Selenoxides as Excellent Chalcogen Bond Donors: Effect of Metal Coordination
Abstract
1. Introduction
2. Results and Discussion
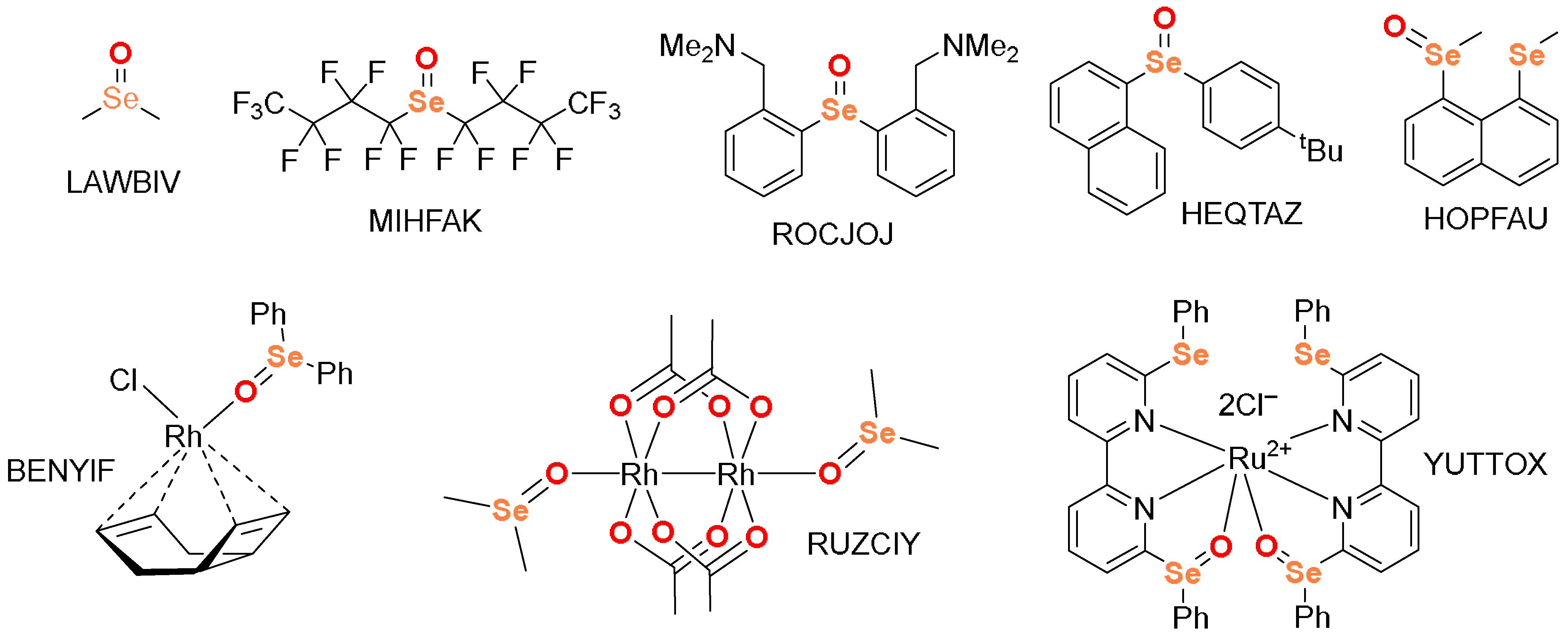
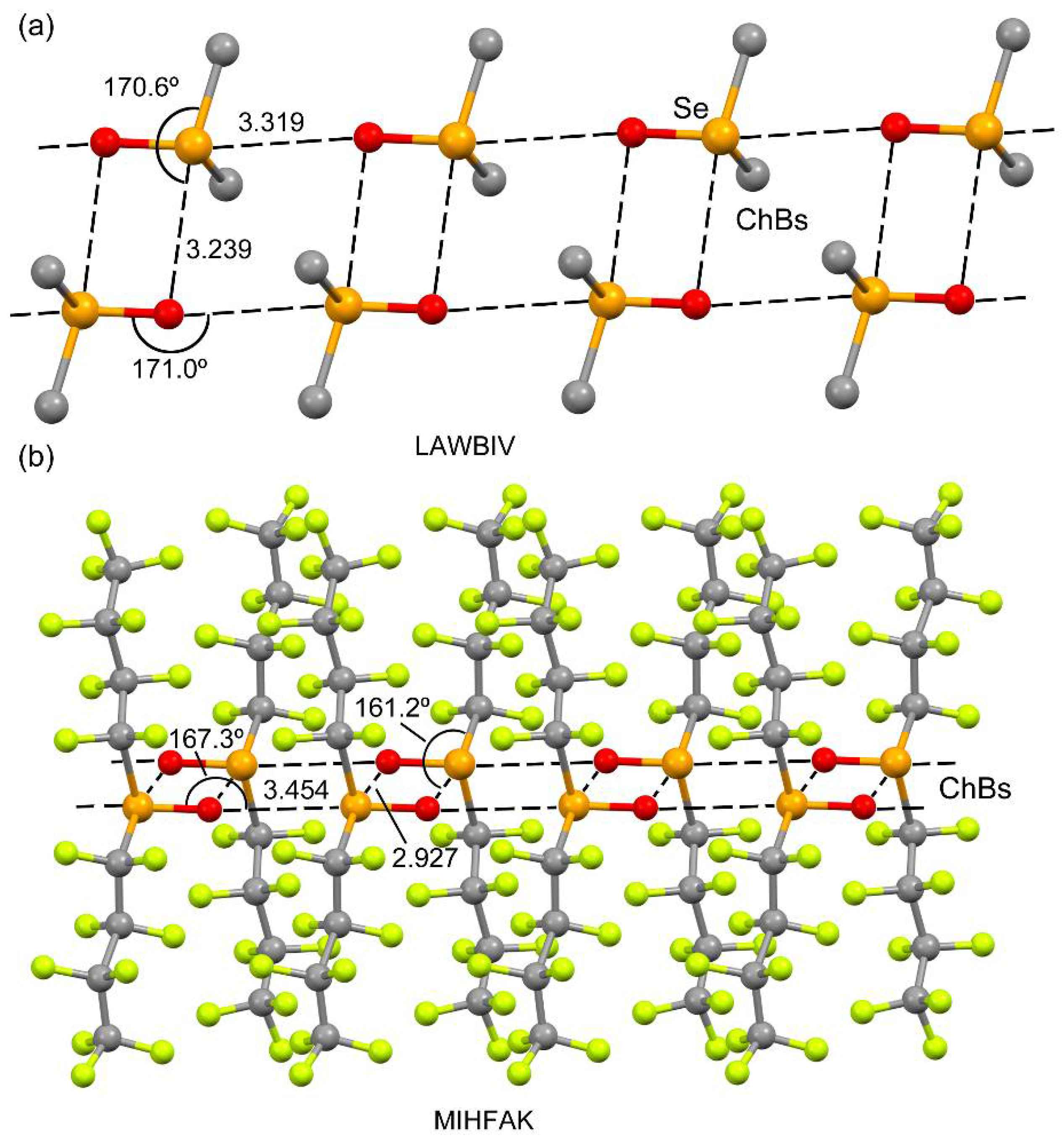
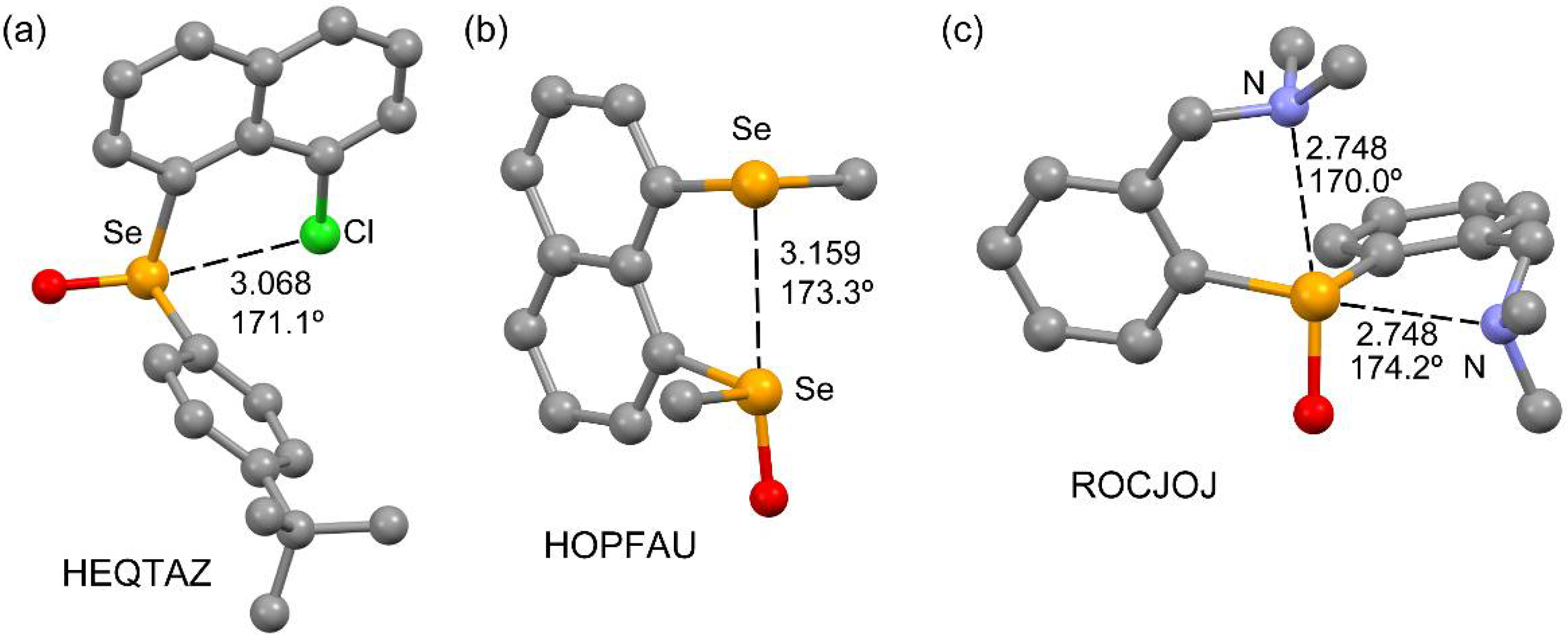
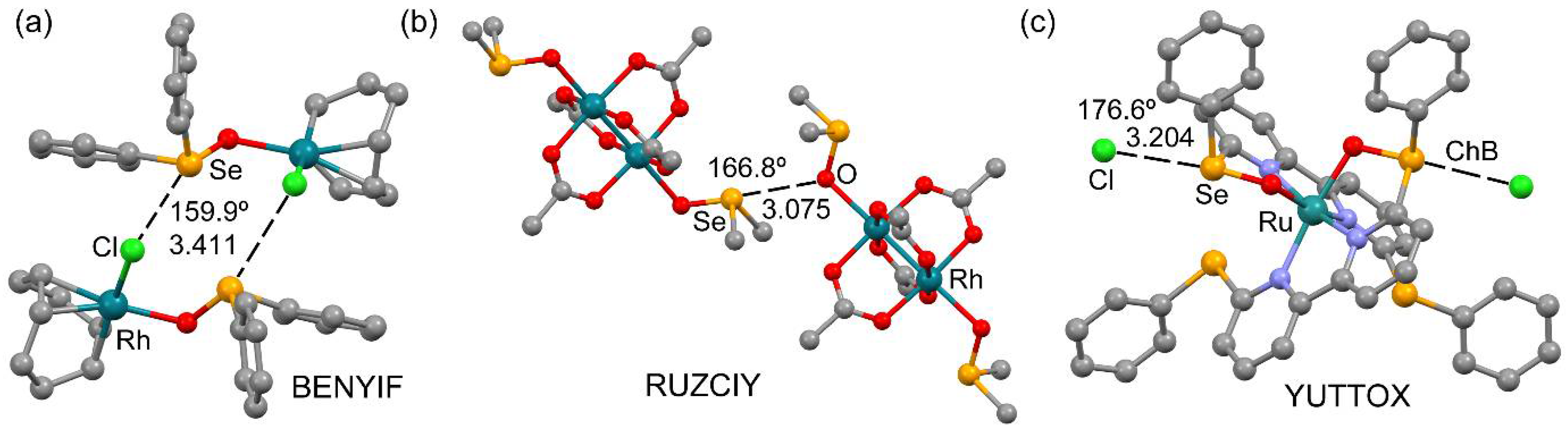
2.1. CSD Survey
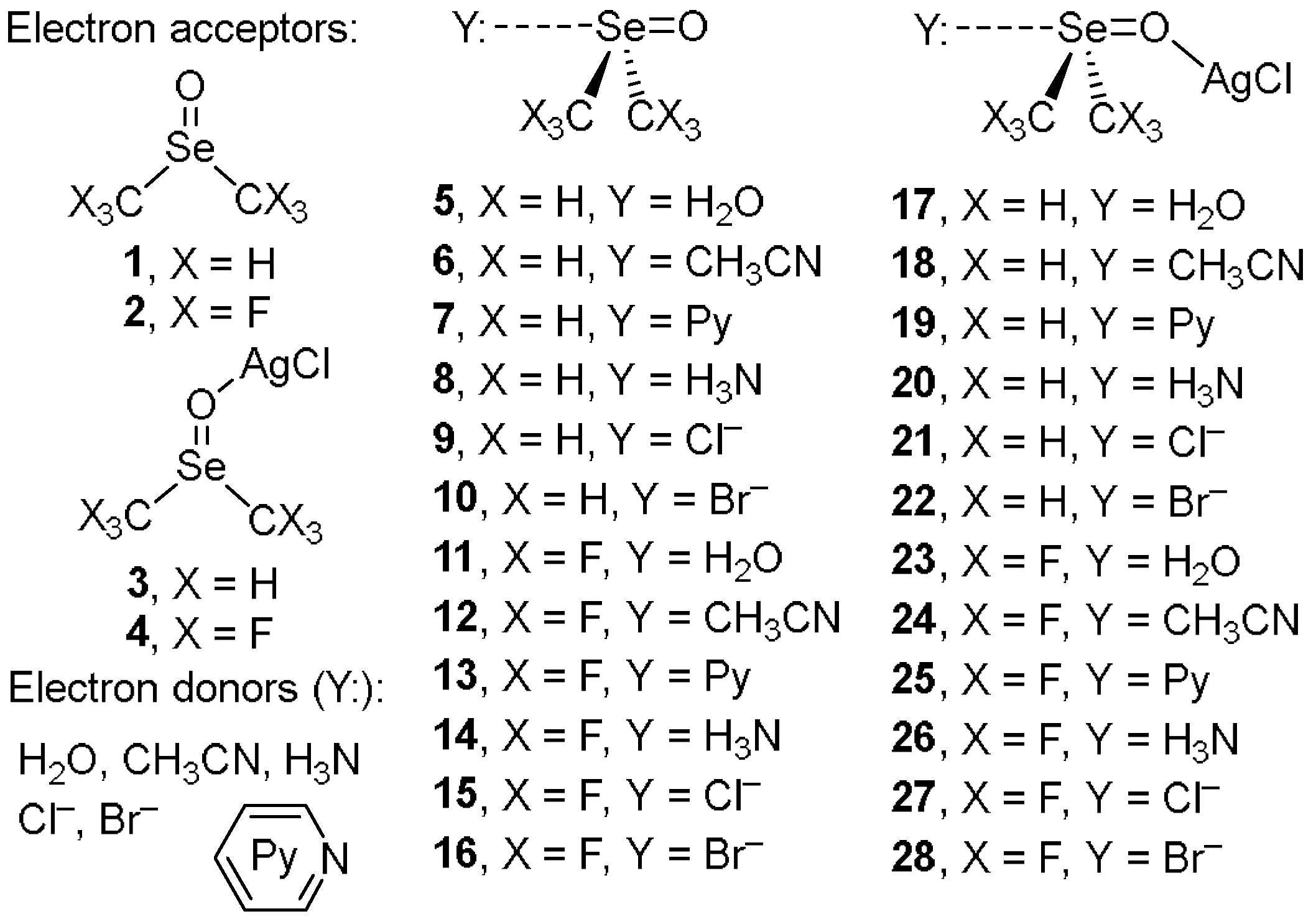
2.2. Theoretical DFT Study
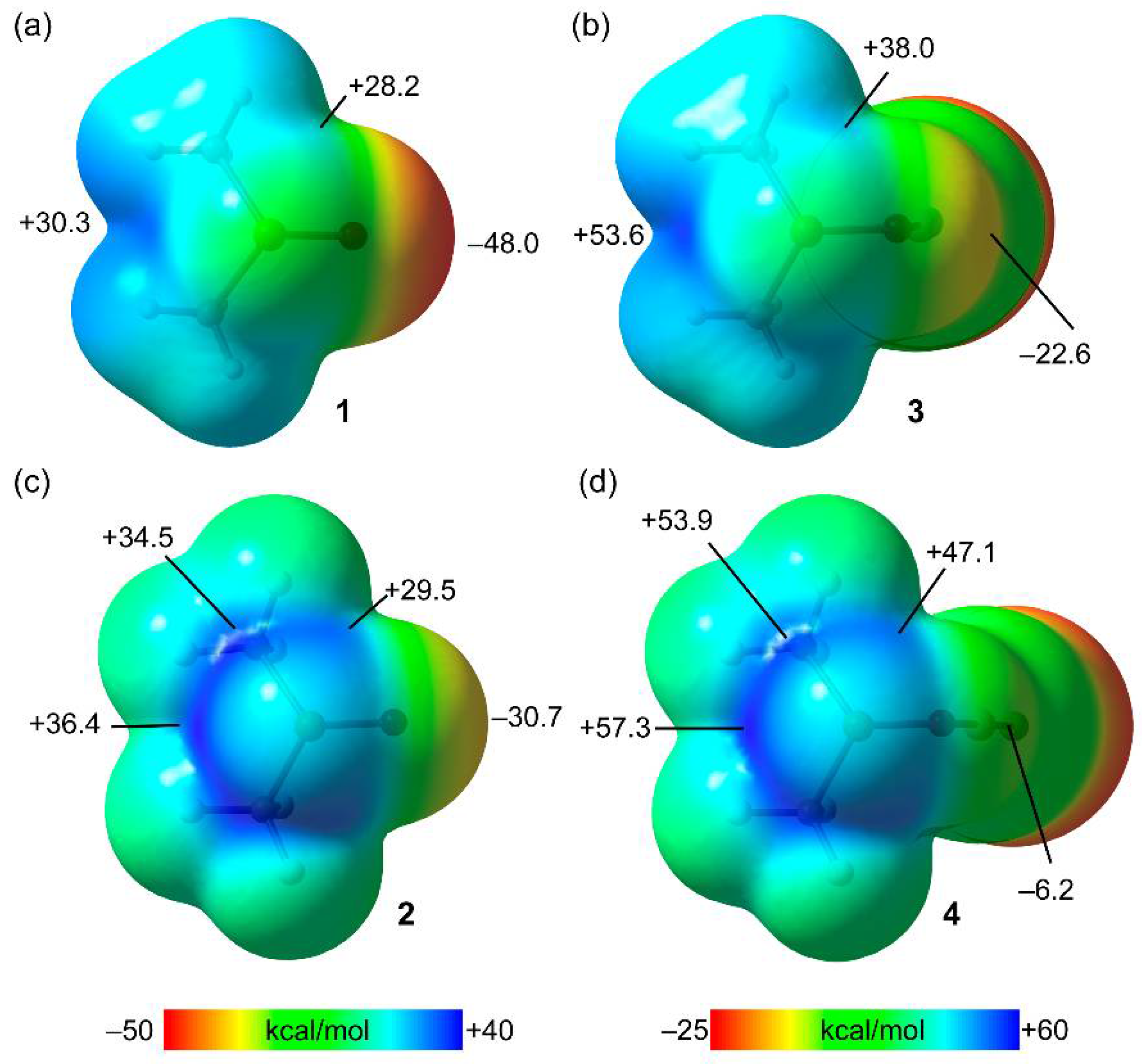
2.2.1. MEP Analysis
2.2.2. DFT Energetic and Geometric Studies
2.2.3. QTAIM and NCIPlot Study
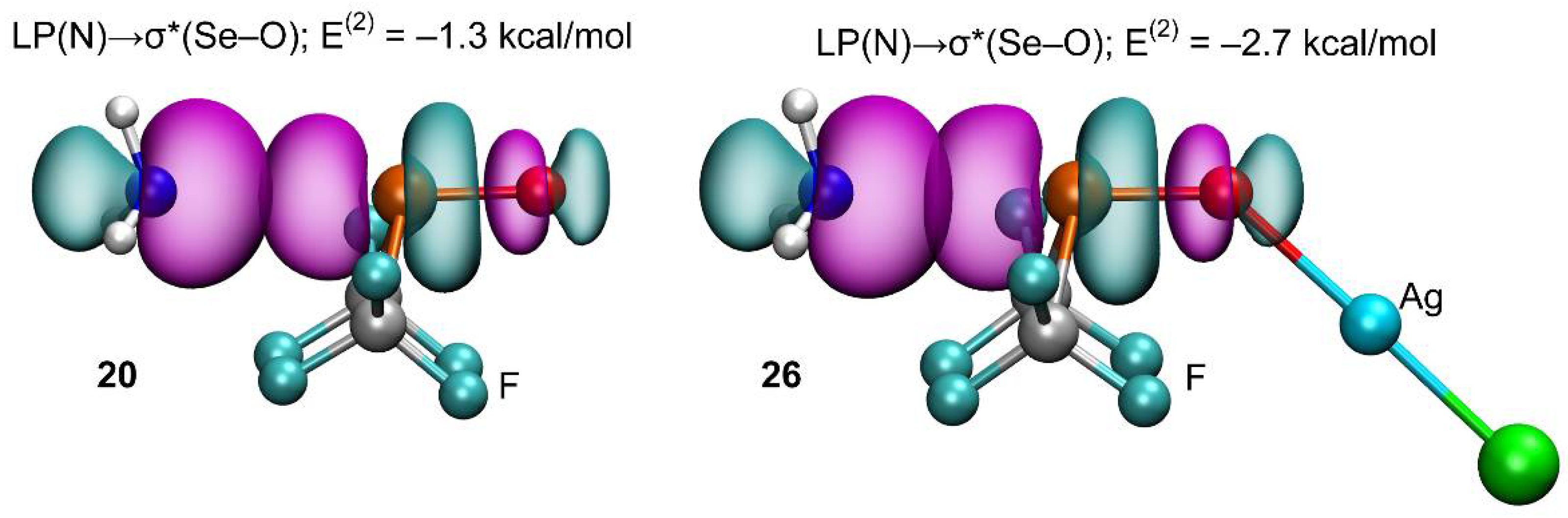
2.2.4. Natural Bond Orbital (NBO) Analysis
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fourmigué, M.; Dhaka, A. Chalcogen bonding in crystalline diselenides and selenocyanates: From molecules of pharmaceutical interest to conducting materials. Coord. Chem. Rev. 2020, 403, 213084. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not only hydrogen bonds: Other noncovalent interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen bonding: An overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef]
