Phytochemistry and Biological Activities of Guarea Genus (Meliaceae)
Abstract
1. Introduction
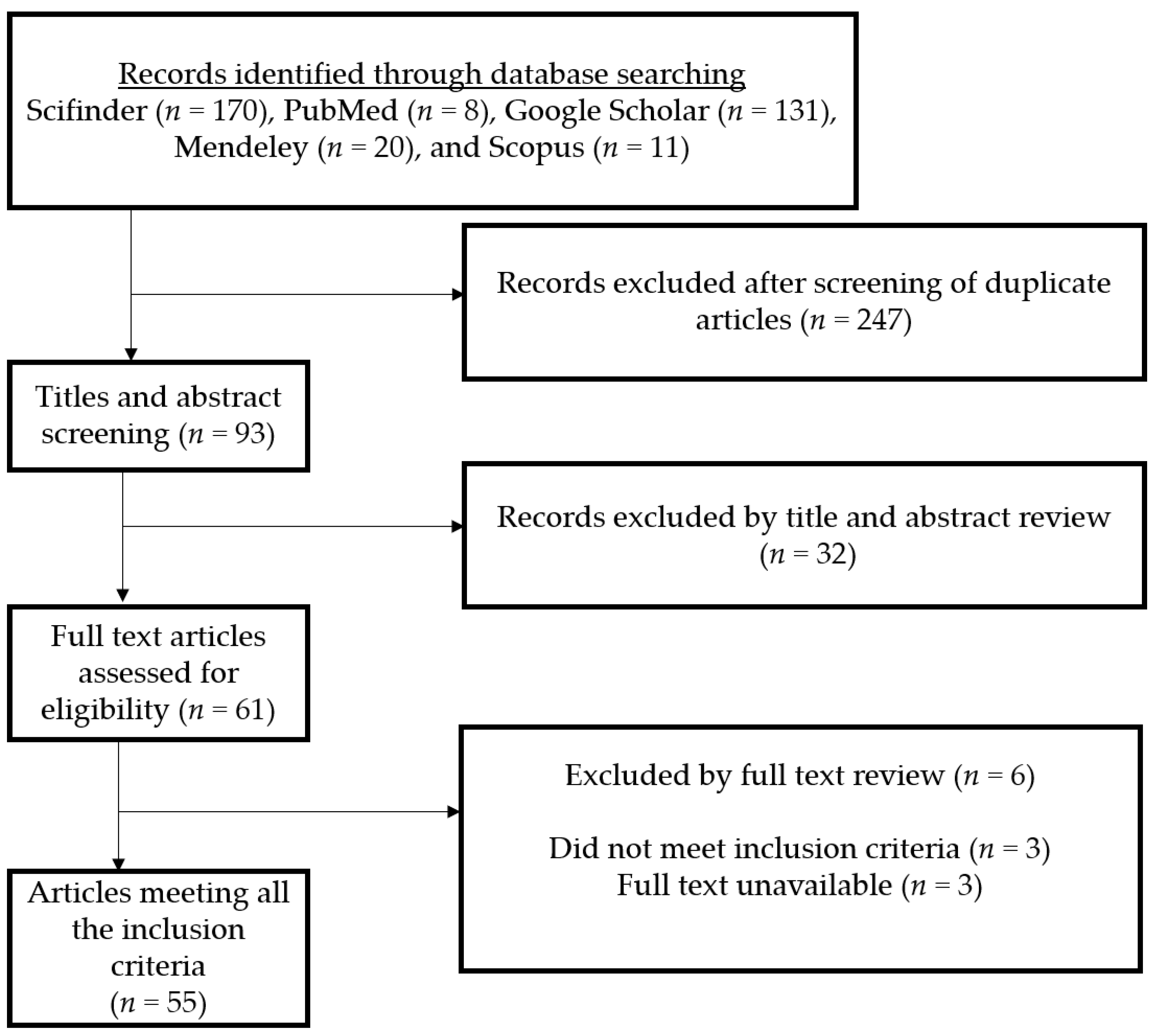
2. Methodology and Botany
3. Phytochemistry
3.1. Overview of Isolated Compounds Derived from Guarea Genus
3.2. Sesquiterpenoid
3.3. Diterpenoid
3.4. Triterpenoid
3.5. Limonoid
3.6. Steroid
3.7. Other Compounds
4. Guarea Bioactivity
4.1. Cytotoxic
4.2. Anti-Inflamation
4.3. Antimalarial
4.4. Antiprotozoal
4.5. Antiviral
4.6. Antimicrobial
4.7. Insecticidal Activity
4.8. Antioxidant and Phosphorylation Inhibitor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, R.; Pednekar, A.; Avalaskar, A.; Rathi, M.; Rewachandani, Y. A Comprehensive Review on Meliaceae Family. World J. Pharm. Sci. 2015, 3, 1572–1577. [Google Scholar]
- Harneti, D.; Supriadin, A.; Ulfah, M.; Safari, A.; Supratman, U.; Awang, K.; Hayashi, H. Cytotoxic Constituents from the Bark of Aglaia eximia (Meliaceae). Phytochem. Lett. 2014, 8, 28–31. [Google Scholar] [CrossRef]
- An, F.L.; Wang, X.B.; Wang, H.; Li, Z.R.; Yang, M.H.; Luo, J.; Kong, L.Y. Cytotoxic Rocaglate Derivatives from Leaves of Aglaia perviridis. Nature 2016, 6, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Song, Y.; Li, H.; Yang, B.; Mao, X.; Zhao, Y.; Shi, X. Cytotoxic and Anti-Inflammatory Tirucallane Triterpenoids from Dysoxylum binectariferum. Fitoterapia 2014, 99, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Liou, M.J.; Kuoh, C.S.; Teng, C.M.; Nagao, T.; Lee, K.H. Cytotoxic and Antiplatelet Aggregation Principles from Aglaia elliptifolia. J. Nat. Prod. 1997, 60, 606–608. [Google Scholar] [CrossRef]
- Yan, H.J.; Wang, J.S.; Kong, L.Y. Cytotoxic Dammarane-Type Triterpenoids from the Stem Bark of Dysoxylum Binecteriferum. J. Nat. Prod. 2014, 77, 234–242. [Google Scholar] [CrossRef]
- Esimone, C.O.; Eck, G.; Nworu, C.S.; Hoffmann, D.; Überla, K.; Proksch, P. Dammarenolic acid, a secodammarane triterpenoid from Aglaia sp. shows potent anti-retroviral activity in vitro. Phytomedicine 2010, 17, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Michaelis, M.; Hsu, H.K.; Tsai, C.C.; Yang, K.D.; Wu, Y.C.; Cinatl, J.; Doerr, H.W. Toona Sinensis Roem Tender Leaf Extract Inhibits SARS Coronavirus Replication. J. Ethnopharmacol. 2008, 120, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Puripattanavong, J.; Tungcharoen, P.; Chaniad, P.; Pianwanit, S.; Tewtrakul, S. Anti-HIV-1 Integrase Effect of Compounds from Aglaia andamanica Leaves and Molecular Docking Study with Acute Toxicity Test in Mice. Pharm. Biol. 2016, 54, 654–659. [Google Scholar] [CrossRef]
- You, H.L.; Chen, C.J.; Eng, H.L.; Liao, P.L.; Huang, S.T. The Effectiveness and Mechanism of Toona Sinensis Extract Inhibit Attachment of Pandemic Influenza A (H1N1) Virus. Evid.-Based Complement. Altern. Med. 2013, 2013, 479718. [Google Scholar] [CrossRef]
- Miranda Júnior, R.N.C.; Dolabela, M.F.; Da Silva, M.N.; Póvoa, M.M.; Maia, J.G.S. Antiplasmodial Activity of the Andiroba (Carapa guianensis Aubl., Meliaceae) Oil and Its Limonoid-Rich Fraction. J. Ethnopharmacol. 2012, 142, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Irungu, B.N.; Adipo, N.; Orwa, J.A.; Kimani, F.; Heydenreich, M.; Midiwo, J.O.; Martin Björemark, P.; Håkansson, M.; Yenesew, A.; Erdélyi, M. Antiplasmodial and Cytotoxic Activities of the Constituents of Turraea robusta and Turraea nilotica. J. Ethnopharmacol. 2015, 174, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.L.; Hematpoor, A.; Hazni, H.; Azirun, M.S.; Litaudon, M.; Supratman, U.; Murata, M.; Awang, K. Mosquito larvicidal limonoids from the fruits of Chisocheton erythrocarpus Hiern. Phytochem. Lett. 2019, 30, 69–73. [Google Scholar] [CrossRef]
- Tepongning, R.N.; Lucantoni, L.; Nasuti, C.C.; Dori, G.U.; Yerbanga, S.R.; Lupidi, G.; Marini, C.; Rossi, G.; Esposito, F.; Habluetzel, A. Potential of a Khaya Ivorensis—Alstonia Boonei Extract Combination as Antimalarial Prophylactic Remedy. J. Ethnopharmacol. 2011, 137, 743–751. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Naqvi, A.A.; Cos, P.; Maes, L.; Apers, S.; Hermans, N.; Pieters, L. Anti-Infective, Cytotoxic and Antioxidant Activity of Ziziphus oxyphylla and Cedrela serrata. Asian Pac. J. Trop. Biomed. 2016, 6, 671–676. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; You, L.J.; Fu, X.; Chen, Y.S.; Luo, Y.Q. Structural Identification of Compounds from Toona sinensis Leaves with Antioxidant and Anticancer Activities. J. Funct. Foods 2014, 10, 427–435. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant Activity of Phenolic Components Present in Barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. Trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Rao, A.S.; Ahemad, S.R.; Ibrahim, M. Phytochemical Studies and Antioxidant Activity of Melia azedarach Linn Leaves by Dpph Scavenging Assay. Int. J. Pharm. Appl. 2012, 3, 271–276. [Google Scholar]
- Kavitha, K.S.; Satish, S. Evaluation of Antimicrobial and Antioxidant Activities from Toona ciliata Roemer. J. Anal. Sci. Technol. 2013, 4, 23. [Google Scholar] [CrossRef]
- Aladesanmi, A.J.U.; Odediran, S.A. Antimicrobial Activity of Trichilia heudelotii Leaves. Fitoterapia 2000, 81, 179–182. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Shi, X. Triterpenoids and Limonoids from Dysoxylum lukii with Cytotoxic and Antimicrobial Activities. Eur. J. Org. Chem. 2011, 2011, 7215–7223. [Google Scholar] [CrossRef]
- Joycharat, N.; Thammavong, S.; Voravuthikunchai, S.P.; Plodpai, P.; Mitsuwan, W.; Limsuwan, S.; Subhadhirasakul, S. Chemical Constituents and Antimicrobial Properties of the Essential Oil and Ethanol Extract from the Stem of Aglaia odorata Lour. Nat. Prod. Res. 2014, 28, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Koul, O.; Shankar, J.S.; Mehta, N.; Taneja, S.C.; Tripathi, A.K.; Dhar, K.L. Bioefficacy of Crude Extracts of Aglaia Species (Meliaceae) and Some Active Fractions against Lepidopteran Larvae. J. Appl. Entomol. 1997, 121, 245–248. [Google Scholar] [CrossRef]
- Mayanti, T.; Tjokronegoro, R.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Hadi, A.H.A. Antifeedant Triterpenoids from the Seeds and Bark of Lansium domesticum Cv Kokossan (Meliaceae). Molecules 2011, 16, 2785–2795. [Google Scholar] [CrossRef]
- Qi, S.H.; Wu, D.G.; Zhang, S.; Luo, X.D. A New Tetranortriterpenoid from Dysoxylum lenticellatum. Z. Fur Naturforsch. Sect. B J. Chem. Sci. 2003, 58, 1128–1132. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Isman, M.B. Antifeedant and Toxic Activity of Trichilia Americana Extract against the Larvae of Spodoptera Litura. Entomol. Exp. Appl. 2001, 98, 9–16. [Google Scholar] [CrossRef]
- Yang, M.H.; Wang, J.S.; Luo, J.G.; Wang, X.B.; Kong, L.Y. Chisopanins A-K, 11 New Protolimonoids from Chisocheton Paniculatus and Their Anti-Inflammatory Activities. Bioorg. Med. Chem. 2011, 19, 1409–1417. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Sonprasit, J.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Diterpenoids and Triterpenoids with Potential Anti-Inflammatory Activity from the Leaves of Aglaia odorata. Phytochemistry 2012, 76, 83–91. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, L.L.; Wang, S.F.; Wang, Y.; Li, Y.; Gao, K. Anti-Inflammatory Terpenoids from the Leaves and Twigs of Dysoxylum gotadhora. J. Nat. Prod. 2015, 78, 1037–1044. [Google Scholar] [CrossRef]
- Cao, D.H.; Yao, J.N.; Sun, P.; Ji, K.L.; Li, X.N.; Cai, Q.; Xiao, C.F.; Hu, H.B.; Yu, Z.Y.; Xu, Y.K. Structurally Diverse Limonoids and Bio-Active Evaluation from Trichilia connaroides. Fitoterapia 2021, 153, 105001. [Google Scholar] [CrossRef]
- Mak, K.K.; Shiming, Z.; Balijepalli, M.K.; Dinkova-Kostova, A.T.; Epemolu, O.; Mohd, Z.; Pichika, M.R. Studies on the Mechanism of Anti-Inflammatory Action of Swietenine, a Tetranortriterpenoid Isolated from Swietenia macrophylla Seeds. Phytomed. Plus 2021, 1, 100018. [Google Scholar] [CrossRef]
- Pennington, T.D.; Clarkson, J.J. A revision of Guarea (Meliaceae). Edinb. J. Bot. 2013, 70, 179–362. [Google Scholar] [CrossRef]
- Akinniyi, J.A.; Connolly, J.D.; Rycroft, D.S. Tetranortriterpenoids and Related Compounds. Part 25. Two 3,4-Secotirucallane Derivatives and 2’-Hydroxyrohitukin from the Bark of Guarea cedrata (Meliaceae). Can. J. Chem. 1980, 58, 1865–1868. [Google Scholar] [CrossRef]
- Housley, R.; King, F.E.; King, T.J.; Taylor, P.R. The Chemistry of Hardwood Extractives. Part XXXIV. Constituents of Guarea species. J. Chem. Soc. 1962, 5095–5104. [Google Scholar]
- Pennington, T.D.; Styles, B.T. A Generic Monograph of The Meliaceae. Blumea 1975, 22, 419–540. [Google Scholar]
- Menut, C.; Lamaty, G.; Seuleiman, A.M.; Fendero, P.; Maidou, E.; Dénamganai, J. Aromatic plants of tropical central Africa. XXI. Chemical composition of bark essential oil of Guarea cedrata (A. Chev.) Pellegr. from Central African Republic. J. Essent. Oil Res. 1995, 7, 207–209. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Roque, N.F. Terpenes from the essential oil of the leaves of Guarea macrophylla Vahl. ssp. tuberculata Vellozo (Meliaceae). J. Essent. Oil Res. 2002, 14, 12–13. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Reis, A.A.; Roque, N.F. Chemical composition from volatile oil of the stem bark of Guarea macrophylla Vahl. ssp. tuberculata Vellozo (Meliaceae). Flavour Fragr. J. 2002, 17, 255–257. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Cornélio, M.L.; Moreno, P.R.H.; Apel, A.; Limberger, R.P.; Henriques, A.T.; Roque, N.F. Sesquiterpenes from essential oil from fruits of Guarea macrophylla Vahl ssp. tuberculata (Meliaceae). J. Essent. Oil Res. 2005, 17, 84–85. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Soares, M.G.; Pereira, L.G.; Silva, M.F.; Correa, A.G.; Fernandes, J.B.; Vieria, P.C.; Roque, N.F. Volatile oil from Guarea macrophylla ssp. tuberculata: Seasonal variation and electroantennographic detection by Hypsipyla grandella. Phytochemistry 2006, 67, 589–594. [Google Scholar] [CrossRef]
