Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor
Abstract
1. Introduction
2. Results and Discussion
2.1. Mechanism of the Ratiometric SERS Detection of Enrofloxacin
2.2. Characterization of the SERS Probe
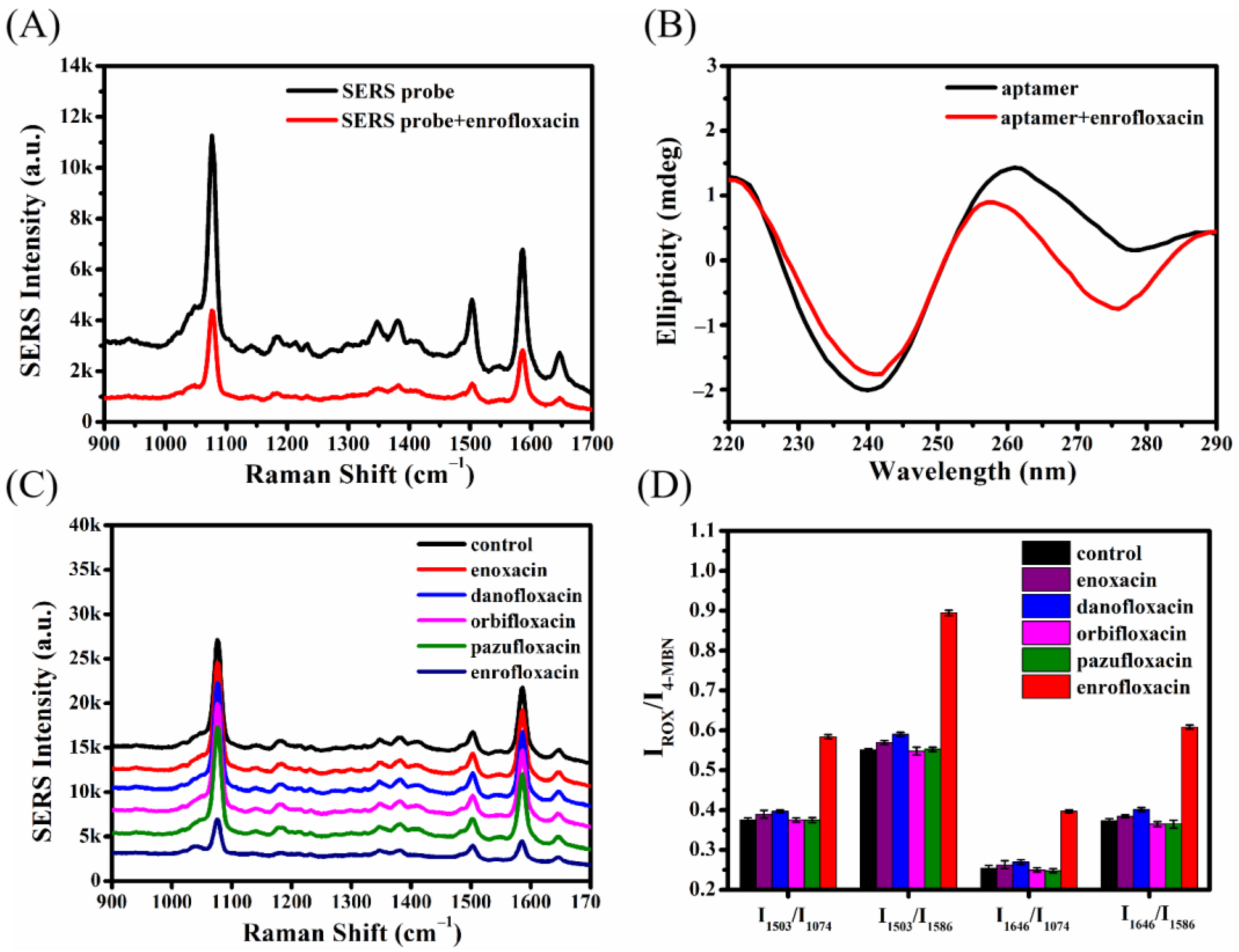
2.3. Feasibility of Detecting Enrofloxacin Using the Ratiometric SERS Aptasensor
2.4. Optimization of Experimental Parameters
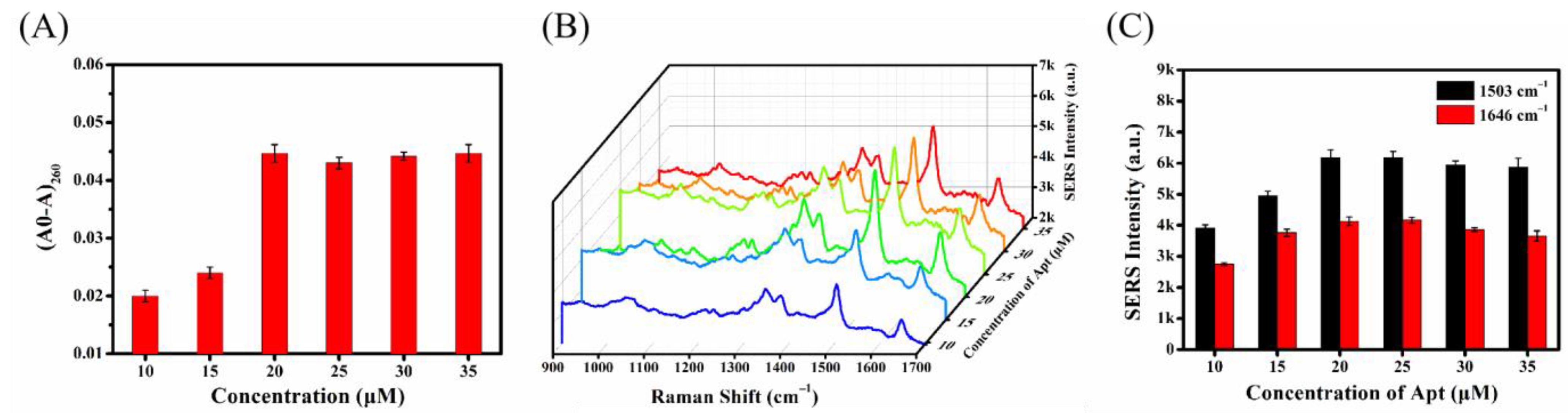
2.4.1. Optimization of Aptamer Concentration
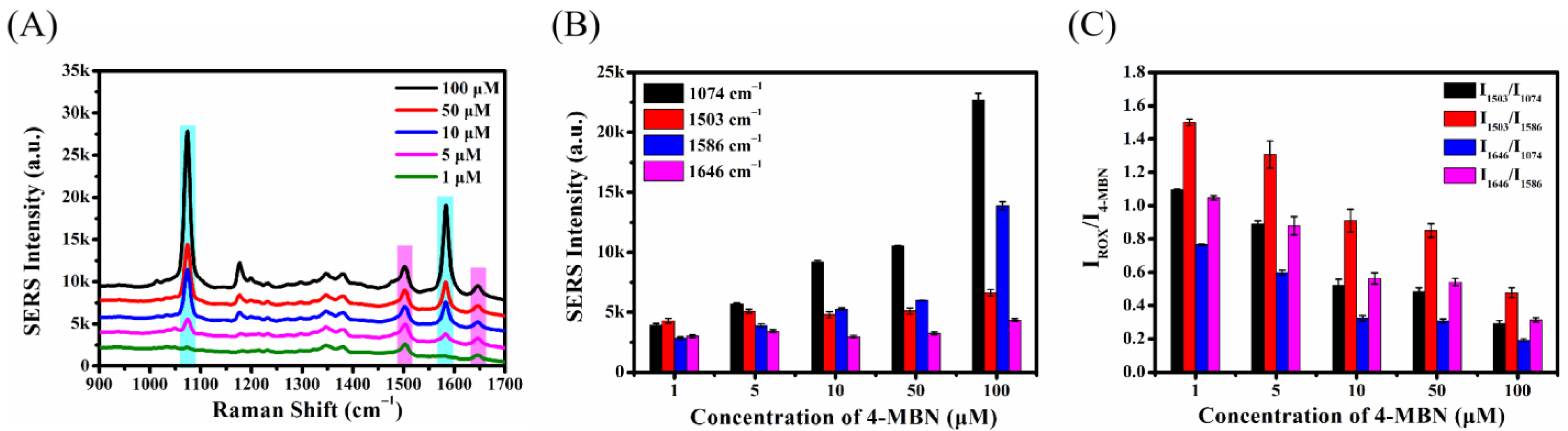
2.4.2. Optimization of 4-MBN Concentration
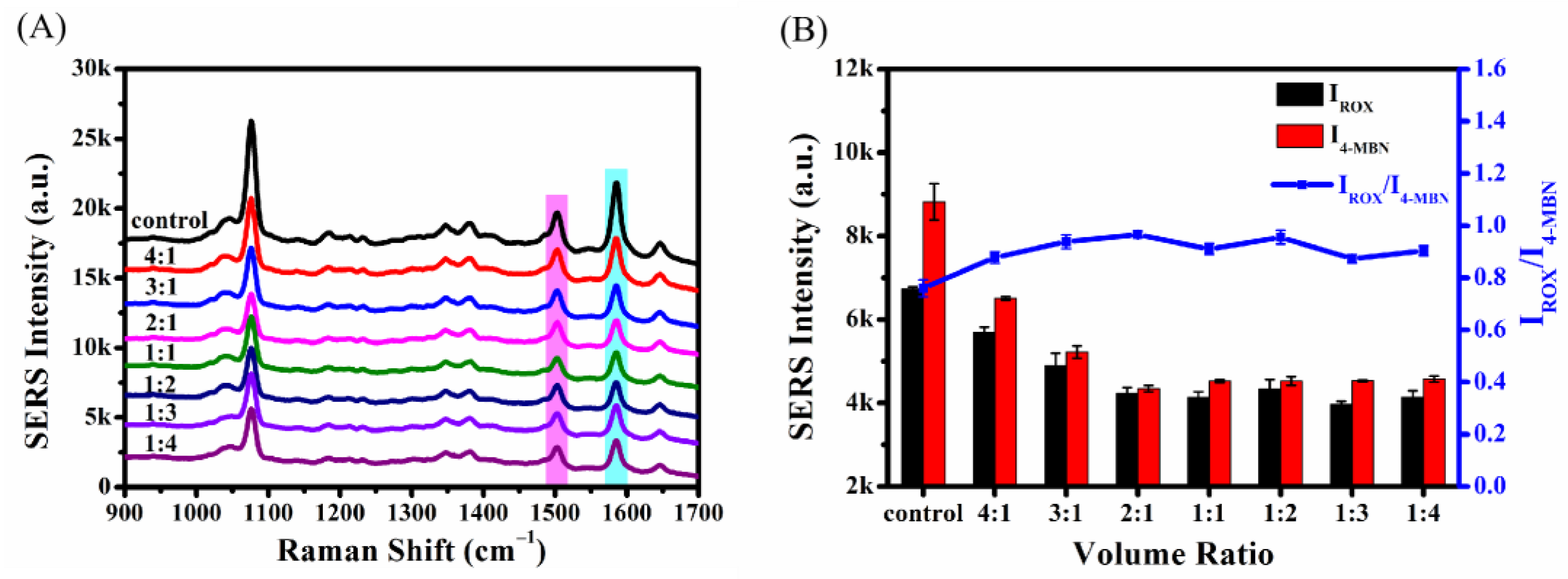
2.4.3. Optimization of the Volume Ratio of SERS Probe to Enrofloxacin
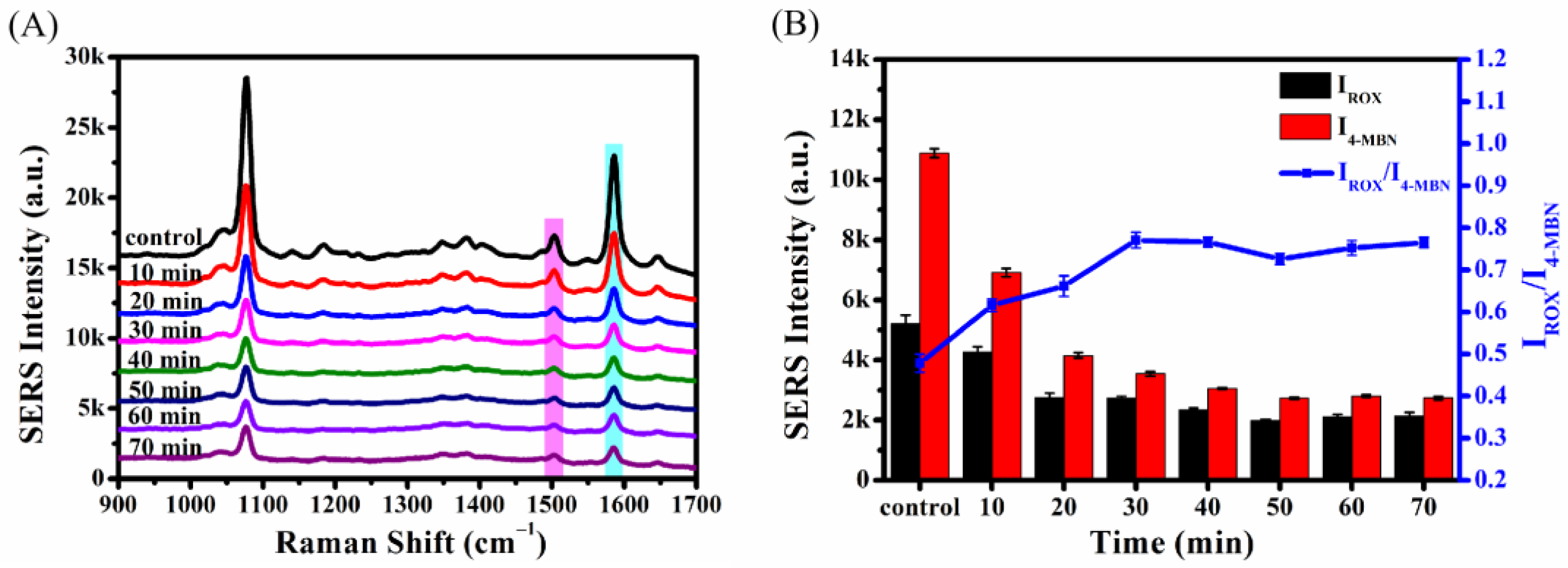
2.4.4. Optimization of Incubation Time
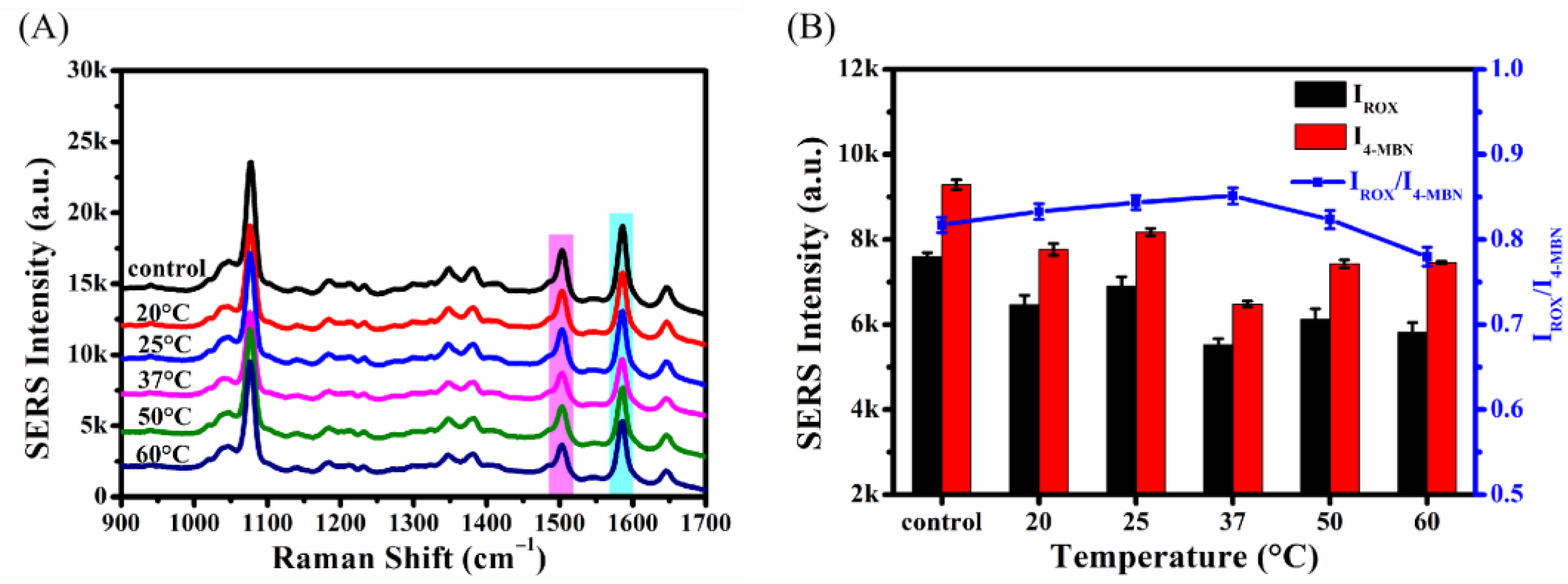
2.4.5. Optimization of Incubation Temperature
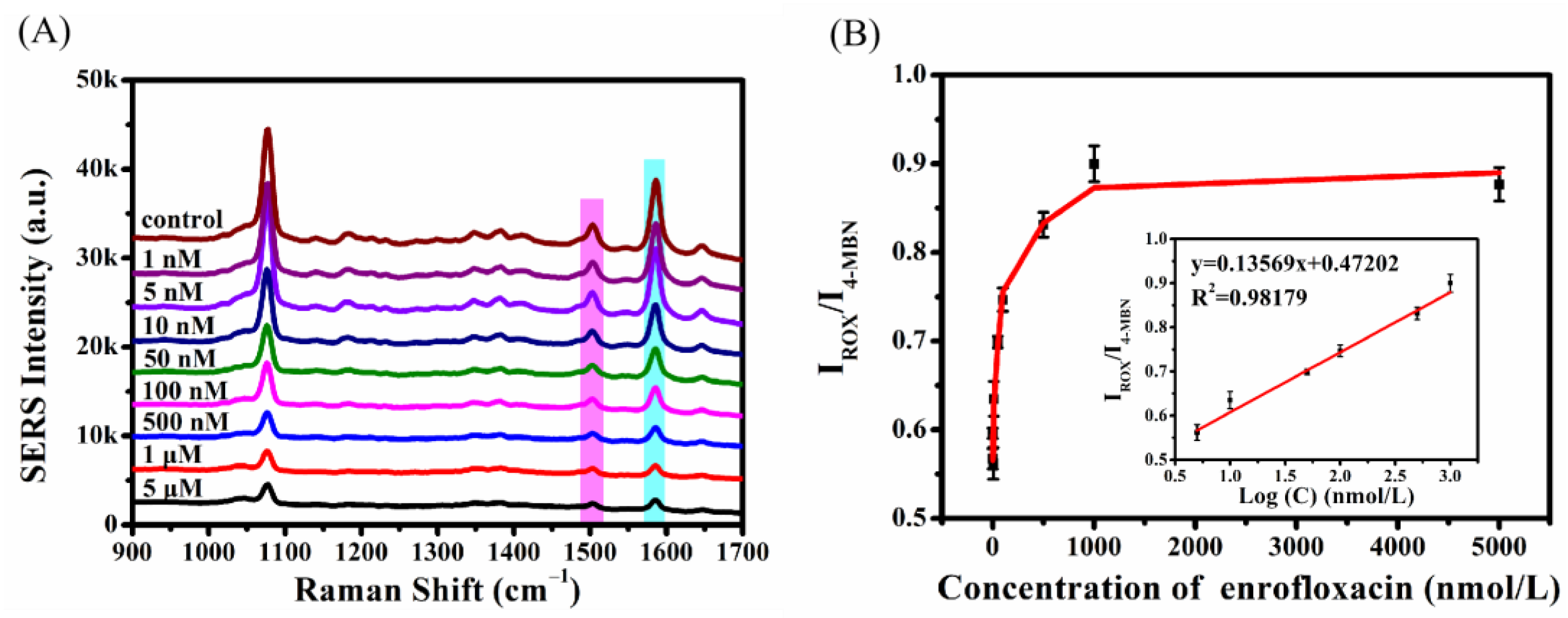
2.5. Detection of Enrofloxacin
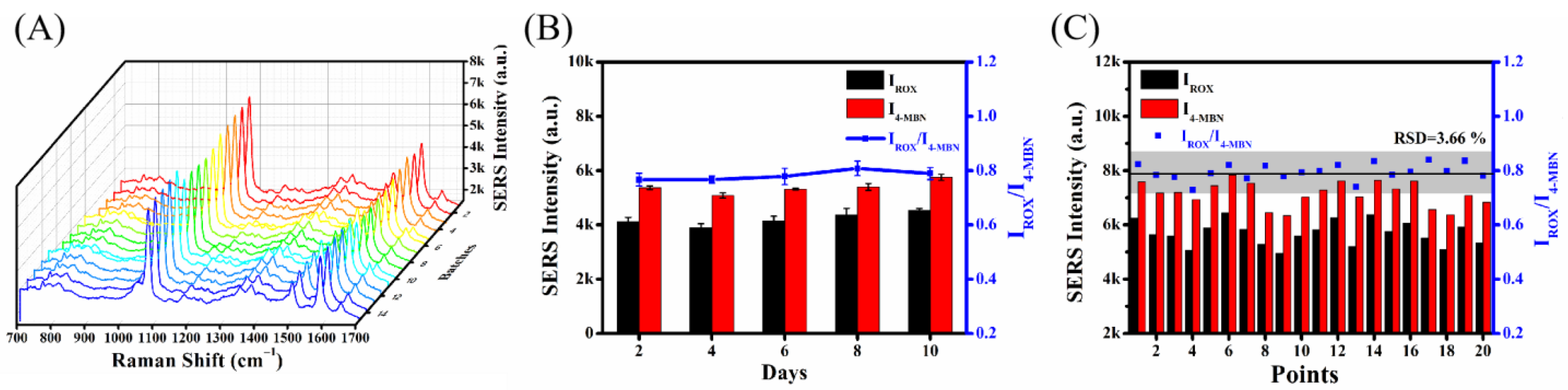
2.6. Reproducibility, Stability and Uniformity of the Established Ratiometric SERS Aptasensor
2.7. Application in the Determination of Enrofloxacin in Real Samples
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Au NPs
3.3. Preparation of the SERS Probe
3.4. SERS Detection of Enrofloxacin
3.5. Detection of Enrofloxacin in Meat Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic Copper-Based Metal Organic Framework as an Effective and Recyclable Adsorbent for Removal of Two Fluoroquinolone Antibiotics from Aqueous Solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Zhou, H.; Zhang, T.; Zhao, X.; Jiang, Z.; Wang, Q. Preparation of Molecularly Imprinted Hybrid Monoliths for the Selective Detection of Fluoroquinolones in Infant Formula Powders. J. Chromatogr. A 2019, 1588, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hyung, S.W.; Lee, C.H.; Kim, B. Development of Certified Reference Materials for Accurate Determination