Multiscale Spectral Analysis on Lysozyme Aqueous Solutions in the Presence of PolyEthyleneGlycol
Abstract
1. Introduction
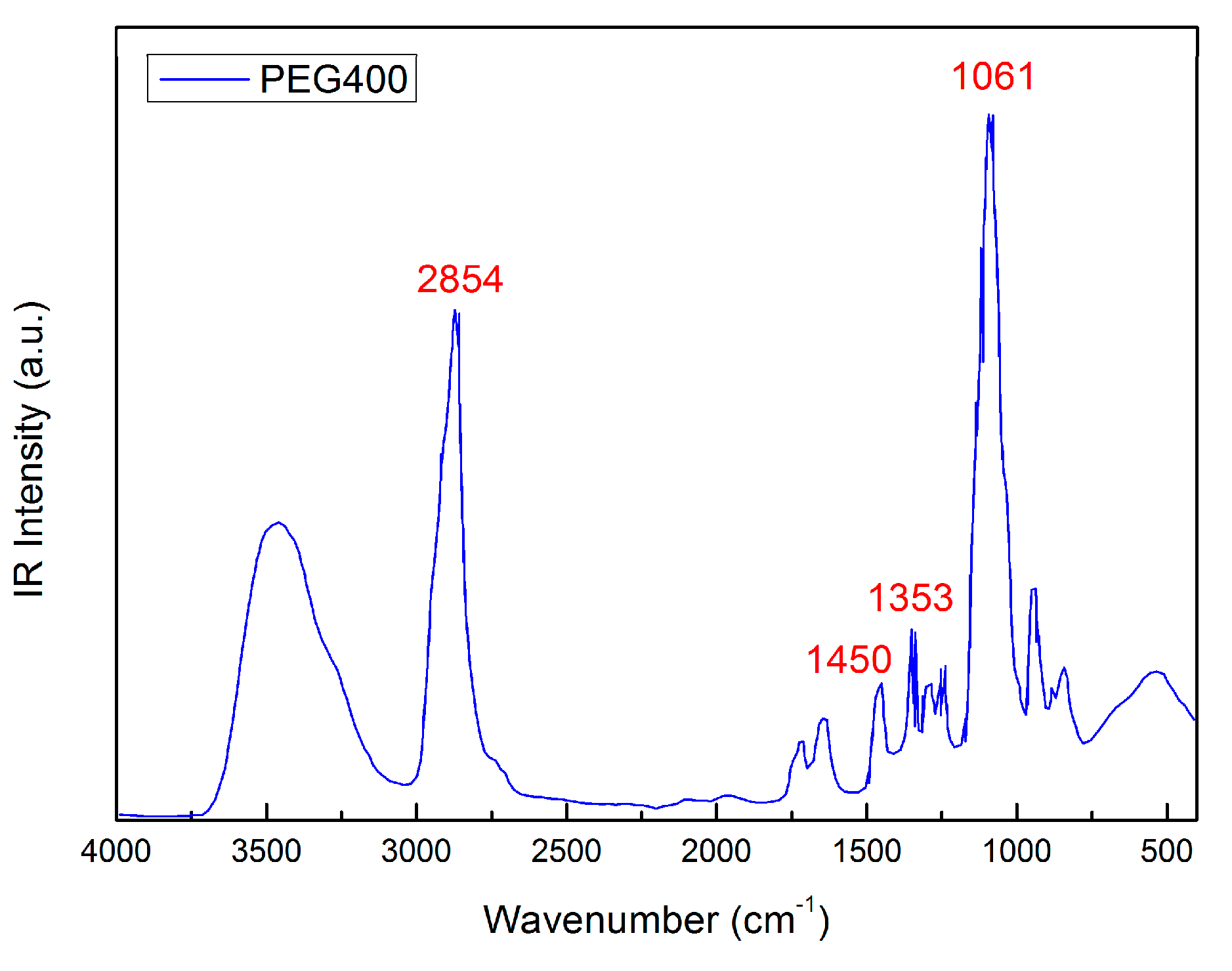
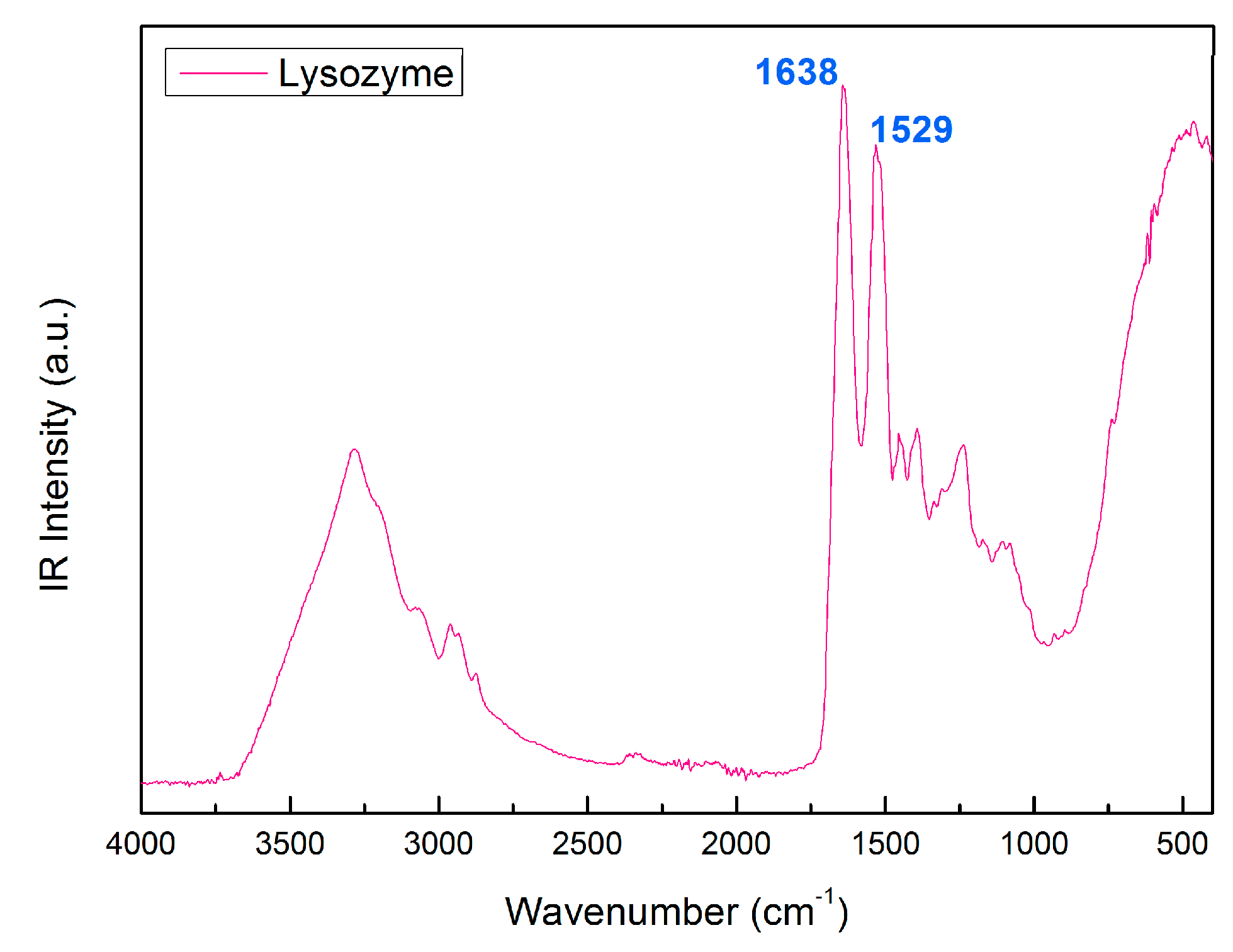
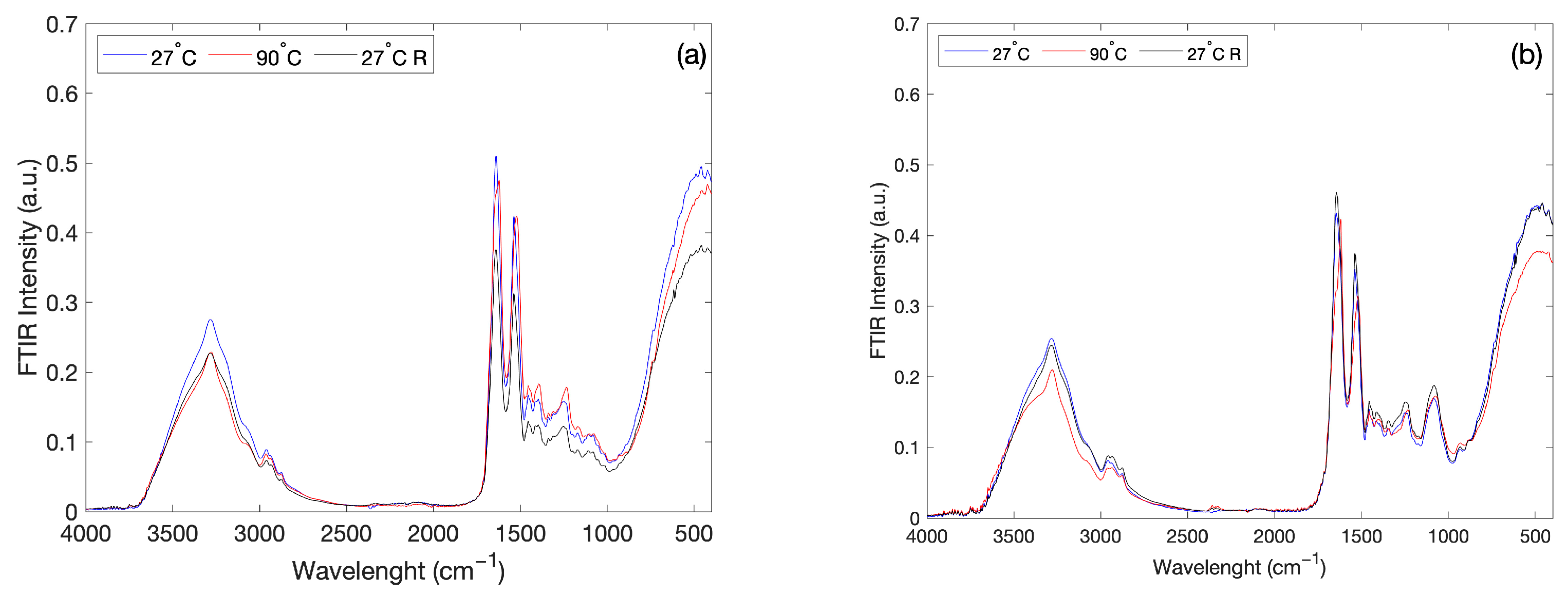
2. Results and Discussion
3. Experimental Setup and Sample Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, Y.; Lane, M.E.; Moore, D.J. An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide. Polymers 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Almásy, L.; Artykulnyi, O.P.; Petrenko, V.I.; Ivankov, O.I.; Bulavin, L.A.; Yan, M.; Haramus, V.M. Structure and Intermolecular Interactions in Aqueous Solutions of Polyethylene Glycol. Molecules 2022, 27, 2573. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Lamprecht, A. Polyethylene glycol as an alternative polymer solvent for nanoparticle preparation. Int. J. Pharm. 2013, 45, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kulkarni, P.S.; Samui, A.B. Polyethylene glycol grafted cotton as phase change polymer. Cellulose 2014, 21, 685–696. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Cannuli, A. PEG Acoustic Levitation Treatment for Historic Wood Preservation investigated by means of FTIR spectroscopy. Curr. Chem. Biol. 2019, 13, 60–72. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Cannuli, A.; Calabrò, E.; Magazù, S. Acoustic Levitator Power Device: Study of Ethylene-Glycol Water Mixtures. IOP Conf. Ser. Mater. Sci. Eng. ACPEE 2017, 199, 012119. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotrib. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Li, Z.; Zhu, X.; Liu, C.; Zhang, D.; Dou, X. Polyethylene Oxide (PEO) and Polyethylene Glycol (PEG) Polymer Sieving Matrix for RNA Capillary Electrophoresis. PLoS ONE 2015, 10, e0123406. [Google Scholar]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef]