- Ribeiro, W.; Arriaga, A.; Neto, M.; Vasconcelos, J.; Santiago, G.M.P.; Nascimento, R.F. Composition of the Essential Oil of Guarea macrophylla Vahl. ssp. tuberculata (Meliaceae) from Northeast of Brazil. J. Essent. Oil Res. 2006, 18, 95–96. [Google Scholar] [CrossRef]
- Oliveira, E.; Martins, E.; Soares, M.; Paula, D.; Passero, L.; Satorelli, P.; Baldim, J.; Lago, J.H.G. A Comparative Study on Chemical Composition, Antileishmanial and Cytotoxic Activities of the Essential Oils from Leaves of Guarea macrophylla (Meliaceae) from Two Different Regions of São Paulo State, Brazil, Using Multivariate Statistical Analysis. J. Braz. Chem. Soc. 2019, 30, 1395–1405. [Google Scholar] [CrossRef]
- Núñez, C.V.; Roque, N.F. Sesquiterpenes from the stem bark of Guarea guidonia (L.) Sleumer (Meliaceae). J. Essent. Oil Res. 1999, 11, 439–440. [Google Scholar] [CrossRef]
- Nunez, C.; Lago, J.H.G.; Roque, N.F. Variation on the chemical composition of the oil from damaged branches of Guarea guidonia (L.) Sleumer (Meliaceae). J. Nat. Prod. 2005, 17, 626–627. [Google Scholar] [CrossRef]
- Magalhães, L.A.M.I.; Da Paz Lima, M.; Marques, M.O.M.; Facanali, R.; Da Silva Pinto, A.C.; Tadei, W.P. Chemical Composition and Larvicidal Activity against Aedes aegypti Larvae of Essential Oils from Four Guarea Species. Molecules 2010, 15, 5734–5741. [Google Scholar] [CrossRef]
- Pandini, J.A.; Pinto, F.G.S.; Scur, M.C.; Santana, C.B.; Costa, W.F.; Temponi, L.G. Chemical Composition, Antimicrobial and Antioxidant Potential of the Essential Oil of Guarea kunthiana A. Juss. Braz. J. Biol. 2018, 78, 53–60. [Google Scholar] [CrossRef]
- Garcez, F.R.; Núñez, C.V.; Garcez, W.S.; Almeida, R.M.; Roque, N.F. Sesquiterpenes, Limonoid and Coumarin from the Wood Bark of Guarea Guidonia. Planta Med. 1998, 64, 79–80. [Google Scholar] [CrossRef]
- Brochini, C.B.; Roque, N.F. Two new cneorubin related diterpenes from the leaves of Guarea guidonia (Meliaceae). J. Braz. Chem. Soc. 2000, 11, 361–364. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Brochini, C.B.; Roque, N.F. Analysis of the essential oil from leaves of three different specimens of Guarea guidonia (L.) Sleumer (Meliaceae). J. Essent. Oil Res. 2005, 17, 271–273. [Google Scholar] [CrossRef]
- Brochini, C.B.; Roque, N.F.; Lago, J.H.G. Natural Product Research: Formerly Natural Product Letters Minor Sesquiterpenes from the Volatile Oil from Leaves of Guarea guidonia Sleumer (Meliaceae). Nat. Prod. Res. 2009, 23, 37–41. [Google Scholar] [CrossRef]
- Soares, L.R.; Silva, A.C.; Freire, T.V.; Garcez, F.R.; Garcez, W.S. Sesquiterpenos de sementes de Guarea guidonia (Meliaceae). Quim. Nov. 2012, 35, 323–326. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Brochini, Â.B.; Roque, N.F. Terpenes from Leaves of Guarea macrophylla ( Meliaceae). Phytochemistry 2000, 55, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.H.G.; Roque, N.F. Estudo fitoquímico da madeira de Guarea macrophylla (Meliaceae). Quim. Nov. 2009, 32, 2351–2354. [Google Scholar] [CrossRef]
- Garcez, F.R.; Garcez, W.S.; Francisca, A.; Silva, G.; Bazzo, R.D.C. Terpenoid Constituents from Leaves of Guarea kunthiana. J. Braz. Chem. Soc. 2004, 15, 767–772. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Roque, N.F. New Diterpenoids from Leaves of Guarea macrophylla (Meliaceae). J. Braz. Chem. Soc. 2005, 16, 643–646. [Google Scholar] [CrossRef]
- Conserva, G.A.; Girola, N.; Figueiredo, R.C.; Azevedo, R.A.; Mousdell, S.; Lago, J.H.G. Terpenoids from Leaves of Guarea macrophylla Display In Vitro Cytotoxic Activity and Induce Apoptosis In Melanoma Cells Authors. Planta Med. 2017, 83, 1289–1296. [Google Scholar]
- Furlan, M.; Lopes, M.N.; Fernandes, J.B.; Pirani, J.R. Diterpenes from Guarea trichilioides. Phytochemistry 1996, 41, 1159–1161. [Google Scholar] [CrossRef]
- Hernandez, V.; De Leo, M.; Cotugno, R.; Braca, A.; De Tommasi, N.; Severino, L. New Tirucallane-Type Triterpenoids from Guarea guidonia Authors. Planta Med. 2018, 84, 716–720. [Google Scholar]
- Camacho, R.; Phillipson, J.D.; Croft, S.L.; Kirby, C.; Warhurst, D.C.; Solis, P.N. Terpenoids from Guarea rhophalocarpa. Phytochemistry 2001, 56, 203–210. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Brochini, C.B.; Roque, N.F. Terpenoids from Guarea guidonia. Phytochemistry 2002, 60, 333–338. [Google Scholar] [CrossRef]
- Furlan, M.; Roque, N.F.; Filho, W.W. Guarea trichilioides Is a Large Tree Occurring in the Amaz. Phytochemistry 1993, 32, 1519–1522. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Roque, N.F. Cycloartane Triterpenoids from Guarea macrophylla. Phytochemistry 2002, 60, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.; Gunn, A.; Marsh, W.; Mcrindle, R.; Restivo, R.; Connolly, J.D.; Fulke, J.; Henderson, M. Triterpenoids from Guarea glabra (meliaceae): A new skeletal class identified by chemical, spectroscopic, and X-ray evidence. J. Chem. Soc. Chem. Commun. 1973, 35, 159–160. [Google Scholar] [CrossRef]
- Harding, W.W.; Jacobs, H.; Mclean, S.; Reynolds, W.F. Structural and 1 H and 13 C NMR Analysis of Two New Glabretal Triterpenoid Derivatives from Guarea Jamaicensis. Magn. Reson. Chem. 2001, 39, 719–722. [Google Scholar] [CrossRef]
- Jimenez, A.; Villaarreal, C.; Toscano, R.; Cook, M.; Arnason, J.; Bye, R.; Mata, R. Limonoids from swietenia humilis and Guarea grandiflora (Meliacea). Phytochemistry 1998, 49, 1981–1988. [Google Scholar] [CrossRef]
- Miguita, C.H.; Silva Da Barbosa, C.; Hamerski, L.; Sarmento, U.C.; Do Nascimento, J.N.; Garcez, W.S.; Garcez, F.R. 3β-O-Tigloylmelianol from Guarea kunthiana: A New Potential Agent to Control Rhipicephalus (Boophilus) Microplus, a Cattle Tick of Veterinary Significance. Molecules 2015, 20, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Hayasida, W.; Oliveira, L.M.; Ferreira, A.G. Ergostane steroids, tirucallane and apotirucallane triterpenes from Guarea convergens. Chem. Nat. Compd. 2017, 53, 312–317. [Google Scholar] [CrossRef]
- Tan, Q.G.; Luo, X.D. Meliaceous Limonoids: Chemistry and Biological Activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Fang, X.; Di, Y.; Hao, X. The Advances in the Limonoid Chemistry of the Meliaceae Family. Curr. Org. Chem. 2011, 15, 1363–1391. [Google Scholar]
- Connolly, J.D.; Okorie, D.A.; Taylor, D.A.H. Limonoid extractives from species of Guarea. An unusual shielding effect on an acetyl group. J. Chem. Soc. Perkin Trans. 1972, 1, 19711. [Google Scholar] [CrossRef]
- Miguita, C.H.; Sarmento, U.C.; Hamerski, L.; Garcez, W.S.; Garcez, F.R. Mexicanolide- and Andirobine-Type Limonoids from the Fruits of Guarea kunthiana. Rec. Nat. Prod. 2014, 8, 290–293. [Google Scholar]
- Mootoo, S.; Reynolds, F. Ecuadorin, a Novel Tetranortriterpenoid of Guarea kunthiana: Structure Elucidation by 2-D NMR Spectroscopy. Can. J Chem 1991, 70, 1260–1264. [Google Scholar] [CrossRef]
- Lukacova, V.; Polonsky, J.; Moretti, C. Isolation and structure of 14,15β-Epoxyprieurianin from the south American tree Guarea guidona. J. Nat. Prod. 1982, 45, 288–294. [Google Scholar] [CrossRef]
- Zelnik, R.; Rosito, C. Le Fissinolide. Tetrahedron Lett. 1966, 6, 6441–6444. [Google Scholar] [CrossRef]
- Zelnik, R.; Rosito, C. The Isolation of Angustinolide From Guarea trichilioides L. Phytochemistry 1971, 10, 1166–1167. [Google Scholar] [CrossRef]
- Bellone, M.L.; Mun, C.; Chini, M.G.; Piaz, F.D.; Hernandez, V.; Bifulco, G.; De Tommasi, N.; Braca, A. Limonoids from Guarea guidonia and Cedrela odorata: Heat Shock Protein 90 (Hsp90) Modulator Properties of Chisomicine D. J. Nat. Prod. 2021, 84, 724–737. [Google Scholar] [CrossRef]
- Djeukeu, C.; Tala, M.F.; Akak, C.M.; Guy, A.; Azebaze, B.; Francois, A.; Wafo, K.; Wansi, J.D.; Vardamides, J.C.; Laatsch, H.; et al. Mayombensin, a new azadirachtin i derivative with unusual structure from Guarea mayombensis. Planta Med. 2017, 4, 104–107. [Google Scholar] [CrossRef]
- Ferguson, B.G.; Gunn, P.A.; Marsh, W.C.; Mccrindle, R.; Restivo, R. Tetranortriterpenoids and related substances. Part XVII. A new skeletal class of triterpenoids from Guarea glabra (Meliaceae). J. Chem. Soc. Perkin Trans. 1975, 491–497. [Google Scholar] [CrossRef]
- Garcez, W.S.; Garcez, F.R.; Soares, L.R. 16,17-Seco- and 2,3:16,17-Di-Seco-Pregnanes from Guarea guidonia. J. Braz. Chem. Soc. 2008, 19, 1073–1077. [Google Scholar] [CrossRef]
- Pereira, C.; Kuster, C. Flavonoids and A Neolignan Flucoside from Guarea macrophylla (Meliacea). Quim. Nov. 2012, 35, 1123–1126. [Google Scholar] [CrossRef]
- Correa, M. Dicionário de Plantas Úteis e Das Exóticas Cultivadas; Ministério da Agricultura: Rio de Janeiro, Brazil, 1984.
