Dinuclear Lanthanide Compound as a Promising Luminescent Probe for Al3+ Ions
Abstract
1. Introduction
2. Results
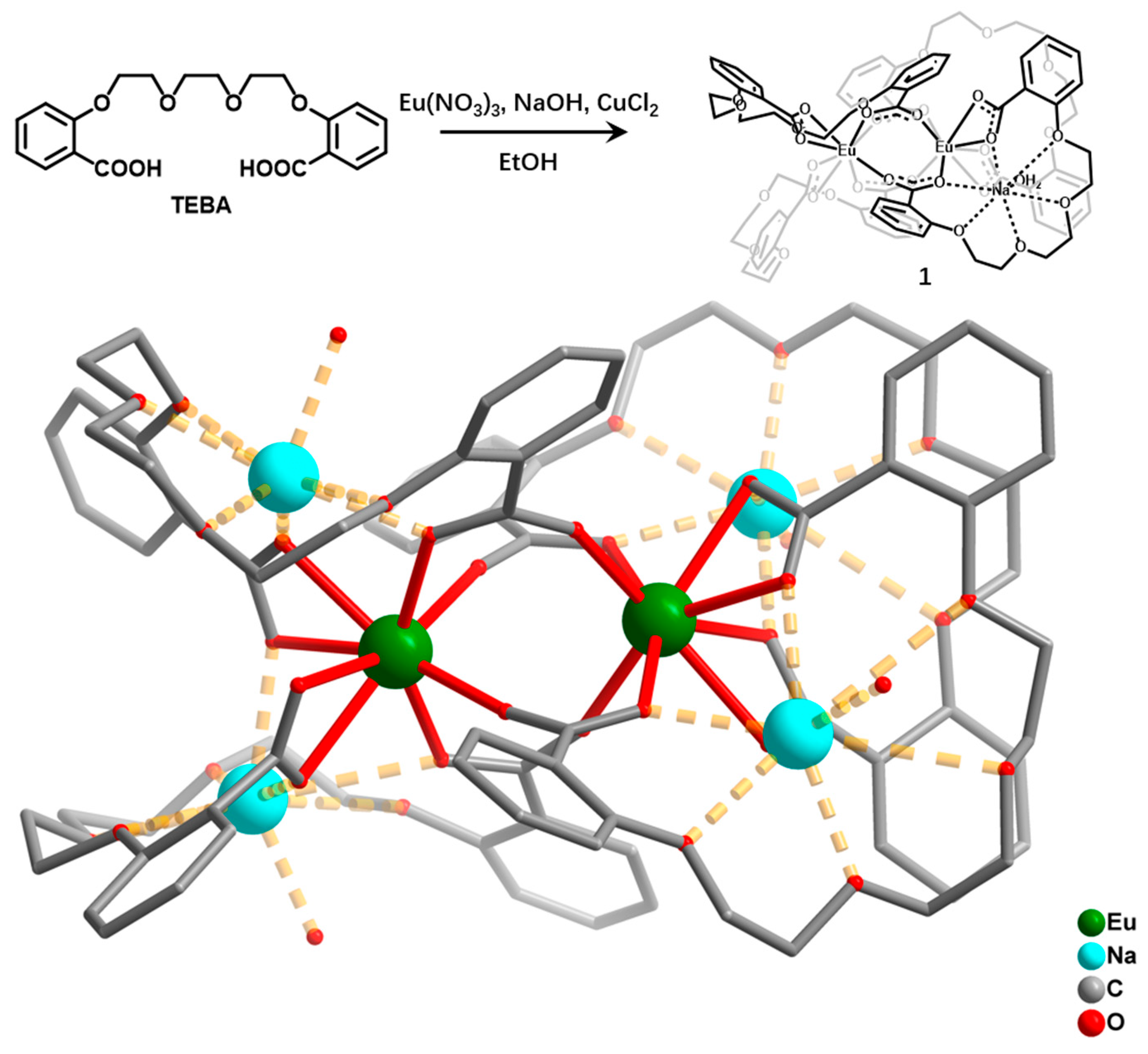
2.1. Synthesis and Structure of Compound 1
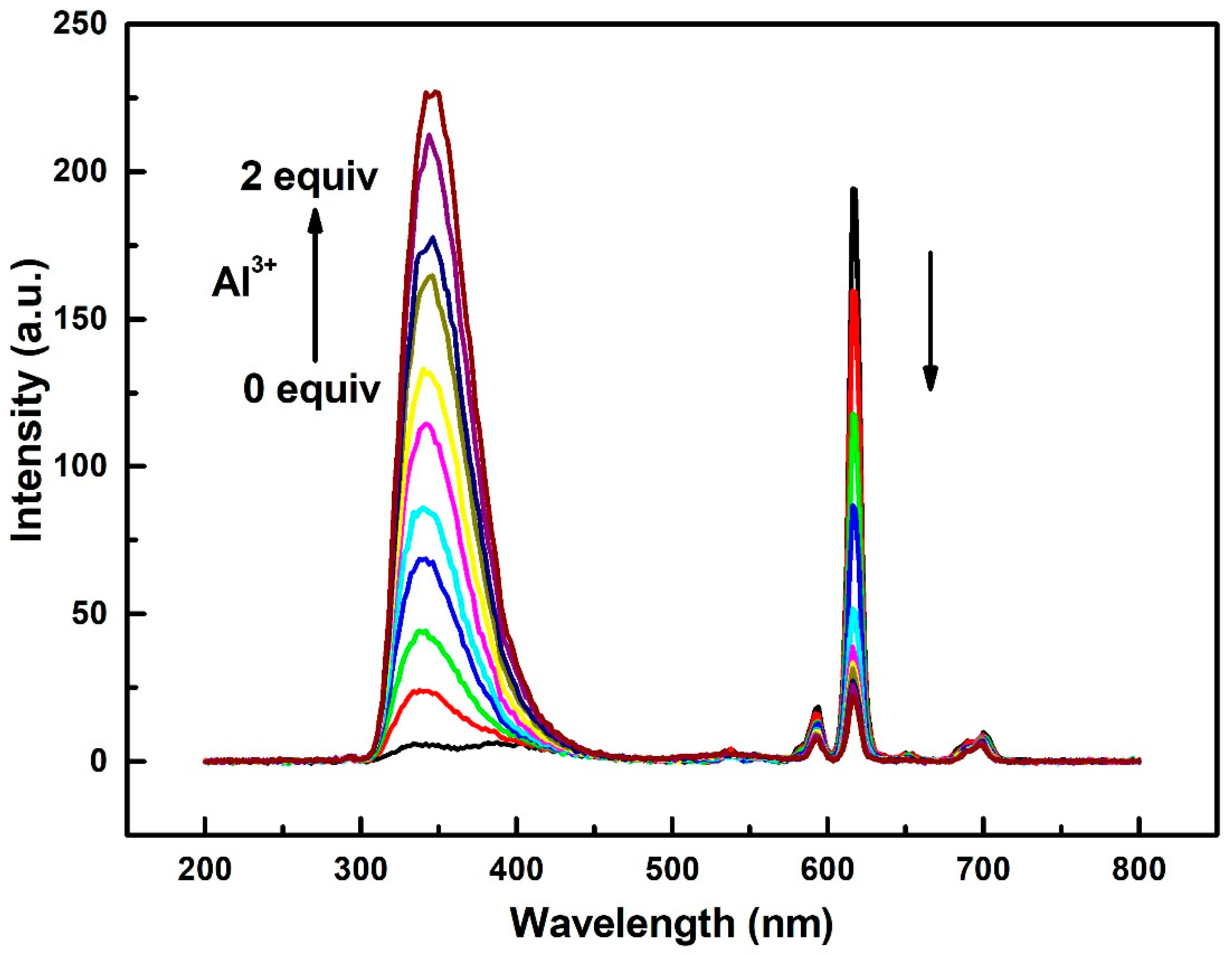
2.2. Luminescent and Sensing Properties of Compound 1
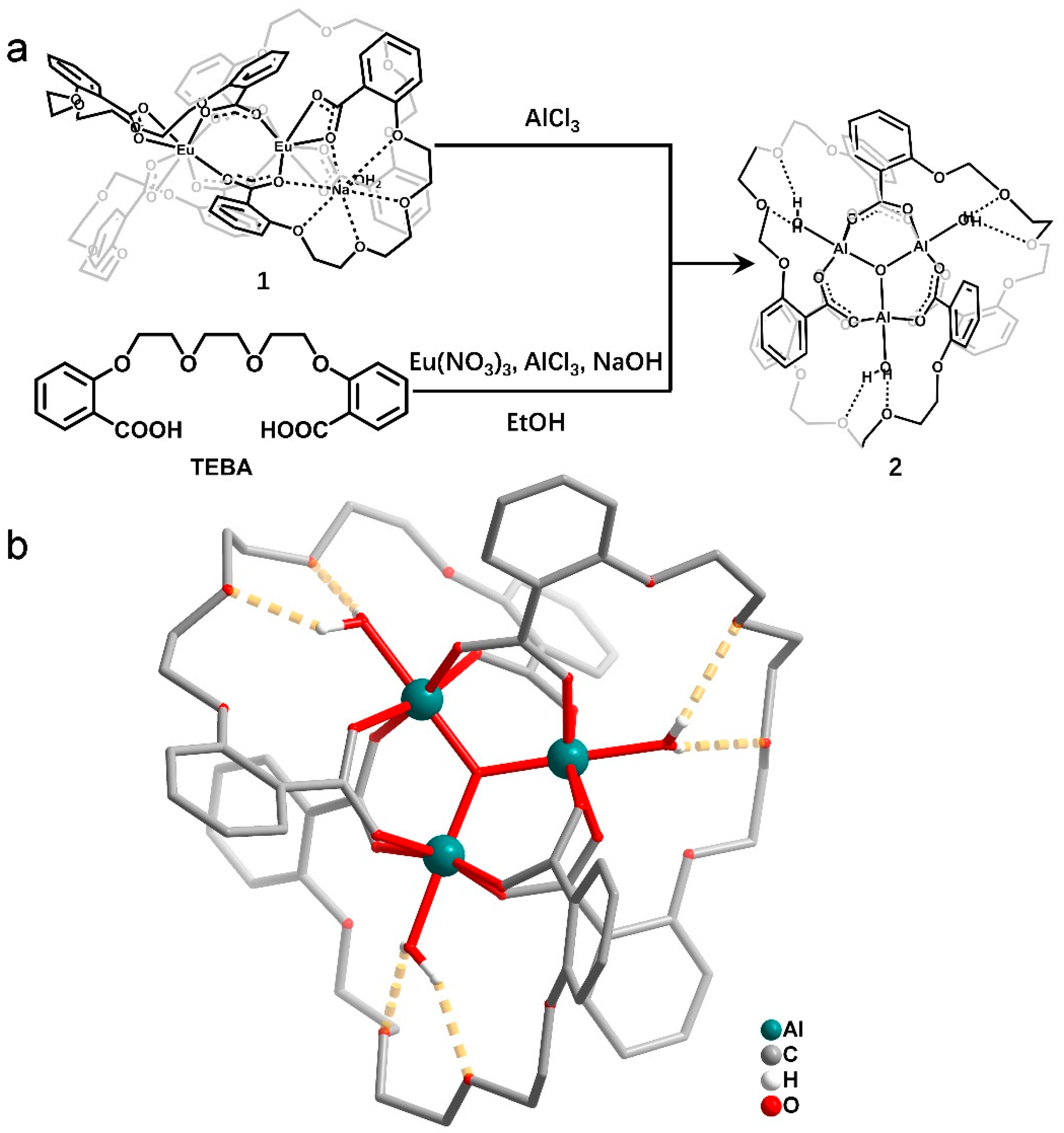
2.3. Sensing Mechanism Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bunzli, J.C. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J. Recent advances in luminescence imaging of biological systems using lanthanide(III) luminescent complexes. Molecules 2020, 25, 2089. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H. Recent progress in the lanthanide-complexes based luminescent hybrid materials. Coord. Chem. Rev. 2021, 441, 213988. [Google Scholar] [CrossRef]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef]
- Parker, D.; Fradgley, J.D.; Wong, K.L. The design of responsive luminescent lanthanide probes and sensors. Chem. Soc. Rev. 2021, 50, 8193–8213. [Google Scholar] [CrossRef]
- Bodman, S.E.; Butler, S.J. Advances in anion binding and sensing using luminescent lanthanide complexes. Chem. Sci. 2021, 12, 2716–2734. [Google Scholar] [CrossRef]
- Reddy, M.L.P.; Bejoymohandas, K.S.; Divya, V. Luminescent lanthanide coordination compounds as potential mitochondria-targeting probes: Molecular engineering to bioimaging. Dyes Pigm. 2022, 205, 110528. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D. Lanthanide-functionalized metal–organic frameworks as ratiometric luminescent sensors. J. Mater. Chem. C 2020, 8, 12739–12754. [Google Scholar] [CrossRef]