- Faraone, A.; Magazù, S.; Maisano, G.; Ponterio, R.; Villari, V. Experimental Evidence of Slow Dynamics in Semidilute Polymer Solutions. Macromolecules 1999, 32, 1128–1133. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Tagging the oligomer-to-polymer crossover on EG and PEGs by infrared and Raman spectroscopies and by wavelet cross-correlation spectral analysis. Vib. Spectr. 2016, 85, 222–227. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Athanasiou, K.A.; Le Baron, R.G.; Mikos, A.G. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J. Biomed. Mater. Res. 2002, 59, 429–437. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Irving, C.S.; Lifschitz, C.H.; Marks, L.M.; Nichols, B.L.; Klein, P.; Lisa, M.; Buford, L. Peter, D. Polyethylene glycol polymers of low molecular weight as probes of intestinal permeability. I. Innovations in analysis and quantitation. J. Lab. Clin. Med. 1986, 107, 290–298. [Google Scholar] [PubMed]
- Caccamo, M.T.; Mavilia, G.; Mavilia, L.; Lombardo, D.; Magazù, S. Self-assembly Processes in Hydrated Montmorillonite by FTIR Investigations. Materials 2020, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Zubair, M.; Rahman, S.S.; Ullah, A. Chapter 14-Polymers for advanced applications. In Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 325–340. [Google Scholar]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Progress Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Ma, T.Y.; Hollander, D.; Krugliak, P.; Katz, K. PEG 400, a hydrophilic molecular probe for measuring intestinal permeability. Gastroent 1990, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hermansky, S.J.; Neptun, D.A.; Loughran, K.A.; Leung, H.W. Effects of polyethylene glycol 400 (PEG 400) following 13 weeks of gavage treatment in fischer-344 rats. Food Chem. Toxic. 1995, 33, 139–149. [Google Scholar] [CrossRef]