- Oga, S.; Sertlé, J.A.; Brasile, A.C.; Hanada, S. Antiinflammatory Effect of Crude Extract from Guarea guidonia. Planta Med. 1981, 42, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Bray, D.H.; Warhurst, D.C.; Connolly, J.D.; O’Neill, M.J.; Phillipson, J.D. Plants as Sources of Antimalarial Drugs. Part 7. Activity of Some Species of Meliaceae Plants and Their Constituent Limonoids. Phyther. Res. 1990, 4, 29–35. [Google Scholar] [CrossRef]
- De Mesquita, M.L.; Desrivot, J.; Bories, C.; Fournet, A.; De Paula, J.E.; Grellier, P.; Espindola, L.S. Antileishmanial and Trypanocidal Activity of Brazilian Cerrado Plants. Mem. Inst. Oswaldo Cruz 2005, 100, 783–787. [Google Scholar] [CrossRef]
- Simoni, I.; Munford, V.; Felicio, J.; Lins, A. Antiviral Activity of Crude Extracts of Guarea guidona. Braz. J. Med. Biol. Res. 1996, 29, 647–650. [Google Scholar]
- Jerjomiceva, N.; Seri, H.; Yaseen, R.; Buhr, N.; Setzer, W.; Naim, H.; Blickwede, M. Guarea Kunthiana Bark Extract Enhances the Antimicrobial Activities of Human and Bovine Neutrophils. Nat. Prod. Commun. 2016, 11, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, U.C.; Miguita, C.H.; Almeida, L.H.D.O.; Gaban, C.R.G.; Silva, L.M.G.E.; Souza, A.S.D.; Garcez, W.S.; Garcez, F.R. Larvicidal Efficacies of Plants from Midwestern Brazil: Melianodiol from Guarea kunthiana as a Potential Biopesticide against Aedes aegypti. Mem. Inst. Oswaldo Cruz 2016, 111, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Pandini, J.A.; Gisele, F.; Scur, M.C.; Francisco, L.; Alves, A.; Martins, C.C. Antimicrobial, Insecticidal, and Antioxidant Activity of Essential Oil and Extracts of Guarea kunthiana A. Juss. J. Med. Plants Res. 2015, 9, 48–55. [Google Scholar] [CrossRef]
- Achnine, L.; Mata, R.; Lotina-hennsen, B. Interference of the Natural Product 7-Oxo-7-deacetoxygedunin with CF0of H+-ATPase of Spinach Chloroplasts. Pestic. Biochem. Physiol. 1999, 63, 139–149. [Google Scholar] [CrossRef]
- Weniger, B.; Robledo, S.; Jaime, G.; Deharo, E.; Callapa, J.; Lobstein, A.; Anton, R. Antiprotozoal Activities of Colombian Plants. J. Etnopharmacol. 2001, 78, 193–200. [Google Scholar] [CrossRef]
- Barbosa, C.d.S.; Borges, L.M.F.; Louly, C.C.B.; Rocha, T.L.; de Sabóia-Morais, S.M.T.; Miguita, C.H.; Garcez, W.S.; Garcez, F.R. In Vitro Activity of 3β-O-Tigloylmelianol from Guarea kunthiana A. Juss (Meliaceae) on Oogenesis and Ecdysis of the Cattle Tick Rhipicephalus (Boophilus) Microplus (Canestrini) (Acari: Ixodidae). Exp. Parasitol. 2016, 164, 5–11. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Romoff, P.; Pirani, J.R.; Roque, N.F. Essential Oil from of Guarea macrophylla Vahl var. tuberculata vellozo (Meliaceae) Leaves—Variation in the Chemical Component Proportions. J. Essent. Oil Res. 2007, 19, 338–341. [Google Scholar] [CrossRef]
- Bevan, C.W.L.; Powell, J.W.; Taylor, D.A.H. West African Timbers. Part VI. Petroleum Extracts from Species of the Genera Khaya, Guarea, Carapa, and Cedrela. J. Chem. Soc. 1963, 180, 980–982. [Google Scholar] [CrossRef]

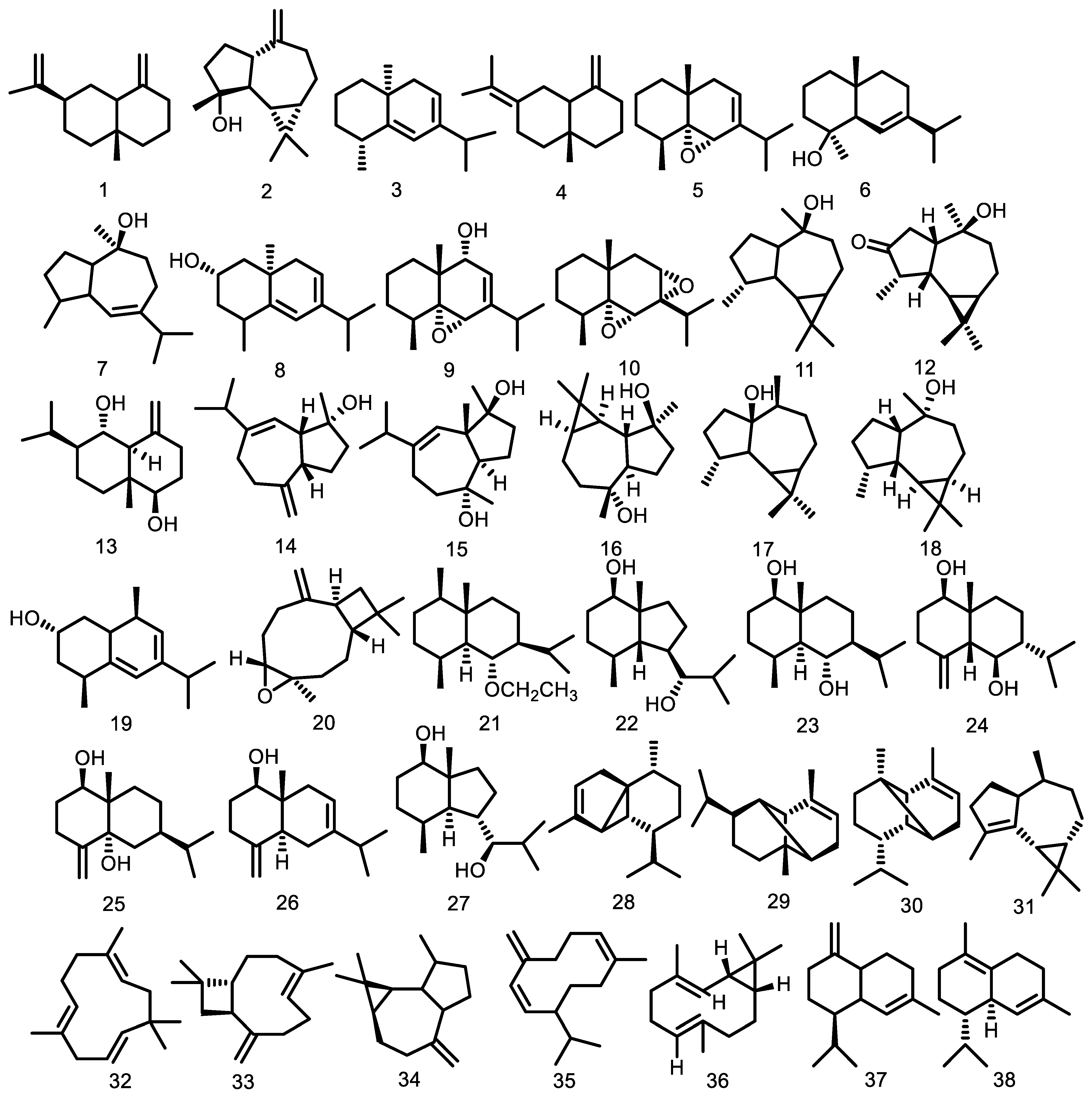
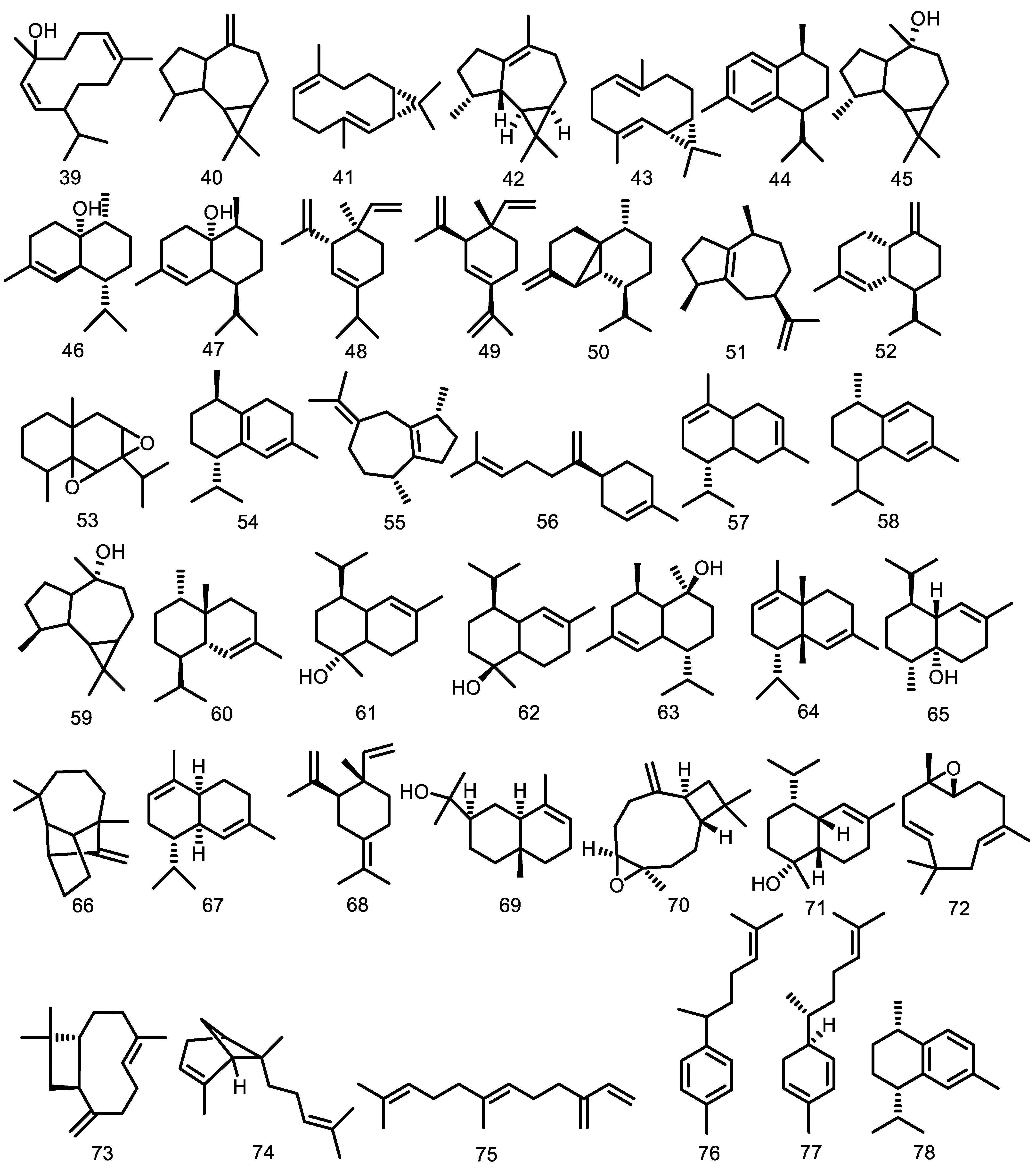
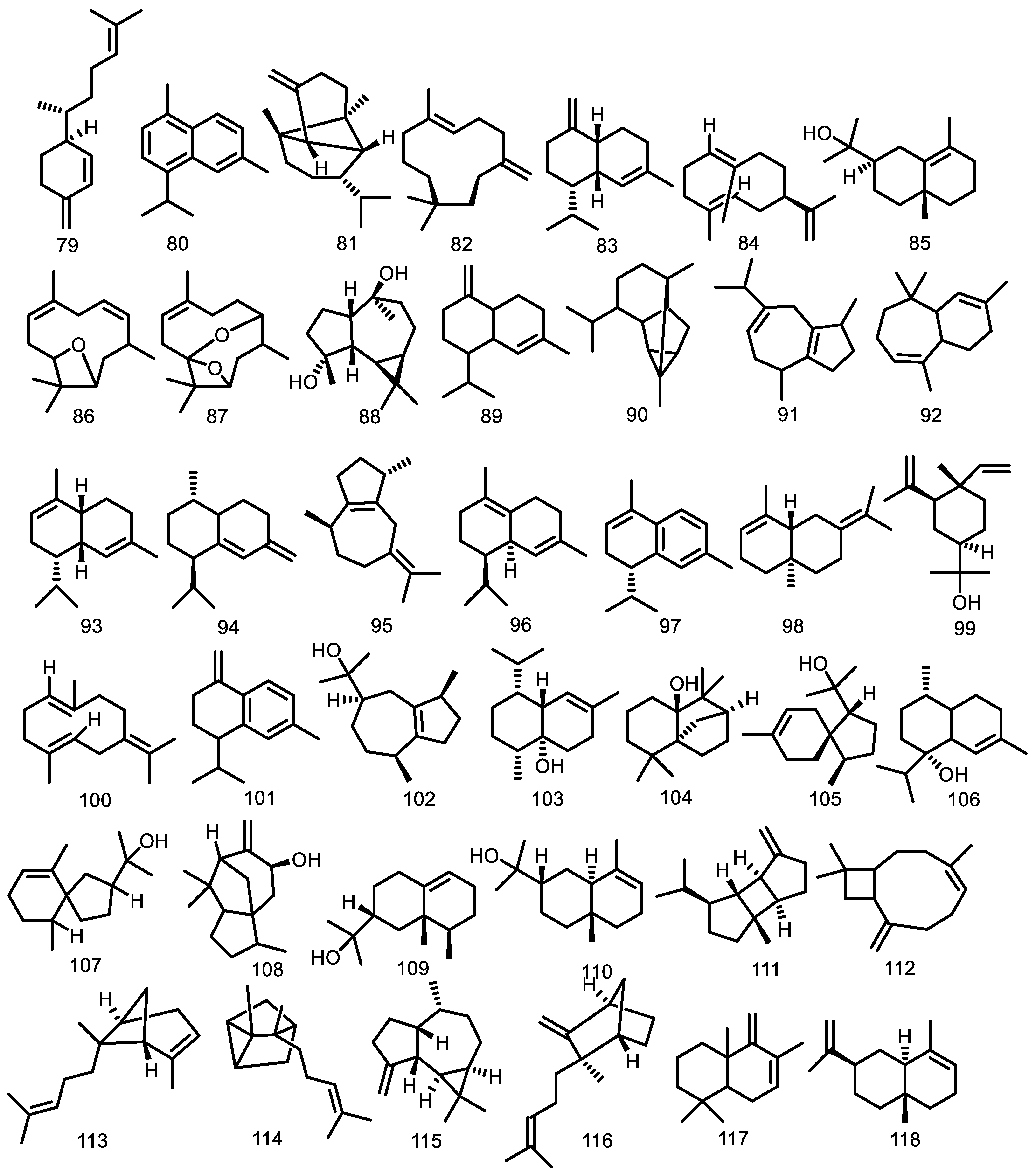
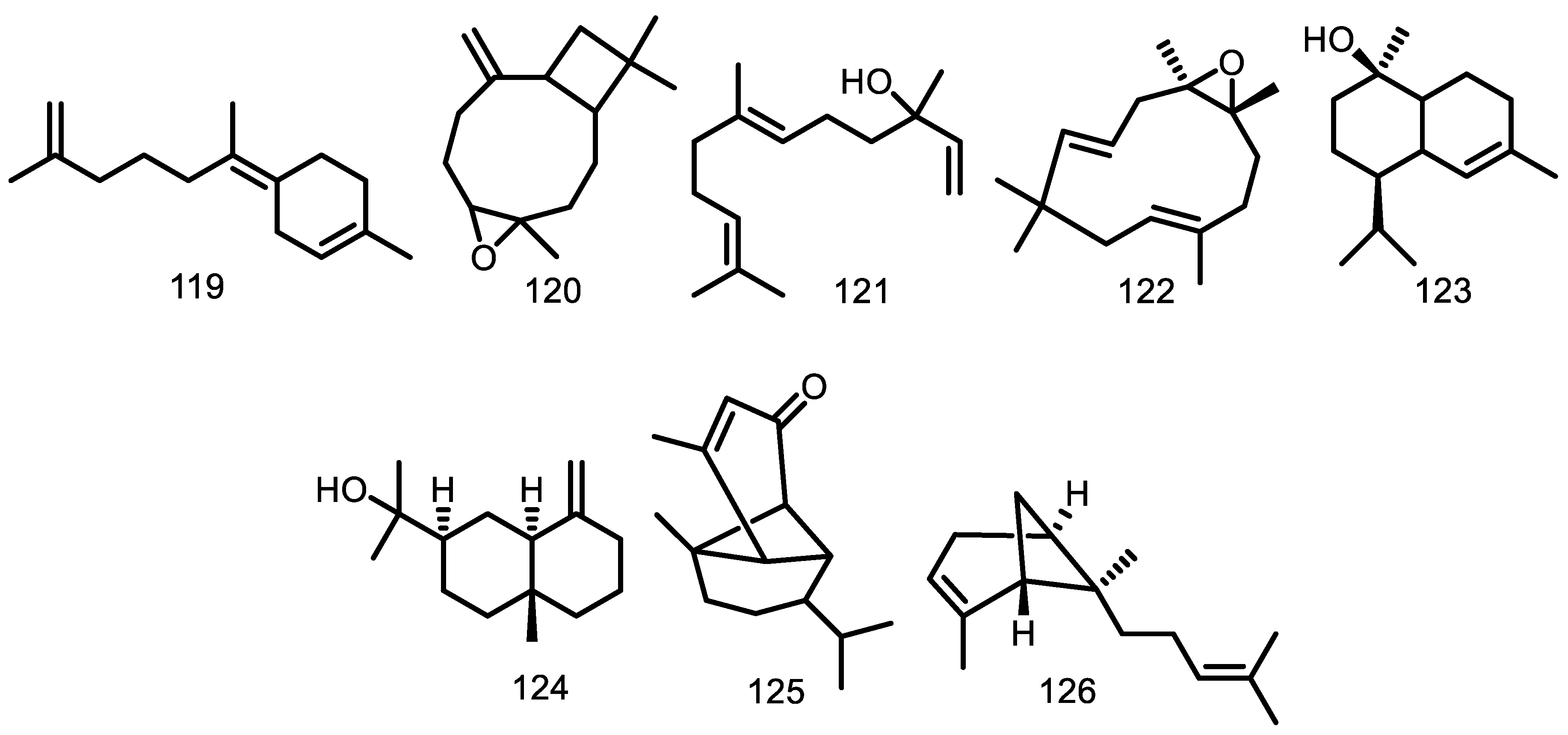
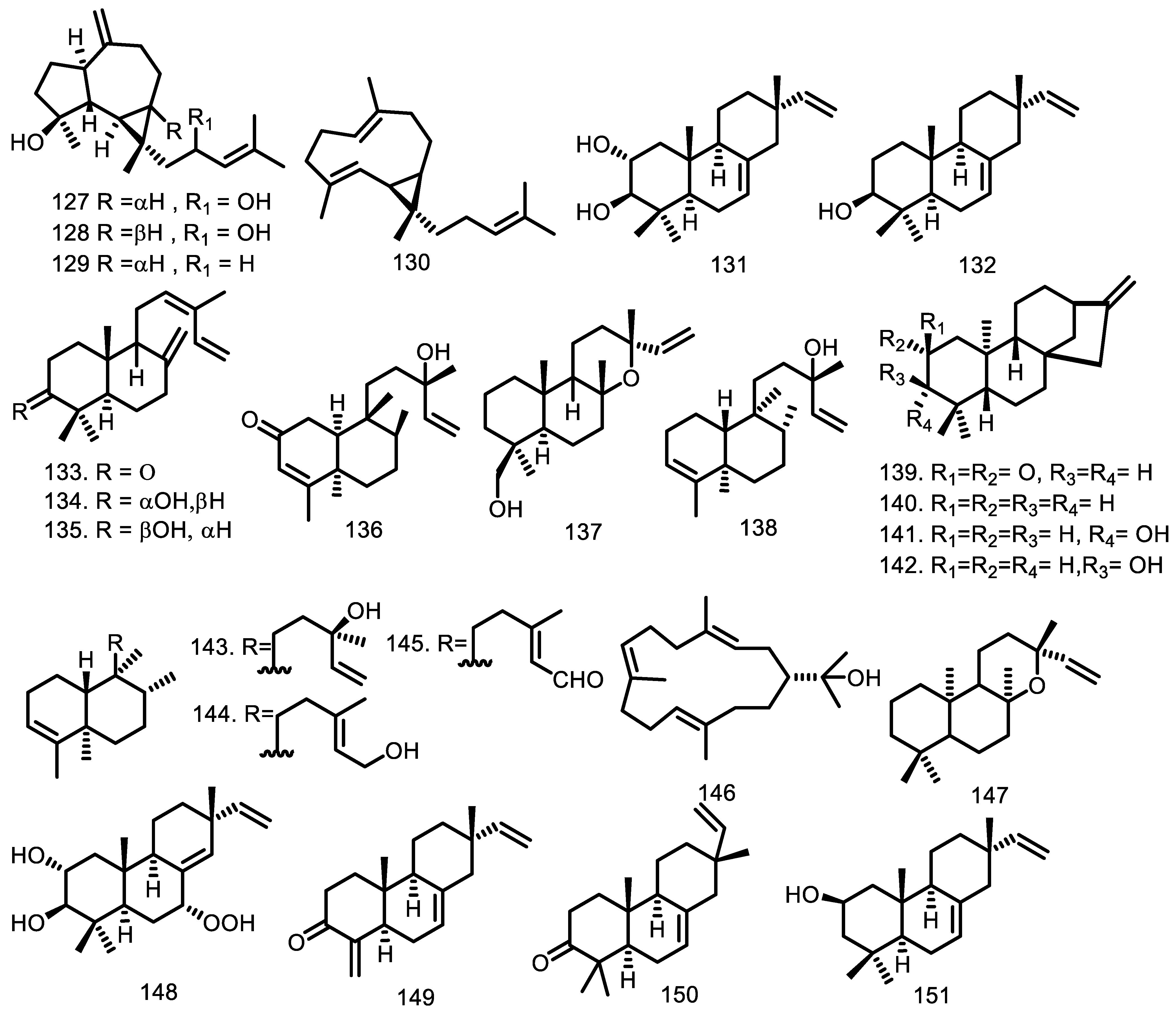
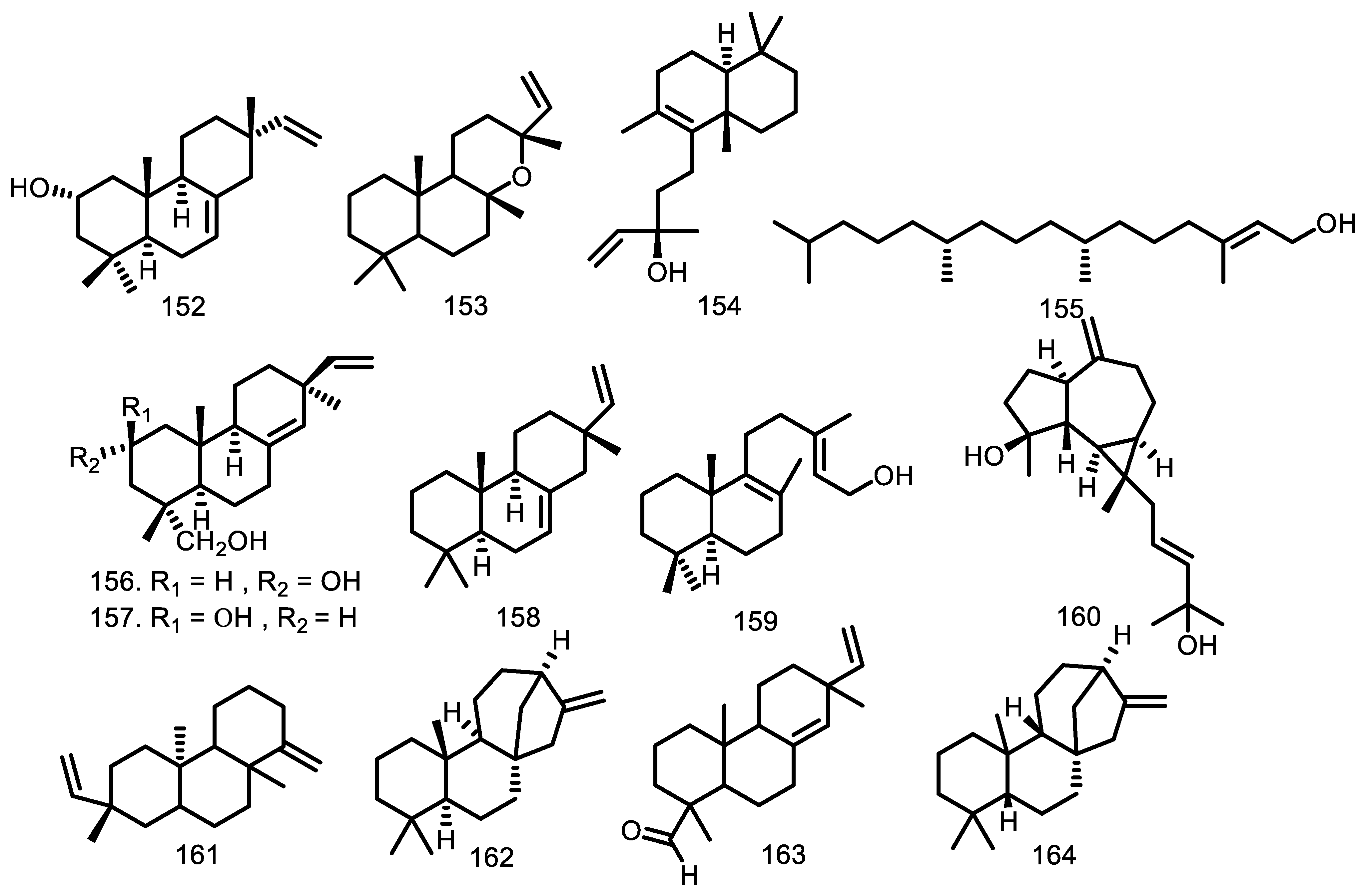

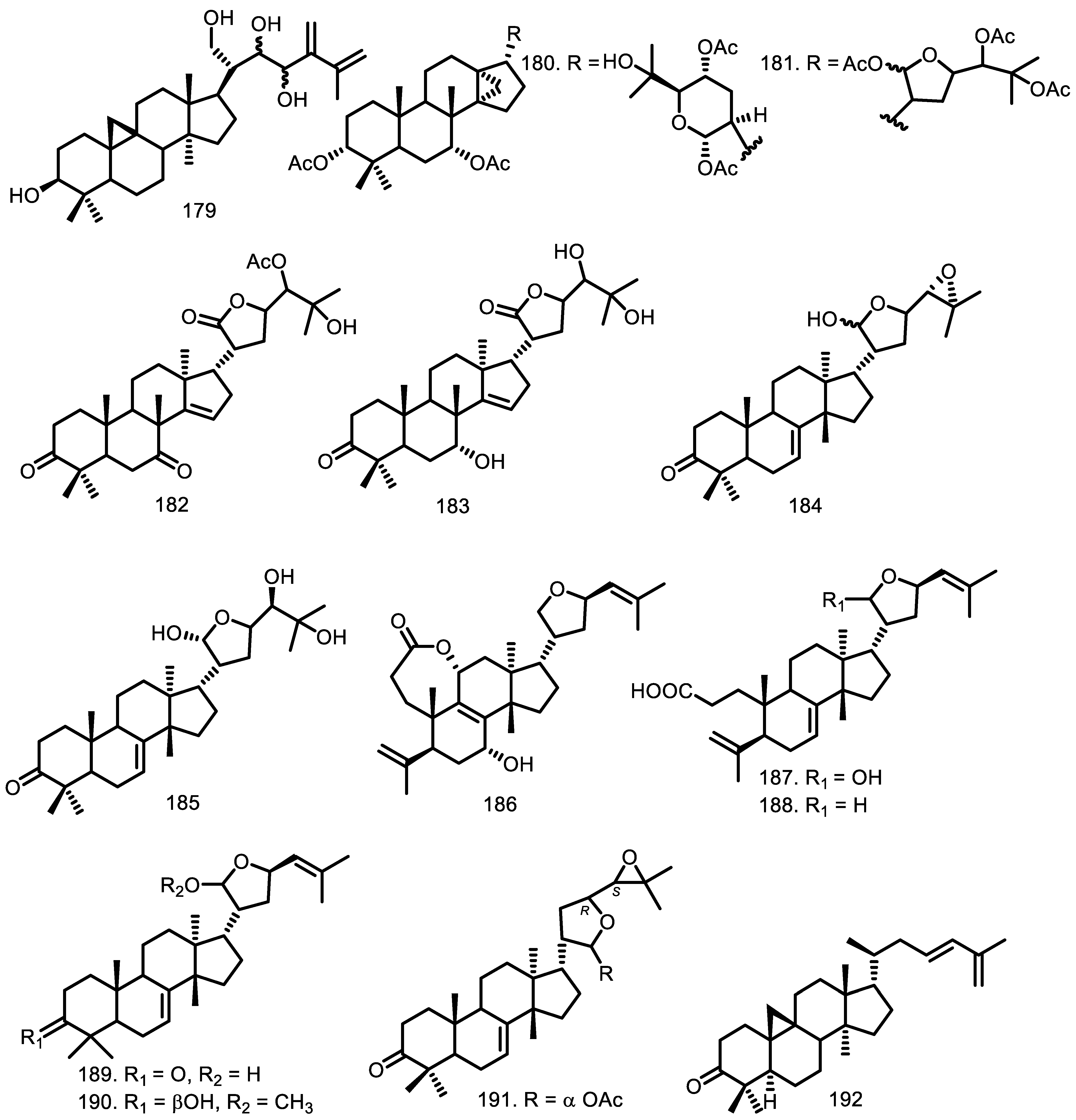

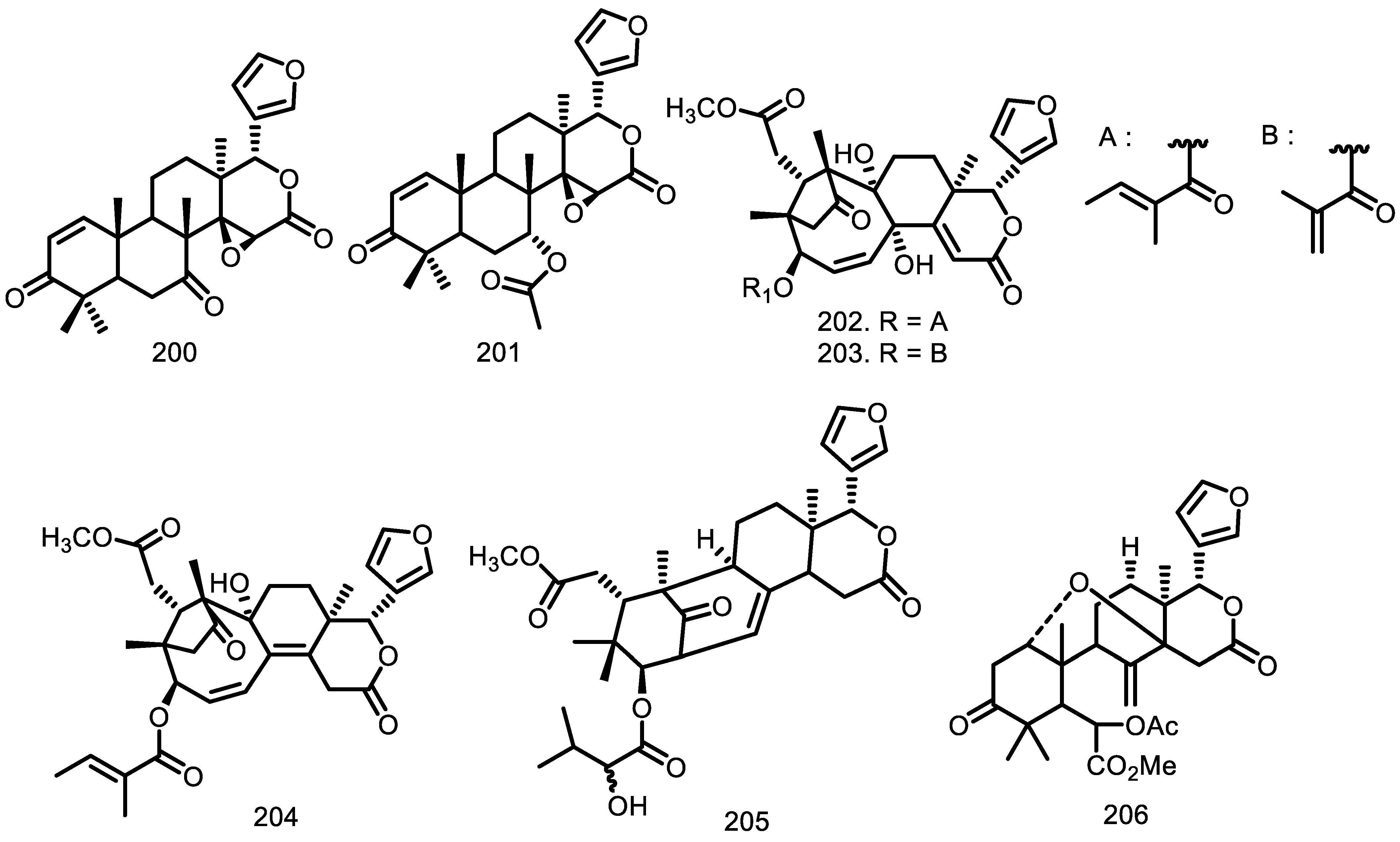
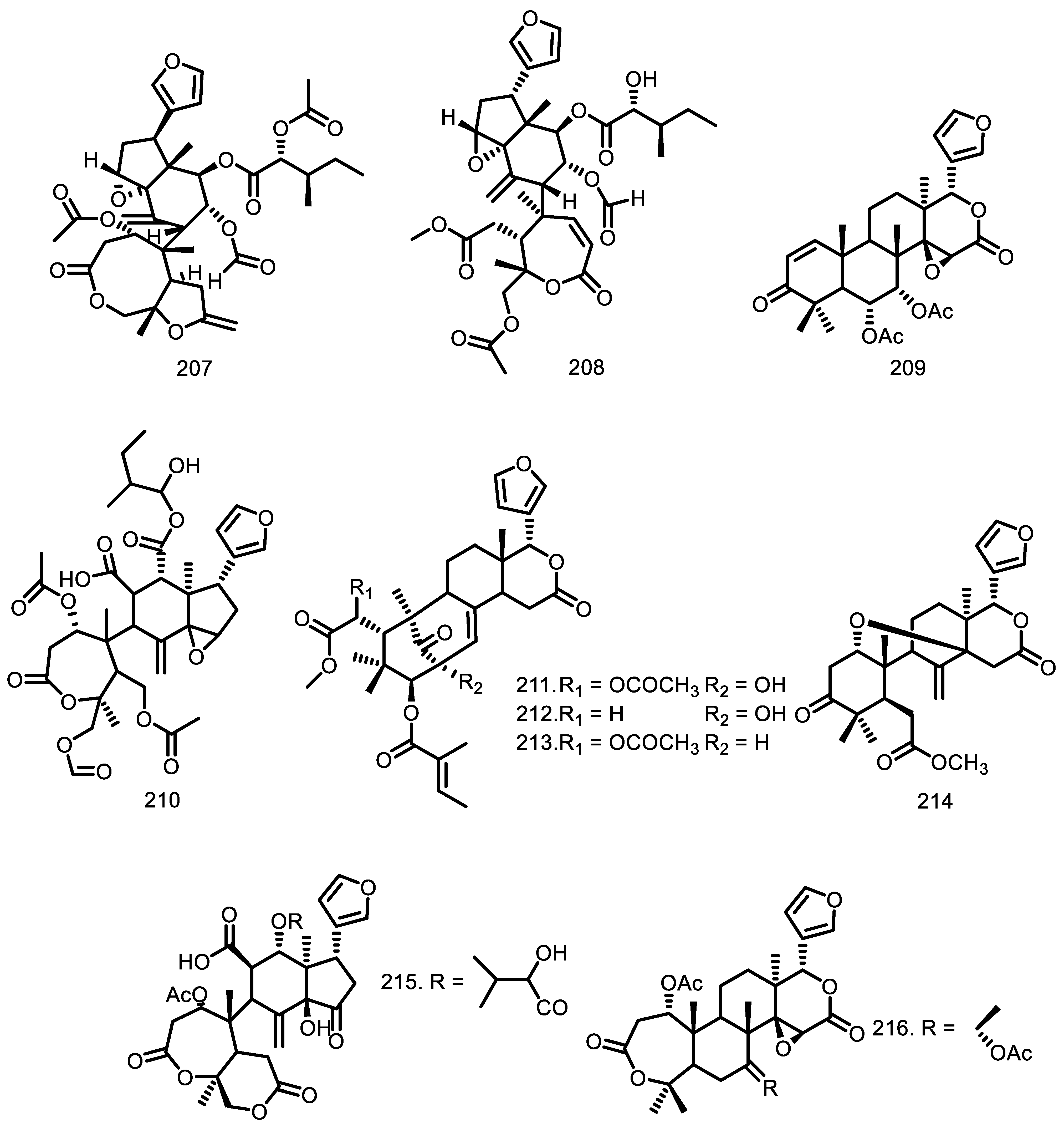

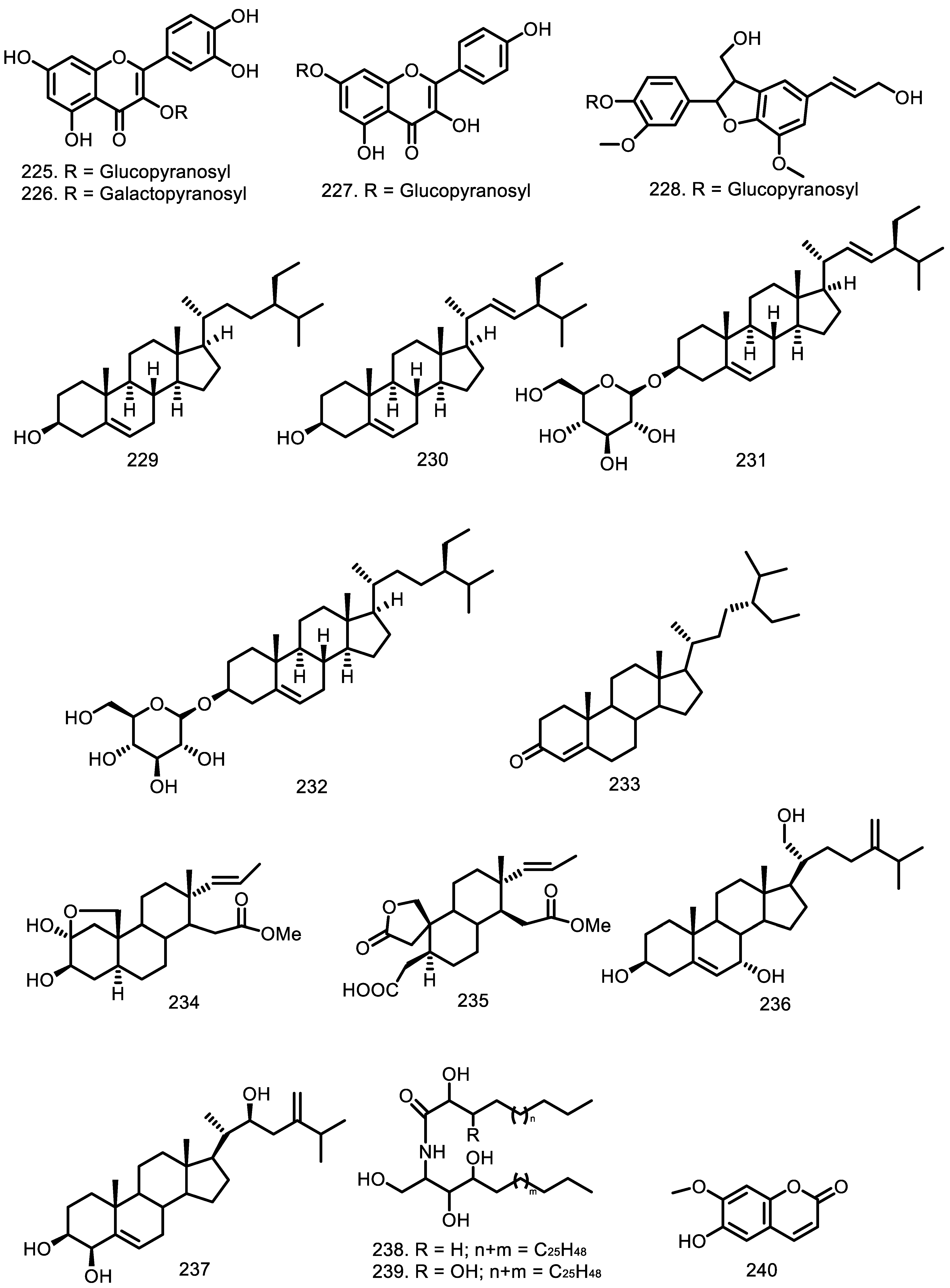
| Compounds | Species | Sources | References |
|---|---|---|---|
| Sesquiterpenoid | |||
| β-selinene (1) | G. guidonia | Leaves Leaf essential oil | [48,60] [49,50] |
| Spathulenol (2) | G. guidonia G. kunthiana G. macrophylla | Leaves Leaves Leaf essential oil Leaves Wood Leaf essential oil Fruit essential oil | [48] [54] [46] [52] [53] [37,40] [39] |
| eudesm-5,7-dien (3) | G. guidonia | Leaf essential oil Leaves | [49,50] [60] |
| Eudesm-4,11-diene (4) | G. guidonia | Leaf essential oil Leaves | [49,50] [60] |
| 5α,6α-epoxy-eudesm-7-ene (5) | G. guidonia | Leaf essential oil | [50] |
| Eudesm-6-en-4β-ol (6) | G. guidonia | Leaf essential oil Leaves | [49,50] [60] |