- Smith, D.G.; McMahon, B.K.; Pal, R.; Parker, D. Live cell imaging of lysosomal pH changes with pH responsive ratiometric lanthanide probes. Chem. Commun. 2012, 48, 8520–8522. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Zapata, F.; Qian, G.; Lobkovsky, E.B. A luminescent microporous metal-organic framework for the recognition and sensing of anions. J. Am. Chem. Soc. 2008, 130, 6718–6719. [Google Scholar] [CrossRef] [PubMed]
- Thibon, A.; Pierre, V.C. A highly selective luminescent sensor for the time-gated detection of potassium. J. Am. Chem. Soc. 2009, 131, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiao, T.; Li, Y.; Liu, Q.; Tan, M.; Wang, H.; Wang, L. Lanthanide coordination polymers and their Ag+-modulated fluorescence. J. Am. Chem. Soc. 2004, 126, 2280–2281. [Google Scholar] [CrossRef]
- Lu, W.-G.; Jiang, L.; Feng, X.-L.; Lu, T.-B. Three-dimensional lanthanide anionic metal-organic frameworks with tunable luminescent properties induced by cation exchange. Inorg. Chem. 2009, 48, 6997–6999. [Google Scholar] [CrossRef]
- Isaac, M.; Raibaut, L.; Cepeda, C.; Roux, A.; Boturyn, D.; Eliseeva, S.V.; Petoud, S.; Seneque, O. Luminescent Zinc fingers: Zn-responsive neodymium near-infrared emission in water. Chem. Eur. J. 2017, 23, 10992–10996. [Google Scholar] [CrossRef]
- Cai, Y.-P.; Zhou, X.-X.; Zhou, Z.-Y.; Zhu, S.-Z.; Thallapally, P.K.; Liu, J. Single-crystal-to-single-crystal transformation in a one-dimensional Ag-Eu helical system. Inorg. Chem. 2009, 48, 6341–6343. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Song, B.; Wang, L.; Tang, Z.; Luo, T.; Yuan, J. Extending the excitation wavelength from UV to visible light for a europium complex-based mitochondria targetable luminescent probe for singlet oxygen. Dalton Trans. 