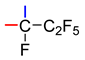Abstract
We describe the carbonylation of a series of mono and dihydroxy derivatives of polyfluorinated alkylbenzenes and benzocycloalkenes with OH groups at benzylic positions using carbon monoxide in the presence of a superacid (TfOH, a TfOH–SbF5 mixture, or a FSO3H–SbF5 mixture). It was shown that the superacid-catalyzed addition of CO to various primary and secondary polyfluorinated alcohols and diols gives the corresponding mono- and dicarboxylic acids or lactones. The efficiency of various superacids depending on alcohol structure was evaluated, and FSO3H–SbF5 yielded the best results in most transformations. The addition of CO to secondary 1-arylalkan-1-ols containing vicinal fluorine atoms was found to be accompanied by elimination of HF with the formation of α,β-unsaturated aryl-carboxylic acids. In contrast to primary and secondary alcohols, conversion of tertiary perfluoro-1,1-diarylalkan-1-ols into carbonylation products is not complete, and the resulting carboxylic acids are easily decarboxylated after water treatment of the reaction mixture.
1. Introduction
Organofluorine compounds are important for basic research in organic chemistry and for its applications, particularly to materials science, biomedicine, and agriculture [1,2,3,4,5,6,7,8,9,10]. Therefore, the development of new approaches to their synthesis is of obvious interest. Currently in hydrocarbon chemistry, there are many known acid-catalyzed carbonylation reactions of alcohols, alkyl halides, and other compounds proceeding via CO addition to carbocations generated from these compounds in acidic systems and leading to the formation of carboxylic-acid derivatives [11,12,13]. In spite of the variety of reactions of fluorinated cations, acid-catalyzed carbonylation of polyfluorinated compounds had not been known before our recent reports [14,15,16,17], and a reverse reaction—decarbonylation of fluorinated acyl halides under the action of Lewis acids—is commonly observed [18,19,20]. Known examples of acid-catalyzed carbonylation of organofluorine compounds (structures where a fluorine atom or polyfluoroalkyl group is separated from the reaction center by no more than 4–5 bonds and can influence it) are limited to reactions of alcohols Ar2RCOH (R = H, CH3) containing no more than two fluorine atoms [21] as well as mono-(polyfluoroalkyl)-substituted adamantyl halides [22,23] with a mixture of formic and sulfuric acids resulting in carboxylic acids.
Transition metal complex–catalyzed carbonylation at a C(sp3)–X bond (X = Hal, OR) or hydrocarboxylation and hydroesterification of alkenes, as an alternative to acid-catalyzed carbonylation, has more examples for some fluorinated compounds, but the range of substrates is quite small. For instance, there is evidence of Pd- or Co-catalyzed carbonylation of primary benzyl derivatives ArCH2X (X = Cl, Br, I, OH, OCOOR) [24,25,26,27,28,29,30,31,32,33] or esters of allylic alcohols [34,35] with only one fluorine atom or CF3 group in the aryl group or at the double bond. Pd-, Ru-, or Co-catalyzed carbonylation of polyfluorinated alkyl iodides [36,37,38,39,40,41,42] or Pd-catalyzed hydrocarboxylation and hydroesterification of perfluoroalkyl-substituted ethylenes using CO [43,44,45,46,47] can produce aliphatic carboxylic acids and their derivatives with a saturated perfluorinated moiety attached to a β-carbon atom or to carbon atoms more distant from the COOH group. For the synthesis of esters of α-CF2H-substituted arylacetic acids, Pd-catalyzed hydroesterification of 1,1-difluoro-2-arylethylenes has been proposed [48].
In our previous research, we have found that perfluorinated alkyl- and phenyl-benzocyclobutenes [14,15] and their carbonyl derivatives [17] undergo carbonylation/four-membered–ring opening tandem reactions under the action of carbon monoxide at atmospheric pressure in the presence of SbF5. In these reactions, irreversible four-membered–ring transformations promote the conversion of polyfluorobenzocyclobutenes into the desired products. In the CO–SbF5 system, the possibility of carbonylation of per- and polyfluorinated indanes and tetralins giving rise to fluorocarbonyl derivatives and corresponding acids after hydrolysis has been demonstrated too [16], and the transformation proceeds as an equilibrium process without skeletal rearrangements, while the degree of conversion substantially depends on the substrate structure. The discovered transformations are first examples of the functionalization of perfluorinated compounds via a carbonylation reaction.
In this research field, we have studied the possibility of H+-catalyzed carbonylation of polyfluorinated alcohols. The current work describes the carbonylation of a series of mono and dihydroxy derivatives of polyfluorinated alkylbenzenes and benzocycloalkenes with OH groups at benzylic positions by means of carbon monoxide in the presence of such a superacid as TfOH, a TfOH–SbF5 mixture, or an FSO3H–SbF5 mixture as a way to transform the above compounds into carboxy derivatives. Polyfluorinated α-aryl-substituted aliphatic carboxylic acids are of interest because this class of compounds with fluorine atoms in both aliphatic and aromatic moieties has been investigated as bioactive compounds or used for synthesis thereof [49,50,51,52,53,54], as is the case for halogenated derivatives of benzocycloalkene-1-carboxylic acids [55,56,57,58,59]. The presence of a polyfluorinated aromatic ring in the molecule gives an opportunity for further synthetic modification via SnAr substitution. Additionally, the perfluorinated aryl group has unique physicochemical properties that can be used in the design of materials. For instance, derivatives of pentafluorophenyl acetic acid have been utilized to create semiconductor and photoluminescent materials [60,61], gas sensors [62], and supramolecular associates for biological applications [63,64].
2. Results and Discussion
2.1. Carbonylation of Primary and Secondary Alcohols C6F5CH(OH)R (R = H, C6F5, CF3), and Selection of a Superacid
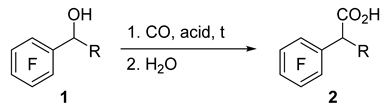
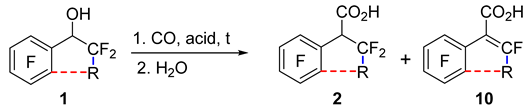
It was shown that 1-phenylalkan-1-ols 1a–c (Table 1) can be converted to corresponding acids 2 by carbonylation in superacids using carbon monoxide under atmospheric pressure, followed by water treatment of the reaction mixture. In TfOH, pentafluorobenzyl alcohol 1a adds CO, and heating to 50 °C is required for a fast and complete reaction, whereas diphenylmethanol 1b easily reacts at room temperature (r.t.). In contrast, alcohol 1c failed to be carbonylated in TfOH, and its acidity is apparently insufficient to generate the respective CF3-substituted intermediate carbocation. The use of a mixture of TfOH and SbF5 (1:1), which has higher acidity, made it possible to obtain the carbonylation product, acid 2c, at r.t. Two equiv. of this mixture allowed to achieve 95% conversion of the starting alcohol into acid 2c in 2 h. As the reaction time was extended, the conversion approached 100%. Reducing the amount of the acid mixture to 1.5 equiv. considerably reduced the content of acid 2c. An FSO3H–SbF5 mixture (1:1) compared to TfOH–SbF5 (1:1) in the amount of 2 equiv. worked more efficiently, yielding complete conversion of alcohol 1c to acid 2c in 2 h, probably due to its higher acidity [65].

Table 1.
Carbonylation of primary and secondary alcohols C6F5CH(OH)R 1a–c in superacids.
2.2. Carbonylation of Tertiary Perfluorinated 1,1-Diarylalkan-1-ols
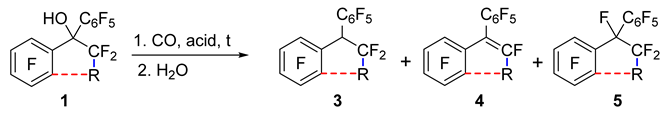
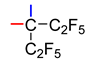
Alcohols 1d–f (Table 2) did not react with CO in TfOH at r.t. despite the presence of two aryl moieties that can stabilize the intermediate cation. With an increase in the reaction temperature, the substitution of fluorine atoms in the aryl moiety began to occur instead of carbonylation. It was noted that TfOH–SbF5 (1:1) enables us to carry out the carbonylation of alcohol 1d; however, after hydrolysis of the reaction mixture, the resulting acid 2d was easily decarboxylated, giving hydro derivative 3d and alkene 4d (see Scheme 1). Replacing the hydrolysis with treatment of the reaction mixture with methanol led to the formation of methyl ester 2dMe; however, decarboxylation products 3d and 4d also formed. Another difference from the carbonylation of alcohols 1a–c was the incomplete conversion of tertiary alcohol 1d into carbonylation products: the reaction mixtures also contained perfluorodiphenylethane 5d and the starting alcohol. The highest conversion of compound 1d to carbonylation products in the TfOH–SbF5 medium was achieved with the help of 2 equiv. of the superacid (total content of products 3d and 4d: ~30%). Extension of the reaction time from 5 to 24 h barely changed the reaction outcome, whereas an increase in the amount of the superacid to 4 equiv. or its decrease to 1.2 equiv. substantially diminished the concentration of products 3d and 4d in the reaction mixture. Using 2 equiv. of the mixture of FSO3H and SbF5 (1:1) instead of TfOH–SbF5 afforded a slightly higher conversion of alcohol 1d to carbonylation products (~45%); in this case, the introduction of a solvent (C6F6) was required to reduce the viscosity of the reaction mixture for efficient mixing.

Table 2.
Carbonylation of tertiary perfluorinated 1,1-diarylalkan-1-ols 1d–f in superacids.
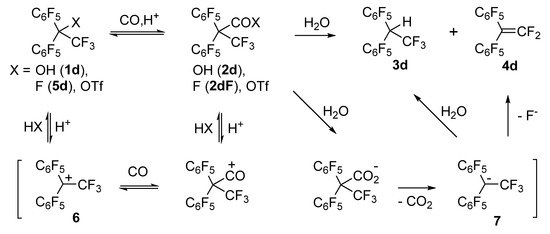
Scheme 1.
Transformations of compound 1d in the reaction with CO in TfOH–SbF5 followed by water treatment.
The results on carbonylation of compound 1d can be explained (Scheme 1) by the presence (in the TfOH–SbF5 medium) of an equilibrium between noncarbonylated forms (alcohol 1d or/and its triflate: fluoro derivative 5d) and carbonylation products (acid 2d, the corresponding acyl fluoride 2dF, or/and acyl triflate). The feasibility of carbonylation of perfluorodiphenylethane 5d in this medium was confirmed by a separate experiment. The equilibrium state depended on the amount of the superacid: its excess shifted the equilibrium toward decarbonylation, whereas its deficit hindered the generation of cation 6, which interacts with CO. After water treatment of the reaction mixture, all carbonylation products were hydrolyzed into acid 2d. In an aqueous medium, its deprotonation was possible, and the resulting carboxylate anion—owing to steric hindrance and the electron-withdrawing effect of substituents—easily eliminated CO2 with the formation of carbanion 7. The latter either added a proton or eliminated a fluoride ion, thereby yielding 3d and 4d, respectively. The formation of alkene 4d as a result of the transformation of hydro derivative 3d during treatment of the reaction mixture is unlikely; it was found that such elimination of HF, even in the presence of a base, is possible only under forcing conditions (NEt3, CaH2, 130 °C). The emergence of acid 2d and acyl fluoride 2dF, along with compounds 3d and 4d, could be detected after water treatment during the extraction of products with a mixture of CH2Cl2 and Et2O; the concentration of acid 2d in the extract gradually declined with time, while the concentration of products 3d and 4d went up. Extraction with pure Et2O gave only compounds 3d and 4d.
The reaction of phenylindanol 1e with CO in FSO3H–SbF5 (Table 2) followed by hydrolysis of the reaction mixture also generated products 3e and 4e; the conversion of 1e into carbonylation products was slightly higher than that for compound 1d (the total content of products 3e and 4e: ~60%) but also incomplete. In a similar reaction of phenyltetralinol 1f, the total level of products 3f and 4f was much lower (~20%), which can be explained by large steric hindrances for CO addition. The conversion to carbonylation products 3e and 4e for alcohol 1e was close to that obtained in the carbonylation of perfluorophenylindane 5e in SbF5 (55% conversion). On the other hand, for alcohol 1f, the conversion to carbonylation products was substantially higher than that in the reaction of perfluorophenyltetralin 5f with CO-SbF5 (5%) [16].
Aside from compounds 3f and 4f, the reaction of alcohol 1f generated lactone 8 as another carbonylation product (Scheme 2). The content of lactone 8 went up with increasing reaction time, temperature, and amount of the superacid. On the contrary, the concentration of products 3f and 4f strongly decreased (Table 2). Lactone 8 emerged via the cyclization of acid 2f; apparently, cation 9 generated from it in the FSO3H–SbF5 medium undergoes an intramolecular attack by the oxygen atom of the carboxyl group. In the carbonylation of phenylindanol 1e, lactone formation was not observed.
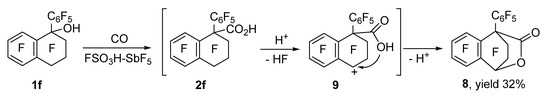
Scheme 2.
Lactone 8 formation during the carbonylation of compound 1f in FSO3H–SbF5.
2.3. Carbonylation of Secondary Polyfluorinated 1-Arylalkan-1-ols and Concomitant Elimination of HF
It was demonstrated that carbonylation of secondary polyfluorinated 1-arylalkan-1-ols with fluorine atoms at the β-position toward the hydroxyl group (in superacid FSO3H–SbF5 or TfOH–SbF5) can be accompanied by partial or complete elimination of HF, thus giving rise to α,β-unsaturated carboxylic acids 10 (Table 3). For instance, the reaction of phenylpropanol 1g with CO in TfOH–SbF5 at r.t., followed by hydrolysis of the reaction mixture, produced acid 2g with a small admixture of unsaturated acid 10g. A higher reaction temperature promoted more complete elimination of HF; acid 10g became the main product at 70 °C. In the FSO3H–SbF5 medium, the proportion of elimination product 10g was higher than that in TfOH–SbF5. An important factor is also the nature of the substituents at the nascent double bond; this nature affects the energy of its formation and accordingly the ease of elimination. Thus, in contrast to phenylpropanol 1g, carbonylation of phenylethanol 1c after heating under similar conditions did not give appreciable amounts of the corresponding unsaturated acid 10c with two fluorine atoms at the double bond.

Table 3.
Carbonylation of secondary polyfluorinated 1-arylalkan-1-ols 1g–n in superacids.
Some structural features can improve the efficiency of conjugation of the resulting double bond with the aryl moiety, for example, its fixation in the plane of the aryl moiety probably contributed to the elimination; as a result, carbonylation of tetralinol 1h gave considerable amounts of acid 10h already at r.t. A greater amount of the superacid caused more complete elimination (Table 3, entries 5 and 7), whereas extension of the time of the process had little effect (Table 3, entries 5 and 6).
A decrease in the size of the aliphatic ring from the six-membered to five-membered hindered the elimination; for example, carbonylation of indanol 1i in the FSO3H–SbF5 medium did not give HF elimination products at r.t., in contrast to its homolog 1h. On the other hand, under these conditions, a partial transformation of the CF2 group into a carbonyl group was observed; as a consequence, the reaction mixture also contained—alongside acid 2i—keto acid 2j and apparently other 3-R-2,2,4,5,6,7-hexafluorindan-1-ones (R = F, OSO2F), which are not carbonylation products. Previously, carbonylation of 1,1,2,2,3,4,5,6,7-nonafluoroindane was conducted in a CO–SbF5 system, and acid 2i was the only product obtained [16]. The transformation of the CF2 group of indanol 1i or of products of its transformation into a carbonyl group is mediated by elimination of the fluoride ion in the superacid and a reaction of the emerging carbocation with O-nucleophiles contained in the reaction medium (for example, RCOOH [66]), thereby leading to the carbonyl derivatives. Lower stability of polyfluorinated tetralin-1-yl cations compared to respective indan-1-yl cations [67] explains the absence of transformations of the benzyl CF2 group during the carbonylation of tetralinol 1h at r.t. When the temperature of the reaction of indanol 1i with CO in the FSO3H–SbF5 medium was raised to 70 °C, both the carbonylation and transformation of the CF2 group into the carbonyl group proceeded completely; futher, the elimination of HF also occurred, which produced a mixture of acids 2j and 10j. A mixture of acids 2j and 10j also formed in the reaction of hydroxyketone 1j with CO in the FSO3H–SbF5 medium at 70 °C.
In contrast to compound 1i, indanols 1k and 1l (in which one or both benzyl fluorine atoms are replaced by pentafluoroethyl groups) in the reaction with CO in FSO3H–SbF5 at r.t. smoothly converted into corresponding acids 2k and 2l. Elimination of HF with the formation of unsaturated acid 10l from alcohol 1l, as in the case of indanol 1i, happened only at a higher reaction temperature.
The presence of hydrocarbon groups in the aliphatic part of the molecule in some cases required lower acidity of the medium for successful carbonylation. For instance, for indanol 1m, carbonylation generating acid 2m in the FSO3H–SbF5 medium was accompanied by considerable resinification, which could be avoided in TfOH–SbF5 (5:1) at 50 °C; in pure TfOH, almost no carbonylation of indanol 1m occurred.
The formation of an aromatic system through the elimination of HF accompanying the carbonylation could ensure rapid and complete elimination even at r.t. For example, in the reaction of heterocyclic alcohol 1n with CO in the FSO3H–SbF5 medium (in the TfOH–SbF5 medium, the outcome was similar), after hydrolysis of the reaction mixture, benzofurancarboxylic acid 10n arose, and acid 2n was not detectable. The formation of a cationic species with a benzofuran moiety (acid 10n protonated at carbonyl oxygen or its acyl cation) was registered before the water treatment of the reaction mixture, thus confirming that the elimination of HF took place in the FSO3H–SbF5 medium.
It should be noted that in most of the carbonylation reactions mentioned above and involving the concomitant elimination of HF, there was no full conversion to elimination product 10; furthermore, in contrast to some factors such as reaction temperature or the amount of the superacid, the increase in the reaction time had little effect on the ratio of acids 2 and 10. It can be theorized that the elimination of HF is a reversible process, but when we introduced product 10j into the reaction mixture obtained after the carbonylation of alcohol 1n and kept the resulting mixture either at r.t. or at 70 °C, we failed to detect (after water treatment) acid 2j as an HF addition product.
The interaction of tetralinol 1h with CO in FSO3H–SbF5 at 70 °C (Scheme 3), in contrast to the analogous reaction of indanol 1i (Table 3, entry 9), did not generate the corresponding ketoacids. The main product was naphthalenecarboxylic acid 11; in addition, the reaction mixture contained acids 2h and 10h, perfluorotheralin (12), and tetralon 13. Acid 11 was isolated as dimethylated derivative 11Me after treatment of the reaction mixture with diazomethane. Carbonylation of hydroxyketone 1o under similar conditions resulted in a complex mixture of compounds, among which acid 11 was the main one as well (Scheme 3).
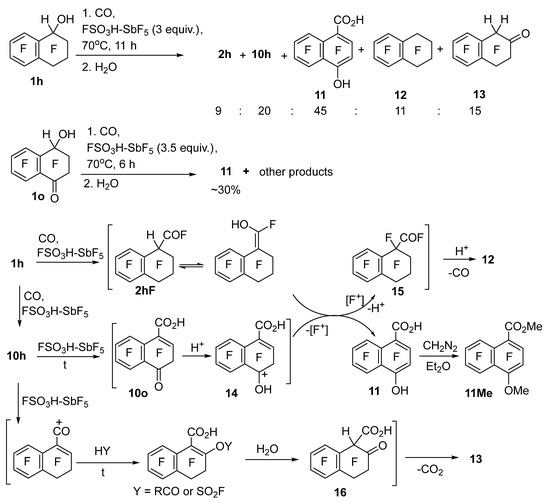
Scheme 3.