| Guai-6-en-10β-ol (7) | G. guidonia G. macrophylla | Leaf essential oil Leaves Leaf essential oil Stem bark essential oil Wood | [49,50] [52,62] [40,92] [38] [53] |
| Eudesm-5,7-dien-2α-ol (8) | G. guidonia | Leaf essential oil | [49,50] |
| 5α,6α-epoxy-eudesm-7-en-9-ol (9) | G. guidonia | Leaf essential oil | [50] |
| 5α,6α,7α,8α-diepoxy-eudesmane (10) | G. guidonia | Leaf essential oil Leaves | [50] [60] |
| Viridiflorol (11) 3-oxo-10-alloaromadendranol (12) Voleneol (13) Alismol (14) Alismoxide (15) (-)-4β,10α-aromadendranediol (16) Palustrol (17) Ledol (18) | G. guidonia G. macrophylla G. guidonia G. guidonia G. kunthiana G. kunthiana G. kunthiana G. macrophylla G. macrophylla G. macrophylla | Wood bark Branch essential oil Stem bark essential oil Stem bark essential oil Wood bark Wood bark Leaves Leaves Leaves Wood Leaves Leaf essential oil Leaves Leaf essential oil Stem bark essential oil | [47] [44] [43] [38] [47] [47] [54] [54] [54] [53] [52] [37,40,92] [52] [37,40,92] [38] |
| (2S*)-eudesm-5,7-dien-2-ol (19) Caryophyllene oxide (20) 6α-ethoxyeudesm-4(15)-en-1β-ol (21) (7R*)-5-epi-opposit-4(15)-ene-1β,7-diol (22) Eudesm-4(15)-ene-1β,6α-diol (23) 5-epi-eudesm-4(15)-ene-1β,6β-diol (24) Eudesm- 4(15)-ene-1β,5α-diol (25) Eudesm-4(15),7-dien-1β-ol (26) (7R*)-opposit-4(15)-ene-1β,7-diol (27) α-cubebene (28) α-ylangene (29) α-copaene (30) α-gurjunene (31) α-humulene (32) β-caryophyllene (33) Allo-aromadendrene (34) Germacrene D (35) | G. guidonia G. macrophylla G. cedrata G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. macrophylla G. guidonia G. macrophylla G. cedrata G. macrophylla G. guidonia G. cedrata G. kunthiana G. macrophylla G. macrophylla G. guidonia G. macrophylla G. guidonia G. cedrata G. macrophylla G. guidonia G. cedrata G. macrophylla G. guidonia G. kunthiana | Leaves Wood Bark essential oil Branch essential oil Stem bark essential oil Seeds Seeds Seeds Seeds Seeds Seeds Seeds Leaf essential oil Stem bark essential oil Fruit essential oil Leaf essential oil Leaf essential oil Bark essential oil Leaf essential oil Stem bark essential oil Fruit essential oil Leaf essential oil Bark essential oil Leaf essential oil Leaf essential oil Leaf essential oil Stem bark essential oil Fruit essential oil Branch essential oil Stem bark essential oil Leaf essential oil Stem bark essential oil Fruit essential oil Leaf essential oil Branch essential oil Stem bark essential oil Bark essential oil Leaf essential oil Stem bark essential oil Fruit essential oil Leaf essential oil Bark essential oil Leaf essential oil Fruit essential oil Branch essential oil Stem bark essential oil | [60] [36] [44] [43] [53] [51] [51] [51] [51] [51] [51] [51] [37,40,92] [38] [39] [49] [37,40,41,92] [36] [37,40,92] [38] [39] [49] [36] [46] [37,40,92] [37,40,41,92] [38] [39] [44] [43] [37,40,41,92] [38] [39] [49] [44] [43] [36] [37,40,92] [38] [39] [49] [36] [37,40,92] [39] [44] [43] |
Bicyclogermacrene (36) γ-cadinene (37) δ-cadinene (38) Germacrene-D-4-ol (39) Aromadendrene (40) cis-bicyclogermacradiene (41) Viridiflorene (42) trans-bicyclogermacradiene (43) cis-calamenene (44) Globulol (45) cis-cubenol (46) trans-cubenol (47) δ-elemene (48) β -elemene (49) β-cubebene (50) α-guaiene (51) γ-muurolene (52) 5,6,7,8-diepoxy-eudesmane (53) Cadina-1(6),4-diene (54) cis-β-guaiene (55) β-bisabolene (56) β-cadinene (57) Cadina-1,4-diene (58) epi-globulol (59) 1-epi-cubenol (60) τ-cadinol (61) τ-muurolol (62) α-cadinol (63) α-cadinene (64) 1-cubenol (65) Longifolene (66) α-muurolene (67) γ-elemene (68) α-eudesmol (69) Isocaryophyllene oxide (70) α-muurolol (71) 6,7-epoxy-2,9-humuladiene (72) E-caryophyllene (73) α-bergamotene (74) β-farnesene (75) α-curcumene (76) α-zingiberene (77) Calamenene (78) β-sesquiphellandrene (79) Cadalene (80) β-copaene (81) 9-epi-β-caryophyllene (82) γ-amorphene (83) Germacrene A (84) γ-eudesmol (85) 1(10)-epoxy-4,7-humuladiene (86) 1(10),4-diepoxy-7-humulene (87) alloaromadendrane-4α,10β-diol (88) trans-4,10(14)-cadinadiene (89) cyclosativene (90) 6,9-guaiadiene (91) γ-himachalene (92) α-amorphene (93) trans-muurola-4(14),5-diene (94) trans-β-guaiene (95) δ-amorphene (96) α-calacorene (97) Selina-3,7(11)-diene (98) Elemol (99) Germacrene B (100) β-calacorene (101) Guaiol (102) 