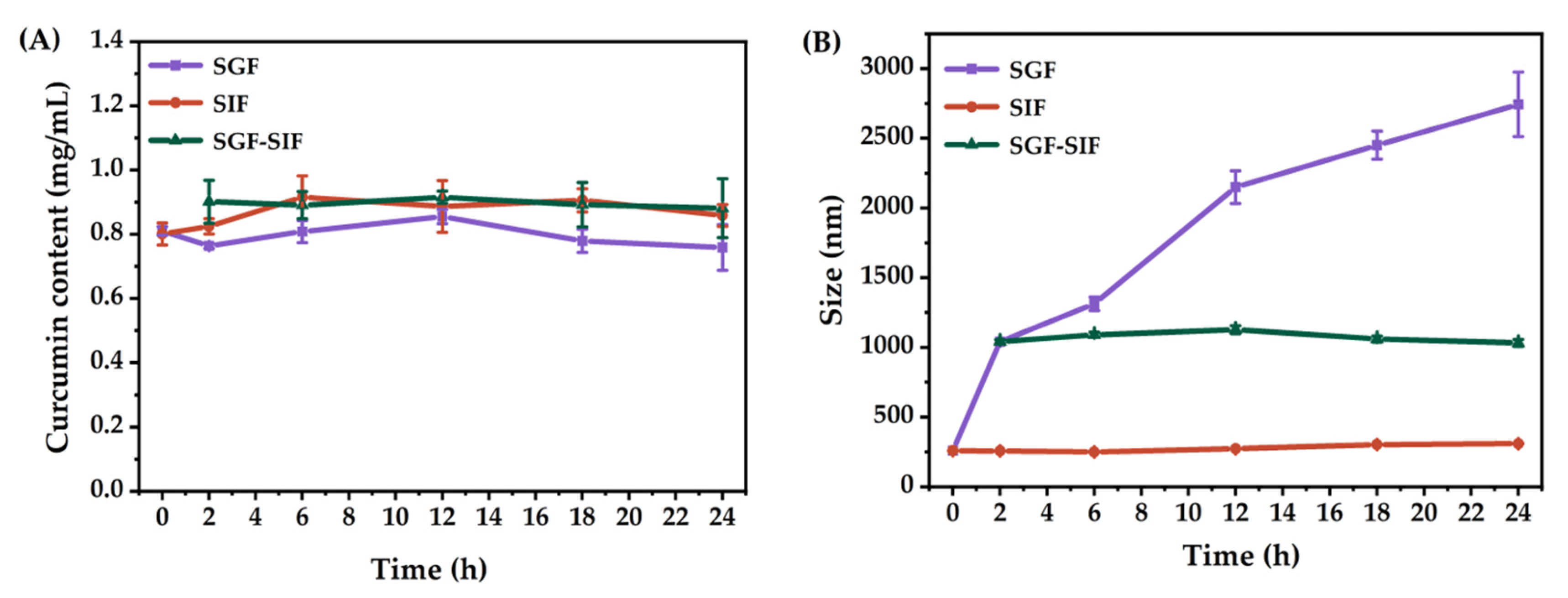
2.5. Stability of Curcumin and the O/W Emulsion during In Vitro Gastrointestinal Digestion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Curcumin O/W Emulsions
3.3. Experimental Design for Optimizing the Preparation of Curcumin O/W Emulsions by RSM
3.4. Stability of Curcumin O/W Emulsions
3.5. Determination of the Droplet Size Distribution of Curcumin O/W Emulsions
3.6. Quantification of Curcumin Encapsulated in O/W Emulsions
3.7. Simulated In Vitro Gastrointestinal Digestion
3.8. Transmission Electron Microscopy (TEM) Observations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976, 25, 1811–1812. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.; Huang, H.; Fenton, M.R.; Fong, D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with Anti-Inflammatory properties. Biochem. Pharmacol. 1998, 55, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The role of curcumin in cancer treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Subramani, P.A.; Panati, K.; Narala, V.R. Curcumin nanotechnologies and its anticancer activity. Nutr. Cancer 2017, 69, 381–393. [Google Scholar] [CrossRef]
- Trošelj, K.G.; Samaržija, I.; Tomljanović, M.; Kujundžić, R.N.; Đaković, N.; Mojzeš, A. Implementing curcumin in translational oncology research. Molecules 2020, 25, 5240. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of Liposome-Encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, X.; Chai, S.; Chen, Z.; An, X.; Shen, W. Effects of curcumin concentration and temperature on the spectroscopic properties of liposomal curcumin. J. Agric. Food Chem. 2012, 60, 1865–1870. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Tech. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef]
- Banaee, F.; Poureini, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Encapsulation of curcumin in gliadin-pectin in a core–shell nanostructure for efficient delivery of curcumin to cancer cells in vitro. Colloid Polym. Sci. 2022, 300, 1063–1073. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; Balamash, K.; Mcclements, D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocolloid. 2016, 58, 160–170. [Google Scholar] [CrossRef]
- Obeid, M.A.; Alsaadi, M.; Aljabali, A.A. Recent updates in curcumin delivery. J. Liposome Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Sukamtoh, E.; Xiao, H.; Mcclements, D.J.; Zhang, G. Curcumin: Recent advances in the development of strategies to improve oral bioavailability. Annu. Rev. Food Sci. Technol. 2019, 10, 597–617. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; Mcclements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; Mcclements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.; Huang, M.; Ho, C.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef]
- Lerche, D.; Sobisch, T. Direct and accelerated characterization of formulation stability. J. Disper. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Development of stable curcumin nanoemulsions: Effects of emulsifier type and surfactant-to-oil ratios. J. Food Sci. Technol. 2018, 55, 3485–3497. [Google Scholar] [CrossRef] [PubMed]