2018, 47, 12852–12857. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; Gschneidtner, T.; Chang, C.J. Lanthanide-based luminescent probes for selective time-gated detection of hydrogen peroxide in water and in living cells. Chem. Commun. 2010, 46, 7510–7512. [Google Scholar] [CrossRef]
- Mailhot, R.; Traviss-Pollard, T.; Pal, R.; Butler, S.J. Cationic europium complexes for visualizing fluctuations in mitochondrial ATP levels in living cells. Chem. Eur. J. 2018, 24, 10745–10755. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of metal-organic frameworks with rod secondary building units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.-S.; Kang, X.-M.; Zhao, B. Lanthanide-based metal-organic frameworks as luminescent probes. Dalton Trans. 2016, 45, 18003–18017. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, H.; Xie, J.; Zhao, B.; Liu, B.; Xu, S.; Pei, W.; Ren, N.; Huang, L.; Huang, W. Recent developments in lanthanide-based luminescent probes. Coord. Chem. Rev. 2014, 273–274, 201–212. [Google Scholar] [CrossRef]
- Yan, B. Luminescence response mode and chemical sensing mechanism for lanthanide-functionalized metal–organic framework hybrids. Inorg. Chem. Front. 2021, 8, 201–233. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal-organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef]
- Sahoo, S.; Mondal, S.; Sarma, D. Luminescent lanthanide metal organic frameworks (LnMOFs): A versatile platform towards organomolecule sensing. Coord. Chem. Rev. 2022, 470, 214707. [Google Scholar] [CrossRef]