Carbonylation of compounds 1h and 1o in FSO3H–SbF5 at 70 °C.
To explain the simultaneous formation of naphthalenecarboxylic acid 11 and tetralin 12 in the reaction of alcohol 1h with CO in FSO3H–SbF5 at 70 °C, the following sequence of transformations can be proposed (Scheme 3). Acid 10h under the reaction conditions is partially converted to carbonyl derivative 10o through the generation of the corresponding cation and its reaction with the O-nucleophiles contained in the reaction medium. Protonation of compound 10o gives naphthalinonium cation 14, the reaction of which with the enol form of acyl fluoride 2hF (or mixed anhydride RCO2SO2F)—as a consequence of a redox process with the transfer of a fluorine atom—gives acid 11 and perfluorinated acyl fluoride 15. The latter is decarbonylated under these reaction conditions, thereby yielding tetralin 12. The possibility of decarbonylation of compound 15 is consistent with the finding that tetralin 12 did not give a carbonylation product in the CO–SbF5 system because the equilibrium of this reaction is shifted toward the starting tetralin [16]. The lower concentration of tetralin 12 in the reaction mixture compared to acid 11 can be attributed to its low solubility in the inorganic superacid in combination with its appreciable volatility; accordingly, it can be partially carried away by the CO flow. Tetralone 13 seems to come from another kind of transformation of acid 10h (Scheme 3, bottom pathway). It is possible that the reaction of the acyl cation deriving from acid 10h with O-nucleophiles present in the reaction medium (RCO2H and FSO3H) drives the replacement of the fluorine atom by oxygen at the conjugated double bond, whereas subsequent hydrolysis during water treatment and decarboxylation of resultant ketocarboxylic acid 16 produce ketone 13.
2.4. Carbonylation of Diols
Carbonylation of benzylic dihydroxy derivatives of polyfluorinated arylalkanes and benzocycloalkenes, depending on the structure, led to the addition of one or two CO molecules. The reaction of indanediol 17a with CO in FSO3H–SbF5 selectively gave diacid 18a as a mixture of cis- and trans-isomers (Scheme 4); no HF elimination products formed at r.t., as in the case of other indanols tested above. On the other hand, in the reaction of tetralindiol 17b with CO in the presence of 5 equiv. of FSO3H–SbF5, the addition of two CO molecules was accompanied by the elimination of two HF molecules already at r.t., resulting in a mixture of acids 18b and 19. Products of the elimination of one HF molecule were absent; apparently, the elimination of the second HF molecule proceeded faster than that of the first one because this led to the aromatic naphthalene system. An increase in the reaction temperature to 70 °C caused complete elimination of HF with the formation of diacid 19; the reaction mixture also contained admixtures of naphthalenecarboxylic acids 20 and 21 arising in side reactions; they were isolated and characterized after esterification as a mixture of ethyl esters 20Et and 21Et. Acid 20 apparently is a consequence of OH group replacement by fluorine in the monocarbonylation product (or in the starting diol) and the elimination of two HF molecules. The pathway to hydro derivative 21 is not clear; it was demonstrated that diacid 19 does not transform into it under the reaction conditions in question.
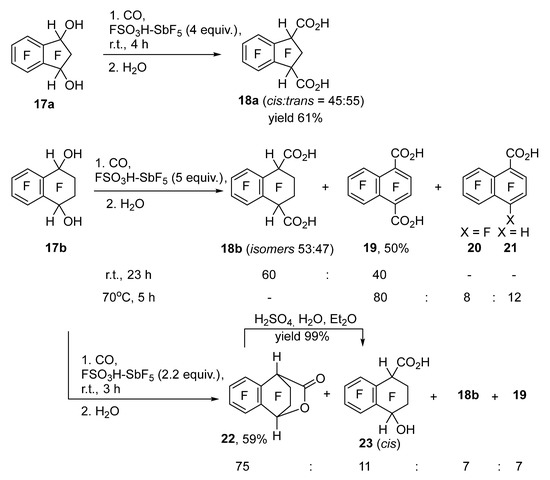
Scheme 4.
Carbonylation of diols 17a and 17b in FSO3H–SbF5.
After a reduction in the amount of FSO3H–SbF5 to 2.2 equiv. in the reaction of diol 17b at r.t., it was possible to achieve selective monocarbonylation, which was accompanied by the assembly of a lactone ring involving the second OH group leading to lactone 22. Hydroxy acid 23 was also present in the reaction mixture, along with dicarbonylation products 18b and 19. In contrast to diacid 18b, hydroxy acid 23 was present in the mixture as a single diastereomer, which apparently has cis-configuration and arises via partial hydrolysis of lactone 22 during water treatment of the reaction mixture. Hydrolysis of lactone 22 by dilute sulfuric acid caused selective formation of the same diastereomer of acid 23. For indandiol 17a, selective preparation of the monocarbonylation product was not achieved.
Monocarbonylation affording lactones also occurred when diols 17c–e were reacted with CO in the presence of a superacid (Table 4). Carbonylation of diol 17c in FSO3H–SbF5 (1:1), as in the case of indanol 1m, was accompanied by considerable resinification. The use of less acidic system TfOH–SbF5 (6:1) helped the carbonylation to generate lactone 24c. Of note, carbonylation of diols 17c–e required more forcing conditions as compared to respective alcohols 1a–c. For instance, diol 17c, carbonylation in pure TfOH proceeded with difficulty, even when heated. Pentafluorophenyl-substituted diol 17d did not react with CO either in TfOH at 50 °C or even in TfOH–SbF5 (7:2) at r.t., giving only the corresponding phthalane as a diol cyclization product. By contrast, in the FSO3H–SbF5 medium at r.t., diol 17d easily added CO, thereby yielding a mixture of isomeric lactones 24d and 25d in a ratio close to 1:1. Carbonylation of diol 17e in FSO3H–SbF5 took place upon heating to 50 °C, and the destabilizing effect of the CF3 group on the cationic center induced regioselective carbonylation in the CH2OH moiety, giving rise to lactone 24e. For ortho-substituted diols 17c–e, products of addition of two CO molecules were not observed, while corresponding diacids 18f and 18g formed from meta- and para-substituted diols 17f and 17g. Meta-isomer 17f readily reacted with CO in TfOH–SbF5 (7:1), whereas para-isomer 17g required the use of FSO3H–SbF5.

Table 4.
Carbonylation of polyfluorinated diols 17c–g in superacids.
2.5. Esterification and Elimination Reactions of the Carbonylation Products
In some cases, esterification was performed to isolate and purify carboxylic acids obtained in carbonylation reactions. Furthermore, the feasibility of conversion of several acids and esters into α,β-unsaturated derivatives by elimination of HF was tested. Thus, mixtures of esters 2gMe + 10gMe and 2hMe + 10hMe were obtained by the reaction of the respective acid mixtures with diazomethane (Scheme 5). Ester 2gMe could be smoothly converted into product 10gMe under the influence of NEt3 in chloroform; elimination in ester 2hMe proceeded fully, even during column chromatography on silica gel. Esterification of a mixture of diacids 18b and 19 with methanol in the presence of H2SO4 made it possible to selectively obtain ester 18bMe because acid 19 became esterified much more slowly. Nonetheless, the purification of ester 18bMe by silica gel chromatography afforded a mixture of esters 18bMe and 19Me, owing to partial elimination of HF; treatment of the obtained mixture with NEt3 in chloroform led to ester 19Me as the only product.
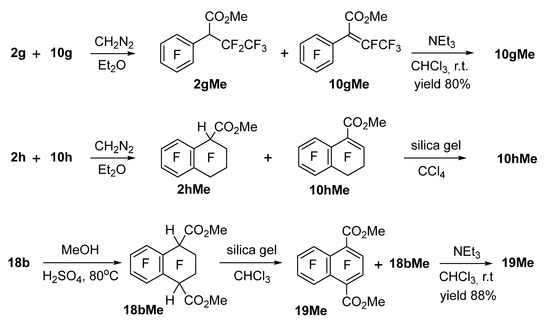
Scheme 5.
Esterification of acids 2g, 2h, 10g, 10h, and 18b in reaction mixtures generated by the carbonylation procedures and HF elimination from esters 2gMe, 2hMe, and 18bMe.
Esterification of acids 2c and 2i with methanol in the presence of H2SO4 gave corresponding esters 2cMe and 2iMe (Scheme 6). The reaction of ester 2iMe with NEt3 in chloroform generated compound 10iMe as the main product, but complete conversion of the starting compound with the increasing reaction time was not achieved. This finding is probably due to the reversibility of this process. When K2CO3 was used as a base, the eliminated HF produced insoluble salts, which helped to achieve full conversion of ester 2iMe.
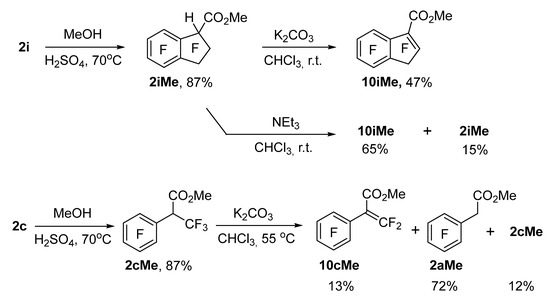
Scheme 6.
Synthesis of esters 2cMe and 2iMe and HF elimination under the action of NEt3 and K2CO3.
An attempt to carry out the conversion of ester 2cMe into product 10cMe in a reaction with NEt3 in chloroform was utterly unsuccessful: ester 2cMe mostly did not change, giving only a small amount of ester 2aMe without a CF3 group (probably owing to a reaction with traces of water). These data are also consistent with the presumed reversibility of the HF elimination process; the equilibrium in the case of ester 2cMe almost completely shifted toward the starting compound because the formation of compound 10cMe with two fluorine atoms at the double bond is thermodynamically less favorable. Nonetheless, it can act as an intermediate in nucleophilic reactions involving CF3 group transformation. The reaction of 2cMe with K2CO3 in chloroform afforded ester 10cMe, but at r.t., it was too slow. When the temperature was raised to 55 °C, ester 2aMe became the main product, which is apparently a consequence of further transformations of compound 10cMe in the reaction with O-nucleophiles.
For acid 2l, it was feasible to implement selective elimination of HF with the formation of acid 10l in the reaction with NEt3 without esterification of the carboxyl group beforehand (Scheme 7). This process is apparently facilitated by the steric effect of the two bulky perfluoroethyl groups, which, on one hand, promotes elimination (because this effect reduces their repulsion from other substituents in the five-membered cycle) and, on the other hand, prevents subsequent nucleophilic addition to the nascent double bond. Attempts to carry out similar selective elimination under the action of NEt3 with a number of other carboxylic acids obtained in this work were futile. The reactions are complicated by concomitant decarboxylation with or without HF elimination. For example, the interaction of acid 2c with NEt3 in Et2O at r.t. or in chloroform at 55 °C predominantly gave ethylbenzene 26 and styrene 27.
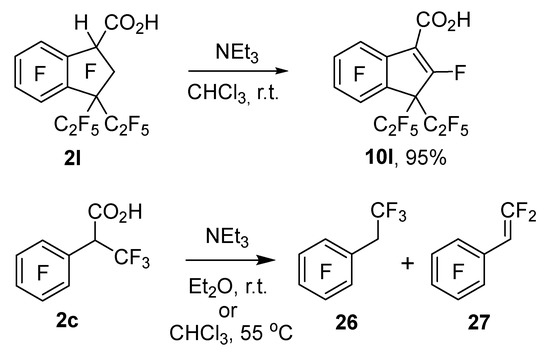
Scheme 7.
Transformation of acids 2c and 2l under the influence of NEt3.
2.6. Starting Compounds
Carbonyl derivatives 1j and 1o were synthesized by the heating of alcohols 1i and 1h with 20% oleum (Scheme 8). Diols 17c,f,g were derived from the corresponding isomers of dicarboxylic acids, which were first converted into diacyl chlorides via a reaction with PCl5 and then reduced with LiBH4. Diols 17d and 17e were obtained by reduction of phthalides 27 and 28 with LiBH4. The other starting compounds were prepared according to literature data: alcohols 1a,b [68], 1c,g,h,i,k,l,m,n and 17a,b [69], 1d [70], 1e [71], 1f [72], and phthalides 28 [73] and 29 [74].
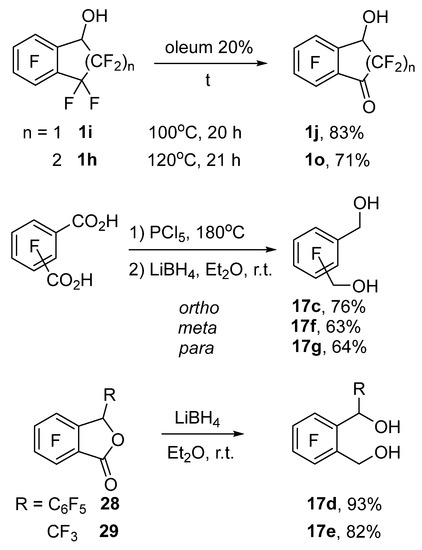
Scheme 8.
Synthesis of starting alcohols 1j,o and diols 17c–g.
2.7. Structural Analysis of Compounds
The structures of the compounds were established by means of high-resolution mass spectrometry (HRMS), elemental analysis, and spectral characteristics. Signals in NMR spectra of compounds were assigned on the basis of chemical shifts of the signals, their fine structure, and integral intensities. Compounds 2a [75], 2i, 3e, 4e [16], 4f [76], 5d [70], 5e [71], 5f [72], 12 [77], 17d [69], 20 [78], 26 [79], and 27 [80] were identified by comparison of 19F NMR findings with the literature data (NMR spectra in Supplementary Materials). Structures of E- and Z-isomers of compounds 10g and 10gOMe were determined by means of through-space JF,F values between closely located fluorine atoms. For instance, for the Z-isomers, JCF3,F(ortho) = 2 Hz, whereas for the E-isomers, it is not observed; on the other hand, J3,ortho < 2 Hz for the Z-isomers and 11–12 Hz for the E-isomers. The position of the substituent (C6F5 or CF3) in lactones 24d, 25d, and 24e was identified with the help of 1H NMR spectra. Chemical shifts of CH2 protons in the ArFCH2CO moiety of compounds 24d and 24e were found to not exceed 4 ppm, whereas in the ArFCH2O moiety of compound 25d, they are more than 5 ppm.
3. Materials and Methods
Analytical measurements and spectral analyses were carried out at the Multi-Access Chemical Research Center SB RAS. IR spectra were acquired on a Bruker Vector 22 IR spectrophotometer. 19F, 1H, and 13C NMR spectra were recorded on a Bruker AV 300 instrument (282.4 MHz, 300 MHz, and 75.5 MHz, respectively). Chemical shifts are given in δ ppm from CCl3F (19F) and TMS (1H, 13C). C6F6 (19F, −162.9 ppm), CHCl3 (1H, 7.24 ppm), acetone-d5 (1H, 2.04 ppm), DMSO-d5 (1H, 2.50 ppm), and CDCl3 (13C, 76.9 ppm) served as internal standards. Gas chromatography coupled with mass spectrometry (GC-MS) was performed on Hewlett Packard G1081A combined with Hewlett Packard 5890 with mass selective detector HP 5971 (EI 70 eV). Molecular masses of the compounds were determined by HRMS with a Thermo Electron Corporation DFS instrument (EI 70 eV). The progress of reactions and levels of products in reaction mixtures were monitored with the help of 19F NMR spectroscopic data.
FSO3H and TfOH were purchased from commercial sources (99% purity); antimony pentafluoride was distilled under atmospheric pressure (bp 142–143 °C); carbon monoxide was prepared by decomposition of formic acid in concentrated sulfuric acid and was additionally dried by passing it through a layer of concentrated sulfuric acid. Et2O and CHCl3 for HF elimination reactions were dehydrated over 3A molecular sieves.
3.1. A Typical Carbonylation Procedure
A mixture of an alcohol and superacid was intensively stirred in a round-bottom glass flask (10 mL) in a slow flow of CO under atmospheric pressure. The mixture was poured into 10–15 mL of 5% hydrochloric acid and extracted three times with 4 mL of a CH2Cl2–Et2O mixture (3:1) (if not specified otherwise). The extract was dried over MgSO4 and analyzed by 19F NMR spectroscopy. Reagent amounts, reaction conditions, and procedures for separation of mixtures and product isolation are detailed in the experiments below.
3.2. Carbonylation of (Pentafluorophenyl)methanol (1a)
Alcohol 1a (0.600 g, 3.03 mmol) and TfOH (2.502 g, 16.68 mmol) (molar ratio 1.0:5.5), according to the typical procedure (Section 3.1) (6 h, 50 °C, extraction with CH2Cl2), after evaporation of the solvent and sublimation (120 °C, 3 Torr), gave 0.613 g of acid 2a (yield 90%).
3.3. Carbonylation of Bis(pentafluorophenyl)methanol (1b)
Alcohol 1b (0.620 g, 1.70 mmol) and TfOH (1.790 g, 11.93 mmol) (molar ratio 1:7), according to the typical procedure (Section 3.1) (3 h, r.t., extraction with CH2Cl2), after evaporation of the solvent, afforded 0.650 g of acid 2b (yield 97%).
2,2-bis(pentafluorophenyl)acetic acid (2b). White solid, mp 109.5–111.8 °C. IR (KBr) ν, cm−1: 1732 (C=O), 1527, 1508 [fluorinated aromatic ring (FAR)]. 1H NMR (CDCl3): δ 11.87 (s, 1H, COOH), 5.60 (s, 1H, CH). 19F NMR (CDCl3): δ −140.8 (m, 4F, F-ortho), −153.0 (tt, 2F, Jpara,meta = 21.0 Hz, Jpara,ortho = 2.5 Hz, F-para), −161.7 (m, 4F, F-meta). HRMS, m/z: 391.9892 (M+). Calcd. for C14H2F10O2: M = 391.9890.
3.4. Carbonylation of 2,2,2-Trifluoro-1-(pentafluorophenyl)ethan-1-ol (1c)
a. Alcohol 1c (0.493 g, 1.84 mmol) and TfOH (1.350 g, 9.00 mmol) (molar ratio 1:5), according to the typical procedure (Section 3.1) (11 h, 50 °C, extraction with CH2Cl2), after evaporation of the solvent, gave 0.484 g of starting compound 1c.
b. Alcohol 1c (0.500 g, 1.88 mmol), TfOH (0.580 g, 3.87 mmol), and SbF5 (0.827 g, 3.83 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (2 h, r.t.), produced a mixture of compounds containing 95% of acid 2c.
c. Alcohol 1c (0.356 g, 1.34 mmol), TfOH (0.400 g, 2.67 mmol), and SbF5 (0.581 g, 2.67 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (20 h, r.t.), after evaporation of the solvent and sublimation (120 °C, 1 Torr), gave 0.358 g of acid 2c (yield 91%).
d. Alcohol 1c (0.560 g, 2.11 mmol), TfOH (0.482 g, 3.21 mmol), and SbF5 (0.684 g, 3.16 mmol) (molar ratio 1.0:1.5:1.5), according to the typical procedure (Section 3.1) (21 h, r.t.), gave a mixture of compounds containing 20% of acid 2c.
e. Alcohol 1c (0.500 g, 1.88 mmol), FSO3H (0.363 g, 3.63 mmol), and SbF5 (0.782 g, 3.61 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (2 h, r.t.), after evaporation of the solvent and sublimation (120 °C, 1 Torr), afforded 0.490 g of acid 2c (yield 89%).
f. Alcohol 1c (0.250 g, 0.94 mmol), FSO3H (0.295 g, 2.95 mmol), and SbF5 (0.655 g, 3.02 mmol) (molar ratio 1:3:3), according to the typical procedure (Section 3.1) (7 h, 70 °C), gave a mixture of compounds containing 93% of acid 2c; the content of 10c did not exceed 2%.