- Celis, M.; Contreras, B.; Forgiarini, A.; Rosenzweig, L.P.; Garcia-Rubio, L.H. Effect of emulsifier type on the characterization of O/W emulsions using a spectroscopy technique. J. Disper. Sci. Technol. 2016, 37, 512–518. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, S. Poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int. J. Pharmaceut. 2007, 342, 208–214. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, F.; Zhang, J.; Yu, L.; Zhang, G.; Liu, C.; Wang, N.; Xu, B. Development of polyglycerol fatty acid ester-based low-energy nanoemulsion for the improvement of curcumin stability. J. Disper. Sci. Technol. 2020, 43, 605–611. [Google Scholar] [CrossRef]
- Nikolic, I.; Jasmin Lunter, D.; Randjelovic, D.; Zugic, A.; Tadic, V.; Markovic, B.; Cekic, N.; Zivkovic, L.; Topalovic, D.; Spremo-Potparevic, B.; et al. Curcumin-loaded low-energy nanoemulsions as a prototype of multifunctional vehicles for different administration routes: Physicochemical and in vitro peculiarities important for dermal application. Int. J. Pharmaceut. 2018, 550, 333–346. [Google Scholar] [CrossRef]
- Borrin, T.R.; Georges, E.L.; Moraes, I.C.F.; Pinho, S.C. Curcumin-loaded nanoemulsions produced by the emulsion inversion point (EIP) method: An evaluation of process parameters and physico-chemical stability. J. Food Eng. 2016, 169, 1–9. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Pan, Y.; Nitin, N. Fate of curcumin encapsulated in silica nanoparticle stabilized Pickering emulsion during storage and simulated digestion. Food Res. Int. 2013, 51, 370–377. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, Y.; Jin, W.; An, Y.; He, L.; Li, Z.; Xu, W.; Li, B. Preparation and optimization of Pickering emulsion stabilized by chitosan-tripolyphosphate nanoparticles for curcumin encapsulation. Food Hydrocolloid. 2016, 52, 369–377. [Google Scholar] [CrossRef]
- Chen, C.; Johnston, T.D.; Jeon, H.; Gedaly, R.; Mchugh, P.P.; Burke, T.G.; Ranjan, D. An in vitro study of liposomal curcumin: Stability, toxicity and biological activity in human lymphocytes and Epstein-Barr virus-transformed human B-cells. Int. J. Pharmaceut. 2009, 366, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a nonionic surfactant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Zhang, G.; Mcclements, D.J. Stability of curcumin in oil-in-water emulsions: Impact of emulsifier type and concentration on chemical degradation. Food Res. Int. 2018, 111, 178–186. [Google Scholar] [CrossRef] [PubMed]



| Run | X1 | X2 | X3 | X4 | TSI |
|---|---|---|---|---|---|
| 1 | 0 | −1 | 1 | 0 | 0.80 |
| 2 | −1 | 0 | 0 | −1 | 1.02 |
| 3 | 0 | −1 | −1 | 0 | 0.92 |
| 4 | 0 | 0 | −1 | −1 | 0.95 |
| 5 | 1 | 1 | 0 | 0 | 0.99 |
| 6 | 0 | 0 | 0 | 0 | 0.78 |
| 7 | 1 | 0 | −1 | 0 | 0.97 |
| 8 | 0 | −1 | 0 | 1 | 0.96 |
| 9 | 0 | 1 | 1 | 0 | 0.94 |