- Li, X.-Z.; Tian, C.-B.; Sun, Q.-F. Coordination-directed self-assembly of functional polynuclear lanthanide supramolecular architectures. Chem. Rev. 2022, 122, 6374–6458. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; He, C.; Wang, J.; Duan, C. Coordination-driven nanosized lanthanide ‘Molecular Lanterns’ as luminescent chemosensors for the selective sensing of magnesium ions. Dalton Trans. 2014, 43, 335–343. [Google Scholar] [CrossRef]
- Zhu, X.; He, C.; Dong, D.; Liu, Y.; Duan, C. Cerium-based triple-stranded helicates as luminescent chemosensors for the selective sensing of magnesium ions. Dalton Trans. 2010, 39, 10051–10055. [Google Scholar] [CrossRef]
- Liu, C.-L.; Zhang, R.-L.; Lin, C.-S.; Zhou, L.-P.; Cai, L.-X.; Kong, J.-T.; Yang, S.-Q.; Han, K.-L.; Sun, Q.-F. Intraligand charge transfer sensitization on self-assembled europium tetrahedral cage leads to dual-selective luminescent sensing toward anion and cation. J. Am. Chem. Soc. 2017, 139, 12474–12479. [Google Scholar] [CrossRef]
- Bell, D.J.; Natrajan, L.S.; Riddell, I.A. Design of lanthanide based metal–organic polyhedral cages for application in catalysis, sensing, separation and magnetism. Coord. Chem. Rev. 2022, 472, 214786. [Google Scholar] [CrossRef]
- Perl, D.; Brody, A. Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 1980, 208, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Perl, D.; Gajdusek, D.; Garruto, R.; Yanagihara, R.; Gibbs, C. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism-dementia of Guam. Science 1982, 217, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Fernández-Marcos, M.L.; Monterroso, C.; Fernández-Sanjurjo, M.J. Application of aluminium toxicity indices to soils under various forest species. For. Ecol. Manage. 2005, 211, 227–239. [Google Scholar] [CrossRef]