1,10-di-epi-cubenol (103) Isolongifolan-7-α-ol (104) α-acorenol (105) cis-cadin-4-en-7-ol (106) Hinesol (107) Cedr-8(15)-en-9α-ol (108) Valerianol (109) 7-epi-α-eudesmol (110) β-bourbonene (111) cis-caryophyllene (112) α-cis-bergamoteme (113) α-santalene (114) β-gurjunene (115) β-santalene (116) Drima-7,9(11)-diene (117) α-selinene (118) (E)-iso-γ-bisabolene (119) Caryophyllene epoxide (120) trans-nerolidol (121) Humulene epoxide II (122) epi-α-cadinol (123) β-eudesmol (124) Mustakone (125) α-trans-bergamotene (126) | G. kunthiana G. macrophylla G. macrophylla G. guidonia G. macrophylla G. guidonia G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. cedrata G. macrophylla G. macrophylla G. macrophylla G. cedrata G. macrophylla G. macrophylla G. guidonia G. guidonia G. macrophylla G. guidonia G. macrophylla G. guidonia G. guidonia G. macrophylla G. cedrata G. guidonia G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. cedrata G. macrophylla G. cedrata G. macrophylla G. macrophylla G. cedrata G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. cedrata G. cedrata G. cedrata G. macrophylla G. cedrata G. cedrata G. guidonia G. guidonia G. guidonia G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. guidonia G. guidonia G. macrophylla G. guidonia G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. scabra G. scabra G. scabra G. convergens G. scabra G. convergens G. convergens G. convergens G. silvatica G. humatensis G. scabra G. silvatica G. scabra G. silvatica G. silvatica G. scabra | Stem bark essential oil Leaf essential oil Leaf essential oil Leaf essential oil Fruit essential oil Leaf essential oil Branch essential oil Leaf essential oil Stem bark essential oil Leaf essential oil Branch essential oil Stem bark essential oil Leaf essential oil Fruit essential oil Stem bark essential oil Stem bark essential oil Stem bark essential oil Fruit essential oil Bark essential oil Stem bark essential oil Stem bark essential oil Fruit essential oil Stem bark essential oil Fruit essential oil Bark essential oil Stem bark essential oil Stem bark essential oil Leaf essential oil Leaf essential oil Branch essential oil Stem bark essential oil Leaf essential oil Leaf essential oil Fruit essential oil Leaf essential oil Leaf essential oil Branch essential oil Stem bark essential oil Fruit essential oil Leaf essential oil Bark essential oil Leaf essential oil Fruit essential oil Fruit essential oil Fruit essential oil Fruit essential oil Fruit essential oil Bark essential oil Fruit essential oil Bark essential oil Fruit essential oil Leaf essential oil Fruit essential oil Leaf essential oil Bark essential oil Fruit essential oil Leaf essential oil Fruit essential oil Leaf essential oil Leaf essential oil Bark essential oil Bark essential oil Bark essential oil Leaf essential oil Bark essential oil Bark essential oil Branch essential oil Stem bark essential oil Branch essential oil Stem bark essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Wood bark Wood bark Wood Stem bark essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil Branch essential oil Leaf essential oil Branch essential oil Branch essential oil Branch essential oil Branch essential oil Branch essential oil Leaf essential oil Branch essential oil Leaf essential oil Branch essential oil Branch essential oil Leaf essential oil | [43] [46] [37,40,92] [37,40,41,92] [39] [49] [44] [37,41] [38] [49] [44] [43] [37,40,92] [39] [38] [38] [38] [39] [36] [38] [38] [39] [38] [39] [36] [38] [38] [49] [49] [44] [43] [41] [49] [39] [49] [49] [44] [43] [39] [41] [36] [49] [39] [39] [39] [39] [39] [36] [39] [36] [39] [40] [39] [40] [36] [39] [40] [39] [40] [40] [36] [36] [36] [41] [36] [36] [44] [43] [44] [43] [46] [46] [46] [46] [46] [46] [46] [46] [41] [41] [41] [41] [41] [47] [47] [53] [43] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [42] [45] [45] [45] [45] [45] [45] [45] [45] [45] [45] [45] [45] [45] [45] [45] [45] |
| Diterpenoid | |||