3,3,3-Trifluoro-2-(pentafluorophenyl)propanoic acid (2c). White solid, mp 133.0–136.0 °C (CCl4). IR (KBr) ν, cm−1: 3427 (OH); 1749 (CO); 1489, 1471 (FAR). 1H NMR (CDCl3): δ 7.5 (br s, 1H, COOH), 4.76 (q, 1H, JH,CF3 = 8 Hz, CH). 19F NMR (CDCl3): δ −68.0 (dt, 3F, JCF3,H = 8 Hz, JCF3,F(ortho) = 8 Hz, CF3), −140 (m, 2F, F-ortho), −151.1 (tt, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 3 Hz, F-para), −161.1 (m, 2F, F-meta). Anal. Calcd for C9H2F8O2: C, 36.76; H, 0.69; F, 51.68%. Found: C, 37.25; H, 0.42; F, 51.42%. HRMS, m/z: 293.9922 (M+). Calcd. M = 293.9922.
3.5. Carbonylation of Perfluoro-1,1-diphenylethan-1-ol (1d)
a. Alcohol 1d (0.618 g, 1.42 mmol), TfOH (0.422 g, 2.81 mmol), and SbF5 (0.610 g, 2.82 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (5 h, r.t. extraction with Et2O), gave a mixture of compounds containing 18% of 3d, 12% of 4d, and 65% of 5d.
b. A mixture of alcohol 1d (0.517 g, 1.20 mmol), TfOH (0.360 g, 2.40 mmol), and SbF5 (0.519 g, 2.40 mmol) (molar ratio 1:2:2) was vigorously stirred in a 10 mL round-bottom glass flask (5 h, r.t.) in a slow flow of CO, then poured into 5 mL of MeOH. The resulting solution was kept at r.t. for 1 h, poured into 5% hydrochloric acid (30 mL), extracted with CH2Cl2 (3 × 4 mL), and the extract was dried over MgSO4. The mixture of compounds in the extract contained 8% of ester 2dMe, 20% of 3d, 2% of 4d, and 64% of 5d.
c. Alcohol 1d (0.506 g, 1.17 mmol), TfOH (0.380 g, 2.53 mmol), and SbF5 (0.503 g, 2.32 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (24 h, r.t.), gave a mixture of compounds containing 21% of 2d, 9% of 2dF, 2% of 3d, 1% of 4d, and 60% of 5d (1 h after extraction) or 17% of 2d, 8% of 2dF, 5% of 3d, 3% of 4d, and 60% of 5d (72 h after extraction). After solvent evaporation, the residue was dissolved in Et2O (5 mL), then 5% hydrochloric acid (5 mL) was introduced, and the mixture was kept at r.t. for 24 h. The mixture of compounds (0.452 g) in the organic layer contained 22% of 3d, 11% of 4d, and 60% of 5d. Silica gel column chromatography (hexane as the eluent) gave 12 mg of compound 3d, 14 mg of alkene 4d, and 0.405 g of mixed fractions containing compounds 3d, 4d, and 5d.
d. Alcohol 1d (0.349 g, 0.81 mmol), TfOH (0.484 g, 3.23 mmol), and SbF5 (0.700 g, 3.23 mmol) (molar ratio 1:4:4), according to the typical procedure (Section 3.1) (24 h, r.t. extraction with Et2O), afforded a mixture of compounds containing 21% of 1d, 8% of 3d, 4% of 4d, and 63% of 5d.
e. Alcohol 1d (0.322 g, 0.75 mmol), TfOH (0.135 g, 0.90 mmol), and SbF5 (0.194 g, 0.90 mmol) (molar ratio 1:1.2:1.2), according to the typical procedure (Section 3.1) (24 h, r.t. extraction with Et2O), gave a mixture of compounds containing 5% of 1d, 6% of 3d, 3% of 4d, and 75% of 5d.
f. Alcohol 1d (0.400 g, 0.93 mmol), FSO3H (0.182 g, 1.82 mmol), SbF5 (0.404 g, 1.86 mmol) (molar ratio 1:2:2), and C6F6 (0.5 mL), according to the typical procedure (Section 3.1) (3 h, r.t., extraction with Et2O), gave a mixture of compounds containing 1% of 1d, 36% of 3d, 8% of 4d, and 51% of 5d. After evaporation of Et2O, the residue was dissolved in CH2Cl2 (5 mL) and washed with a saturated aqueous solution of NaHCO3 (5 mL) to remove acidic impurities and dried over MgSO4. Evaporation of CH2Cl2 yielded 0.358 g of a mixture of compounds 1d, 3d, 4d, and 5d, to which NEt3 (0.200 g), CaH2 (0.360 g), and toluene (4 mL) were added. The resultant mixture was heated with stirring in a sealed glass ampoule at 125–130 °C for 12 h; then, the organic layer was filtered and treated with 5% hydrochloric acid (10 mL). The mixture of compounds (0.350 g) in the organic layer contained 30% of 4d and 50% of 5d. Silica gel column chromatography (hexane as eluent) gave 25 mg of alkene 4d and 0.151 g of a mixed fraction containing compounds 4d and 5d at the 30:70 ratio.
1,1,1-Trifluoro-2,2-bis(pentafluorophenyl)ethane (3d). Colorless liquid. 1H NMR (CDCl3): δ 5.39 (q, 1H, JH,CF3 = 9.5 Hz, CH). 19F NMR (CDCl3): δ −67.2 (quintet d, 3F, JCF3,F(ortho) = 11.5 Hz, JCF3,H = 9.5 Hz, CF3), −139.9 (m, 4F, F-ortho), −151.8 (tt, 2F, Jpara,meta = 21 Hz, Jpara,ortho = 3 Hz, F-para), −161.2 (m, 4F, F-meta). HRMS, m/z: 415.9862 (M+). Calcd. for C14H1F13: M = 415.9865.
Perfluoro-1,1-diphenylethene (4d). White solid, mp 91.5–93.5 °C (hexane). IR (KBr) ν, cm−1: 1738 (C=CF2), 1525, 1504 (FAR). 19F NMR (CO(CD3)2): δ −75.4 (m, 2F, CF2), −139.2 (m, 4F, F-ortho), −152.3 (tt, 2F, Jpara,meta = 20.5 Hz, Jpara,ortho = 2.5 Hz, F-para), −161.7 (m, 4F, F-meta). HRMS m/z, 395.9808 (M+). Calcd. for C14F12: M = 395.9803.
Perfluoro-2,2-diphenylpropionic acid (2d) in the mixture with compounds 2dF, 3d, 4d, and 5d. 19F NMR (CH2Cl2-Et2O 3:1): δ −63.1 (quintet, 3F, JCF3,F(ortho) = 19.5 Hz, CF3), −134.1 (m, 4F, F-ortho), −152.1 (tt, 2F, Jpara,meta = 21.5 Hz, Jpara,ortho = 5.5 Hz, F-para), −161.8 (m, 4F, F-meta).
Perfluoro-2,2-diphenylpropionyl fluoride (2dF) in the mixture with compounds 2d, 3d, 4d, and 5d. 19F NMR (CH2Cl2-Et2O 3:1): δ 34.4 (octet, 1F, JCOF,F(ortho) = JCOF,CF3 = 14.5 Hz, COF), −64.2 (quintet d, 3F, JCF3,F(ortho) = 18.5 Hz, JCF3,COF = 14.5 Hz, CF3), −134.3 (m, 4F, F-ortho), −148.8 (tt, 2F, Jpara,meta = 21.5 Hz, Jpara,ortho = 5.5 Hz, F-para), −159.6 (m, 4F, F-meta).
Methyl perfluoro-2,2-diphenylpropionate (2dMe) in the mixture with compounds 3d, 4d, and 5d. 1H NMR (CDCl3): δ 3.80 (s, 3H, CH3). 19F NMR (CDCl3): δ −61.8 (quintet, 3F, JCF3,F(ortho) = 19.5 Hz, CF3), −134.8 (m, 4F, F-ortho), −151.3 (tt, 2F, Jpara,meta = 21.5 Hz, Jpara,ortho = 5 Hz, F-para), −161.3 (m, 4F, F-meta). GC-MS, m/z: 474 (M+).
3.6. Carbonylation of Perfluoro-1,1-diphenylethane (5d)
Alcohol 1d (0.510 g, 1.18 mmol), TfOH (0.343 g, 2.29 mmol), and SbF5 (0.510 g, 2.35 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (24 h, r.t.), gave a mixture of compounds containing 9% of 1d, 9% of 2dF, and 81% of 5d; levels of 3d and 4d were >1%.
3.7. Carbonylation of Perfluoro-1-phenylindan-1-ol (1e)
Alcohol 1e (0.350 g, 0.79 mmol), FSO3H (0.155 g, 1.55 mmol), SbF5 (0.345 g, 1.59 mmol) (molar ratio 1:2:2), and C6F6 (0.5 mL), according to the typical procedure (Section 3.1) (3 h, r.t., extraction with Et2O), after evaporation of the solvent, afforded a mixture of compounds (0.332 g) containing 35% of 1e, 47% of 3e, 10% of 4e, and 4% of 5e.
3.8. Carbonylation of Perfluoro-1-phenyltetralin-1-ol (1f)
a. Alcohol 1f (0.350 g, 0.71 mmol), FSO3H (0.139 g, 1.39 mmol), SbF5 (0.310 g, 1.43 mmol) (molar ratio 1:2:2), and C6F6 (0.5 mL), according to the typical procedure (Section 3.1) (3 h, r.t., extraction with Et2O), after evaporation of the solvent, gave a mixture of compounds (0.336 g) containing 13% of 1f, 8% of 3f, 14% of 4f, 41% of 5f, and 20% of lactone 8. 2,2,3,3,4,4,5,6,7,8-decafluoro-1-(pentafluorophenyl)tetralin (3f) was detected in the reaction mixture by GC-MS (M = 478) and 1H NMR (CDCl3): δ 5.25 (dd, JH,F(2) = 17 and 10 Hz, H-1).
b. Alcohol 1f (0.600 g, 1.21 mmol), FSO3H (0.477 g, 4.77 mmol), SbF5 (1.062 g, 4.90 mmol) (molar ratio 1:4:4), and C6F6 (1 mL), according to the typical procedure (Section 3.1) (10 h, 50 °C, extraction with CH2Cl2), after evaporation of the solvent, gave a mixture of compounds (0.580 g) containing 15% of 1f, 1% of 3f, 2% of 4f, 27% of 5f, and 47% of lactone 8. Silica gel column chromatography (CCl4 as the eluent) and recrystallization from hexane afforded 0.112 g of lactone 8 (yield 32%).
Perfluoro-4-phenyl-1,4-(epoxymethano)tetralin-9-one (8). White solid, mp 86–87.5 °C (hexane). IR (KBr) ν, cm−1: 1821 (C=O), 1537, 1520, 1508, 1493 (FAR). 19F NMR (CDCl3): δ −111.5 (dddd, 1F, JA3,B3 = 237 Hz, JA3,ortho-2 = 64.2 Hz, JA3,B2 = 12.3 Hz, JA3,F1 = 4.2 Hz, FA-3), −115.4 (dddd, 1F, JB3,A3 = 237 Hz, JB3,ortho-2 = 69.3 Hz, JB3,A2 = 9.3 Hz, JB3,1 = 5.5 Hz, FB-3), −122.8 (ddd, 1F, JA2,B2 = 230 Hz, JA2,1 = 16.3 Hz, JA2,B3 = 9.2 Hz, FA-2), −130.4 (ddq, 1F, Jortho,meta = 22.5 Hz, J = 10 Hz, J = 5 Hz, F-ortho-1), −133.8 (ddm, 1F, Jortho-2,B3 = 69.3 Hz, Jortho-2,A3 = 64.2 Hz, F-ortho-2), −134.9 (ddd, 1F, JB2,A2 = 230 Hz, JB2,A3 = 12.3 Hz, JB2,1 = 12.1 Hz, FB-2), −139.6 (ddddd, 1F, J5,6 = 20 Hz, J5,8 = 15.2 Hz, J5,7 = 8.6 Hz, J5,1 = 5 Hz, J5,ortho = 5 Hz, F-5), −140.5 (dddd, 1F, J8,1 = 43.4 Hz, J8,7 = 21.6 Hz, J8,5 = 15.2 Hz, J8,6 = 8.2 Hz, F-8), −144.6 (ddd, 1F, J6,7 = 20.2 Hz, J6,5 = 20 Hz, J6,8 = 8.2 Hz, F-6), −146.5 (ddd, 1F, J7,8 = 21.2 Hz, J7,6 = 20.2 Hz, J7,5 = 8.6 Hz, F-7), −149.3 (tt, 1F, Jpara,ortho = 21.5 Hz, Jpara,meta = 5.5 Hz, F-para), −159.2 (dddddd, 1F, J1,8 = 43.4 Hz, J1,A2 = 16.3 Hz, J1,B2 = 12.1 Hz, J1,B3 = 5.5 Hz, J1,5 = 5 Hz, J1,A3 = 4.2 Hz, F-1), −159.8 (ddd, 1F, Jmeta,ortho = 21.3 Hz, Jmeta,para = 21.3 Hz, J = 5.6 Hz, F-meta-1), −161.3 (ddd, 1F, Jmeta,ortho = 22.1 Hz, Jmeta,para = 22.1 Hz, J = 7.6 Hz, F-meta-2). 13C NMR (CDCl3): δ 155.8 (dd, 3JCF = 10 Hz, 3JCF = 5.5 Hz, C=O), 141.4–147.8 (components of seven signals, dm, 1JCF ≈ 250–260 Hz, CFAr), 138.6 (dm, 1JCF = 252.5, CFAr), 137.5 (dm, 1JCF = 253.5, CFAr), 111.4 (dd, 2JCF = 22, 2JCF = 12, CAr), 110.5 (m, CAr), 110.2 (ttd, 1JCF = 282, 2JCF = 22, 3JCF = 6, C-3), 109.4 (ddtd, 1JCF = 282, 1JCF = 272, 2JCF = 23.5, 2JCF = 20.5, C-2), 104.9 (dt, 1JCF = 256, 2JCF = 26, C-1), 102.4 (t, 2JCF = 14, CAr), 57.5 (t, 2JCF = 25, C-4). Anal. Calcd for C17F14O2: C, 40.66; H, 0.00; F, 52.97%. Found: C, 41.28; H, 0.22; F, 52.95%. HRMS m/z, 501.9665 (M+). Calcd: M = 501.9669.
3.9. Carbonylation of 2,2,3,3,3-Pentafluoro-1-(pentafluorophenyl)propan-1-ol (1g)
a. Alcohol 1g (0.442 g, 1.40 mmol), TfOH (0.460 g, 3.07 mmol), and SbF5 (0.606 g, 2.80 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (2 h, r.t.), gave a mixture of acids 2g and 10g in the 98:2 ratio. Evaporation of the solvent and sublimation (95 °C, 3 Torr) afforded 0.423 g of acid 2g (yield 88%).
b. Alcohol 1g (0.192 g, 0.61 mmol), TfOH (0.294 g, 1.96 mmol), and SbF5 (0.410 g, 1.89 mmol) (molar ratio 1:3:3), according to the typical procedure (Section 3.1) (5.5 h, 70 °C), gave a mixture of compounds containing 42% of 2g and 52% of 10g (E:Z = 18:82).
c. Alcohol 1g (0.205 g, 0.65 mmol), FSO3H (0.214 g, 2.14 mmol), and SbF5 (0.473 g, 2.18 mmol) (molar ratio 1:3:3), according to the typical procedure (Section 3.1) (5.5 h, 70 °C) gave a mixture of compounds containing 13% of 2g and 80% of 10g (E:Z = 15:85). Evaporation of the solvent and sublimation (95 °C, 3 Torr) resulted in a mixture (0.154 g, yield 73%) of acids 2g and 10g (E:Z = 20:80) at the 16:84 ratio. The mixture was treated with an Et2O solution of CH2N2. Evaporation of the solvent gave a mixture (0.152 g) of esters 2gMe and 10gMe (E:Z = 25:75) in the 16:84 ratio.
The mixture of esters 2gMe and 10gMe (0.100 g) was dissolved in dry CHCl3 (3 mL), then NEt3 (0.3 mL) was added. The mixture was kept at r.t. for 1 h and washed with 5% hydrochloric acid (2 × 7 mL). Silica gel column chromatography (CCl4–CHCl3 [1:1] as the eluent) gave 80 mg (yield 80%) of compound 10gMe (E:Z = 46:54).
3,3,4,4,4-Pentafluoro-2-(pentafluorophenyl)butanoic acid (2g). White solid, mp 74.5–75.2 °C (CCl4). IR (KBr) ν, cm−1: 1751 (C=O), 1531, 1510 (FAR). 1H NMR (CDCl3): δ 10.2 (br.s, 1H, COOH), 4.81 (dd, 1H, JH,F(B3) = 16.4 Hz, JH,F(A3) = 12.4 Hz, CH). 19F NMR (CDCl3): δ −83.4 (t, 3F, JCF3,F(ortho) = 2.3 Hz, CF3), −114.4 (ddt, 1F, JA3,B3 = 276 Hz, JF(A3),H = 12.4 Hz, JA3,ortho = 7.7 Hz, FA-3), −117.5 (dq, 1F, JA3,B3 = 276 Hz, JF(B3),H = JB3,ortho = 16.4 Hz, FB-3), −139.3 (br.s, 2F, F-ortho), −150.6 (tt, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 3.3 Hz, F-para), −161.0 (m, 2F, F-meta). Anal. Calcd for C10H2F10O2: C, 34.90; H, 0.59; F, 55.21%. Found: C, 34.75; H, 0.56; F, 55.04%. HRMS m/z, 343.9895 (M+). Calcd. M = 343.9890.
Methyl 3,3,4,4,4-pentafluoro-2-(pentafluorophenyl)butanoate (2gMe) in the mixture with 10gMe (2gMe:10gMe = 16:84). 1H NMR (CDCl3): δ 4.74 (dd, 1H, JH,F(B3) = 16 Hz, JH,F(A3) = 13 Hz, CH), 3.81 (s, 3H, CH3). 19F NMR (CDCl3): δ −83.4 (t, 3F, JCF3,F(ortho) = 2.3 Hz, CF3), −114.6 (ddt, 1F, JA3,B3 = 275 Hz, JF(A3),H = 13 Hz, JA3,ortho = 8.2 Hz, FA-3), −117.2 (ddt, 1F, JA3,B3 = 275 Hz, JF(B3),H = 16 Hz, JB3,ortho = 16 Hz, FB-3), −139.6 (br.s, 2F, F-ortho), −151.5 (tt, 1F, Jpara,meta = 21.0 Hz, Jpara,ortho = 3.1 Hz, F-para), −161.4 (m, 2F, F-meta). HRMS m/z, 358.0038 (M+). Calcd. for C11H4F10O2: M = 358.0046.
Perfluoro-2-phenylbut-2-enoic acid (10g) (E:Z =20:80) in the mixture with 2g (2g:10g = 16:84). (E)-10g: 1H NMR (CDCl3): δ 9.94 (s, COOH). 19F NMR (CDCl3): δ −67.6 (d, 3F, JCF3,F(3) = 6.3 Hz, CF3), −100.1 (tq, 1F, J3,ortho = 11 Hz, JF(3),CF3 = 6.3 Hz, F-3), −137.9 (m, 2F, F-ortho), −150.3 (tt, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 2.5 Hz, F-para), −161.2 (m, 2F, F-meta). (Z)-10g: 1H NMR (CDCl3): δ 9.94 (s, COOH). 19F NMR (CDCl3): δ −70.3 (dt, 3F, JCF3,F(3) = 7.3 Hz, JCF3,F(ortho) = 2 Hz, CF3), −97.5 (qt, 1F, JF(3),CF3 = 7.3 Hz, J3,ortho = 2 Hz, F-3), −139.2 (m, 2F, F-ortho), −150.4 (tt, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 2.5 Hz, F-para), −161.5 (m, 2F, F-meta).