- Chadwich, V.S.; Phillips, S.F.; Hoffman, A.F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). I. Chemical analysis and biological properties of PEG 400. Gastroent 1977, 73, 241–246. [Google Scholar] [CrossRef]
- Cho, C.H.; Hui, W.M.; Liao, N.X.; Liu, X.G.; Lam, S.K.; Ogle, C.W. Polyethylene glycol: Its adverse gastric effects in rats. J. Pharm. Pharmacol. 1992, 44, 518–520. [Google Scholar] [CrossRef]
- Bončina, M.; Reščič, J.; Vlachy, V. Solubility of lysozyme in polyethylene glycol-electrolyte mixtures: The depletion interaction and ion-specific effects. Biophys. J. 2008, 95, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Baumann, P.; Brunner, C.; Hubbuch, J. Effect of PEG molecular weight and PEGylation degree on the physical stability of PEGylated lysozyme. Int. J. Pharm. 2017, 519, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Malzert, A.; Boury, F.; Renard, D.; Robert, P.; Lavenant, L.; Benoît, J.P.; Proust, J.E. Spectroscopic studies on poly(ethylene glycol)-lysozyme interactions. Int. J. Pharm. 2003, 260, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hamsa Priya, M.; Pratt, L.R.; Paulaitis, M.E. Effect of PEG End-Group Hydrophobicity on Lysozyme Interactions in Solution Characterized by Light Scattering. Langmuir 2011, 27, 13713–13718. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Lin, W.; Chen, S. Investigation of the interaction between poly(ethylene glycol) and protein molecules using low field nuclear magnetic resonance. Acta Biomat. 2013, 9, 6414–6420. [Google Scholar] [CrossRef]