of Fluoroquinolone Antibiotics in Chicken Meat. Food Chem. 2017, 229, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Y.; Xu, X.; Zhang, L. Metal-Organic Framework-Derived Three-Dimensional Porous Graphitic Octahedron Carbon Cages-Encapsulated Copper Nanoparticles Hybrids as Highly Efficient Enrichment Material for Simultaneous Determination of Four Fluoroquinolones. J. Chromatogr. A 2018, 1533, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, J.; Chen, L.; Qiu, J.; Liu, Y.; Ouyang, G. Rapid in Vivo Determination of Fluoroquinolones in Cultured Puffer Fish (Takifugu Obscurus) Muscle by Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry. Talanta 2017, 175, 550–556. [Google Scholar] [CrossRef]
- Pugajeva, I.; Avsejenko, J.; Judjallo, E.; Bērziņš, A.; Bartkiene, E.; Bartkevics, V. High Occurrence Rates of Enrofloxacin and Ciprofloxacin Residues in Retail Poultry Meat Revealed by an Ultra-Sensitive Mass-Spectrometric Method, and Antimicrobial Resistance to Fluoroquinolones in Campylobacter Spp. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1107–1115. [Google Scholar] [CrossRef]
- Ha, M.S.; Chung, M.S.; Bae, D.H. Simple Detection of Residual Enrofloxacin in Meat Products Using Microparticles and Biochips. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 817–823. [Google Scholar] [CrossRef]
- Tian, Y.; Li, G.; Zhang, H.; Xu, L.; Jiao, A.; Chen, F.; Chen, M. Construction of Optimized Au@Ag Core-Shell Nanorods for Ultralow SERS Detection of Antibiotic Levofloxacin Molecules. Opt. Express 2018, 26, 23347. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Kwon, D.; Yim, C.; Jeon, S. Microbial Respiration-Based Detection of Enrofloxacin in Milk Using Capillary-Tube Indicators. Sens. Actuators B Chem. 2017, 244, 559–564. [Google Scholar] [CrossRef]