| 10 | 0 | 0 | −1 | 1 | 0.92 |
| 11 | 0 | −1 | 0 | −1 | 0.85 |
| 12 | 0 | 1 | 0 | 1 | 0.89 |
| 13 | −1 | −1 | 0 | 0 | 1.03 |
| 14 | 0 | 0 | 1 | −1 | 0.94 |
| 15 | 1 | −1 | 0 | 0 | 0.92 |
| 16 | 0 | 0 | 0 | 0 | 0.74 |
| 17 | 1 | 0 | 0 | −1 | 0.96 |
| 18 | −1 | 0 | −1 | 0 | 1.04 |
| 19 | 0 | 0 | 0 | 0 | 0.73 |
| 20 | −1 | 0 | 0 | 1 | 1.02 |
| 21 | −1 | 1 | 0 | 0 | 1.05 |
| 22 | 1 | 0 | 0 | 1 | 0.97 |
| 23 | 0 | 1 | −1 | 0 | 0.89 |
| 24 | 0 | 0 | 1 | 1 | 0.84 |
| 25 | 0 | 0 | 0 | 0 | 0.72 |
| 26 | 1 | 0 | 1 | 0 | 0.88 |
| 27 | 0 | 1 | 0 | −1 | 0.97 |
| 28 | −1 | 0 | 1 | 0 | 1.01 |
| 29 | 0 | 0 | 0 | 0 | 0.75 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.2644 | 14 | 0.0189 | 30.53 | <0.0001 | significant |
| X1 | 0.0192 | 1 | 0.0192 | 31.03 | <0.0001 | |
| X2 | 0.0052 | 1 | 0.0052 | 8.42 | 0.0116 | |
| X3 | 0.0065 | 1 | 0.0065 | 10.56 | 0.0058 | |
| X4 | 0.0007 | 1 | 0.0007 | 1.09 | 0.3139 | |
| X1X2 | 0.0006 | 1 | 0.0006 | 1.01 | 0.3319 | |
| X1X3 | 0.0009 | 1 | 0.0009 | 1.45 | 0.2478 | |
| X1X4 | 0.0000 | 1 | 0.0000 | 0.0404 | 0.8436 | |
| X2X3 | 0.0072 | 1 | 0.0072 | 11.68 | 0.0042 | |
| X2X4 | 0.0090 | 1 | 0.0090 | 14.59 | 0.0019 | |
| X3X4 | 0.0012 | 1 | 0.0012 | 1.98 | 0.1812 | |
| X1² | 0.1732 | 1 | 0.1732 | 279.98 | <0.0001 | |
| X2² | 0.0438 | 1 | 0.0438 | 70.78 | <0.0001 | |
| X3² | 0.0304 | 1 | 0.0304 | 49.07 | <0.0001 | |
| X4² | 0.0551 | 1 | 0.0551 | 89.06 | <0.0001 | |
| Residual | 0.0087 | 14 | 0.0006 | |||
| Lack of Fit | 0.0065 | 10 | 0.0007 | 1.23 | 0.4536 | not significant |
| Pure Error | 0.0021 | 4 | 0.0005 | |||
| Cor Total | 0.2731 | 28 |
| Parameters | Factor Code | Level of Factors | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Concentration of triglycerol monolaurate (wt%) | X1 | 1.0 | 1.2 | 1.4 |
| Oil phase fraction (%) | X2 | 20 | 30 | 40 |
| Homogenization pressure (MPa) | X3 | 60 | 80 | 100 |
| Processing cycles (number) | X4 | 6 | 8 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhang, Q.; Wang, L.; Ji, L.; Han, P.; Zhao, F.; Su, Q. Preparation and Optimization of O/W Emulsions Stabilized by Triglycerol Monolaurate for Curcumin Encapsulation. Molecules 2022, 27, 8861. https://doi.org/10.3390/molecules27248861
Zhang G, Zhang Q, Wang L, Ji L, Han P, Zhao F, Su Q. Preparation and Optimization of O/W Emulsions Stabilized by Triglycerol Monolaurate for Curcumin Encapsulation. Molecules. 2022; 27(24):8861. https://doi.org/10.3390/molecules27248861
Chicago/Turabian StyleZhang, Guiqiong, Qiang Zhang, Lan Wang, Lei Ji, Pengbing Han, Fengju Zhao, and Qun Su. 2022. "Preparation and Optimization of O/W Emulsions Stabilized by Triglycerol Monolaurate for Curcumin Encapsulation" Molecules 27, no. 24: 8861. https://doi.org/10.3390/molecules27248861
APA StyleZhang, G., Zhang, Q., Wang, L., Ji, L., Han, P., Zhao, F., & Su, Q. (2022). Preparation and Optimization of O/W Emulsions Stabilized by Triglycerol Monolaurate for Curcumin Encapsulation. Molecules, 27(24), 8861. https://doi.org/10.3390/molecules27248861