- Biot, N.; Bonifazi, D. Chalcogen-bond driven molecular recognition at work. Coord. Chem. Rev. 2020, 413, 213243. [Google Scholar] [CrossRef]
- Navarro-García, E.; Galmés, B.; Velasco, M.D.; Frontera, A.; Caballero, A. Anion Recognition by Neutral Chalcogen Bonding Receptors: Experimental and Theoretical Investigations. Chem. Eur. J. 2020, 26, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, E.; Galmés, B.; Esquivel, J.L.; Velasco, M.D.; Bastida, A.; Zapata, F.; Caballero, A.; Frontera, A. Host–guest complexes vs. supramolecular polymers in chalcogen bonding receptors: An experimental and theoretical study. Dalton Trans. 2022, 51, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Frontera, A.; Bauza, A. Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations. Int. J. Mol. Sci. 2022, 23, 4188. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton. Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef]
- Romito, D.; Fresta, E.; Cavinato, L.M.; Kählig, H.; Amenitsch, H.; Caputo, L.; Chen, Y.; Samorì, P.; Charlier, J.-C.; Costa, R.D.; et al. Supramolecular Chalcogen-Bonded Semiconducting Nanoribbons at Work in Lighting Devices. Angew. Chem. Int. Ed. 2022, 61, e202202137. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. Effects of Charge and Substituent on the S···N Chalcogen Bond. J. Phys. Chem. A 2014, 118, 3183–3192. [Google Scholar] [CrossRef]
- Azofra, L.M.; Scheiner, S. Substituent Effects in the Noncovalent Bonding of SO2 to Molecules Containing a Carbonyl Group. The Dominating Role of the Chalcogen Bond. J. Phys. Chem. A 2014, 118, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Young, M.W.; Lauer, R.F. Reactions of Selenoxides: Thermal Syn-elimination and H218O Exchange. Tetrahedron Lett. 1973, 14, 1979–1982. [Google Scholar] [CrossRef]
- Goodman, M.A.; Detty, M.R. Selenoxides as Catalysts for the Activation of Hydrogen Peroxide. Bromination of Organic Substrates with Sodium Bromide and Hydrogen Peroxide. Organometallics 2004, 23, 3016–3020. [Google Scholar] [CrossRef]