- Aymard, C.; Kanso, H.; Serrano, M.J.; Pagán, R.; Noguer, T.; Istamboulie, G. Development of a New Dual Electrochemical Immunosensor for a Rapid and Sensitive Detection of Enrofloxacin in Meat Samples. Food Chem. 2022, 370, 131016. [Google Scholar] [CrossRef]
- Aslam, B.; Kousar, N.; Javed, I.; Raza, A.; Ali, A.; Khaliq, T.; Muhammad, F.; Khan, J.A. Determination of Enrofloxacin Residues in Commercial Broilers Using High Performance Liquid Chromatography. Int. J. Food Prop. 2016, 19, 2463–2470. [Google Scholar] [CrossRef]
- Schneider, M.J.; Braden, S.E.; Reyes-Herrera, I.; Donoghue, D.J. Simultaneous Determination of Fluoroquinolones and Tetracyclines in Chicken Muscle Using HPLC with Fluorescence Detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 846, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, L.; Sun, L.; Sun, X.; Du, X.; Wang, J.; Wang, T.; Zeng, Q.; Wang, H.; Xu, Y.; et al. Microwave-Assisted Extraction and in Situ Clean-up for the Determination of Fluoroquinolone Antibiotics in Chicken Breast Muscle by LC-MS/MS. J. Sep. Sci. 2011, 34, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Denadai, M.; Cass, Q.B. Simultaneous Determination of Fluoroquinolones in Environmental Water by Liquid Chromatography—Tandem Mass Spectrometry with Direct Injection: A Green Approach. J. Chromatogr. A 2015, 1418, 177–184. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pinacho, D.G.; Pascual, N.; Granados, M.; Companyó, R.; Marco, M.P. Development and Validation of an Enzyme Linked Immunosorbent Assay for Fluoroquinolones in Animal Feeds. Food Control 2015, 57, 195–201. [Google Scholar] [CrossRef]
- Tian, R.; Ji, J.; Zhou, Y.; Du, Y.; Bian, X.; Zhu, F.; Liu, G.; Deng, S.; Wan, Y.; Yan, J. Terminal-Conjugated Non-Aggregated Constraints of Gold Nanoparticles on Lateral Flow Strips for Mobile Phone Readouts of Enrofloxacin. Biosens. Bioelectron. 2020, 160, 112218. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Su, L.; Gao, X.; Tang, Y.; Ma, T.; Zhu, L.; Li, J. Novel Hybrid Probe Based on Double Recognition of Aptamer-Molecularly Imprinted Polymer Grafted on Upconversion Nanoparticles for Enrofloxacin Sensing. Biosens. Bioelectron. 2017, 87, 203–208. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, S.; Li, H.; Xu, J.; Tang, H.; Wang, Y.; Wang, Y.; Sun, F.; Zhao, X. Ultrasensitive and Facile Detection of Multiple Trace Antibiotics with Magnetic Nanoparticles and Core-Shell Nanostar SERS Nanotags. Talanta 2022, 237, 122955. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Su, Z.; Xu, Q.; Xi, L.; Li, L.; Wu, Y.; Li, G. Artificial Clickase-Triggered Fluorescence “Turn on” Based on a Click Bio-Conjugation Strategy for the Immunoassay of Food Allergenic Protein. Food Chem. 2023, 398, 133882. [Google Scholar] [CrossRef]
- Shen, X.; Chen, J.; Lv, S.; Sun, X.; Dzantiev, B.B.; Eremin, S.A.; Zherdev, A.V.; Xu, J.; Sun, Y.; Lei, H. Fluorescence Polarization Immunoassay for Determination of Enrofloxacin in Pork Liver and Chicken. Molecules 2019, 24, 4462. [Google Scholar] [CrossRef]
- Sha, J.; Lin, H.; Timira, V.; Sui, J. The Construction and Application of Aptamer to Simultaneous Identification of Enrofloxacin and Ciprofloxacin Residues in Fish. Food Anal. Methods 2021, 14, 957–967. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, L.; Ma, X.; Xie, L.; Lin, M.; Chen, H.; He, B. Au@ZnNi-MOF Labeled Electrochemical Aptasensor for Detection of Enrofloxacin Based on AuPt@h-CeO2/MoS2 and DNAzyme-Driven DNA Walker Triple Amplification Signal Strategy. Biosens. Bioelectron. 2022, 210, 114296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, J.; Wei, H.; Wu, Z.; Wei, Y.; Wang, X.; Pei, Y. Fabrication of Ag NPs Decorated on Electrospun PVA/PEI Nanofibers as SERS Substrate for Detection of Enrofloxacin. J. Food Meas. Charact. 2022, 16, 2314–2322. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.; Li, X.; Shan, J.; Xu, Y.; Li, G. One-Step Chemical Reaction Triggered Surface Enhanced Raman Scattering Signal Conversion Strategy for Highly Sensitive Detection of Nitrite. Vib. Spectrosc. 2021, 113, 103221. [Google Scholar] [CrossRef]