| Cneorubin A (127) Cneorubin B (128) Cneorubin X (129) Cneorubin Y (130) Isopimara-7,15-dien-2α,3β-diol (131) Isopimara-7,15-dien-3β-ol (132) 3-oxo-labd-8(17),12Z,14-triene (133) 3α-hydroxylabd-8(17),12Z,14-triene (134) 3β-hydroxylabd-8(17),12Z,14-triene (135) (-)-2-oxo-13-hydroxy,3,14-clerodandiene (136) 19-hydroxymanoyloxide (137) 13-hydroxy-3,14-clerodandiene (138) ent-kaur-16-en-2-one (139) ent-kaur-16-ene (140) ent-3β-hydroxykaur-16-ene (141) ent-3α-hydroxykaur-16-ene (142) Kolavelool (143) Kolavenol (144) Kolavenal (145) (-)-nephthenol (146) ent-13-epimanoyloxide (147) 7α-hydroperoxy-isopimara-8(14),15-diene-2α,3β-diol (148) 19-nor-isopimara-7,15,4(18)-trien-3-one (149) Isopimara-7,15-dien-3-one (150) Isopimara-7,15-dien-2β-ol (151) Isopimara-7,15-dien-2α-ol (152) Manoyl oxide (153) Labda-8,14-dien-13-ol (154) phytol (155) ent-8(14),15-sandaracopimaradiene-2α,18-diol (156) ent-8(14),15-sandaracopimaradine-2β,18-diol (157) Isopimara-7,15-diene (158) Labda-8,13-(E)-dien-15-ol (159) Boscartol C (160) 13-epi-dolabradiene (161) Phyllocladane (162) Sandaracopimarinal (163) Kaurene (164) | G. guidonia G. guidonia G. guidonia G. guidonia G. macrophylla G. macrophylla G. trichilioides G. trichilioides G. trichilioides G. trichilioides G. trichilioides G. trichilioides G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. guidonia G. rhophalocarpa G. rhophalocarpa G. macrophylla G. macrophylla G. guidonia G. macrophylla G. macrophylla G. macrophylla G. silvatica | Leaves The aerial parts Leaves The aerial parts Leaves The aerial parts Leaves Leaves Leaves Leaf essential oil Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaf essential oil Leaves Leaves Leaf essential oil Leaves Leaf essential oil Stem bark essential oil Leaves Leaves Leaves Leaves Leaves Leaf essential oil Leaves Leaf essential oil The aerial parts Leaf essential oil Leaf essential oil Leaf essential oil Leaf essential oil | [48] [58] [48] [58] [48] [58] [48] [56] [55,56] [37,40,92] [57] [57] [57] [57] [57] [57] [54] [54] [54] [54] [54] [54] [54] [54] [54] [55] [55] [52,55] [37,40,92] [52] [55] [40,92] [52,55] [37,40,92] [38] [55] [55] [60] [59] [59] [37,40,92] [52] [37,40,92] [58] [42] [42] [42] [45] |
| Triterpenoid | |||
| 3,4-secotirucalla-4(28),7,24-trien-3,21-dioic-acid (165) 3,4-secotirucalla-4(28),7,24-trien-3,21-dioic-acid-3-methyl ester (166) 3β-O-tigloylmelianol (167) 23-hydroxy-5α-lanosta7,9(11),24-triene-3-one (168) 5α-lanosta-7,9(11),24-triene-3α,23-diol (169) cycloart-23E-ene-3β,25-diol (170) (23S*,24S*)-dihydroxycicloart-25-en-3-one (171) Glabretal (172) Cycloart-24-en-3,23-dione (173) 23-hydroxycycloart-24-en-3-one(epimers) (174 & 175) 3β-hydroxycycloart-24-en-23-one (176) 25-hydroxycycloart-23-en-3-one (177) 3β-21-dihydroxycycloartane (178) 3β,21,22,23tetrahydroxycycloartane-24(31),25-diene (179) 21,24-epoxy-3α,7α,21,23tetraacetoxy-25-hydroxy-4α,4β,8β-trimethyl-14,18-cyclo-5α,13α,14α,17α-cholestane (180) 21,23-epoxy-3α,7α,21,24,25pentaacetoxy-4α,4β,8β-trimethyl-14,18-cyclo-5α,13α,14α,17α-cholestane (181) 24-acetoxy-25-hydroxy-3,7-dioxoapotirucalla-14-en-21,23-olide (182) 7α,24,25-trihydroxy-3-oxoapotirucalla-14-en-21,23-olide (183) Melianone (184) Melianodiol (185) Guareolide (186) Guareoic acid A (187) Guareoic acid B (188) Flindissone (189) Picroquassin E (190) 21-α-acetylmelianone (191) cycloarta-23,25-dien-3-one (192) (23S*)-cycloart-24-ene-3β,23-diol (193) (23R*)-cycloart-24-ene-3β,23-diol (194) Meliantriol (195) 22,25-dihydroxycycloart-23E-en-3-One (196) 24-methylenecycloartane-3β,22-diol (197) 3β-O-tigloylmeliantriol (198) Melianol (199) | G. cedrata G. cedrata G. kunthiana G. rhophalocarpa G. rhophalocarpa G. macrophylla G. humaitensis G. macrophylla G. glabra G. trichilioides G. macrophylla G. guidonia G. trichilioides G. macrophylla G. trichilioides G. macrophylla G. guidonia G. trichilioides G. macrophylla G. trichilioides G. trichilioides G. jamicensis G. jamicensis G. convergens G. convergens G. convergens G. grandiflora G. convergens G. grandiflora G. kunthiana G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. grandiflora G. macrophylla G. guidonia G. guidonia G. kunthiana G. macrophylla G. macrophylla G. kunthiana G. kunthiana | Bark Bark Fruits Leaves Leaves Leaves wood Leaves Heartwood Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves Leaves and twigs Leaves and twigs Leaves and branches Leaves and branches Leaves and branches Seeds Leaves and branches Seeds The aerial parts The aerial parts The aerial parts The aerial parts The aerial parts The aerial parts Seeds Leaves Leaves wood Leaves Wood The aerial parts Leaves Leaves Fruits Fruits | [33] [33] [91] [59] [59] [56,62] [53] [56] [63] [61] [62] [60] [61] [62] [61] [62] [60] [61] [62] [61] [61] [64] [64] [67] [67] [67] [65] [67] [65] [87] [58] [58] [58] [58] [58] [65] [52,62] [60] [53] [60] [53] [87] [62] [62] [66] [66] |