Methyl perfluoro-2-phenylbut-2-enoate (10gMe) (E:Z = 46:54). Colorless liquid. IR (film) ν, cm−1: 2962 (CH), 1753 (C=O), 1523, 1506 (FAR). (E)-10g: 1H NMR (CDCl3): δ 3.85 (s, 3H, CH3). 19F NMR (CDCl3): δ −68.3 (d, 3F, JCF3,F(3) = 7.0 Hz, CF3), −106.4 (tqd, 1F, J3,ortho = 12.3 Hz, JF(3),CF3 = 7 Hz, JF3,para = 1.2 Hz, F-3), −138.0 (m, 2F, F-ortho), −150.9 (ttd, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 3.5 Hz, Jpara,F3 = 1.2 Hz, F-para), −161.5 (m, 2F, F-meta). (Z)-10g: 1H NMR (CDCl3): δ 3.85 (s, 3H, CH3). 19F NMR (CDCl3): δ −70.3 (dt, 3F, JCF3,F(3) = 7.7 Hz, JCF3,F(ortho) = 2.1 Hz, CF3), −102.6 (qt, 1F, JF(3),CF3 = 7.7 Hz, J3,ortho = 2 Hz, F-3), −139.4 (m, 2F, F-ortho), −151.2 (tt, 1F, Jpara,meta = 21 Hz Jpara,ortho = 2.5 Hz, F-para), −161.9 (m, 2F, F-meta). HRMS m/z, 337.9986 (M+). Calcd. for C11F9H3O2: M= 337.9984.
3.10. Carbonylation of 2,2,3,3,4,4,5,6,7,8-Decafluorotetralin-1-ol (1h)
a. Alcohol 1h (0.417 g, 1.28 mmol), FSO3H (0.260 g, 2.60 mmol), and SbF5 (0.580 g, 2.68 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (2 h, r.t.), gave a mixture of compounds containing 69% of 2h and 23% of 10h. Evaporation of the solvent, sublimation (110 °C, 1 Torr), and recrystallization from CCl4 resulted in 0.150 g of acid 2h (yield 35%).
b. Alcohol 1h (0.310 g, 0.95 mmol), FSO3H (0.201 g, 2.01 mmol), and SbF5 (0.439 g, 2.03 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (24 h, r.t.), gave a mixture of compounds containing 61% of 2h and 25% of 10h. Evaporation of the solvent and sublimation (110 °C, 1 Torr) afforded a mixture (0.268 g, yield 80%) containing >95% of acids 2h and 10h in the 75:25 ratio. The mixture was treated with an Et2O solution of CH2N2 to produce a mixture of esters 2hMe and 10hMe at the same ratio. Silica gel column chromatography (CCl4 as eluents) gave 0.197 mg (yield 59%) of compound 10hMe.
c. Alcohol 1h (0.560 g, 1.71 mmol), FSO3H (0.690 g, 6.90 mmol), and SbF5 (1.470 g, 6.79 mmol) (molar ratio 1:4:4), according to the typical procedure (Section 3.1) (20 h, r.t.), after evaporation of the solvent and sublimation (120 °C, 3 Torr), gave a mixture of compounds (0.500 g) containing 48% of 2h and 45% of 10h.
d. Alcohol 1h (0.400 g, 1.22 mmol), FSO3H (0.368 g, 3.68 mmol), and SbF5 (0.782 g, 3.61 mmol) (molar ratio 1:3:3), according to the typical procedure (Section 3.1) (11 h, 70 °C), gave a mixture of compounds 2h, 10h, 11, 12, and 13 in the 9:20:45:11:15 ratio. The extract was washed with a saturated aqueous solution of NaHCO3 (20 mL). The organic layer was dried over MgSO4 and evaporated to obtain 76 mg of a mixture of compounds containing (according to GC-MS) 41% of tetralin 12 and 51% of tetralon 13. The aqueous solution was acidified with HCl (pH < 1) and extracted with Et2O (3 × 4 mL), and the solvent was evaporated to obtain 0.248 g of a mixture of acids 2h, 10h, and 11 in the 11:29:60 ratio. The mixture was treated with an Et2O solution of CH2N2. Silica gel column chromatography (first CCl4 and then CCl4–CHCl3 [1:1] as an eluent) resulted in 83 mg of compound 11Me (yield 21%).
2,2,3,3,4,4,5,6,7,8-decafluorotetralin-1-carboxylic acid (2h). White solid, mp 99.2–101.5 °C (CCl4). IR (KBr) ν, cm−1: 2935 (CH), 1740 (C=O), 1533, 1500 (FAR). 1H NMR (CDCl3): δ 7.99 (br.s, 1H, COOH), 4.67 (t, 1H, JH,F(2) = 11.0 Hz, CH). 19F NMR (CDCl3): δ −106.3 (dm, 1F, JA4,B4 = 291 Hz, FA-4), −109.7 (dm, 1F, JA4,B4 = 291 Hz, FB-4), −114.0 (dm, 1F, JA2,B2 = 271 Hz, FA-2), −123.0 (dm, 1F, JA2,B2 = 271 Hz, FB-2), −135.1 (dm, 1F, JA3,B3 = 270 Hz, FA-3), −136.7 (dddm, 1F, J8,7 = 20.8 Hz, J8,5 = 11.6 Hz, J8,6 = 5.6 Hz, F-8), −137.2 (dtdd, 1F, J5,6 = 20.8 Hz, J5,4 = 18.7 Hz, J5,8 = 11.6 Hz, J5,7 = 8.2 Hz, F-5), −140.1 (dm, 1F, 1F, JA3,B3 = 270 Hz, FB-3), −147.3 (dddt, 1F, J7,6 = 20.8 Hz, J7,8 = 20.8 Hz, J7,5 = 8.2 Hz, J7,4 = 2 Hz, F-7), −150.5 (dddt, 1F, J6,5 = 20.8 Hz, J6,7 = 20.8 Hz, J6,8 = 5.6 Hz, J = 1 Hz, F-6). Anal. Calcd for C11H2F10O2: C, 37.10; H, 0.57; F, 53.35%. Found: C, 36.70; H, 0.62; F, 53.43%. HRMS m/z, 355.9893 (M+). Calcd. M = 355.9890.
Methyl 2,2,3,3,4,4,5,6,7,8-decafluorotetralin-1-carboxylate (2hMe) in the mixture with acid 2hMe (2hMe:10Me = 75:25). 19F NMR (Et2O-CDCl3 4:1): δ −106.2 (dm, 1F, JA4,B4 = 291 Hz, FA-4), −108.3 (dm, 1F, JA4,B4 = 291 Hz, FB-4), −113.8 (dm, 1F, JA2,B2 = 271 Hz, FA-2), −122.3 (dm, 1F, JA2,B2 = 271 Hz, FB-2), −135.4 (dm, 1F, JA3,B3 = 270 Hz, FA-3), −136.4 (m, 1F, F-8), −137.8 (m, 1F, F-5), −138.9 (dm, 1F, 1F, JA3,B3 = 270 Hz, FB-3), −148 (m, 1F, F-7), −151.3 (m, 1F, F-6).
Perfluoro-3,4-dihydronaphthalene-1-carboxylic acid (10h) in the mixture with acid 2h (2h:10h = 75:25). 19F NMR (CDCl3): δ −119.8 (dm, 2F, J4,5 = 32.6 Hz, F-4), −121.0 (m, 1F, F-2), −130.3 (m, 2F, F-3), −133.6 (m, 1F, F-8), −136.6 (tm, 1F, J5,4 = 32.6 Hz, F-5), −146.9 (m, 1F, F-7), −149.4 (m, 1F, F-6).
Methyl perfluoro-3,4-dihydronaphthalene-1-carboxylate (10hMe). Colorless liquid. IR (film) ν, cm−1: 2962 (CH), 1753 (C=O), 1520, 1497 (FAR). 1H NMR (CDCl3): δ 3.95 (s, 3H, CH3). 19F NMR (CDCl3): δ −120.6 (dm, 2F, J4,5 = 32.6 Hz, F-4), −123.9 (tdm, 1F, J2,3 = 18.5 Hz, J2,8 = 7 Hz, J2,6 = 6.3 Hz, F-2), −131.0 (dm, 2F, J3,2 = 18.5 Hz, F-3), −136.3 (ddddm, 1F, J8,7 = 20 Hz, J8,5 = 11.5 Hz, J8,2 = 7 Hz, J8,6 = 6.3 Hz, F-8), −137 (tdddd, 1F, J5,4 = 32.6 Hz, J5,6 = 21 Hz, J5,8 = 11.5 Hz, J5,7 = 8.9 Hz, J5,2 = 2 Hz, F-5), −147.8 (dddm, 1F, J7,6 = 20 Hz, J7,8 = 20 Hz, J7,5 = 8.7 Hz, F-7), −150.3 (dddd, 1F, J6,5 = 21 Hz, J6,7 = 20 Hz, J6,8 = 6.3 Hz, J6,2 = 6.3 Hz, F-6). HRMS m/z, 349.9979 (M+). Calcd. for C12H3F9O2: M = 349.9984.
2,3,5,6,7,8-hexafluoro-4-hydroxy-1-naphthoic (11) in the mixture with acids 2h and 10h (11:2h:10h = 60:11:29). 19F NMR (Et2O-CDCl3 4:1): δ −135.4 (dm, 1F, J2,3 = 21.6 Hz, F-2), −142.7 (m, 1F, F-5 or F-8), −142.9 (m, 1F, F-5 or F-8), −157.6 (m, 1F, F-6 or F-7), −160.5 (m, 1F, F-6 or F-7), −161.8 (dm, 1F, J3,2 = 21.6 Hz, F-3).
Methyl 2,3,5,6,7,8-hexafluoro-4-methoxy-1-naphthoate (11Me). White solid, mp 43.8–45.0 °C. IR (film) ν, cm−1: 2960 (CH), 1747 (C=O), 1529, 1506 (FAR). 1H NMR (CDCl3): δ 4.12 (d, 3H, JCH3,F(3) = 2 Hz, OCH3), 4.00 (s, 3H, COOCH3). 19F NMR (CDCl3): δ −133.6 (ddddd, 1F, J2,3 = 20.3 Hz, J2,6 = 7.9 Hz, J2,8 = 5 Hz, J2,5 = 4 Hz, J2,7 = 1.4 Hz, F-2), −143.7 (ddddd, J8,7 = 18.6 Hz, J8,5 = 14.8 Hz, J8,2 = 5 Hz, J8,3 = 4 Hz, J8,6 = 1.9 Hz, 1F, F-8), −146.1 (ddddd, 1F, J5,6 = 17.8 Hz, J5,8 = 14.5 Hz, J5,3 = 6 Hz, J5,2 = 4 Hz, J5,7 = 3.2 Hz, F-5), −152.9 (dddddq, 1F, J3,2 = 20.3 Hz, J3,7 = 8.4 Hz, J3,5 = 6 Hz, J3,8 = 4 Hz, J3,6 = 2.9 Hz, JF(3),CH3 = 2 Hz, F-3), −155.8 (ddddd, 1F, J7,6 = 20.3 Hz, J7,8 = 18.6 Hz, J7,3 = 8.4 Hz, J7,5 = 3.2 Hz, J7.2 = 1.4 Hz, F-7), −157.4 (ddddd, 1F, J6,7 = 20.3 Hz, J6,5 = 17.8 Hz, J6,2 = 7.9 Hz, J6,3 = 2.9 Hz, J6,8 = 1.9 Hz, F-6). HRMS m/z, 324.0214 (M+). Calcd. for C13H6F6O3: M = 324.0216.
3,3,4,4,5,6,7,8-Octafluorotetralin-2-one (13) in the mixture with tetralin 12. 1H NMR (CDCl3): δ 4.00 (s, 2H, H-1). 19F NMR (CDCl3): δ −109.3 (dt, 2F, J4,5 = 28 Hz, J4,3 = 12 Hz, F-4), −133.6 (t, 2F, J3,4 = 12 Hz, F-3), −138.3 (tddd, 1F, J5,4 = 28 Hz, J5,6 = 20.5 Hz, J5,8 = 12.7 Hz, J5,7 = 8.0 Hz, F-5), −138.8 (dddt, 1F, J8,7 = 21 Hz, J8,5 = 12.7 Hz, J8,6 = 4 Hz, J = 2 Hz, F-8), −148.0 (dddt, 1F, J7,8 = 21 Hz, J7,6 = 20 Hz, J7,5 = 8 Hz, J7,4 = 2.5 Hz, F-7), −153.3 (dddm, 1F, J6,5 = 20.5 Hz, J6,7 = 20 Hz, J6,8 = 4 Hz, F-6). GC-MS, m/z: 290 (M+).
3.11. Carbonylation of 2,2,3,3,4,5,6,7-Octafluoroindan-1-ol (1i)
a. Alcohol 1i (0.405 g, 1.47 mmol), FSO3H (0.287 g, 2.87 mmol), and SbF5 (0.580 g, 2.87 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (2.5 h, r.t.), gave a mixture of compounds containing (judging by 19F NMR and GC-MS) 50% of 2i, 5% of 10i, 20% of 2,2,3,4,5,6,7-heptafluoroindan-1-one (M = 258), and 50% of 2,2,4,5,6,7-hexafluoro-3-oxoindan-1-yl sulfurofluoridate (M = 338).
b. Alcohol 1i (0.413 g, 1.49 mmol), FSO3H (0.452 g, 4.52 mmol), and SbF5 (0.984 g, 4.55 mmol) (molar ratio 1:3:3), according to the typical procedure (Section 3.1) (6 h, 70 °C), gave a mixture of compounds containing 63% of 2j and 34% of 10j. The solvent was evaporated, and the residue was dissolved in Et2O (10 mL), after which the solution was saturated with gaseous ammonia until precipitation of yellow crystals stopped. Crystals of an ammonium salt of acid 10j were filtered off and washed with Et2O (5 mL). Next, 5% hydrochloric acid (5 mL) and Et2O (5 mL) were added to the crystals, the organic layer was separated and dried over MgSO4; evaporation and recrystallization from CCl4–acetone gave 0.100 g (yield 25%) of acid 10j. The ether solution obtained after the filtration of crystals of the ammonium salt of acid 10j was washed with 5% hydrochloric acid (10 mL) and dried over MgSO4. Evaporation of the solvent and sublimation (130 °C, 1 Torr) afforded 0.170 g of acid 2j (yield 40%).
2,2,4,5,6,7-Hexafluoro-3-oxoindan-1-carboxylic acid (2j). White solid, mp 136.2–137.0 °C (CCl4 + CH2Cl2). IR (KBr) ν, cm−1: 2966 (CH), 1794 (C=O), 1525, 1504 (FAR). 1H NMR (CDCl3): δ 7.85 (br.s, 1H, COOH), 4.64 (dd, 1H, JH,F(A2) = 15.7 Hz, JH,F(B2) = 5 Hz, CH). 19F NMR (CDCl3): δ −107.3 (dd, 1F, JA2,B2 = 284 Hz, JF(A2),H = 15.7 Hz, FA-2), −116.4 (dd, 1F, JA2,B2 = 284 Hz, JF(B2),H = 5 Hz, FB-2), −134.5 (ddd, 1F, J4,5 = 20.5 Hz, J4,7 = 17.9 Hz, J4,6 = 12 Hz, F-4), −138.1 (ddd, 1F, J6,7 = 20 Hz, J6,5 = 19 Hz, J6,4 = 12 Hz, F-6), −138.6 (ddd, 1F, J7,6 = 20.5 Hz, J7,4 = 17.9 Hz, J7,5 = 4.5 Hz, F-7), −149.8 (dddd, 1F, J5,4 = 20.5 Hz, J5,6 = 19 Hz, J5,7 = 4.5 Hz, JF(5)-H = 2.1 Hz, F-5). Anal. Calcd for C10H2F6O3: C, 42.28; H, 0.71; F, 40.12%. Found: C, 42.06; H, 0.83; F, 40.24%.). HRMS m/z, 283.9900 (M+). Calcd. M = 283.9903.
Perfluoro-1-oxo-1H-indene-3-carboxylic acid (10j). Yellow solid, mp 183.0–185.0 °C (with decomposition) (CCl4 + acetone). IR (KBr) ν, cm−1: 1751, 1736 (C=O), 1506, 1485 (FAR). 1H NMR (CO(CD3)2): δ 3.8 (br.s, 1H, COOH). 19F NMR (CO(CD3)2): δ −128.1 (dddd, 1F, J2,6 = 14.8 Hz, J2,4 = 8.5 Hz, J2,5 = 2.6 Hz, J2,7 = 2.6 Hz, F-2), −134.8 (dddd, 1F, J4,5 = 19.1 Hz, J4,7 = 13.2 Hz, J4,2 = 8.5 Hz, J4,6 = 4 Hz, F-4), −136.0 (dddd, 1F, J7,6 = 20.5 Hz, J7,4 = 13.2 Hz, J7,5 = 10.2 Hz, J7,2 = 2.6 Hz, F-7), −143.7 (dddd, 1F, J5,4 = 19.1 Hz, J5,6 = 16 Hz, J5,7 = 10.2 Hz, J5,2 = 2.6 Hz, F-5), −153.5 (dddd, 1F, J6,7 = 20.5 Hz, J6,5 = 16 Hz, J6,2 = 14.8 Hz, J6,4 = 2.6 Hz, F-6). Anal. Calcd for C10H1F5O3: C, 45.48; H, 0.38; F, 35.97%. Found: C, 44.89; H, 0.57; F, 35.77%. HRMS m/z, 263.9845 (M+). Calcd. M = 263.9840.
3.12. Carbonylation of 2,2,4,5,6,7-Hexafluoro-3-Hydroxyindan-1-one (1j)
Alcohol 1j (0.192 g, 1.80 mmol), FSO3H (0.618 g, 6.18 mmol), and SbF5 (1.345 g, 6.21 mmol) (molar ratio 1.0:3.5:3.5), according to the typical procedure (Section 3.1) (9.5 h, 70 °C), gave a mixture of compounds containing 35% of 2j and 55% of 10j.
3.13. Carbonylation of 2,2,3,4,5,6,7-Heptafluoro-3-(Pentafluoroethyl)indan-1-ol (1k)
Alcohol 1k (0.340 g, 0.90 mmol), FSO3H (0.18 g, 1.80 mmol), and SbF5 (0.390 g, 1.80 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (2.5 h, r.t.), after evaporation of the solvent and sublimation (120 °C, 1 Torr), gave 0.300 g of acid 2k (yield 82%).