- Fenimore, P.W.; Frauenfelder, H.; Magazù, S.; McMahon, B.H.; Mezei, F.; Migliardo, F.; Young, R.D.; Stroe, I. Concepts and problems in protein dynamics. Chem. Phys. 2013, 424, 2–6. [Google Scholar] [CrossRef]
- Jollès, P.; Jollès, J. What’s new in lysozyme research? Mol. Cell. Biochem. 1984, 63, 165–189. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Boikova, A.S.; D’yakova, Y.A.; Il’ina, K.B.; Konarev, P.V.; Kryukova, A.E.; Marchenkova, M.A.; Blagov, A.E.; Pisarevskii, Y.V.; Koval’chuk, M.V. Small-angle X-ray scattering study of the influence of solvent replacement (from H2O to D2O) on the initial crystallization stage of tetragonal lysozyme. Crystallogr. Rep. 2017, 62, 837–842. [Google Scholar] [CrossRef]
- Phillips, D.C. The three-dimensional structure of an enzyme molecule. Sci. Am. 1966, 215, 78–90. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Q.; Wu, D.; Hu, Y.; Chen, S.; Ding, T.; Ye, X.; Liu, D.; Chen, J. What is new in lysozyme research and its application in food industry? A review. Food Chem. 2019, 274, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Leśnierowski, G. Lysozyme and its modified forms: Properties, potential for its production and application. In Handbook of Eggs in Human Function; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; pp. 624–630. [Google Scholar]
- Silvetti, T.; Brasca, M.; Lodi, R.; Vanoni, L.; Chiolerio, F.; De Groot, M.; Bravi, A. Effects of lysozyme on the microbiological stability and organoleptic properties of unpasteurized beer. J. Inst. Brew. 2010, 116, 33–40. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Intramolecular OH stretching analysis of hydrated lysozyme in presence of trehalose by IR spectroscopy. Atti Accad. Pelor. Peric. 2019, S1, A2. [Google Scholar]