- Xiong, J.; Dong, C.; Zhang, J.; Fang, X.; Ni, J.; Gan, H.; Li, J.; Song, C. DNA Walker-Powered Ratiometric SERS Cytosensor of Circulating Tumor Cells with Single-Cell Sensitivity. Biosens. Bioelectron. 2022, 213, 114442. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.; Li, X.; Wang, L.; Xu, Y.; He, L.; Li, G. Recent Advances in Dual Recognition Based Surface Enhanced Raman Scattering for Pathogenic Bacteria Detection: A Review. Anal. Chim. Acta 2021, 1157, 338279. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, X.; Lv, Y.; Wu, Z.; Chen, F.; Chen, Z. Excellent Surface Enhanced Raman Scattering of SiO2 Fiber Membrane Embedded with Ag Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2018, 28, 251–257. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.; Kong, L. Flexible Ag/Nanocellulose Fibers SERS Substrate and Its Applications for in-Situ Hazardous Residues Detection on Food. Appl. Surf. Sci. 2020, 533, 147454. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Z.; Ren, Z.; Qin, Y.; Pan, Z.; Shao, K.; She, Y.; Huang, W. Interface-Induced Ag Monolayer Film for Surface-Enhanced Raman Scattering Detection of Water-Insoluble Enrofloxacin. Plasmonics 2021, 16, 349–358. [Google Scholar] [CrossRef]
- Ma, P.; Duan, N.; Ye, H.; Xia, Y.; Ding, Z.; Wang, Z. Selection, Truncation and Fluorescence Polarization Based Aptasensor for Weissella Viridescens Detection. Talanta 2022, 246, 123499. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Yang, X.; Wang, K.; Chen, N.; Zhou, C.; Luo, B.; Du, S. Evaluation of Medicine Effects on the Interaction of Myoglobin and Its Aptamer or Antibody Using Atomic Force Microscopy. Anal. Chem. 2015, 87, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhou, G.; Wang, X.; Zhang, Y.; Li, Z.; Liu, P.; Yu, B.; Zhang, J. A Metal-Organic Framework/Aptamer System as a Fluorescent Biosensor for Determination of Aflatoxin B1 in Food Samples. Talanta 2020, 219, 121342. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, N.; Su, Y.; Wang, H.; He, Y. Reusable Silicon-Based Surface-Enhanced Raman Scattering Ratiometric Aptasensor with High Sensitivity, Specificity, and Reproducibility. Anal. Chem. 2017, 89, 10279–10285. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, T.; Wu, Z.; Yu, R. Internal Standard-Based SERS Aptasensor for Ultrasensitive Quantitative Detection of Ag+ Ion. Talanta 2018, 185, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ma, P.; Li, K.; Zhang, S.; Zhang, Y.; Guo, H.; Wang, Z. A Novel Ratiometric Aptasensor Based on Dual-Emission Fluorescent Signals and the Conformation of G-Quadruplex for OTA Detection. Sens. Actuators B Chem. 2022, 358, 131484. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, S.; Chen, Z.; Jiang, J.; Sun, J. Rapid Nanomolar Detection of Methamphetamine in Biofluids via a Reagentless Electrochemical Aptamer-Based Biosensor. Anal. Chim. Acta 2022, 1207, 339742. [Google Scholar] [CrossRef]
- Duan, N.; Yao, T.; Li, C.; Wang, Z.; Wu, S. Surface-Enhanced Raman Spectroscopy Relying on Bimetallic Au–Ag Nanourchins for the Detection of the Food Allergen β-Lactoglobulin. Talanta 2022, 245, 123445. [Google Scholar] [CrossRef]
- Pham, X.H.; Hahm, E.; Huynh, K.H.; Son, B.S.; Kim, H.M.; Jeong, D.H.; Jun, B.H. 4-Mercaptobenzoic Acid Labeled Gold-Silver-Alloy-Embedded Silica Nanoparticles as an Internal Standard Containing Nanostructures for Sensitive Quantitative Thiram Detection. Int. J. Mol. Sci. 2019, 20, 4841. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Liao, M.; Kidd, M.T.; Li, Y. Rapid Detection of Enrofloxacin Using a Localized Surface Plasmon Resonance Sensor Based on Polydopamine Molecular Imprinted Recognition Polymer. J. Food Meas. Charact. 2021, 15, 3376–3386. [Google Scholar] [CrossRef]