- Jeong, J.W.; Rao, B.A.; Son, Y.-A. Rhodamine-chloronicotinaldehyde-based “OFF–ON” chemosensor for the colorimetric and fluorescent determination of Al3+ ions. Sens. Actuators B 2015, 208, 75–84. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Chen, C.-H.; Wu, A.-T. A turn-on and reversible fluorescence sensor for Al3+ ion. Analyst 2012, 137, 5201–5203. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Yu, M.-X.; Yu, Y.-H.; Bai, Z.-P.; Shen, Z.; Li, F.-Y.; You, X.-Z. A colorimetric and fluorescent turn-on chemosensor for Al3+ and its application in bioimaging. Tetra. Lett. 2009, 50, 6169–6172. [Google Scholar] [CrossRef]
- Le, T.-N.; Lin, K.-Y.; Valaboju, A.; Lee, C.-K.; Jiang, J.-C.; Rao, N.V. The fluorescence turn-off mechanism of a norbornene-derived homopolymer—An Al3+ colorimetric and fluorescent chemosensor. Mater. Adv. 2021, 2, 4685–4693. [Google Scholar] [CrossRef]
- Pungut, N.A.S.; Mat Saad, H.; Sim, K.S.; Tan, K.W. A turn on fluorescent sensor for detecting Al3+ and colorimetric detection for Cu2+: Synthesis, cytotoxicity and on-site assay kit. J. Photochem. Photobiol. A 2021, 414, 113290. [Google Scholar] [CrossRef]
- Chand, S.; Verma, G.; Pal, A.; Pal, S.C.; Ma, S.; Das, M.C. Porous anionic Co(II) metal-organic framework, with a high density of amino groups, as a superior luminescent sensor for turn-on Al(III) detection. Chem. Eur. J. 2021, 27, 11804–11810. [Google Scholar] [CrossRef]
- Xu, H.; Zhai, B.; Cao, C.-S.; Zhao, B. A bifunctional europium-organic framework with chemical fixation of CO2 and luminescent detection of Al3+. Inorg. Chem. 2016, 55, 9671–9676. [Google Scholar] [CrossRef]