- Bloustine, J.; Virmani, T.; Thurston, G.M.; Fraden, S. Light scattering and phase behavior of lysozyme-poly (ethylene glycol) mixtures. Phys. Rev. Lett. 2006, 96, 087803. [Google Scholar] [CrossRef]
- Tan, H.; Jin, D.; Qu, X.; Liu, H.; Chen, X.; Yin, M.; Liu, C. A PEG-Lysozyme hydrogel harvests multiple functions as a fit-to-shape tissue sealant for internal-use of body. Biomaterials 2019, 192, 392–404. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Migliardo, F.; Magazù, S.; Caccamo, M.T. Infrared, Raman and INS Studies of Poly-Ethylene Oxide Oligomers. J. Mol. Struc. 2013, 1048, 261–266. [Google Scholar] [CrossRef]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl. Phys. 2004, 29, 463–487. [Google Scholar] [CrossRef]
- Magazù, S.; Calabrò, E.; Caccamo, M.T. Experimental study of thermal restraint in bio-protectant disaccharides by FTIR spectroscopy. Open Biotech. 2018, 12, 123–133. [Google Scholar] [CrossRef]
- Shi, Z.; Cogdill, R.P.; Short, S.M.; Anderson, C.A. Process characterization of powder blending by near-infrared spectroscopy: Blend end-points and beyond. J. Pharm. Biom. Anal. 2008, 47, 738–745. [Google Scholar] [CrossRef]
- Lavine, B.; Almirall, J.; Muehlethaler, C.; Neumann, C.; Workman, J. Criteria for comparing infrared spectra—A review of the forensic and analytical chemistry literature. Foren. Chem. 2020, 18, 100224. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. A Conic Pendulum of Variable Length Analysed by wavelets. In New Trends in Physics Education Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; pp. 117–131. ISBN 978-1-53613-893-1. [Google Scholar]
