Chemical Composition Profiling and Antifungal Activity of Saffron Petal Extract
Abstract
1. Introduction
2. Results
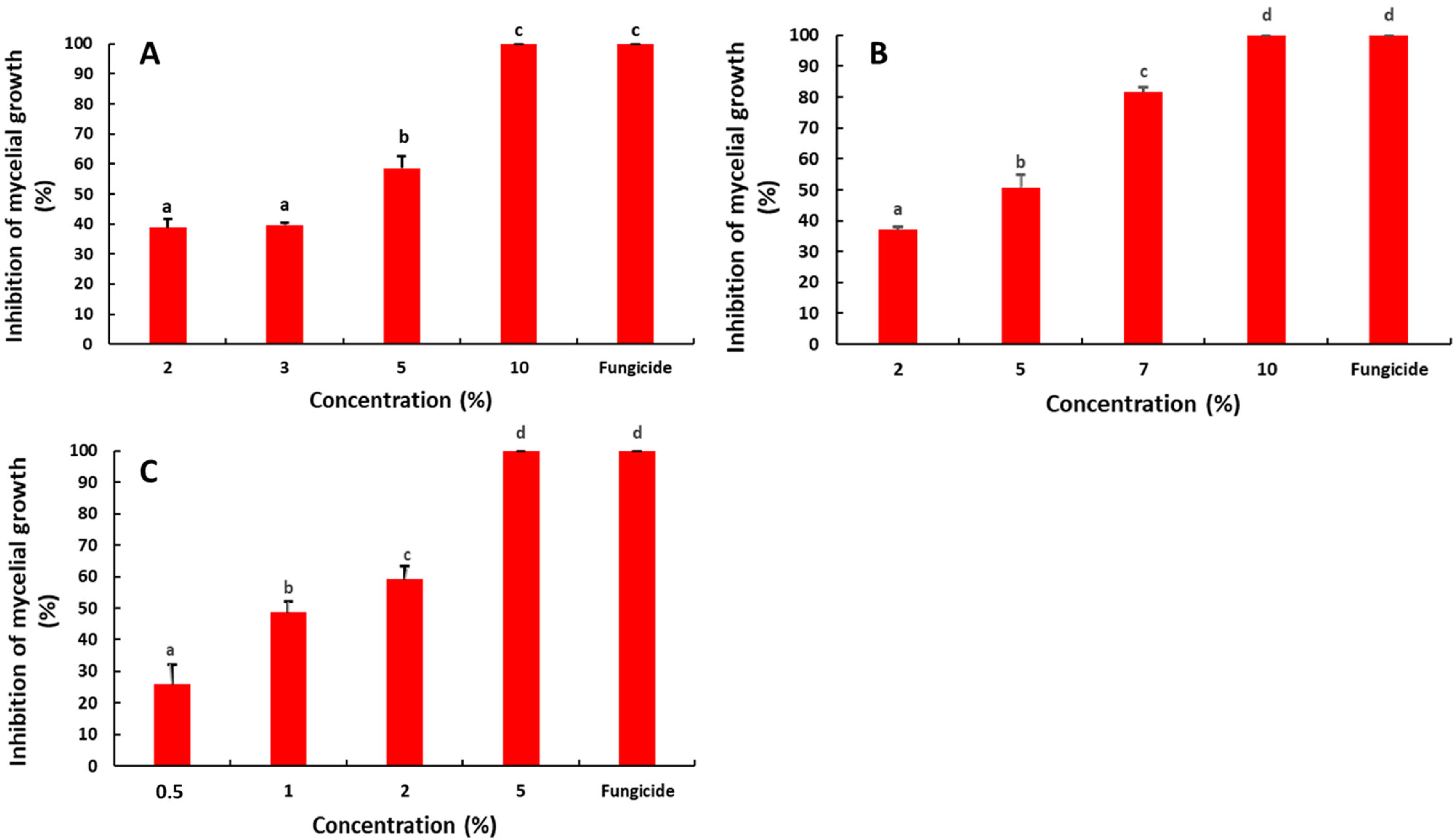
2.1. Antifungal Activity of the Saffron Petal Extract (SPE) on the Mycelial Growth
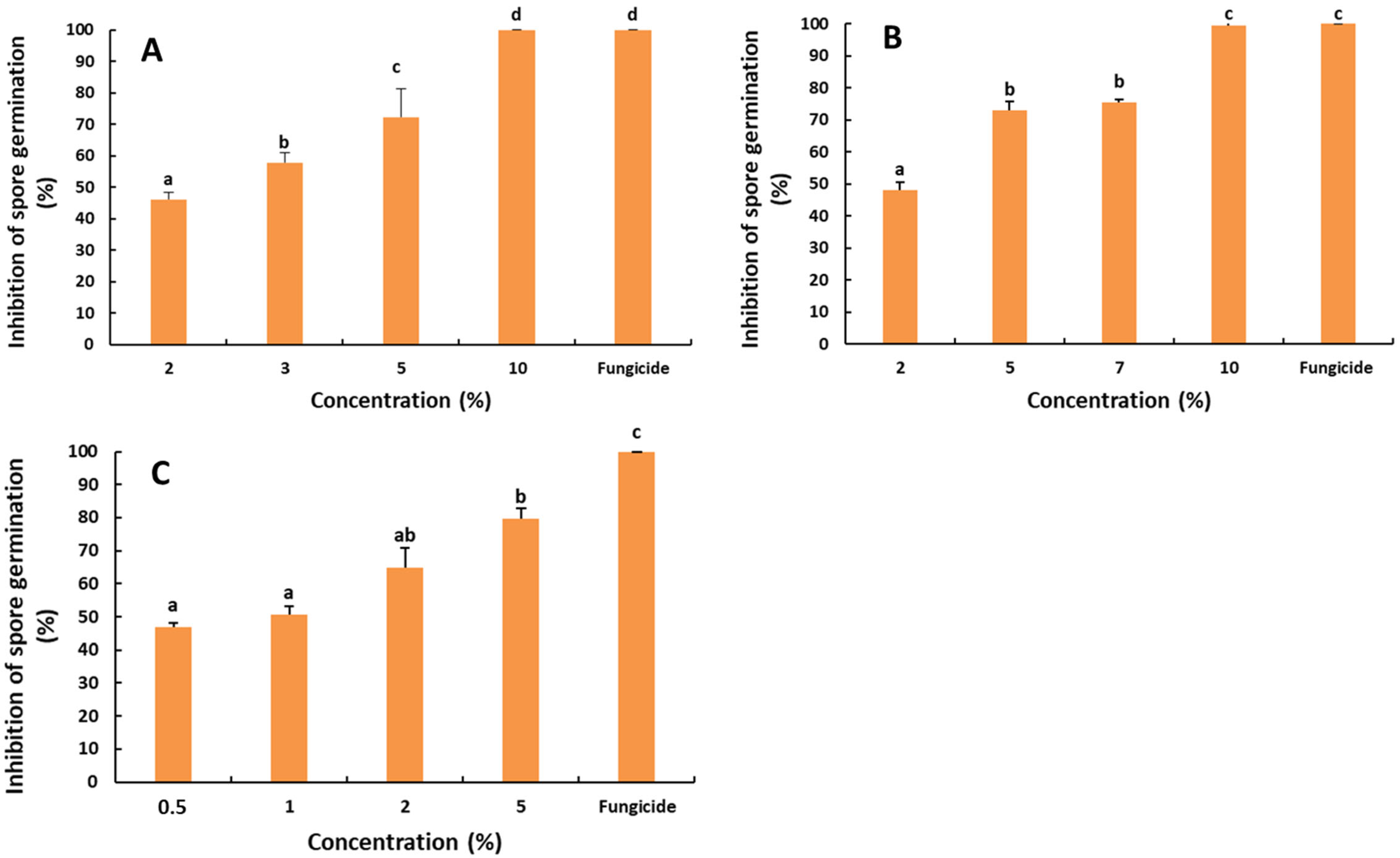
2.2. Antifungal Activity of the Saffron Petal Extract (SPE) on the Spore Germination
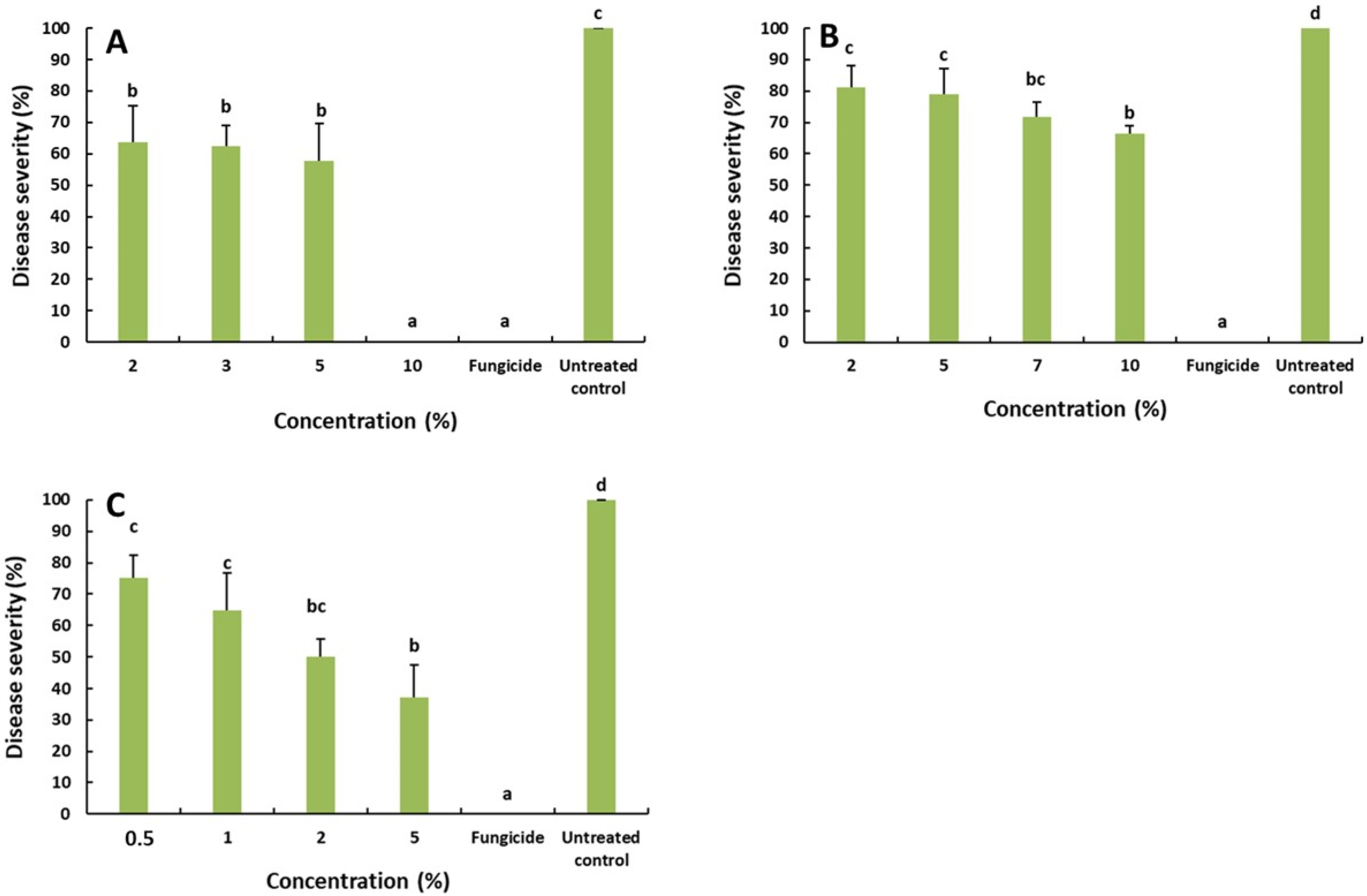
2.3. Effect of the Saffron Petal Extract on the Rot Decay Development
2.4. Chemical Composition
2.4.1. FTIR Analysis of the Organic Composition
2.4.2. Total Phenolic, Flavonoid Contents and the DPPH Radical Scavenging Activity
2.4.3. Volatile Composition: GC-MS
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of the Saffron Petal Aqueous Extract
4.2. Fungal Pathogens
4.3. Fruit Preparation
4.4. Antifungal Activity of the Saffron Petal Extract (SPE) on the Mycelial Growth
4.5. Effect of the Saffron Petal Extract on the Spore Germination
4.6. Effect of the Saffron Petal Extract on the Rot Decay Development
4.7. Chemical Composition Analysis of the Saffron Petal
4.7.1. FTIR Analysis
4.7.2. Determination of the Total Phenolic and Flavonoid Contents and the DPPH Radical Scavenging Activity
4.7.3. GC-MS Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serrano-Diaz, J.; Sanchez, A.M.; Martinez-Tome, M.; Winterhalter, P.; Alonso, G.L. Flavonoid Determination in the Quality Control of Floral Bioresidues from (Crocus