- Farina, M.; Folmer, V.; Bolzan, R.C.; Andrade, L.H.; Zeni, G.; Braga, A.J.; Rocha, J.B.T. Selenoxides inhibit δ-aminolevulinic acid dehydratase. Toxicol. Lett. 2001, 119, 27–37. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Filatov, A.S.; Block, E.; Petrukhina, M.A. Dimethyl selenoxide. Acta Cryst. 2005, C61, o596–o598. [Google Scholar] [CrossRef]
- Gockel, S.; Haas, A.; Probst, V.; Boese, R.; Muller, I. Contributions to bis(perfluoroalkyl) chalkogenide chemistry: Preparation of (Rf)2SeO [Rf = C2F5, (CF3)2CF, n-C4F9], (Rf′)2TeX2 [X = F, Cl: Rf′ = n-C3F7, (CF3)2CF, n-C4F9; X = Br: Rf′ = n-C3F7, n-C4F9], (CF3)2Te(NSO)2 and (C2F5)2Te(OH)NO3. J. Fluor. Chem. 2000, 102, 301–311. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mat. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Hayashi, S.; Wada, H.; Ueno, T.; Nakanishi, W. Structures of 1-(Arylseleninyl)naphthalenes: O, G, and Y Dependences in 8-G-1-[p-YC6H4Se(O)]C10H6. J. Org. Chem. 2006, 71, 5574–5585. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Nakanishi, W.; Furuta, A.; Drabowicz, J.; Sasamori, T.; Tokitoh, N. How does non-covalent Se⋯Se=O interaction stabilize selenoxides at naphthalene 1,8-positions: Structural and theoretical investigations. New J. Chem. 2009, 33, 196–206. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Krumm, B.; Scherr, M. Studies on the Properties of Organoselenium(IV) Fluorides and Azides. Inorg. Chem. 2008, 47, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- Weilbeer, C.; Selent, D.; Dyballa, K.M.; Franke, R.; Spannenberg, A.; Börner, A. Evaluation of Organoselenium Based Compounds as Co-Catalysts in Rhodium-Catalyzed Hydroformylation. ChemistrySelect 2016, 1, 5421–5429. [Google Scholar] [CrossRef]
- Dikarev, E.V.; Petrukhina, M.A.; Li, X.; Block, E. Small Organoselenium Molecules. 1. Dimethyl Selenoxide: Structure, Complexation, and Gas-Phase Transformation. Inorg. Chem. 2003, 42, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Laskavy, A.; Shimon, L.J.W.; Konstantinovski, L.; Iron, M.A.; Neumann, R. Activation of Molecular Oxygen by a Dioxygenase Pathway by a Ruthenium Bis-bipyridine Compound with a Proximal Selenium Site. J. Am. Chem. Soc. 2010, 132, 517–523. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Mertsalov, D.F.; Gomila, R.M.; Zaytsev, V.P.; Grigoriev, M.S.; Nikitina, E.V.; Zubkov, F.I.; Frontera, A. On the Importance of Halogen Bonding Interactions in Two X-ray Structures Containing All Four (F, Cl, Br, I) Halogen Atoms. Crystals 2021, 11, 1406. [Google Scholar] [CrossRef]
- Gomila, R.M.; Frontera, A. Matere Bonds vs. Multivalent Halogen and Chalcogen Bonds: Three Case Studies. Molecules 2022, 27, 6597. [Google Scholar] [CrossRef] [PubMed]
- Roselló, Y.; Benito, M.; Molins, E.; Barceló-Oliver, M.; Frontera, A. Adenine as a Halogen Bond Acceptor: A Combined Experimental and DFT Study. Crystals 2019, 9, 224. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural bond orbital methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO 7.0: New Vistas in Localized and Delocalized Chemical Bonding Theory; Theoretical Chemistry Institute, University of Wisconsin-Madison: Madison, WI, USA, 2018. [Google Scholar]
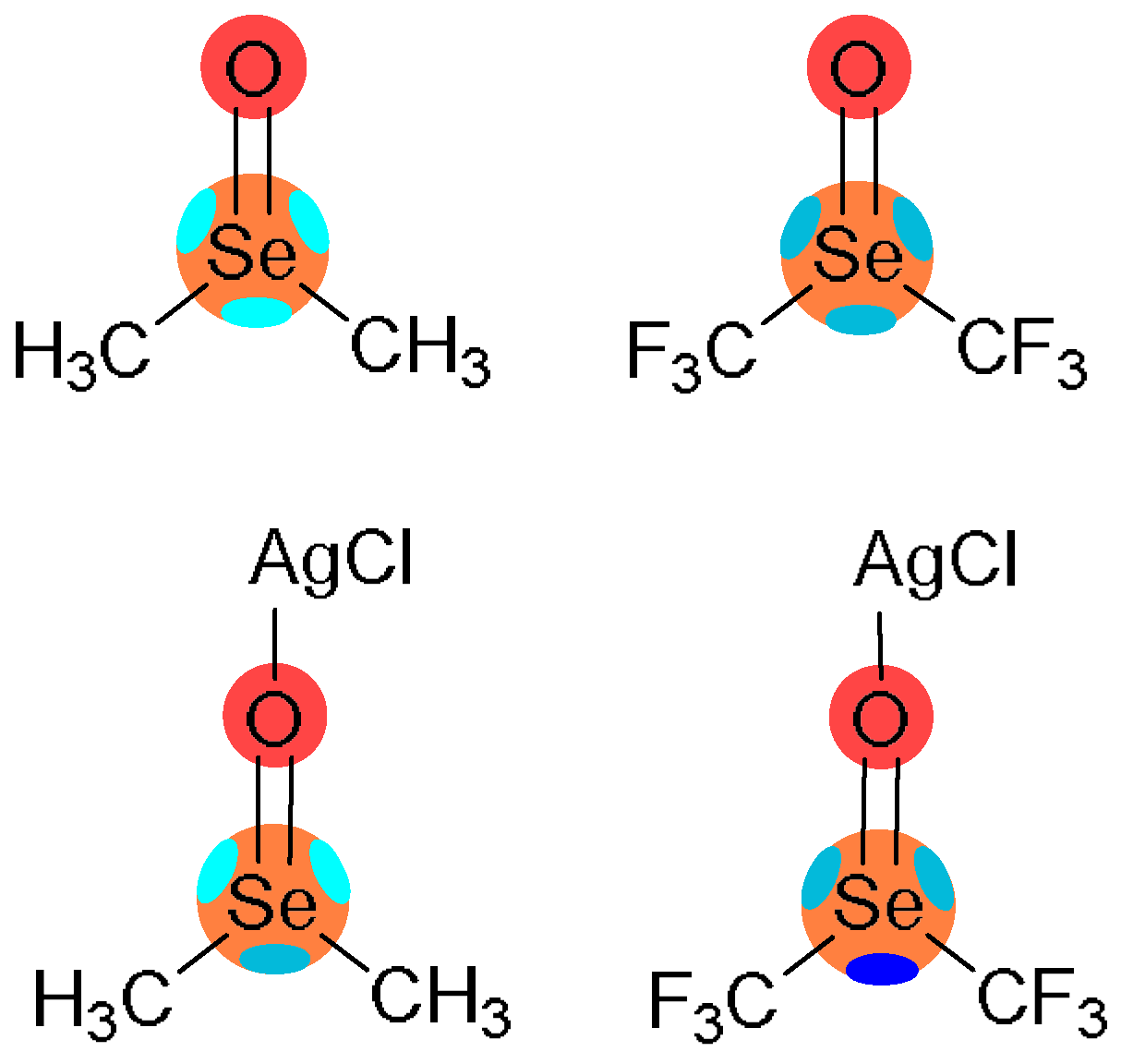


| Complex | E | d | α | ΣRvdw |
|---|---|---|---|---|