- Shah, N.K.; Gemperline, P.J. Combination of the Mahalanobis distance and residual variance pattern recognition techniques for classification of near-infrared reflectance spectra. Analyt. Chem. 1990, 62, 465–470. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, M.T.; Magazù, S. Thermal restraint on PEG-EG mixtures by FTIR investigations and wavelet cross-correlation analysis. Pol. Test. 2017, 62, 311–318. [Google Scholar] [CrossRef]
- Abhinandan, B.; Blasiak, B.; Pasquier, E.; Boguslaw, T.; Trudel, S. Synthesis, characterization, and evaluation of PEGylated first-row transition metal ferrite nanoparticles as T2 contrast agents for high-field MRI. RSC Adv. 2017, 7, 38125–38134. [Google Scholar]
- Kamyar, S.; Mansor, A.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Shahri, M.M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar]
- Kun, D.; Sun, J.; Song, X.; Chen, H.; Wei, A.F.; Peijun, J. Interaction of Ionic Liquid [bmin][CF3SO3] with Lysozyme Investigated by Two-Dimensional Fourier Transform Infrared Spectroscopy. ACS Sust. Chem. Eng. 2014, 2, 1420–1428. [Google Scholar]
- Carlucio, A.C.; Pimenta, M.G.R.; Vieira, R.H.S.F.; Furtado, R.; Guedes, M.I.F.; Rui, S.; Odilio, B.G.A. Practical use of immobilized lysozyme for the remediation process of Escherichia coli in aqueous solution. Electron. J. Biotechnol. 2007, 10. [Google Scholar]
- Lawrence, G.; Baskar, A.V.; El-Newehy, M.H.; Cha, W.S.; Al-Deyab, S.S.; Ajayan, V. Quick high-temperature hydrothermal synthesis of mesoporous materials with 3D cubic structure for the adsorption of lysozyme. Sci. Technol. Adv. Mater. 2015, 16, 024806. [Google Scholar] [CrossRef]