2,2,3,4,5,6,7-heptafluoro-3-(pentafluoroethyl)indan-1-carboxylic acid (2k) (isomers A:B = 67:33). Colorless liquid. IR (film) ν, cm−1: 3033 (OH), 2943 (CH), 1741 (CO), 1516 (FAR). Isomer A. 1H NMR (CDCl3): δ 10.48 (br.s, 1H, COOH), 4.61 (tm, 1H, JH,F(2) ~ 12 Hz, CH). 19F NMR (CDCl3): δ −80.8 (m, 3F, CF3), −110.8 (dm, 1F, JA2,B2 = 242 Hz, FA-2), −113.5 (dm, 1F, JA2,B2 = 242 Hz, FB-2), −119.6 (dm, 1F, JA,B = 297 Hz, FA-CF2CF3), −122.1 (dm, 1F, JA,B = 297 Hz, FB-CF2CF3), −135.7 (m, 1F, F-4), −136.6 (ddd, 1F, J7,6 = 20.6 Hz, J7,4 = 16.1 Hz, J7,5 = 5.6 Hz, F-7), −145.6 (dddd, 1F, J6,7 = 20.6 Hz, J6,5 = 19.4 Hz, J6,4 = 8.7 Hz, J = 3.3 Hz, F-6), −150.1 (dddm, 1F, J5,4 = 20.6 Hz, J5,6 = 19.4 Hz, J5,7 = 5.6 Hz, F-5), −175.2 (br.s, 1F, F-3). Isomer B. 1H NMR (CDCl3): δ 10.48 (br.s, 1H, COOH), 4.71 (dd, 1H, JH-F(2) = 14.2 Hz, JH-F(2) = 6.9 Hz, CH). 19F NMR (CDCl3): δ −80.9 (m, 3F, CF3), −104.7 (dm, 1F, JA2,B2 = 247 Hz, FA-2), −115.4 (dm, 1F, JA2,B2 = 247 Hz, FB-2), −117.9 (dm, 1F, JA,B = 298 Hz, FA-CF2CF3), −120.6 (dm, 1F, JA,B = 298 Hz, FB-CF2CF3), −133.6 (dm, 1F, J = 47 Hz, F-4), −137.6 (ddd, 1F, J7,6 = 20.8 Hz, J7,4 = 15.9 Hz, J7,5 = 5.3 Hz, F-7), −145.6 (dddd, 1F, J6,7 = 20.8 Hz, J6,5 = 19.8 Hz, J6,4 = 8.4 Hz, J = 5 Hz, F-6), −149.9 (dddm, 1F, J5,4 = 20.0 Hz, J5,6 = 19.8 Hz, J5,7 = 5.3 Hz, F-5). Anal. Calcd for C12H2F12O2: C, 35.49; H, 0.50; F, 56.14%. Found: C, 35.30; H, 0.72; F, 56.01%. HRMS m/z, 405.9852 (M+). Calcd. M = 405.9858.
3.14. Carbonylation of 2,2,4,5,6,7-Hexafluoro-3,3-bis(pentafluoroethyl)indan-1-ol (1l)
a. Alcohol 1l (0.384 g, 0.80 mmol), FSO3H (0.162 g, 1.62 mmol), and SbF5 (0.351 g, 1.62 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (3 h, r.t.), after evaporation of the solvent and sublimation (120 °C, 1 Torr), gave 0.290 g of acid 2l (yield 71%).
b. Alcohol 1l (0.258 g, 0.54 mmol), FSO3H (0.177 g, 1.77 mmol), and SbF5 (0.386 g, 1.78 mmol) (molar ratio 1:3:3), according to the typical procedure (Section 3.1) (6.5 h, 70 °C), gave a mixture of compounds containing 71% of 2l and 27% of 10l.
2,2,4,5,6,7-Hexafluoro-3,3-bis(pentafluoroethyl)indan-1-carboxylic acid (2l). White solid, mp 113.6–114.8 °C. IR (film) ν, cm−1: 2926 (CH), 1743 (C=O), 1512 (FAR). 1H NMR (CDCl3): δ 7.5 (br.s, 1H, COOH), 4.72 (t, 1H, JH,F(2) = 13.8 Hz, CH). 19F NMR (CDCl3): δ −76.8 (dm, 3F, J = 35 Hz, CF3), −78.4 (dtm, 3F, J = 31.3 Hz, J = 14.4 Hz, CF3), −96.1 (dm, 1F, JA2,B2 = 258 Hz, FA-2), −99.3 (dm, 1F, JA2,B2 = 258 Hz, FB-2), −102.8 (dm, 1F, JA,B = 293 Hz, FA-CF2CF3), −105.9 (dm, 1F, JA,B = 293 Hz, FB-CF2CF3), −106.1 (dm, 1F, JA,B = 297 Hz, FA-CF2CF3), −109.8 (dm, 1F, JA,B = 297 Hz, FB-CF2CF3), −130.9 (m, 1F, F-4), −136.5 (dddm, 1F, J7,6 = 21.2 Hz, J7,4 = 14.9 Hz, J7,5 = 5 Hz, F-7), −147.1 (dddm, 1F, J6,7 = 21.2 Hz, J6,5 = 19.9 Hz, J6,4 = 8.8 Hz, F-6), −150.4 (dddd, 1F, J5,4 = 19.9 Hz, J5,6 = 19.9 Hz, J5,7 = 5 Hz, JF(5),H = 2.3 Hz F-5). Anal. Calcd for C14H2F16O2: C, 33.22; H, 0.4. Found: C, 33.24; H, 0.53. HRMS m/z, 505.9796 (M+). Calcd. M = 505.9794.
3.15. Carbonylation of 2,2,4,5,6,7-Hexafluoroindan-1-ol (1m)
a. Alcohol 1m (0.324 g, 1.34 mmol), FSO3H (0.284 g, 2.84 mmol), and SbF5 (0.618 g, 2.85 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (1.5 h, r.t.), gave a dark mixture of compounds containing ~70% of acid 2m along with resinification products.
b. Alcohol 1m (0.298 g, 1.23 mmol), TfOH (0.864 g, 5.76 mmol), and SbF5 (0.260 g, 1.20 mmol) (molar ratio 1:5:1), according to the typical procedure (Section 3.1) (1.5 h, 50 °C), after evaporation of the solvent and sublimation (130 °C, 1 Torr), afforded 0.317 g of acid 2m (yield 95%).
2,2,4,5,6,7-Hexafluoroindan-1-carboxylic acid (2m). White solid, mp 79.5–80.8 °C (CCl4). IR (KBr) ν, cm−1: 2939 (CH), 1734 (C=O), 1512, 1502 (FAR). 1H NMR (CDCl3): δ 7.00 (br.s, 1H, COOH), 4.51 (ddm, 1H, J = 16.4 Hz, J = 3.3 Hz, H-1), 3.78–3.41 (m, 2H, H-3). 19F NMR (CDCl3): δ −90.1 (dm, 1F, JA2,B2 = 237 Hz, FA-2), −101.6 (dm, 1F, JA2,B2 = 237 Hz, FB-2), −140.7 (dddm, 1F, J7,6 = 20.5 Hz, J7,4 = 17 Hz, JF(7),H(1) = 3.3 Hz, F-7), −141.8 (ddm, 1F, J4,5 = 20 Hz, J4,7 = 17 Hz, F-4), −154.1 (ddm, 1F, J6,7 = 20.5 Hz, J6,5 = 19.7 Hz, F-6), −155.8 (ddm, 1F, J5,4 = 20 Hz, J5,6 = 19.7 Hz, F-5). Anal. Calcd for C10H4F6O2: C, 44.46; H, 1.49; F, 42.20%. Found: C, 44.13; H, 1.59; F, 42.36%. HRMS m/z, 270.0112 (M+). Calcd. M = 270.0110.
3.16. Carbonylation of 2,2,4,5,6,7-Hexafluoro-2,3-dihydrobenzofuran-3-ol (1n)
a. Alcohol 1n (0.410 g, 1.68 mmol), FSO3H (0.336 g, 3.36 mmol), and SbF5 (0.729 g, 3.37 mmol) (molar ratio 1:2:2), according to the typical procedure (Section 3.1) (2 h, r.t.), gave a mixture of compounds containing ~85% of acid 10n. Evaporation of the solvent and sublimation (125 °C, 1 Torr) resulted in 0.373 g of a mixture of acid 10n and another product (apparently, 4,5,6,7-tetrafluoro benzofuran-2(3H)-one; GC-MS: M 206) in the 92:8 ratio. This solid was dissolved in a mixture of CH2Cl2 (10 mL) and a saturated aqueous solution of NaHCO3 (15 mL). The aqueous layer was separated, acidified with HCl (pH < 1), and extracted with Et2O (2 × 5 mL). Evaporation of the solvent gave 0.300 g of acid 10n (yield 71%).
b. A mixture of alcohol 1n (0.200 g, 0.82 mmol), FSO3H (0.164 g, 1.64 mmol), and SbF5 (0.355 g, 1.64 mmol) (molar ratio 1:2:2) was vigorously stirred (2 h, r.t.) in a round-bottom glass flask (10 mL) in a slow flow of CO at atmospheric pressure. The reaction mixture was diluted with FSO3H (0.295 g, 2.95 mmol) and SbF5 (0.640 g, 2.96 mmol) and then placed into an NMR tube and analyzed by 19F NMR spectroscopy. The signal of FSO3H served as an internal standard (41.1 ppm). The spectrum contained signals of a cationic species with a benzofuran moiety: δ −65.1 (d, 1F, J = 8 Hz, F-2), −143.0 (m, 1F, F-4), −154.8 (td, 1F, J = 18 Hz, J = 8 Hz), −156.5 (t, 1F, J = 19 Hz), −157.6 (t, 1F, J = 16 Hz). The mixture was poured into 5% hydrochloric acid (10 mL), extracted with Et2O (3 × 4 mL), and dried over MgSO4. The resulting solution contained 0.193 g of acid 10n.
c. A mixture of alcohol 1n (0.31 g, 1.27 mmol), FSO3H (0.253 g, 2.53 mmol), and SbF5 (0.551 g, 2.54 mmol) (molar ratio 1:2:2) was intensively stirred (2 h, r.t.) in a round-bottom glass flask (10 mL) in a slow flow of CO under atmospheric pressure. After that, acid 10j (0.046 g, 0.17 mmol) was added to the reaction mixture followed by incubation at r.t. for 20 h. The mixture was poured into 5% hydrochloric acid (10 mL), extracted with a CH2Cl2–Et2O mixture (3:1) (3 × 4 mL), and dried over MgSO4. The resultant solution contained acids 10n and 10j in the 87:13 ratio and did not contain acid 2j. A similar experiment with heating of the reaction mixture (4.5 h, 70 °C) after addition of acid 10j yielded the same result.
Perfluorobenzofuran-3-carboxylic acid (10n). White solid, mp 141.5–142.5 °C (CHCl3 + Et2O). IR (KBr) ν, cm−1: 1705 (C=O), 1489, 1471 (FAR). 1H NMR (CO(CD3)2): δ 11.2 (br.s, 1H, COOH). 19F NMR (CO(CD3)2): δ −92.8 (ddt, 1F, J2,6 = 12.7 Hz, J2,4 = 4 Hz, J2,5 = J2,7 = 1.7 Hz, F-2), −140.5 (ddd, 1F, J4,5 = 19.9 Hz, J4,7 = 15.1 Hz, J4,2 = 4 Hz, F-4), −160.9 (ddd, 1F, J6,7 = 20 Hz, J6,5 = 19.9 Hz, J6,2 = 12.6 Hz, F-6), −161.3 (ddt, 1F, J5,4 = 19.9 Hz, J5,6 = 19.9 Hz, J2,5 = J5,7 = 1.7 Hz, F-5), −161.9 (ddt, 1F, J7,6 = 20 Hz, J7,4 = 15.1 Hz, J2,7 = J5,7 = 1.7 Hz, F-7). HRMS m/z, 251.9837 (M+). Calcd. for C9H1F5O3: M = 251.9840.
3.17. Carbonylation of 2,2,3,3,5,6,7,8-Octafluoro-4-hydroxytetralin-1-one (1o)
Alcohol 1o (0.34 g, 1.11 mmol), FSO3H (0.394 g, 3.94 mmol), and SbF5 (0.856 g, 3.95 mmol) (molar ratio 1.0:3.5:3.5), according to the typical procedure (Section 3.1) (6 h, 70 °C), afforded a complex mixture of compounds containing ~30% of acid 11, ~10% of perfluoronaphthalene-1,4-dione, and ~10% of naphthalen-1(4H)-one.
3.18. Carbonylation of 2,2,4,5,6,7-Hexafluoroindan-1,3-diol (17a)
Diol 17a (0.271 g, 1.05 mmol), FSO3H (0.422 g, 4.22 mmol), and SbF5 (0.919 g, 4.24 mmol) (molar ratio 1:4:4), according to the typical procedure (Section 3.1) (4 h, r.t.), gave a mixture of compounds containing ~95% of diacid 18a (cis:trans = 45:50); judging by 19F NMR with an internal standard: the yield was 91%. The solvent was evaporated, and the residue was washed with CH2Cl2 (3 mL), dissolved in Et2O (5 mL), and filtered; the obtained solution contained isomers of diacid 18a (cis:trans = 50:50). Evaporation of the solvent and sublimation (170 °C, 1 Torr) afforded 0.200 g (yield 61%) of diacid 18a (cis:trans = 38:62).
2,2,4,5,6,7-Hexafluoroindan-1,3-dicarboxylic acid (18a), (cis:trans = 38:62). White solid, mp 215 °C (with decomposition). IR (KBr) ν, cm−1: 1738 (C=O), 1510 (FAR). Cis-18a. 1H NMR (CO(CD3)2): δ 7.3 (br.s, COOH), 4.94 (dd, 2H, JH,F(A2) = 15.2 Hz, J H,F(B2) = 13.1 Hz, CH). 19F NMR (CO(CD3)2): δ −88.4 (dt, 1F, JA2,B2 = 238 Hz, JF(A2),H = 15.2 Hz, FA-2), −100.7 (dt, 1F, JA2,B2 = 238 Hz, JF(B2),H = 13.1 Hz, FB-2), −139.2 (m, 2F, F-4,7), −155.3 (m, 2F, F-5,6). Trans-18a. 1H NMR (CO(CD3)2): δ 7.3 (br.s, COOH), 4.92 (t, 2H, 3JH,F(2) = 12.5 Hz, CH). 19F NMR (CO(CD3)2): δ −96.5 (t, 2F, 3JF(2),H = 12.5 Hz, F-2), −139.5 (m, 2F, F-4,7), −154.8 (m, 2F, F-5,6). Anal. Calcd for C11H4F6O4: C, 42.06; H, 1.28; F, 36.29%. Found: C, 42.70; H, 1.53; F, 36.13%. HRMS m/z, 314.0009 (M+). Calcd. M = 314.0008.
3.19. Carbonylation of 2,2,3,3,5,6,7,8-Octafluorotetralin-1,4-diol (17b)
a. Diol 17b (0.203 g, 0.66 mmol), FSO3H (0.315 g, 3.15 mmol), and SbF5 (0.686 g, 3.17 mmol) (molar ratio 1:5:5), according to the typical procedure (Section 3.1) (23 h, r.t.), gave a mixture of compounds 18b (isomer A), 18b (isomer B), and 19 in the 32:28:40 ratio. Evaporation of the solvent and sublimation (180 °C, 1 Torr) resulted in a mixture (0.170 g) of compounds 18b (isomer A), 18b (isomer B), and 19 at the 28:32:40 ratio. The mixture was heated (52 h, 75–80 °C) with MeOH (5 mL) and 96% H2SO4 (1 mL) in a sealed tube and then diluted with water (20 mL) and extracted with CH2Cl2 (3 × 4 mL). The extract was washed with a saturated aqueous solution of NaHCO3 (15 mL). The organic layer was dried over MgSO4 and evaporated to obtain 89 mg of a mixture of compounds containing diesters 18bMe and 19Me in the 95:5 ratio. Silica gel column chromatography (CHCl3 as eluents) gave 61 mg of diesters 18bMe (isomer A), 18bMe (isomer B), and 19Me in the 20:25:55 ratio.
The resultant mixture of diesters 18bMe and 19Me (50 mg) was dissolved in dry CHCl3 (0.5 mL), after which NEt3 (40 mg) was introduced. The mixture was kept at r.t. for 20 h and washed with 5% hydrochloric acid (2 × 2 mL). Evaporation of the solvent and sublimation (110 °C, 1 Torr) afforded 42 mg of diester 19Me (yield 88%).
b. Diol 17b (0.739 g, 2.40 mmol), FSO3H (1.149 g, 11.49 mmol), and SbF5 (2.500 g, 11.54 mmol) (molar ratio 1:5:5), according to the typical procedure (Section 3.1) (5 h, 70 °C), gave a mixture of compounds 19, 20, and 21 at the 80:8:12 ratio. Evaporation of the solvent and sublimation (150 °C, 1 Torr) resulted in 0.275 g of compounds 19, 20, and 21 in the 40:25:35 ratio; subsequent sublimation (220 °C, 1 Torr) gave 0.390 g (yield 50%) of diacid 19. The first sublimation fraction was heated (14 h, 70 °C) with SOCl2 (0.5 mL) and DMF (one drop). SOCl2 was evaporated, and the obtained mixture was heated (23 h, 70 °C) with EtOH (3 mL). Silica gel column chromatography (CCl4–CHCl3 [1:1] as the eluent) gave 0.153 g of a mixture of esters 20Et and 21Et in the 40:60 ratio.
c. Diol 17b (0.413 g, 1.34 mmol), FSO3H (0.294 g, 2.94 mmol), SbF5 (0.640 g, 2.95 mmol) (molar ratio 1.0:2.2:2.2), and C6F6 (0.5 mL), according to the typical procedure (Section 3.1) (3 h, r.t.), gave a mixture of compounds 18b, 19, 22, and 23 in the 7:7:75:11 ratio. The extract was washed with a saturated aqueous solution of NaHCO3 (15 mL), and the organic layer was dried over MgSO4. Evaporation of the solvent and sublimation (95 °C, 1 Torr) afforded 0.253 g (yield 59%) of lactone 22 (cis:trans = 38:62).
A mixture of lactone 22 (0.140 g), 5% sulfuric acid (5 mL), and Et2O (5 mL) was kept at r.t. for 12 weeks. The organic layer was separated and dried over MgSO4; evaporation of the solvent gave 0.147 g (yield 99%) of acid 23 as one isomer.
2,2,3,3,5,6,7,8-Octafluorotetralin-1,4-dicarboxylic acid (18b) in the mixture with acid 19 [18b(isomer A):18b(isomer B):19 = 28:32:40]. Isomer A: 1H NMR (CO(CD3)2): δ 10.12 (br.s, COOH), 4.96 (tm, 2H, JH,F = 13 Hz, CH). 19F NMR (CO(CD3)2): δ −114.0 (dm, 2F, JA,B ~ 260 Hz, FA-CF2), −120.6 (dm, 2F, JA,B ~ 260 Hz, FB-CF2), −138.3 (m, 2F, F-5,8), −156.1 (m, 2F, F-6,7). Isomer B: 1H NMR (CO(CD3)2): δ 10.12 (br.s, COOH), 4.96 (tm, 2H, JH,F = 13 Hz, CH). 19F NMR (CO(CD3)2): δ −115.1 (dm, 2F, JA,B = 259 Hz, FA-CF2), −122.7 (dm, 2F, JA,B = 259 Hz, FB-CF2), −137.2 (m, 2F, F-5,8), −155.8 (m, 2F, F-6,7).
Dimethyl 1,2,2,3,3,5,6,7,8-nonafluorotetralin-1,4-dicarboxylate (18bMe) in the mixture with diester 19Me [18bMe(isomer A):18bMe(isomer B):19Me = 20:25:55]. Isomer A: 1H NMR (CDCl3): δ 4.53 (m, 2H, CH), 3.82 (s, 6H, CH3). 19F NMR (CDCl3): δ −116.0 (dm, 2F, JA,B ~ 262 Hz, FA-CF2), −122.7 (dm, 2F, JA,B ~ 262 Hz, FB-CF2), −139.6 (m, 2F, F-5,8), −154.5 (m, 2F, F-6,7). Isomer B: 1H NMR (CDCl3): δ 4.53 (m, 2H, CH), 3.83 (s, 6H, CH3). 19F NMR (CDCl3): δ −117.6 (dm, 2F, JA,B = 261 Hz, FA-CF2), −125.0 (dm, 2F, JA,B = 261 Hz, FB-CF2), −138.4 (m, 2F, F-5,8), −154.3 (m, 2F, F-6,7). HRMS m/z, 392.0286 (M+). Calcd. for C14H8F8O4: M = 392.0289.