| Limonoid | |||
| 7-deacetoxy-7-oxogedunin (200) Gedunin (201) Chisomicine D (202) Chisomicine E (203) Chisomicine F (204) 3-(2′hydroxyisovaleroyl) khasenegasin I (205) Methyl-6-acetoxyangolensate (206) Dregeanin (207) Mombasol (208) 6α-acetoxygedunin (209) 14,15β-epoxyprieuriani (210) Humilinolide E (211) Methyl-2-hydroxy-3β-tigloyloxy-1-oxomeliac-8(30)-enate (212) Swietenine acetate (213) Methyl angolensate (214) 2’-hydroxyrohitukin (215) 7-acetyldihydronomilin (216) Ecuadorin (217) 7-oxo-gedunin (218) Prieurianin (219) Fissinolide (220) Dihydrogedunin (221) Mayombensin (222) Azadirachtin I (223) Angustinolide (224) | G. grandiflora G. grandiflora G. guidonia G. guidonia G. guidonia G. guidonia G. thompsonii G. thompsonii G. guidonia G. grandiflora G. guidonia G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. cedrata G. guidonia G. kunthiana G. guidonia G. guidonia G. guidonia G. thompsonii G. mayombensis G. mayombensis G. trichilioides | Seeds Seeds Stem bark Stem bark Stem bark Stem bark Bark Bark Bark Seeds Root Bark Fruits Fruits Fruits Fruits Bark The aerial parts Aerial parts Root bark Root bark Seeds Heartwood Twigs Twigs Seeds | [65] [65] [76] [76] [76] [76] [70] [70] [47] [65] [73] [71] [71] [71] [71] [33] [58] [72] [73] [73] [74] [34] [77] [77] [75] |
| Other Compounds | |||
| Quercetin 3-O-β-D-glucopyranoside (225) Quercetin 3-O-β-D-galactopyranoside (226) Kaempferol-7-O-β-D-glucopyranoside (227) Dehydrodiconiferyl-alcohol-4-β-d-glucoside (228) β-sitosterol (229) Stigmasterol (230) Stigmasterol glucoside (231) β-sitosterol glucoside (232) β-sitostenone (233) 2α,3β-dihydroxy-16,17-seco-pregn-17-ene-16-oic acid methyl ester 2β,19-hemiketal (234) 2,3:16,17-di-seco-pregn-17-ene-3-oic-acid-16-oic acid methyl ester-19-hydroxy-2-carboxylic acid-2,19-lactone (235) Ergosta-5,24(24′)-diene-3β,7α,21-triol (236) Ergosta-5,24(24′)-diene-3β,4β,22S-triol (237) Ceramide A (238) Ceramide B (239) Scopoletin (240) | G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. glabra G. cedrata G. convergens G. trichilioides G. guidonia G. convergens G. mayombensis G. mayombensis G. glabra G. guidonia G. guidonia G. convergens G. convergens G. mayombensis G. mayombensis G. rhopalocarpa | Flowering branches Flowering branches Flowering branches Flowering branches Heartwood Heartwood Leaves and branches Seeds and bark Leaves Leaves and branches Twigs Twigs Heartwood Trunk bark Trunk bark Leaves and branches Leaves and branches Twigs Twigs Leaves | [80] [80] [80] [80] [78] [93] [67] [75] [48,60] [67] [77] [77] [78] [79] [79] [67] [67] [77] [77] [59] |
| Biology Activity | Compound or Extract | Plant Species | Ref. |
|---|---|---|---|
| Cytotoxic: Compounds 210 and 219 are active against leukemia cell line P388 ED50 0.47–0.74 µg/mL and P388 ED50 4.4–7.8 µg/mL; methylene chloride extract evaluated against U-937 cell lines with each LD50 of 6.1 ± 0.5 µg/mL and 6.1 ± 1.2 µg/mL while the seed of G. guidonia had LD50 of 28.8 ± 8.2 µg/mL; 156, 157, 168, 169, 230, and 240 were tested against the KB cell line with IC50 of 48; 75.8; 30.2, 21.2; > 1272; and 130.2 µM, respectively; 170 was tested with EC50 HL-60 (18.3), HeLa (52.1), B16F10-Nex2 (58.9), A2058 (60.7), and MCF-7 (63.5) µM while 131 and 132 against five cell lines over 100 µM; 189 showed activity with EC50 25, 27, 50, and > 100 µM for the Jurkat, HeLa, MCF-7, and PBMC cell lines; 187 with EC50 39 µM against the Jurkat cell line; 202 (U-937 IC50 20 ± 3 µM and HeLa > 50 µM. Anti-inflammation: Anti-inflammation against male Wistar rats showed the effects of 8.0 mL/kg extract dose and the effects increased from time to time by 5.0 mL/kg extract. Antimalarial: Three extracts have IC50 50 µg/mL from petroleum ether extract of