- Phi Van, T.; Nguy, T.P.; Truong, L.T.N. A Highly Sensitive Impedimetric Sensor Based on a MIP Biomimetic for the Detection of Enrofloxacin. Anal. Methods 2022, 14, 2195–2203. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, B.; Bao, Q.; Yang, T.; Wei, T.; Wang, J.; Mao, C.; Zhang, C.; Yang, M. Aptamer-Modified Sensitive Nanobiosensors for the Specific Detection of Antibiotics. J. Mater. Chem. B 2020, 8, 8607–8613. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, L.; Dang, S.; Sun, S.; Zhou, Y.; Yan, P.; Yan, Y.; Li, H. A Sensitive Photoelectrochemical Aptasensor for Enrofloxacin Detection Based on Plasmon-Sensitized Bismuth-Rich Bismuth Oxyhalide. Talanta 2022, 246, 123515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Geng, L.; Zhang, X.; Sang, Y.; Xu, H. A Robust Nanofilm Co-Assembled from Poly(4-Vinylpyridine) Grafted Carbon Nanotube and Gold Nanoparticles at the Water/Oil Interface as Highly Active SERS Substrate for Antibiotics Detection. Appl. Surf. Sci. 2022, 605, 154737. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Z.; Li, Y.; Guo, Y.; Liu, H.; Zhang, W.; Zou, Z.; Zhang, Y.; Liu, Z. Few-Layer NbTe2Nanosheets as Substrates for Surface-Enhanced Raman Scattering Analysis. ACS Appl. Nano Mater. 2020, 3, 11363–11371. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Y.; Song, L.; Deng, Z. Flash Synthesis of Spherical Nucleic Acids with Record DNA Density. J. Am. Chem. Soc. 2021, 143, 3065–3069. [Google Scholar] [CrossRef]


| Analytical Method | Linear Range (M) | R2 | LOD (M) | Ref. |
|---|---|---|---|---|
| Localized surface plasmon resonance (LSPR) | 6.95 × 10−8–2.78 × 10−6 | 0.98 | 1.70 × 10−9 | [39] |