Perfluoronaphthalene-1,4-dicarboxylic acid (19). White solid, mp 265–268 °C (with decomposition). IR (KBr) ν, cm−1: 1720 (C=O), 1535, 1508, 1479, 1454 (FAR). 1H NMR (CO(CD3)2): δ 6.3 (br.s, 2H, COOH). 19F NMR (CO(CD3)2): δ −135.2 (m, 2F, F-2,3), −141.4 (m, 2F, F-5,8), −155.0 (m, 2F, F-6,7). HRMS m/z, 323.9854 (M+). Calcd. for C12H2F6O4: M = 323.9852.
Dimethyl perfluoronaphthalene-1,4-dicarboxylate (19Me). White solid, mp 112.5–114 °C (hexane). IR (KBr) ν, cm−1: 2970 (CH), 1745 (C=O), 1531, 1512 (FAR). 1H NMR (CDCl3): δ 4.03 (s, 6H, CH3). 19F NMR (CDCl3): δ −134.4 (s, 2F, F-2,3), −142.9 (m, 2F, F-5,8), −154.5 (m, 2F, F-6,7). Anal. Calcd for C14H6F6O4: C, 47.75; H, 1.72; F, 32.37%. Found: C, 48.11; H, 2.13; F, 31.87%. HRMS m/z, 352.0168 (M+). Calcd. M = 352.0165.
Ethyl 2,3,4,5,6,7,8-heptafluoro-1-naphthoate (20Et) in the mixture with ester 21Et (20Et:21Et = 40:60). 1H NMR (CDCl3): δ 4.51 (q, 2H, JCH2,CH3 = 7, CH2), 1.40 (t, 3H, JCH2,CH3 = 7, CH3). 19F NMR (CDCl3): δ −132.4 (m, 1F, F-2), −137.3 (dm, 1F, J4,5 = 60.3 Hz, F-4), −142.3 (m, 1F, F-8), −145.7 (dm, 1F, J5,4 = 60.3 Hz, F-5), −153.9 (m, 1F, F-7), −155.4 (m, 1F, F-6), −156.9 (m, 1F, F-3). HRMS, m/z: 326.0174 (M+). Calcd. for C13H5F7O2: M = 326.0172.
Ethyl 2,3,5,6,7,8-hexafluoro-1-naphthoate (21Et) in the mixture with ester 21Et (20Et:21Et = 40:60). 1H NMR (CDCl3): δ 7.84 (ddd, 1H, JH,F = 9.5 Hz, JH,F = 7.7 Hz, JH,F = 1 Hz, H-4), 4.51 (q, 2H, JCH2,CH3 = 7 Hz, CH2), 1.42 (t, 3H, JCH2,CH3 = 7, CH3). 19F NMR (CDCl3): δ −132.4 (m, 1F, F-3), −135.6 (m, 1F, F-2), −143.9 (m, 1F, F-8), −148.0 (m, 1F, F-5), −156.3 (m, 1F, F-7), −156.9 (m, 1F, F-7). HRMS, m/z: 308.0269 (M+). Calcd. for C13H6F6O2: M = 308.0267.
2,2,3,3,5,6,7,8-Octafluoro-1,4-(epoxymethano)tetralin-9-one (22). White solid, mp 100.6–101.6 °C. IR (KBr) ν, cm−1: 2926 (CH), 1809 (C=O), 1512 (FAR). 1H NMR (CO(CD3)2): δ 6.74 (m, 1H, H-1), 5.42 (m, 1H, H-4). 19F NMR (CO(CD3)2): δ −111.2 (dm, 1F, JA,B = 241 Hz, FA-CF2), −112.2 (dm, 1F, JA,B = 241 Hz, FB-CF2), −115.8 (dm, 1F, JA,B = 247 Hz, FA-CF2), −122.8 (dm, 1F, JA,B = 247 Hz, FB-CF2), −141.1 (ddd, 1F, J8,7 = 20.5 Hz, J8,5 = 16 Hz, J8.6 = 6 Hz, F-8), −141.6 (ddd, 1F, J5,6 = 20 Hz, J8,5 = 16 Hz, J5,7 = 5 Hz, F-5), −148.8 (ddd, 1F, J6,5 = 20 Hz, J6,7 = 18.5 Hz, J6,8 = 6 Hz, F-6), −151.1 (ddd, 1F, J7,8 = 20.5 Hz, J7,6 = 18.5 Hz, J7,5 = 5 Hz, F-7). Anal. Calcd for C11F8H2O2: C, 41.53; H, 0.63; F, 47.78%. Found: C, 41.95; H, 0.69; F, 48.16%. HRMS m/z, 317.9921 (M+). Calcd. M = 317.9922.
2,2,3,3,5,6,7,8-Octafluoro-4-hydroxytetralin-1-carboxylic acid (23). White solid, mp 150.1–151.8 °C. IR (KBr) ν, cm−1: 2982 (CH), 1755 (C=O), 1524, 1489 (FAR). 1H NMR (CO(CD3)2): δ 5.95 (br.s, 2H, COOH and OH), 5.43 (td, 1H, JH,F = 8.5 Hz, JH,F = 4.5 Hz, H-4), 4.86 (ddm, 1H, JH,F = 16 Hz, JH,F = 15 Hz, H-1). 19F NMR (CO(CD3)2): δ −114.8 (dm, 1F, JA,B = 263 Hz, FA-CF2), −116.0 (dm, 1F, JA,B = 263 Hz, FB-CF2), −122.2 (dm, 1F, JA,B = 264 Hz, FA-CF2), −133.6 (dm, 1F, JA,B = 264 Hz, FB-CF2), −136.7 (dddm, 1F, J8,7 = 19.8 Hz, J8,5 = 12 Hz, J8,6 = 3.7, J = 12 Hz, F-8), −141.6 (dddm, 1F, J5,6 = 20 Hz, J5,8 = 12 Hz, J6,7 = 3.7, F-5), −155.3 (ddd, 1F, J7,6 = 20 Hz, J8,7 = 19.8 Hz, J7,5 = 3.7 Hz, F-7), −155.5 (dddm, 1F, J6,5 = 20 Hz, J6,7 = 20 Hz, J6,8 = 3.9 Hz, F-6). HRMS, m/z: 336.0023 (M+). Calcd. for C11H4F8O3: M = 336.0027.
3.20. Carbonylation of (2,3,4,5-Tetrafluoro-6-(hydroxymethyl)phenyl)methanol (17c)
a. Diol 17c (0.200 g, 0.95 mmol) and TfOH (1.000 g, 6.67 mmol) (molar ratio 1:7), according to the typical procedure (Section 3.1) (4 h, 70 °C), gave a mixture of compounds containing ~25% of lactone 24c.
b. Diol 17c (0.200 g, 0.95 mmol), TfOH (1.035 g, 6.90 mmol), and SbF5 (0.247 g, 1.14 mmol) (molar ratio 1.0:7.2:1.2), according to the typical procedure (Section 3.1) (2 h, r.t.), afforded a mixture of compounds containing ~80% of lactone 24c. Silica gel column chromatography (CH2Cl2 as eluents) and sublimation (95 °C, 1 Torr) gave 0.115 g (yield 55%) of lactone 24c.
c. Diol 17c (0.300 g, 1.43 mmol), FSO3H (0.570 g, 5.70 mmol), and SbF5 (1.240 g, 5.72 mmol) (molar ratio 1:4:4), according to the typical procedure (Section 3.1) (4 h, r.t.), gave a dark mixture containing ~25% of lactone 24c along with other compounds and resinification products.
5,6,7,8-Tetrafluoroisochroman-3-one (24c). White solid, mp 101.1–102.2 °C. IR (KBr) ν, cm−1: 2974 (CH), 1747, 1730 (C=O), 1516, 1498 (FAR). 1H NMR (CDCl3): δ 5.39 (s, 2H, H-1), 3.73 (s, 2H, H-4). 19F NMR (CDCl3): δ −145.4 (m, 2F), −155.1 (m, 1F), −157.0 (m, 1F). Anal. Calcd for C9F4H4O2: C, 49.11; H, 1.83; F, 34.52%. Found: C, 49.01; H, 2.03; F, 34.58%. HRMS m/z, 220.0145 (M+). Calcd. M = 220.0142.
3.21. Carbonylation of (Pentafluorophenyl)(2,3,4,5-tetrafluoro-6-(hydroxymethyl)phenyl)methanol (17d)
Diol 17d (0.200 g, 0.53 mmol), FSO3H (0.212 g, 2.12 mmol), and SbF5 (0.462 g, 2.13 mmol) (molar ratio 1:4:4), according to the typical procedure (Section 3.1) (5 h, r.t.), gave a mixture of compounds containing 50% of 24d and 45% of 25d. Silica gel column chromatography (CHCl3 as eluents) resulted in 0.150 g (yield 73%) of a mixture of lactones 24d and 25d in the 56:44 ratio.
5,6,7,8-Tetrafluoro-1-(pentafluorophenyl)isochroman-3-one (24d) and 5,6,7,8-tetrafluoro-4-(pentafluorophenyl)isochroman-3-one (25d) (24d:25d = 56:44). White solid. IR (KBr) ν, cm−1: 2926, 2901 (CH), 1753 (C=O), 1512, 1498 (FAR). Compound 24d: 1H NMR (CDCl3): δ 7.00 (s, 1H, H-1), 3.92 (dm, 1H, JA4,B4 = 21.7 Hz, HA-4), 3.84 (dm, 1H, JA4,B4 = 21.7 Hz, HB-4). 19F NMR ((CDCl3): δ −143.3 (m, 1F, F-8), −143.8 (dddt, 1F, J5,6 = 20.8 Hz, J5,8 = 13 Hz, J5,7 = 3.1 Hz, J5,H = 1.5 Hz, F-5), −144.4 (m, 2F, F-ortho), −150.8 (tt, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 3.3 Hz, F-para), −153.9 (ddd, 1F, J6,5 = 20.8 Hz, J6,7 = 20.6 Hz, J6,8 = 4 Hz, F-6), −156.5 (dddt, 1F, J7,6 = 20.6 Hz, J7,8 = 20.6 Hz, J7,5 = 3.1 Hz, JF(7),H = 2 Hz, F-7), −160.8 (m, 2F, F-meta). Compound (25d): 1H NMR (CDCl3): δ 5.64 (dm, 1H, JA1,B1 ~ 17 Hz, HA-1), 5.61 (dm, 1H, JA1,B1 ~ 17 Hz, HB-1), 5.36 (s, 1H, H-4). 19F NMR (CDCl3): δ −143.2 (m, 1F, F-5), −143.3 (m, 2F, F-ortho), −144.4 (m, 1F, F-8), −153.2 (tt, 1F, Jpara,meta = 20.5 Hz, Jpara,ortho = 3.8 Hz, F-para), −154.5 (m, 1F, F-7 or F-6), −154.8 (m, 1F, F-6 or F-7), −161.4 (m, 2F, F-meta). Anal. Calcd for C15H3F9O2: C, 46.65; H, 0.78; F, 44.28%. Found: C, 46.58; H, 0.93; F, 44.32%. HRMS m/z, 385.9986 (M+). Calcd. M = 385.9984.
3.22. Carbonylation of 2,2,2-Trifluoro-1-(2,3,4,5-tetrafluoro-6-(hydroxymethyl)phenyl)ethan-1-ol (17e)
Diol 17e (0.200 g, 0.72 mmol), FSO3H (0.295 g, 2.95 mmol), and SbF5 (0.641 g, 2.96 mmol) (molar ratio 1:4:4), according to the typical procedure (Section 3.1) (7 h, 50 °C), gave a mixture of compounds containing ~65% of lactone 24e. Silica gel column chromatography (CHCl3 as the eluent) and recrystallization from hexane gave 66 mg of lactone 24e (yield 32%).
5,6,7,8-Tetrafluoro-1-(trifluoromethyl)isochroman-3-one (24e). White solid, mp 27.0–29.4 °C. IR (KBr) ν, cm−1: 2985 (CH), 1784 (C=O), 1525, 1506 (FAR). 1H NMR (CDCl3): δ 5.93 (q, 1H, JH,CF3 = 6.5 Hz, H-1), 3.99 (d, 1H, JA4,B4 = 20.9 Hz, HA-4), 3.68 (d, 1H, JA4,B4 = 20.9 Hz, HB-4). 19F NMR (CDCl3): δ −78.3 (dd, 3F, JCF3,F(8) = 7.5 Hz, JCF3,H(1) = 6.5 Hz, CF3), −141.8 (ddqd, 1F, J8,7 = 20.7 Hz, J8,5 = 14.1 Hz, JF(8),CF3 = 7.5 Hz, J8,6 = 5.4 Hz, F-8), −143.4 (ddd, 1F, J5,6 = 20.7 Hz, J5,8 = 14.1 Hz, J5,7 = 3.3 Hz, F-5), −151.0 (ddd, 1F, J6,5 = 20.7 Hz, J6,7 = 20.7 Hz, J6,8 = 5.4 Hz, F-6), −155.3 (dddd, 1F, J7,6 = 20.7 Hz, J7,8 = 20.7 Hz, J7,5 = 3.3 Hz, JF(7),H = 2.5 Hz, F-7). Anal. Calcd for C10H3F7O2: C, 41.69; H, 1.05; F, 46.16%. Found: C, 41.72; H, 1.05; F, 45.79%. HRMS m/z, 288.0015 (M+). Calcd. M = 288.0016.
3.23. Carbonylation of (2,3,4,6-Tetrafluoro-5-(hydroxymethyl)phenyl) (17f)
Diol 17f (0.200 g, 0.95 mmol), TfOH (1.000 g, 6.67 mmol), and SbF5 (0.217 g, 1.00 mmol) (molar ratio 1:7:1), according to the typical procedure (Section 3.1) (5 h, r.t.), after evaporation of the solvent and sublimation (190 °C, 1 Torr), afforded 0.175 g of acid 18f (yield 69%).
[3-(Carboxymethyl)-2,4,5,6-tetrafluorophenyl]acetic acid (18f). White solid, mp 179.1–181.0 °C. IR (KBr) ν, cm−1: 2991 (CH), 1709 (C=O), 1502 (FAR). 1H NMR (CO(CD3)2): δ 9.21 (br.s, 2H, COOH), 3.78 (br.s, 4H, 2CH2). 19F NMR (CO(CD3)2): δ −121.9 (d quintet, 1F, J2,5 = 11.2 Hz, JF(2),CH2 = 1.6 Hz, F-2), −137.7 (d, 2F, J4,5 = J6,5 = 21 Hz, F-4,6), −166.7 (td, 1F, J5,4 = J5,6 = 21 Hz, J5,2 = 11.2, Hz, F-5). Anal. Calcd for C10F4H6O4: C, 45.13; H, 2.27; F, 28.55%. Found: C, 45.05; H, 2.34; F, 28.59%. HRMS m/z, 266.0192 (M+). Calcd. M = 266.0197.
3.24. Carbonylation of (2,3,5,6-Tetrafluoro-4-(hydroxymethyl)phenyl) (17g)
A mixture of diol 17g (0.300 g, 1.43 mmol), FSO3H (0.700 g, 7.00 mmol), and SbF5 (1.520 g, 7.02 mmol) (molar ratio 1:5:5) was intensively stirred (6.5 h, r.t.) in a round-bottom glass flask (10 mL) in a slow flow of CO at atmospheric pressure. The mixture was poured into 5% hydrochloric acid (10 mL), the crystals were filtered off, the aqueous phase was extracted with Et2O (3 × 5 mL), and the extract was combined with the filtered-off crystals. Evaporation of the solvent and sublimation (230 °C, 1 Torr) gave 0.210 g of acid 18g (yield 55%).
[4-(Carboxymethyl)-2,3,5,6-tetrafluorophenyl]acetic acid (18g). White solid, mp 310 °C (with decomposition). IR (KBr) ν, cm−1: 2970 (CH), 1714 (C=O), 1491 (FAR). 1H NMR (DMSO-d6): δ 12.97 (br.s, 2H, 2COOH), 3.78 (s, 4H, 2CH2). 19F NMR (DMSO-d6): δ −144.5 (s, 4F). Anal. Calcd for C10F4H6O4: C, 45.13; H, 2.27; F, 28.55%. Found: C, 45.10; H, 2.27; F, 28.17%. HRMS m/z, 266.0195 (M+). Calcd. M = 266.0197.
3.25. Synthesis of Ester 2iMe and Its Reactions with NEt3 and K2CO3
a. A mixture of acid 2i (1.258 g, 4.11 mmol) with MeOH (15 mL) and 96% H2SO4 (3 mL) was heated (11.5 h, 70 °C) in a sealed tube and then diluted with water (100 mL), extracted with CH2Cl2 (3 × 10 mL), and the extract was dried over MgSO4. Evaporation of the solvent and distillation in vacuum (120–130 °C, 6 Torr) gave 1.144 g of ester 2iMe (yield 87%).
b. Ester 2iMe (0.492 g, 1.55 mmol) was dissolved in a mixture of dry CHCl3 (10 mL) and NEt3 (1 mL). The solution was incubated at r.t. for 15 min, washed with 5% hydrochloric acid (30 mL), and dried over MgSO4. Solvent evaporation yielded a mixture of compounds containing 65% of 10iMe and 15% of 2iMe (chromatographically inseparable).
c. Ester 2iMe (72 mg, 0.225 mmol) was dissolved in a mixture of dry CHCl3 (1.5 mL) and NEt3 (0.15 mL). The solution was kept at r.t. for 1 h, washed with 5% hydrochloric acid (10 mL), and dried over MgSO4. The mixture of compounds in the solution contained 65% of 10iMe and 15% of 2iMe.
d. Ester 2iMe (0.292 g, 0.91 mmol) was dissolved in dry CHCl3 (5 mL) and stirred with K2CO3 (0.750 g) at r.t. for 26 h. The organic layer was filtered, and the mixture of compounds in it contained >90% of ester 10iMe and was free of the starting compound. Silica gel column chromatography (CHCl3 as the eluent) gave 0.129 g (yield 47%) of ester 10iMe.
Methyl 2,2,3,3,4,5,6,7-octafluoroindan-1-carboxylate (2iMe). Colorless liquid. IR (film) ν, cm−1: 2964 (CH), 1759 (C=O), 1520 (FAR). 1H NMR (CDCl3): δ 4.55 (dd, 1H, JH,F(A2) = 14 Hz, JH,F(B2) = 6.6 Hz, H-1), 3.86 (s, 3H, CH3). 19F NMR (CDCl3): δ −106.7 (dm, 1F, JA3,B3 = 259 Hz, FA-3), −109.7 (dm, 1F, JA3,B3 = 259 Hz, FB-3), −112.5 (dddd, 1F, JA2,B2 = 241 Hz, JF(A2),H = 14 Hz, J = 3.6 Hz, J = 2.2 Hz, FA-2), −120.4 (dddd, 1F, JA2,B2 = 241 Hz, JF(B2),H = 6.6 Hz, J = 4.5 Hz, J = 2.9 Hz, FB-2), −139.0 (ddd, 1F, J7,6 = 20.4 Hz, J7,4 = 16.8 Hz, J7,5 = 5.4 Hz, F-7), −140.8 (dddt, 1F, J4,5 = 20.4 Hz, J4,7 = 16.8 Hz, J4.6 = 7.2 Hz, J = 6.8 Hz, F-4), −146.3 (dddm, 1F, J6,7 = 20.4 Hz, J6,5 = 18.8 Hz, J6,4 = 7.2 Hz, F-6), −150.5 (dddm, 1F, J5,4 = 20.4 Hz, J5,6 = 18.8 Hz, J4.6 = 7.2 Hz, F-5). HRMS m/z, 320.0087 (M+). Calcd. for C11H4F8O2: M = 320.0078.