leaves, methanol extract of stem bark and fruits, and also chloroform extract of stem bark. Anti-parasitic Antiprotozoal: Methylene chloride extract of bark and leaves G. polymera has a selectivity index against Leishmania Viannia panamensis LD50/ED50 1.5 µg/mL and the seeds of G. guidonia have activity against Plasmodium falciparum with LD50/IC50 2.9 µg/mL (IC50 156 (16.8); 157 (49.7); 168 (7.2) µg/mL. Antiviral Antimicrobial: The essential oil has been evaluated for MIC and MBC against S. infantris, S. tyrphimurium and S. give with MIC and MBC 54.6 µg/mL. Insectisidal activity: The ethyl acetate extract against Aedes aegyptyi had LC50 and LC90 105.7 and 408.9 µg/mL; 185 with LC50 14.4 and LC90 17.54; and 195 over 100 µg/mL. Antioxidant: The essential oil, alcoholic, aqueous and ethyl acetate extracts showed IC50 15.3; 176.8 µg/mL Phosphorylation inhibitor | Cycloart-23E-ene-3β,25-diol (170) (23S*,24S*)-dihydroxycicloart-25-en-3-one (171) Isopimara-7,15-dien-2α,3β-diol (131) Isopimara-7,15-dien-3β-ol (132) Guareolide (186) Guareoic acid A (187) Guareoic acid B (188) Flindissone (189) Picroquassin E (190) 14,15β-epoxyprieuriani (210) 7-oxo-gedunin (218) Prieurianin (219) Chisomicine D (202) Chisomicine E (203) Chisomicine F (204) 3-(2′-hydroxyisovaleroyl)khasenegasin I (205) ent-8(14),15-sandaracopimaradiene-2α,18-diol (156) ent-8(14),15-sandaracopimaradine-2β,18-diol (157) 23-hydroxy-5α-lanosta 7,9(11),24-triene-3-one (168) 5α-lanosta-7,9(11),24-triene-3α,23-diol (169) Stigmasterol (230) Scopoletin (240) Methylene chloride extract Methylene chloride extract Ethanol extract Petroleum Extract Methanol Extract Water Extract Chloroform Extract Hexane extract ent-8(14),15-sandaracopimaradiene-2α,18-diol (156) ent-8(14),15-sandaracopimaradine-2β,18-diol (157) 23-hydroxy-5α-lanosta 7,9(11),24-triene-3-one (168) 5α-lanosta-7,9(11),24-triene-3α,23-diol (169) Stigmasterol (230) Scopoletin (240) Methylene chloride extract Methylene chloride extract Methanol extract 3β-O-tigloylmelianol (167) Aqueous Extract Essential oil Methanol Extract Melianone (184) Melianodiol (185) 21-α-acetylmelianone (191) 6α-acetoxygedunin (209) Aqueous extract Acetate extract Alcoholic extract Essential oil Ethyl acetate phase Melianodiol (185) Meliantriol (195) Essential oil Alcoholic extract Aqueous extract Ethyl acetate extract 7-deacetoxy-7-oxogedunin (200) Gedunin (201) | G. macrophylla G. macrophylla G. macrophylla G. macrophylla G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. guidonia G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. guidonia G. polymera L G. guidonia G. multiflora G. multiflora G. multiflora G. multiflora G. kunthiana G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. rhophalocarpa G. guidonia G. polymera L G. polymera L G. kunthiana G. guidonia G. kunthiana G. kunthiana G. grandiflora G. grandiflora G. grandiflora G. grandiflora G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. kunthiana G. grandiflora G. grandiflora | [56] [56] [56] [56] [58] [58] [58] [58] [58] [73] [73] [73] [76] [76] [76] [76] [59] [59] [59] [59] [59] [59] [90] [90] [82] [83] [83] [83] [83] [84] [59] [59] [59] [59] [59] [59] [90] [90] [90] [91] [85] [88] [88] [65] [65] [65] [65] [88] [88] [88] [88] [88] [87] [87] [87] [88] [88] [88] [89] [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safriansyah, W.; Sinaga, S.E.; Supratman, U.; Harneti, D. Phytochemistry and Biological Activities of Guarea Genus (Meliaceae). Molecules 2022, 27, 8758. https://doi.org/10.3390/molecules27248758
Safriansyah W, Sinaga SE, Supratman U, Harneti D. Phytochemistry and Biological Activities of Guarea Genus (Meliaceae). Molecules. 2022; 27(24):8758. https://doi.org/10.3390/molecules27248758
Chicago/Turabian StyleSafriansyah, Wahyu, Siska Elisahbet Sinaga, Unang Supratman, and Desi Harneti. 2022. "Phytochemistry and Biological Activities of Guarea Genus (Meliaceae)" Molecules 27, no. 24: 8758. https://doi.org/10.3390/molecules27248758
APA StyleSafriansyah, W., Sinaga, S. E., Supratman, U., & Harneti, D. (2022). Phytochemistry and Biological Activities of Guarea Genus (Meliaceae). Molecules, 27(24), 8758. https://doi.org/10.3390/molecules27248758