- Hédoux, A.; Willart, J.F.; Ionov, R.; Affouard, F.; Guinet, Y.; Paccou, L.; Lerbret, A.; Descamps, M. Analysis of sugar bioprotective mechanisms on the thermal denaturation of lysozyme from Raman scattering and differential scanning calorimetry investigations. J. Phys. Chem. B 2006, 110, 22886–22893. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Caccamo, M.T.; Magazù, S.; Pasqua, L.; Kiselev, M.A. Interdisciplinary approaches to the study of biological membranes. AIMS Biophys. 2020, 7, 267–290. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Multiscaling Wavelet Analysis of Infrared and Raman Data on Polyethylene Glycol 1000 Aqueous Solutions. Spectr. Lett. 2017, 50, 130–136. [Google Scholar] [CrossRef]
- Chambers Jeanne, C.; Allen Craig, R.; Cushman Samuel, A. Operationalizing Ecological Resilience Concepts for Managing Species and Ecosystems at Risk. Front. Ecol. Evol. 2019, 7, 241. [Google Scholar] [CrossRef]
- Feder, A.; Fred-Torres, S.; Southwick, S.M.; Charney, D.S. The Biology of Human Resilience: Opportunities for Enhancing Resilience Across the Life Span. Biol. Psych. 2019, 86, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, C.; Ji, Y.; Yang, L. Biological and Psychological Perspectives of Resilience: Is It Possible to Improve Stress Resistance? Front. Hum. Neurosci. 2018, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Ruud, J.R.; Hartigh, D.; Hill, Y. Conceptualizing and measuring psychological resilience: What can we learn from physics? New Ideas Psych. 2022, 66, 100934. [Google Scholar]
- Saenz de Miera, L.E.; Pinto, R.; Calvo, L.; Ansola, G. A new index of resilience applicable to external pulse-disturbances that considers the recovery of communities in the short term. Ecol. Indic. 2021, 130, 108051. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, Y.; Wang, L.; Hou, C.; Zhou, X.; Ling, X.; Xu, Z. Intraoperative detection of thyroid carcinoma by fourier transform infrared spectrometry. J. Surg. Res. 2011, 171, 650–656. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, W.; Tian, P.; Ling, X.; Xu, Z. Intraoperative diagnosis of thyroid diseases by fourier transform infrared spectroscopy based on support vector machine. Int. J. Clin. Exp. Med. 2016, 9, 2351–2358. [Google Scholar]
- Bhosale, J.S. High signal-to-noise Fourier transform spectroscopy with light emitting diode sources. Rev. Sci. Instrum. 2011, 82, 093103. [Google Scholar] [CrossRef]
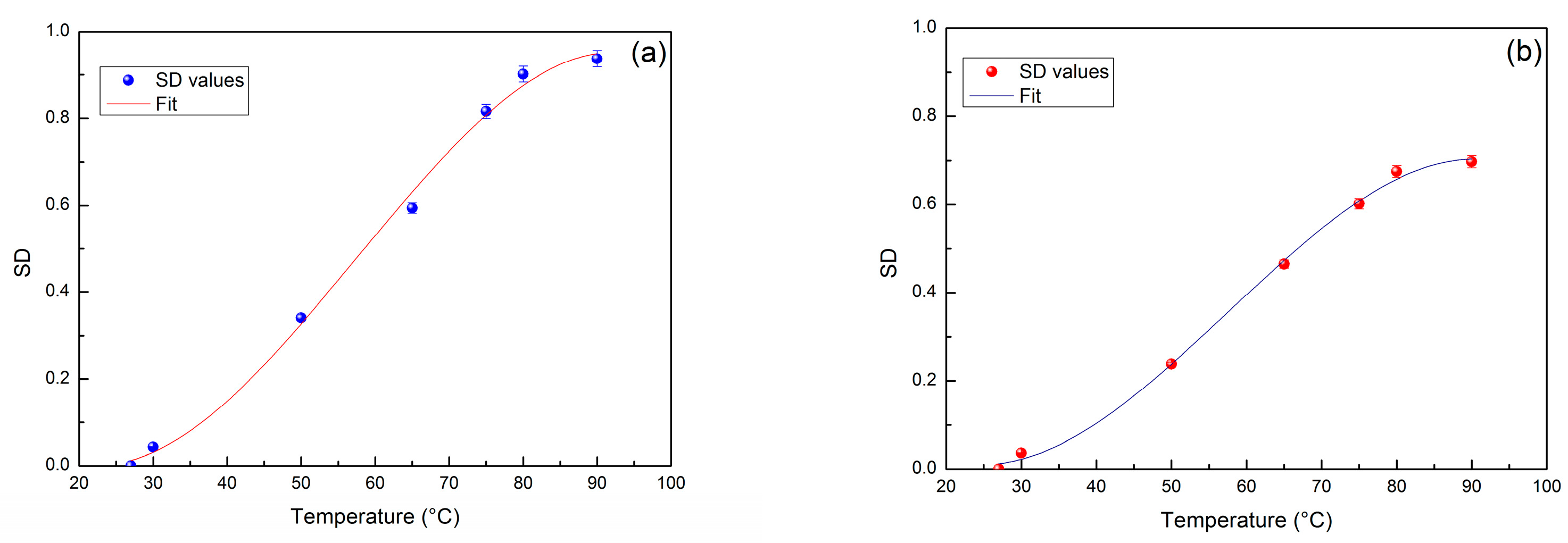
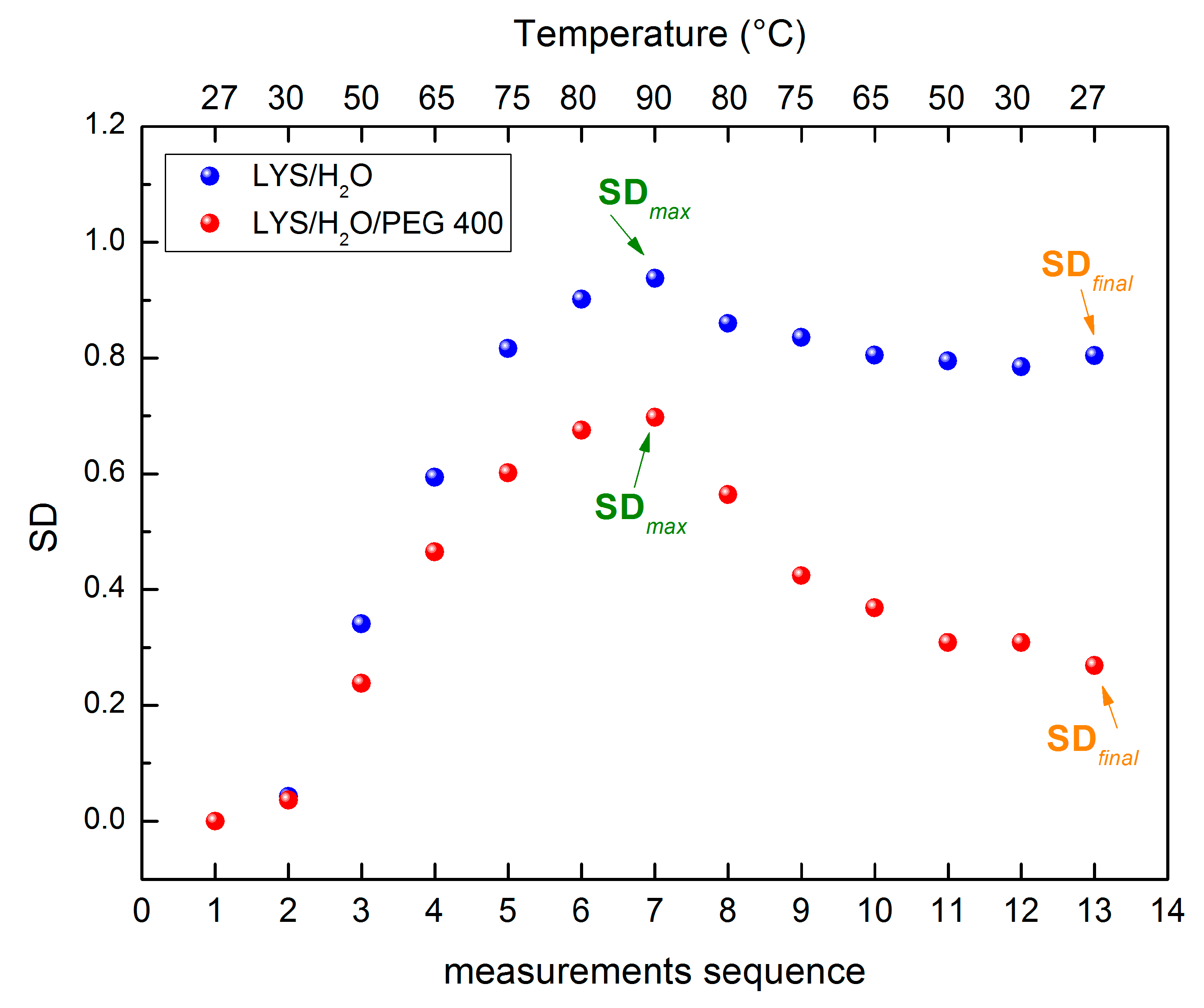
| Amplitude | Steepness | Temperature (°C) | |
|---|---|---|---|
| Lysozyme/H2O | 0.975 | −0.01283 | 55.32 |
| Lysozyme/H2O/PEG 400 | 0.697 | −0.01577 | 58.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccamo, M.T.; Magazù, S. Multiscale Spectral Analysis on Lysozyme Aqueous Solutions in the Presence of PolyEthyleneGlycol. Molecules 2022, 27, 8760. https://doi.org/10.3390/molecules27248760
Caccamo MT, Magazù S. Multiscale Spectral Analysis on Lysozyme Aqueous Solutions in the Presence of PolyEthyleneGlycol. Molecules. 2022; 27(24):8760. https://doi.org/10.3390/molecules27248760
Chicago/Turabian StyleCaccamo, Maria Teresa, and Salvatore Magazù. 2022. "Multiscale Spectral Analysis on Lysozyme Aqueous Solutions in the Presence of PolyEthyleneGlycol" Molecules 27, no. 24: 8760. https://doi.org/10.3390/molecules27248760
APA StyleCaccamo, M. T., & Magazù, S. (2022). Multiscale Spectral Analysis on Lysozyme Aqueous Solutions in the Presence of PolyEthyleneGlycol. Molecules, 27(24), 8760. https://doi.org/10.3390/molecules27248760