Sativus, L.). J. Agric. Food Chem. 2014, 62, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus, L.), the King of Spices: An Overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Kafi, M.; Kamili, A.N.; Husaini, A.M.; Ozturk, M.; Altay, V. An Expensive Spice Saffron (Crocus sativus, L.): A Case Study from Kashmir, Iran, and Turkey. In Global Perspectives on Underutilized Crops; Springer: Berlin, Germany, 2018; pp. 109–149. [Google Scholar]
- Armellini, R.; Peinado, I.; Pittia, P.; Scampicchio, M.; Heredia, A.; Andres, A. Effect of Saffron (Crocus sativus, L.) Enrichment on Antioxidant and Sensorial Properties of Wheat Flour Pasta. Food Chem. 2018, 254, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Soleimani, A.; Singh, G.; Amin, A.; Tabtabaei, S.; Latella, G.; Stein, U.; Akhondzadeh, S.; Solanki, N.; Gondré-Lewis, M.C.; et al. Saffron: The Golden Spice with Therapeutic Properties on Digestive Diseases. Nutrients 2019, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Roshanravan, N. Saffron: An Updated Review on Biological Properties with Special Focus on Cardiovascular Effects. Biomed. Pharmacother. 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D. Efficacy of Curcumin, and a Saffron/Curcumin Combination for the Treatment of Major Depression: A Randomised, Double-Blind, Placebo-Controlled Study. J. Affect. Disord. 2017, 207, 188–196. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Kalani, M.R.; Avan, A. The Antileukemic Effects of Saffron (Crocus sativus L.) and Its Related Molecular Targets: A Mini Review. J. Cell. Biochem. 2019, 120, 4732–4738. [Google Scholar] [CrossRef]