| Molecular imprinting technique (MIT) | 2.70 × 10−10–2.70 × 10−8 | 0.996 | 1.39 × 10−10 | [40] |
| Colorimetry | 5.0 × 10−9–1.0 × 10−7 | 0.99702 | 1.89 × 10−9 | [21] |
| Fluorescence | 2.70 × 10−9–1.70 × 10−7 | 0.98 | 1.30 × 10−9 | [41] |
| Photoelectrochemical (PEC) | 2.0 × 10−12–1.0 × 10−7 | 0.994 | 8.30 × 10−10 | [42] |
| Electrochemical immunosensor (ECI) | 1.39 × 10−8–2.78 × 10−8 | 0.975 | 8.30 × 10−9 | [10] |
| SERS | 2.78 × 10−5–1.39 × 10−3 | 0.9976 | 1.98 × 10−6 | [29] |
| SERS | 2.80 × 10−10–2.80 × 10−5 | 0.97 | 1.0 × 10−10 | [43] |
| SERS | 1.0 × 10−7–1.0 × 10−3 | 0.99 | 1.0 × 10−7 | [44] |
| Ratiometric SERS aptasensor | 5.0 × 10−9–1.0 × 10−6 | 0.98 | 1.20 × 10−10 | This work |
| Sample | Spiked Concentration (nM) | Detected Concentration (nM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Fish | - | - | ||
| 10.0 | 9.4 ± 0.9 | 94.2 | 1.2 | |
| 100.0 | 112.0 ± 7.9 | 112.0 | 1.0 | |
| 500.0 | 424.5 ± 46.4 | 93.6 | 2.7 | |
| Chicken | - | - | ||
| 10.0 | 9.5 ± 0.8 | 95.3 | 0.7 | |
| 100.0 | 102.9 ± 16.9 | 102.9 | 2.1 | |
| 500.0 | 517.3 ± 35.6 | 103.4 | 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, L.; Li, C.; Li, X.; Li, G. Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor. Molecules 2022, 27, 8764. https://doi.org/10.3390/molecules27248764
Wang P, Wang L, Li C, Li X, Li G. Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor. Molecules. 2022; 27(24):8764. https://doi.org/10.3390/molecules27248764
Chicago/Turabian StyleWang, Panxue, Li Wang, Cen Li, Xiang Li, and Guoliang Li. 2022. "Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor" Molecules 27, no. 24: 8764. https://doi.org/10.3390/molecules27248764
APA StyleWang, P., Wang, L., Li, C., Li, X., & Li, G. (2022). Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor. Molecules, 27(24), 8764. https://doi.org/10.3390/molecules27248764