Methyl perfluoro-1H-indene-3-carboxylate (10iMe). Colorless liquid. IR (film) ν, cm−1: 2964 (CH), 1749 (C=O), 1516, 1497 (FAR). 1H NMR (CDCl3): δ 3.93 (s, 3H, CH3). 19F NMR (CDCl3): δ −120.6 (tdddd, 1F, J2,1 = 14.4 Hz, J2,6 = 14.4 Hz, J2,4 = 7.2 Hz, J2,5 = 2.6 Hz, J2,7 = 2.6 Hz, F-2), −125.2 (ddm, 2F, J1,2 = 14.4 Hz, J1,7 = 4.4 Hz, F-1), −136.4 (ddddt, 1F, J4,5 = 19.4 Hz, J4,7 = 14.8 Hz, J4,2 = 7.2 Hz, J4,6 = 4.4 Hz, J4,1 = 2 Hz, F-4), −140.0 (dddtd, 1F, J76 = 21.3 Hz, J7,4 = 14.4 Hz, J7,5 = 7.3 Hz, J7,1 = 4.3 Hz, J7,2 = 2.6 Hz, F-7), −146.9 (dddd, 1F, J5,4 = 19.4 Hz, J5,6 = 17.9 Hz, J5,7 = 7.3 Hz, J5,2 = 2.6 Hz, F-5), −153.7 (dddd, 1F, J6,7 = 21.1 Hz, J6,5 = 17.4 Hz, J6,2 = 14.5 Hz, J6,4 = 4.7 Hz, F-6). HRMS m/z, 300.0014 (M+). Calcd. for C11H3F7O2: M = 300.0016.
3.26. Synthesis of Ester 2cMe and Its Reactions with NEt3 and K2CO3
a. A mixture of acid 2c (1.170 g, 3.98 mmol) with MeOH (14 mL) and 96% H2SO4 (2.9 mL) was heated (16 h, 70 °C) in a sealed tube and then diluted with water (150 mL), extracted with CH2Cl2 (3 × 10 mL), and the extract was then washed with a saturated aqueous solution of NaHCO3 (20 mL), and finally dried over MgSO4. Evaporation of the solvent gave 1.100 g of ester 2cMe (yield 90%).
b. Ester 2cMe (55 mg, 0.18 mmol) was dissolved in a mixture of dry CHCl3 (0.5 mL) and NEt3 (36 mg, 0.36 mmol). The solution was kept at r.t. for 5 days, washed with 5% hydrochloric acid (1.5 mL), and dried over MgSO4. The mixture of compounds in the solution contained (as evidenced by GC-MS) 87% of starting ester 2cMe and 6% of ester 2aMe along with a number of unidentified compounds (M = 340−604).
c. Ester 2cMe (27 mg, 0.088 mmol) was dissolved in dry CHCl3 (0.6 mL) and stirred with K2CO3 (90 mg) at r.t. for 20 h. A mixture of compounds in the solution contained 97% of the starting compound and 2% of ester 10cMe. The reaction mixture was heated (7 h, 55 °C), treated with 5% hydrochloric acid (5 mL), and dried over MgSO4. The mixture of compounds in the solution contained (judging by GC-MS) 12% of the starting ester 2cMe, 13% of ester 10cMe, and 72% of ester 2aMe along with two unidentified compounds (M = 564).
Methyl 3,3,3-trifluoro-2-(pentafluorophenyl)propanoate (2cMe)
White solid, mp 42.5–43.5 °C. IR (film) ν, cm−1: 2962 (CH); 1770 (C=O); 1527, 1510 (FAR). 1H NMR (CDCl3): δ 4.71 (q, 1H, JH,CF3 = 8 Hz, CH), 3.84 (s, 3H, CH3). 19F NMR (CDCl3): δ −68.0 (dt, 3F, JCF3,H = 8 Hz, JCF3-F(ortho) = 8 Hz, CF3), −140.5 (m, 2F, F-ortho), −151.8 (tt, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 3 Hz, F-para), −161.5 (m, 2F, F-meta). HRMS, m/z: 308.0064 (M+). Calcd. for C10H4F8O2: M = 308.0078.
Methyl perfluoro-2-phenylacrylate (10cMe) in the mixture with compounds 2cMe and 2aMe. 19F NMR (CDCl3): δ −62.8 (dt, 1F, J3A,3B = 29.7 Hz, J3A, ortho = 1.7 Hz, F-3A), −63.8 (dt, 1F, J3A,3B = 29.7 Hz, J3B,ortho = 6.2 Hz, F-3B), −139.1 (m, 2F, F-ortho), −152.8 (tt, 1F, Jpara,meta = 21 Hz, Jpara,ortho = 2.5 Hz, F-para), −162.5 (m, 2F, F-meta). GC-MS, m/z: 288 (M+).
3.27. The Reaction of Acid 2l with NEt3
Acid 2l (185 mg, 0.37 mmol) was dissolved in a mixture of dry CHCl3 (3 mL) and NEt3 (0.186 g). The solution was kept at r.t. for 5 h, washed with 5% hydrochloric acid (2 × 10 mL), and dried over MgSO4. Evaporation of the solvent resulted in 0.170 g of acid 10l (yield 95%).
Perfluoro-1,1-diethyl-1H-indene-3-carboxylic acid (10l). White solid, mp 120.2–121.3 °C (CHCl3). IR (KBr) ν, cm−1: 2929(CH), 1730(C=O), 1514, 1502(FAR). 1H NMR (CDCl3): δ 7.1 (br.s, 1H, COOH). 19F NMR (CDCl3): δ −81.8 (d, 6F, J = 3.6 Hz, CF3), −101.3 (m, 1F, F-2), −109.3 (dm, 4F, JA,B ~ 285 Hz, FA-CF2), −112.0 (dm, 4F, JA,B ~ 285 Hz, FB-CF2), −132.5 (tdddd, 1F, J = 49 Hz, J7,6 = 20.6 Hz, J7,4 = 14.6 Hz, J7,5 = 8.7 Hz, J7,2 = 2 Hz, F-7), −136.8 (dddd, 1F, J4,5 = 20.8 Hz, J4,7 = 14.6 Hz, J4,2 = 5.8 Hz, J4,6 = 4 Hz, F-4), −147.2 (dddd, 1F, J5,4 = 20.8 Hz, J5,6 = 18.9 Hz, J5,7 = 8.7 Hz, J5,2 = 1.7 Hz, F-5), −153.0 (dddd, 1F, J6,7 = 20.6 Hz, J6,5 = 18.9 Hz, J6,2 = 14.9 Hz, J6,4 = 4 Hz, F-6). Anal. Calcd for C14H1F15O2: C, 34.59%; H, 0.21%; F, 58.62%. Found: C, 35.12%; H, 0.43%; F, 58.48%. HRMS m/z, 485.9729 (M+). Calcd. M = 485.9732.
3.28. The Reaction of Acid 2c with NEt3
a. Acid 2c (50 mg, 0.17 mmol) was dissolved in a mixture of dry Et2O (0.5 mL) and NEt3 (55 mg). The solution was kept at r.t. for 14 days, washed with 5% hydrochloric acid (3 mL), and dried over MgSO4. The mixture of compounds in the extract contained 20% of starting acid 2c, 75% of ethylbenzene 26, and 3% of styrene 27.
b. Acid 2c (30 mg, 0.10 mmol) was dissolved in a mixture of dry CDCl3 (0.5 mL) and NEt3 (50 mg). The solution was heated (27 h, 55 °C), treated with 5% hydrochloric acid (5 mL), and dried over MgSO4. The mixture of compounds in the extract contained 17% of starting acid 2c, 34% of ethylbenzene 26, and 35% of styrene 27.
3.29. Synthesis of Alcohol 1j
A mixture of alcohol 1i (3.000 g, 10.79 mmol) with 20% oleum (10 mL) was heated (20 h, 100 °C) in a sealed tube and poured into ice water (100 mL). The resulting mixture was heated (5 h, 80 °C), extracted with Et2O (4 × 20 mL), and dried over MgSO4. Evaporation of the solvent and distillation in vacuum (150−160 °C, 1 Torr) gave 2.283 g of compound 1j (yield 83%).
2,2,4,5,6,7-Hexafluoro-3-hydroxyindan-1-one (1j). Colorless liquid. IR (film) ν, cm−1: 2945 (CH), 1761 (C=O), 1506 (FAR). 1H NMR (CDCl3): δ 5.42 (d, 1H, JH,F(A2) = 12.5 Hz, CH), 3.20 (s, 1H, OH). 19F NMR (CDCl3): δ −114.7 (dd, 1F, JA2,B2 = 285 Hz, J F(A2),H = 12.5 Hz, FA-2), −126.8 (d, 1F, JA2,B2 = 285 Hz, FB-2), −135.0 (ddd, 1F, J7,6 = 19.8 Hz, J7,4 = 19 Hz, J7,5 = 11.7 Hz, F-7), −138.2 (ddd, 1F, J5,4 = 19.5 Hz, J5,6 = 19.5 Hz, J5,7 = 11.7 Hz, F-5), −140.7 (ddd, 1F, J4,5 = 19.5 Hz, J4,7 = 19 Hz, J4,6 = 5.4 Hz, F-4), −149.4 (ddd, 1F, J6,7 = 19.8 Hz, J6,5 = 19.5 Hz, J6,4 = 5.4 Hz, F-6). HRMS m/z, 255.9950 (M+). Calcd. for C9H2F6O2: M = 255.9954.
3.30. Synthesis of Alcohol 1o
A mixture of alcohol 1h (3.000 g, 9.15 mmol) with 20% oleum (10 mL) was heated (21 h, 120 °C) in a sealed tube and poured into ice water (100 mL). The resultant mixture was heated (2.5 h, 80 °C), extracted with Et2O (4 × 20 mL), and the extract was dried over MgSO4. Evaporation of the solvent and distillation in vacuum (160−180 °C, 1 Torr) afforded 2.000 g of compound 1j (yield 71%).
2,2,3,3,5,6,7,8-Octafluoro-4-hydroxy-3,4-tetralin-1-one (1o). Colorless liquid. IR (film) ν, cm−1: 2933 (CH), 1735 (C=O), 1520 (FAR). 1H NMR (CDCl3): δ 5.50 (q, 1H, JH,F ~ 6.7 Hz, CH), 3.00 (br.s, 1H, OH). 19F NMR (CDCl3): δ −118.1 (dd, 1F, JA2,B2 = 290 Hz, JF-F = 16 Hz, FA-2), −134.5 (dm, 1F, JA2,B2 = 290 Hz, FB-2), −120.9 (dm, 1F, JA3,B3 = 273 Hz, FA-3), −131.6 (dm, 1F, JA3,B3 = 273 Hz, FB-3), −134.8 (ddd, 1F, J8,7 = 19.8 Hz, J8,5 = 13.3 Hz, J8,6 = 13.3 Hz, F-8), −139.4 (ddd, 1F, J5,6 = 21 Hz, J5,8 = 13.3 Hz, J5,7 = 5.9 Hz, F-5), −140.7 (ddd, 1F, J6,5 = 21 Hz, J6,7 = 20 Hz, J6,8 = 13.3 Hz, F-6), −150.1 (ddd, 1F, J7,6 = 20 Hz, J7,8 = 19.8 Hz, J7,5 = 5.9 Hz, F-7). Anal. Calcd for C10H2F8O2: C, 39.24%; H, 0.66%; F, 49.65%. Found: C, 39.17%; H, 0.67%; F, 49.63%. HRMS m/z, 305.9920 (M+). Calcd. M = 305.9922.
3.31. Synthesis of Diols 17c, 17f, and 17g
a. A mixture of tetrafluorophthalic acid (3.000 g, 12.61 mmol) with PCl5 (6.600 g, 31.65 mmol) was heated (2 h, 180 °C) with distillation of volatile products. Organic products were dissolved in hexane (2 × 10 mL) and filtered. The solvent was evaporated; the residue was dissolved in Et2O (10 mL) and added dropwise to a mixture of LiBH4 (1.200 g, 55.05 mmol) and Et2O (20 mL) cooled on ice. The obtained mixture was stirred at r.t. for 2 h, poured into 5% hydrochloric acid (80 mL), and extracted with Et2O (4 × 20 mL). The extract was washed with a 10% aqueous solution of K2CO3 (80 mL) and dried over MgSO4. Evaporation of the solvent and sublimation (130−140 °C, 1 Torr) gave 2.000 g of compound 17c (yield 76%).
b. An analogous procedure for tetrafluoroisophthalic acid afforded compound 17f (yield 63%).
c. An analogous procedure for tetrafluoroterephthalic acid produced compound 17g (yield 64%).
3.32. Synthesis of Diols 17d and 17e
a. A solution of 4,5,6,7-tetrafluoro-3-(pentafluorophenyl)phthalide (28) (1.430 g, 3.84 mmol) in Et2O (15 mL) was added to a mixture of LiBH4 (0.344 g, 15.78 mmol) and Et2O (10 mL). The resulting mixture was stirred at r.t. for 8.5 h, poured into 5% hydrochloric acid (20 mL), extracted with Et2O (3 × 10 mL), and the extract was dried over MgSO4. Evaporation of the solvent and sublimation (170 °C, 1 Torr) gave 1.342 g of compound 17d (yield 93%).
b. A solution of 4,5,6,7-tetrafluoro-3-(trifluoromethyl)phthalide (29) (0.818 g, 2.96 mmol) in Et2O (8 mL) was introduced into a mixture of LiBH4 (0.300 g, 13.76 mmol) and Et2O (4 mL). The obtained mixture was stirred at r.t. for 6 h, poured into 5% hydrochloric acid (10 mL), extracted with Et2O (3 × 5 mL), and the extract was dried over MgSO4. Evaporation of the solvent and sublimation (100 °C, 1 Torr) resulted in 0.682 g of compound 17e (yield 82%).
2,2,2-Trifluoro-1-(2,3,4,5-tetrafluoro-6-(hydroxymethyl)phenyl)ethan-1-ol (17e). White solid, mp 94.9–96.9 °C. IR (KBr) ν, cm−1: 3201 (OH), 2928 (CH), 1520, 1485 (FAR). 1H NMR (CO(CD3)2): δ 6.56 (d, 1H, JOH,CH = 6.7 Hz, CHOH), 5.84 (qd, 1H, JCH,CF3 = 7.8 Hz, JCH,OH = 6.7 Hz, CH), 4.91 (m, 1H, JAB = 12.5 Hz, HA-CH2), 4.76 (m, 1H, JAB = 12.5 Hz, HB-CH2), 4.49 (t, 1H, JOH,CH2 = 6 Hz, CH2OH). 19F NMR (CO(CD3)2): δ −75.7 (dd, 3F, JCF3,F(2) = 12.3 Hz, JCF3,CH = 7.8 Hz, CF3), −137.7 (dqdd, 1F, J2,3 = 20.3 Hz, JF(2),CF3 = 12.3 Hz, J2,5 =12.1 Hz, J2,4 = 4.3 Hz, F-2), −142.2 (ddd, 1F, J5,4 = 21.3 Hz, J5,2 = 12.1 Hz, J5,3 = 3 Hz, F-5), −154.8 (ddd, 1F, J4,5 = 21.3 Hz, J4,3 = 20.3 Hz, J4,2 = 4.3 Hz, F-4), −156.8 (ddd, 1F, J3,2 = 20.3 Hz, J3,4 = 20.3 Hz, J3,5 = 3 Hz, F-3). Anal. Calcd for C9H5F7O2: C, 39.26; H, 1.88; F, 47.91%. Found: C, 38.87; H, 1.81; F, 47.82%. HRMS m/z, 278.0176 (M+). Calcd. M = 278.0172.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248757/s1, Figures S1−S73: NMR spectra of the obtained compounds.
Author Contributions
Investigation and visualization, S.W.; conceptualization and writing—original draft preparation, Y.V.Z.; conceptualization and writing—review and editing, V.M.K.; writing—review and editing, T.V.M. and O.A.L.; project administration, T.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw data of this study are available within this article.