- Xu, H.; Fang, M.; Cao, C.-S.; Qiao, W.-Z.; Zhao, B. Unique (3,4,10)-connected lanthanide-organic framework as a recyclable chemical sensor for detecting Al3+. Inorg. Chem. 2016, 55, 4790–4794. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Choi, S.H.; Lee, J.; Huh, J.O.; Do, Y.; Churchill, D.G. Highly selective fluorescence detection of Cu2+ in water by chiral dimeric Zn2+ complexes through direct displacement. Inorg. Chem. 2009, 48, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Royzen, M.; Dai, Z.; Canary, J.W. Ratiometric displacement approach to Cu(II) sensing by fluorescence. J. Am. Chem. Soc. 2005, 127, 1612–1613. [Google Scholar] [CrossRef] [PubMed]
- Comby, S.; Tuck, S.A.; Truman, L.K.; Kotova, O.; Gunnlaugsson, T. New trick for an old ligand! The sensing of Zn(II) using a lanthanide based ternary Yb(III)-cyclen-8-hydroxyquinoline system as a dual emissive probe for displacement assay. Inorg. Chem. 2012, 51, 10158–10168. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Xie, Y.; Li, Z.; Lin, T. Dinuclear Lanthanide Compound as a Promising Luminescent Probe for Al3+ Ions. Molecules 2022, 27, 8761. https://doi.org/10.3390/molecules27248761
Chen Z, Xie Y, Li Z, Lin T. Dinuclear Lanthanide Compound as a Promising Luminescent Probe for Al3+ Ions. Molecules. 2022; 27(24):8761. https://doi.org/10.3390/molecules27248761
Chicago/Turabian StyleChen, Zhi, Yinghao Xie, Zhanbo Li, and Tao Lin. 2022. "Dinuclear Lanthanide Compound as a Promising Luminescent Probe for Al3+ Ions" Molecules 27, no. 24: 8761. https://doi.org/10.3390/molecules27248761
APA StyleChen, Z., Xie, Y., Li, Z., & Lin, T. (2022). Dinuclear Lanthanide Compound as a Promising Luminescent Probe for Al3+ Ions. Molecules, 27(24), 8761. https://doi.org/10.3390/molecules27248761