- Mentis, A.F.A.; Dalamaga, M.; Lu, C.; Polissiou, M.G. Saffron for “Toning down” COVID-19-Related Cytokine Storm: Hype or Hope? A Mini-Review of Current Evidence. Metab. Open 2021, 11, 100111. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Sánchez, A.M.; Maggi, L.; Martínez-Tomé, M.; García-Diz, L.; Murcia, M.A.; Alonso, G.L. Increasing the Applications of Crocus Sativus Flowers as Natural Antioxidants. J. Food Sci. 2012, 77, C1162–C1168. [Google Scholar] [CrossRef]
- Nasab, B.F. Evaluation of Antibacterial Activities of Hydroalcoholic Extract of Saffron Petals on Some Bacterial Pathogens. J. Med. Bacteriol. 2019, 8, 8–20. [Google Scholar]
- Moshiri, E.; Basti, A.A.; Noorbala, A.A.; Jamshidi, A.H.; Abbasi, S.H.; Akhondzadeh, S. Crocus sativus L. (Petal) in the Treatment of Mild-to-Moderate Depression: A Double-Blind, Randomized and Placebo-Controlled Trial. Phytomedicine 2006, 13, 607–611. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Rodríguez-Conde, M.F.; Reina-Ureña, J.V.; Escolano-Tercero, M.A.; Herraiz-Peñalver, D.; Santana-Méridas, O. In Vitro Antioxidant and Metal Chelating Properties of Corm, Tepal and Leaf from Saffron (Crocus sativus, L.). Ind. Crops Prod. 2012, 39, 149–153. [Google Scholar] [CrossRef]