Acknowledgments
The authors are grateful to the Multi-Access Chemical Research Center of SB RAS for spectral and analytical measurements. Siqi Wang thanks the China Scholarship Council for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chambers, R.D. Fluorine in Organic Chemistry; Blackwell Pub: New York, NY, USA; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2004; pp. 203–277. [Google Scholar]
- Banks, R.E.; Smart, B.E.; Tatlow, J.C. (Eds.) Organofluorine Chemistry: Principles and Commercial Applications; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Tang, M.L.; Bao, Z. Halogenated Materials as Organic Semiconductors. Chem. Mater. 2011, 23, 446–455. [Google Scholar] [CrossRef]
- Babudri, F.; Farinola, G.M.; Naso, F.; Ragni, R. Fluorinated Organic Materials for Electronic and Optoelectronic Applications: The Role of the Fluorine Atom. Chem. Commun. 2007, 10, 1003–1022. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I. (Ed.) Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, UK, 2009; pp. 291–311. [Google Scholar]
- Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Leroux, F.; Jeschke, P.; Schlosser, M. α-Fluorinated Ethers, Thioethers, and Amines: Anomerically Biased Species. Chem. Rev. 2005, 105, 827–856. [Google Scholar] [CrossRef] [PubMed]
- Prchalová, E.; Štěpánek, O.; Smrček, S.; Kotora, M. Medicinal Applications of Perfluoroalkylated Chain-Containing Compounds. Future Med. Chem. 2014, 6, 1201–1229. [Google Scholar] [CrossRef] [PubMed]
- Eidus, Y.T.; Lapidus, A.L.; Puzitskii, K.V.; Nefedov, B.K. Carbonylation of Polyunsaturated Hydrocarbons and of Saturated and Unsaturated Alcohols and Halogeno-Derivatives. Russ. Chem. Rev. 1973, 42, 199–213. [Google Scholar] [CrossRef]
- Falbe, J. (Ed.) New Syntheses with Carbon Monoxide; Reactivity and Structure; Springer: Berlin, Germany; New York, NY, USA, 1980. [Google Scholar]
- Farooq, O.; Marcelli, M.; Prakash, G.K.S.; Olah, G.A. Electrophilic Reactions at Single Bonds. 22. Superacid-Catalyzed Electrophilic Formylation of Adamantane with Carbon Monoxide Competing with Koch-Haaf Carboxylation. J. Am. Chem. Soc. 1988, 110, 864–867. [Google Scholar] [CrossRef]
- Zonov, Y.V.; Karpov, V.M.; Platonov, V.E. The First Carbonylation of Perfluoroorganic Compounds: The Reactions of Perfluorobenzocyclobutene and Its Perfluoroalkyl and Pentafluorophenyl Derivatives with CO in SbF5 Medium. J. Fluor. Chem. 2014, 162, 71–77. [Google Scholar] [CrossRef]
- Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V.; Rybalova, T.V.; Gatilov, Y.V.; Platonov, V.E. Transformations of Perfluorinated 1,2-Dialkyl-, 1,1- and 1,2-Alkylphenylbenzocyclobutenes to Indan-2-one and Isochromene Derivatives under the Action of CO/SbF5. J. Fluor. Chem. 2016, 188, 117–125. [Google Scholar] [CrossRef]
- Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V.; Platonov, V.E. Carbonylation of Polyfluorinated Indans, Tetralins and Perfluoro-2,3-Dihydrobenzofuran under the Action of CO/SbF5. J. Fluor. Chem. 2018, 214, 24–34. [Google Scholar] [CrossRef]
- Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V. Carbonylation of Polyfluorobenzocyclobutenones in a SbF5 Medium. Russ. J. Org. Chem. 2019, 55, 1103–1111. [Google Scholar] [CrossRef]
- Olah, G.A.; Germain, A.; Lin, H.C. Stable Carbocations. CLXXXIII. Haloacetylium Ions. J. Am. Chem. Soc. 1975, 97, 5481–5488. [Google Scholar]
- Krespan, C.G.; Petrov, V.A. The Chemistry of Highly Fluorinated Carbocations. Chem. Rev. 1996, 96, 3269–3302. [Google Scholar] [CrossRef]
- Bardin, V.V.; Furin, G.G.; Yakobson, G.G. Aromatic fluoroderivatives XCVIII. Heptafluorophenylacetic acid. Synthesis and some properties. J. Org. Chem. USSR 1984, 20, 514–519. [Google Scholar]
- Takahashi, Y.; Yoneda, N.; Nagai, H. Carboxylation of alcohols with carbon monoxide supersaturated in strong acid. Facile synthesis of 2,2-bis(4-halophenyl)acetic, -propionic, and related acids. Chem. Lett. 1985, 14, 1733–1734. [Google Scholar] [CrossRef]
- Zhyhadlo, Y.Y.; Gaidai, A.V.; Levandovskiy, I.A.; Bezdudny, A.V.; Rassukana, Y.V. Facile Synthesis of 3-(Trifluoromethyl)Adamantane Derivatives. J. Fluor. Chem. 2017, 201, 11–14. [Google Scholar] [CrossRef]
- Nazarova, M.P.; Morozov, V.A.; Podkhalyuzin, A.T. Reactivity of (fluoroalkyl)adamantanes and their derivatives. III. Synthesis of (fluoroalkyl)adamantanecarboxylic acids. J. Org. Chem. USSR 1983, 19, 499–504. [Google Scholar] [CrossRef]
- Wakuluk-Machado, A.-M.; Dewez, D.F.; Baguia, H.; Imbratta, M.; Echeverria, P.-G.; Evano, G. Pd(OH)2/C, a Practical and Efficient Catalyst for the Carboxylation of Benzylic Bromides with Carbon Monoxide. Org. Process Res. Dev. 2020, 24, 713–723. [Google Scholar] [CrossRef]
- Rilvin-Derrick, E.; Oram, N.; Richardson, J. An Efficient Palladium-Catalysed Aminocarbonylation of Benzyl Chlorides. Synlett 2020, 31, 369–372. [Google Scholar]
- Li, H.; Zhang, Y.; Liu, D.; Liu, X. An Improved Method for the Synthesis of Phenylacetic Acid Derivatives via Carbonylation. J. Chem. Res. 2019, 43, 548–552. [Google Scholar] [CrossRef]
- She, M.; Xiao, D.; Yin, B.; Yang, Z.; Liu, P.; Li, J.; Shi, Z. An Efficiently Cobalt-Catalyzed Carbonylative Approach to Phenylacetic Acid Derivatives. Tetrahedron 2013, 69, 7264–7268. [Google Scholar] [CrossRef]
- Karimi, F.; Långström, B. Synthesis of 11C-Amides Using [11C]Carbon Monoxide and in Situ Activated Amines by Palladium-Mediated Carboxaminations. Org. Biomol. Chem. 2003, 1, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Langström, B. Synthesis of 11C-Labelled Amides by Palladium-Mediated Carboxamination Using [11C]Carbon Monoxide, in Situ Activated Amines and 1,2,2,6,6-Pentamethylpiperidine. Eur. J. Org. Chem. 2003, 2003, 2132–2137. [Google Scholar] [CrossRef]
- Karimi, F.; Långström, B. Palladium-Mediated Carboxylation of Aryl Halides (Triflates) or Benzyl Halides Using [13C]/[11C]Carbon Monoxide with Tetrabutylammonium Hydroxide or Trimethylphenylammonium Hydroxide. J. Chem. Soc. Perkin Trans. 1 2002, 20, 2256–2259. [Google Scholar] [CrossRef]
- Wang, W.; Qi, X.; Wu, X. Palladium-Catalyzed Thiocarbonylation of Benzyl Chlorides with Sulfonyl Chlorides for the Synthesis of Arylacetyl Thioesters. Adv. Synth. Catal. 2021, 363, 2541–2545. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wu, X.-F. A Sustainable Procedure toward Alkyl Arylacetates: Palladium-Catalysed Direct Carbonylation of Benzyl Alcohols in Organic Carbonates. Green Chem. 2018, 20, 969–972. [Google Scholar] [CrossRef]
- Xu, J.-X.; Wu, X.-F. Palladium-Catalyzed Decarboxylative Carbonylative Transformation of Benzyl Aryl Carbonates: Direct Synthesis of Aryl 2-Arylacetates. Org. Lett. 2018, 20, 5938–5941. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, T.; Dandois, C.; Walliser, B. The Hydrogenation of Fluoroolefins. Tetrahedron Lett. 1991, 32, 2735–2736. [Google Scholar] [CrossRef]
- Inokuchi, E.; Narumi, T.; Niida, A.; Kobayashi, K.; Tomita, K.; Oishi, S.; Ohno, H.; Fujii, N. Efficient Synthesis of Trifluoromethyl and Related Trisubstituted Alkene Dipeptide Isosteres by Palladium-Catalyzed Carbonylation of Amino Acid Derived Allylic Carbonates. J. Org. Chem. 2008, 73, 3942–3945. [Google Scholar] [CrossRef]
- Ai, H.-J.; Yuan, Y.; Wu, X.-F. Ruthenium Pincer Complex-Catalyzed Heterocycle Compatible Alkoxycarbonylation of Alkyl Iodides: Substrate Keeps the Catalyst Active. Chem. Sci. 2022, 13, 2481–2486. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, H.; Wu, X. Palladium-Catalyzed Perfluoroalkylative Carbonylation of Unactivated Alkenes: Access to Β-Perfluoroalkyl Esters. Angew. Chem. Intl. Ed. 2021, 60, 24292–24298. [Google Scholar] [CrossRef] [PubMed]
- Urata, H.; Kinoshita, Y.; Asanuma, T.; Kosukegawa, O.; Fuchikami, T. A Facile Synthesis of. Alpha.,.Omega.-Dicarboxylic Acids Containing Perfluoroalkylene Groups. J. Org. Chem. 1991, 56, 4996–4999. [Google Scholar] [CrossRef]
- Urata, H.; Kosukegawa, O.; Ishii, Y.; Yugari, H.; Fuchikami, T. Carbonylation of 1-Perfluoroalkyl-Substituted 2-Iodoalkanes Catalyzed by Transition-Metal Complexes. Tetrahedron Lett. 1989, 30, 4403–4406. [Google Scholar]
- Urata, H.; Ishii, Y.; Fuchikami, T. Palladium-Catalyzed Double Carbonylation of Alkyl Iodides Bearing Perfluoroalkyl Group. Tetrahedron Lett. 1989, 30, 4407–4410. [Google Scholar] [CrossRef]
- Ai, H.-J.; Rabeah, J.; Brückner, A.; Wu, X.-F. Rhodium-Catalyzed Carbonylative Coupling of Alkyl Halides with Thiols: A Radical Process Faster than Easier Nucleophilic Substitution. Chem. Commun. 2021, 57, 1466–1469. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, H.; Wu, X. Palladium-Catalyzed Carbonylative Four-Component Synthesis of Β-Perfluoroalkyl Amides. Chem. A Eur. J. 2021, 27, 17682–17687. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Z.; Shen, C.; Tian, X.; Tang, L.; Ji, X.; Dong, K. Asymmetric Alkoxy- and Hydroxy-Carbonylations of Functionalized Alkenes Assisted by Β-Carbonyl Groups. Angew. Chem. Int. Ed. 2021, 60, 17693–17700. [Google Scholar] [CrossRef]
- Liu, J.; Dong, K.; Franke, R.; Neumann, H.; Jackstell, R.; Beller, M. Development of Efficient Palladium Catalysts for Alkoxycarbonylation of Alkenes. Chem. Commun. 2018, 54, 12238–12241. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Ge, Y.; Huang, W.; Schneider, C.; Dühren, R.; Franke, R.; Neumann, H.; Jackstell, R.; Beller, M. A General Platinum-Catalyzed Alkoxycarbonylation of Olefins. Chem. Commun. 2020, 56, 5235–5238. [Google Scholar] [CrossRef]
- Sang, R.; Kucmierczyk, P.; Dühren, R.; Razzaq, R.; Dong, K.; Liu, J.; Franke, R.; Jackstell, R.; Beller, M. Synthesis of Carboxylic Acids by Palladium-Catalyzed Hydroxycarbonylation. Angew. Chem. Int. Ed. 2019, 58, 14365–14373. [Google Scholar] [CrossRef]
- Fuchikami, T.; Ohishi, K.; Ojima, I. Regioselective Hydroesterification and Hydrocarboxylation of 3,3,3-Trifluoropropene and Pentafluorostyrene Catalyzed by Phosphine-Palladium Complex. J. Org. Chem. 1983, 48, 3803–3807. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Ferretti, F.; Jackstell, R.; Beller, M. Pd-Catalyzed Selective Carbonylation of Gem-Difluoroalkenes: A Practical Synthesis of Difluoromethylated Esters. Angew. Chem. Int. Ed. 2019, 58, 4690–4694. [Google Scholar] [CrossRef] [PubMed]
- Daśko, M.; Przybyłowska, M.; Rachon, J.; Masłyk, M.; Kubiński, K.; Misiak, M.; Składanowski, A.; Demkowicz, S. Synthesis and Biological Evaluation of Fluorinated N-Benzoyl and N -Phenylacetoyl Derivatives of 3-(4-Aminophenyl)-Coumarin-7-O-Sulfamate as Steroid Sulfatase Inhibitors. Eur. J. Med. Chem. 2017, 128, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Hara, S.; Senboku, H. Synthesis of 2-Aryl-3,3,3-Trifluoropropanoic Acids Using Electrochemical Carboxylation of (1-Bromo-2,2,2-Trifluoroethyl)Arenes and Its Application to the Synthesis of β,β,β-Trifluorinated Non-Steroidal Anti-Inflammatory Drugs. Tetrahedron 2010, 66, 473–479. [Google Scholar] [CrossRef]
- Yan, S.-J.; Huang, C.; Zeng, X.-H.; Huang, R.; Lin, J. Solvent-Free, Microwave Assisted Synthesis of Polyhalo Heterocyclic Ketene Aminals as Novel Anti-Cancer Agents. Bioorganic Med. Chem. Lett. 2010, 20, 48–51. [Google Scholar] [CrossRef]
- Nguyen, J.-T.; Kato, K.; Kumada, H.-O.; Hidaka, K.; Kimura, T.; Kiso, Y. Maintaining Potent HTLV-I Protease Inhibition without the P3-Cap Moiety in Small Tetrapeptidic Inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 1832–1837. [Google Scholar] [CrossRef]
- Ye, S.; Yoshida, S.; Fröhlich, R.; Haufe, G.; Kirk, K.L. Fluorinated Phenylcyclopropylamines. Part 4: Effects of Aryl Substituents and Stereochemistry on the Inhibition of Monoamine Oxidases by 1-Aryl-2-Fluoro-Cyclopropylamines. Bioorganic Med. Chem. 2005, 13, 2489–2499. [Google Scholar] [CrossRef]
- Su, H.; Xie, Y.; Liu, W.-B.; You, S.-L. Methyl-Monofluorination of Ibuprofen Selectively Increases Its Inhibitory Activity toward Cyclooxygenase-1 Leading to Enhanced Analgesic Activity and Reduced Gastric Damage in Vivo. Bioorganic Med. Chem. Lett. 2011, 21, 3578–3582. [Google Scholar] [CrossRef]
- Fujibayashi, K.; Sakamoto, K.; Watanabe, M.; Iizuka, Y. Pharmacological Properties of R-84760, a Novel κ-Opioid Receptor Agonist. Eur. J. Pharmacol. 1994, 261, 133–140. [Google Scholar] [CrossRef]
- Guo, T.; Gan, Z.; Chen, J.; Wang, D.; He, L.; Song, Q.; Xu, Y. Design, Synthesis and Biological Evaluation of Novel Tetrahydroisoquinoline Quaternary Derivatives as Peripheral κ-Opioid Receptor Agonists. Bioorganic Med. Chem. 2016, 24, 2964–2970. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Chen, J.; Tao, Y.; Wang, Y.; Xu, X.; Chen, J.; Xu, Y.; Xi, T.; Hu, X.; et al. Novel κ-Opioid Receptor Agonist MB-1C-OH Produces Potent Analgesia with Less Depression and Sedation. Acta Pharm. Sin. 2015, 36, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Bachar, S.C.; Nahar, L.; Sarker, S.D. Synthesis and Structure-Activity-Relationships of Indan Acid Derivatives as Analgesic and Anti-Inflammatory Agents. Ref. J. Chem. 2016, 6, 125–138. [Google Scholar] [CrossRef][Green Version]
- Kiyoi, T.; Reid, M.; Francis, S.; Davies, K.; Laats, S.; McArthur, D.; Easson, A.-M.; Kiyoi, Y.; Tarver, G.; Caulfield, W.; et al. Synthesis of Hexahydro[2]Benzopyrano[3,4-c]Pyrroles as Serotonin 5-HT2C Receptor Agonists via Intramolecular Hetero Diels–Alder Reactions. Tetrahedron Lett. 2011, 52, 3413–3416. [Google Scholar] [CrossRef]
- Stoll, I.; Brockhinke, R.; Brockhinke, A.; Böttcher, M.; Koop, T.; Stammler, H.-G.; Neumann, B.; Niemeyer, A.; Hütten, A.; Mattay, J. 2-Aminopyrimidine-Silver(I) Based Hybrid Organic Polymers: Self-Assembly and Phase Transitions of a Novel Class of Electronic Material. Chem. Mater. 2010, 22, 4749–4755. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Kiskin, M.A.; Sidoruk, A.V.; Varaksina, E.A.; Shmelev, M.A.; Gogoleva, N.V.; Taydakov, I.V.; Eremenko, I.L. Monometallic Ln3+ and heterometallic Ln3+ −Cd2+ complexes based on pentafluorophenylacetic acid: Efficient control of dimension and luminescent properties. Aust. J. Chem. 2022, 75, 572–580. [Google Scholar] [CrossRef]
- Im, J.; Sterner, E.; Swager, T. Integrated Gas Sensing System of SWCNT and Cellulose Polymer Concentrator for Benzene, Toluene, and Xylenes. Sensors 2016, 16, 183. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Wu, F.-Y.; Lai, T.-S.; Lin, Y.-C.; Lin, H.-C. Self-Assembly and Hydrogelation from Multicomponent Coassembly of Pentafluorobenzyl-Phenylalanine and Pentafluorobenzyl-Diphenylalanine. RSC Adv. 2015, 5, 22943–22946. [Google Scholar] [CrossRef]
- Talloj, S.K.; Cheng, B.; Weng, J.-P.; Lin, H.-C. Glucosamine-Based Supramolecular Nanotubes for Human Mesenchymal Cell Therapy. ACS Appl. Mater. Interfaces 2018, 10, 15079–15087. [Google Scholar] [CrossRef]
- Olah, G.A.; Olah, G.A. (Eds.) Superacid Chemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 35–82. [Google Scholar]
- Zonov, Y.V.; Karpov, V.M.; Platonov, V.E. Transformation of Perfluorinated Benzocycloalkenes and Alkylbenzenes to Their Carbonyl Derivatives under the Action of CF3COOH/SbF5. J. Fluor. Chem. 2007, 128, 1058–1064. [Google Scholar] [CrossRef]
- Karpov, V.M.; Mezhenkova, T.V.; Platonov, V.E.; Yakobson, G.G. Interaction of Perfluorobenzocycloalkenes with Tetrafluoroethylene in the Presence of SbF5. J. Fluor. Chem. 1985, 28, 121–137. [Google Scholar] [CrossRef]
- Barbour, A.K.; Buxton, M.W.; Coe, P.L.; Stephens, R.; Tatlow, J.C. 173. Aromatic Polyfluoro-Compounds. Part VIII. Pentafluorobenzaldehyde and Related Pentafluorophenyl Ketones and Carboxylic Acids. J. Chem. Soc. 1961, 808–817. [Google Scholar] [CrossRef]
- Wang, S.; Golokhvastova, D.S.; Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V.; Gatilov, Y.V. Reduction of Perfluorinated Benzocycloalkenones and Other Polyfluoroaryl Ketones to Alcohols with LiBH4. Russ. J. Org. Chem. 2022, 58, 780–790. [Google Scholar] [CrossRef]
- Brovko, V.V.; Sokolenko, V.A.; Yakobson, G.G. Alkylation of pentafluorobenzene with 1,1,2-trichlorotrifluoroethane and fluoroform in the presence of antimony pentafluoride. J. Org. Chem. USSR 1974, 10, 300–302. [Google Scholar]
- Karpov, V.M.; Mezhenkova, T.V.; Platonov, V.E.; Sinyakov, V.R. Skeletal Transformations of Perfluoro-1-Phenylindan under the Action of Antimony Pentafluoride. J. Fluor. Chem. 2001, 107, 53–57. [Google Scholar] [CrossRef]
- Karpov, V.M.; Mezhenkova, T.V.; Platonov, V.E.; Sinyakov, V.R.; Shchegoleva, L.N. Pentafluorophenylation of Perfluorinated Benzocyclobutene, Indan, and Tetralin by Reaction with Pentafluorobenzene in SbF5. Russ. J. Org. Chem. 2002, 38, 1158–1165. [Google Scholar] [CrossRef]
- Zonov, Y.V.; Wang, S.; Karpov, V.M.; Mezhenkova, T.V. The Aliphatic Ring-Opening and SNAr Substitution in the Reactions of Perfluorobenzocycloalkenones with K2CO3 in Water and Methanol. J. Fluor. Chem. 2021, 249, 109851. [Google Scholar] [CrossRef]
- Zonov, Y.V.; Karpov, V.M.; Platonov, V.E.; Rybalova, T.V.; Gatilov, Y.V. Oxygen Replacement by Fluorine in Carbonyl Derivatives of Perfluoroaromatic Compounds and Isomerization of Perfluoroindan-1,3-Dione to Perfluoro-3-Methylenephthalide under the Action of HF/SbF5. J. Fluor. Chem. 2006, 127, 1574–1583. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Kiskin, M.A. On the Hydrolysis of Diethyl 2-(Perfluorophenyl)Malonate. Beilstein J. Org. Chem. 2020, 16, 1863–1868. [Google Scholar] [CrossRef]
- Pozdnyakovich, Y.V.; Chuikova, T.V.; Shteingarts, V.D. Fluorine-containing carbocations. IX. Alkylation of pentafluorobenzene by perfluorinated arenonium ions and generation of perfluorinated 3-phenylbenzenonium 3- and 6-phenylnaphthalenonium ions. J. Org. Chem. USSR 1975, 11, 1681–1690. [Google Scholar]
- Malyuta, N.G.; Platonov, V.E.; Furin, G.G.; Yakobson, G.G. Thermolytic Reactions of Polyfluoroaromatic Compounds—XI. Tetrahedron 1975, 31, 1201–1207. [Google Scholar] [CrossRef]
- Kobrina, L.S.; Shteingarts, V.D.; Shchegoleva, L.N. Chemical shifts in fluorine-19 NMR spectra of polyfluorinated naphthalene derivatives. Izv. Sib. Otd. Akad. Nauk SSSR Ser. Khim. Nauk 1974, 1, 68–77. [Google Scholar]
- Oksenenko, B.G.; Sokolenko, V.A.; Vlasov, V.M.; Yakobson, G.G. Fluorinated aromatics. XLI. Reaction of polyfluorinated aromatic compounds containing oxygen with sulfur tetrafluoride. Izv. Sib. Otd. Akad. Nauk SSSR Ser. Khim. Nauk 1970, 1, 102–106. [Google Scholar]
- Nguyen, B.V.; Burton, D.J. A New Route for the Preparation of Substituted 2,2-Difluorostyrenes and a Convenient Route to Substituted (2,2,2-Trifluoroethyl)Benzenes. J. Org. Chem. 1997, 62, 7758–7764. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).