| 5 (1 + H2O) | −4.2 | 3.303 | 179.6 | 3.45 |
| 6 (1 + CH3CN) | −4.7 | 3.501 | 176.1 | 3.50 |
| 7 (1 + Py) | −5.3 | 3.341 | 179.6 | 3.50 |
| 8 (1 + NH3) | −4.9 (−3.5) | 3.475 (3.453) | 179.6 (178.5) | 3.50 |
| 9 (1 + Cl−) | −24.0 | 3.772 | 163.3 | 3.70 |
| 10 (1 + Br−) | −20.2 | 4.007 | 163.4 | 3.80 |
| 11 (2 + H2O) | −5.1 | 3.074 | 173.8 | 3.45 |
| 12 (2 + CH3CN) | −5.0 | 3.221 | 171.9 | 3.50 |
| 13 (2 + Py) | −7.0 | 2.973 | 176.3 | 3.50 |
| 14 (2 + NH3) | −6.7 (−4.4) | 3.012 (3.017) | 177.0 (176.6) | 3.50 |
| 15 (2 + Cl−) | −30.1 | 2.793 | 178.4 | 3.70 |
| 16 (2 + Br−) | −24.0 | 3.028 | 175.3 | 3.80 |
| 17 (3 + H2O) | −6.5 | 3.177 | 178.5 | 3.45 |
| 18 (3 + CH3CN) | −7.6 | 3.220 | 178.5 | 3.50 |
| 19 (3 + Py) | −8.3 | 3.076 | 176.3 | 3.50 |
| 20 (3 + NH3) | −7.3 (−5.8) | 3.184 (3.202) | 174.2 (173.8) | 3.50 |
| 21 (3 + Cl−) | −43.1 | 2.896 | 178.3 | 3.70 |
| 22 (3 + Br−) | −37.2 | 3.143 | 177.5 | 3.80 |
| 23 (4 + H2O) | −7.2 | 2.911 | 172.3 | 3.45 |
| 24 (4 + CH3CN) | −8.1 | 2.936 | 175.2 | 3.50 |
| 25 (4 + Py) | −10.8 | 2.810 | 175.1 | 3.50 |
| 26 (4 + NH3) | −10.3 (−7.1) | 2.797 (2.802) | 178.8 (178.0) | 3.50 |
| 27 (4 + Cl–) | −53.3 | 2.618 | 177.6 | 3.70 |
| 28 (4 + Br–) | −45.3 | 2.829 | 175.2 | 3.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burguera, S.; Gomila, R.M.; Bauzá, A.; Frontera, A. Selenoxides as Excellent Chalcogen Bond Donors: Effect of Metal Coordination. Molecules 2022, 27, 8837. https://doi.org/10.3390/molecules27248837
Burguera S, Gomila RM, Bauzá A, Frontera A. Selenoxides as Excellent Chalcogen Bond Donors: Effect of Metal Coordination. Molecules. 2022; 27(24):8837. https://doi.org/10.3390/molecules27248837
Chicago/Turabian StyleBurguera, Sergi, Rosa M. Gomila, Antonio Bauzá, and Antonio Frontera. 2022. "Selenoxides as Excellent Chalcogen Bond Donors: Effect of Metal Coordination" Molecules 27, no. 24: 8837. https://doi.org/10.3390/molecules27248837
APA StyleBurguera, S., Gomila, R. M., Bauzá, A., & Frontera, A. (2022). Selenoxides as Excellent Chalcogen Bond Donors: Effect of Metal Coordination. Molecules, 27(24), 8837. https://doi.org/10.3390/molecules27248837