- Goupy, P.; Vian, M.A.; Chemat, F.; Caris-Veyrat, C. Identification and Quantification of Flavonols, Anthocyanins and Lutein Diesters in Tepals of Crocus Sativus by Ultra Performance Liquid Chromatography Coupled to Diode Array and Ion Trap Mass Spectrometry Detections. Ind. Crops Prod. 2013, 44, 496–510. [Google Scholar] [CrossRef]
- Li, C.Y.; Lee, E.J.; Wu, T.S. Antityrosinase Principles and Constituents of the Petals of Crocus Ativus. J. Nat. Prod. 2004, 67, 437–440. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and Anti-Inflammatory Effects of Crocus sativus L. Stigma and Petal Extracts in Mice. BMC Pharmacol. 2002, 2, 1–8. [Google Scholar]
- Zheng, C.J.; Li, L.; Ma, W.H.; Han, T.; Qin, L.P. Chemical Constituents and Bioactivities of the Liposoluble Fraction from Different Medicinal Parts of Crocus sativus. Pharm. Biol. 2011, 49, 756–763. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Montoro, P.; Fenu, M.A.; Pizza, C. Antioxidant Activity, Cytotoxic Activity and Metabolic Profiling of Juices Obtained from Saffron (Crocus sativus L.) Floral by-Products. Food Chem. 2016, 199, 18–27. [Google Scholar] [CrossRef]
- Ahmadi Shadmehri, A.; Namvar, F.; Miri, H.; Yaghmaei, P.; Nakhaei Moghaddam, M. Cytotoxicity, Antioxidant and Antibacterial Activities of Crocus Sativus Petal Extract. J. Res. Appl. Basic Med. Sci. 2019, 5, 69–76. [Google Scholar]
- Karimi, G.H.; Taiebi, N.; Hosseinzadeh, A.; Shirzad, F. Evaluation of Subacute Toxicity of Aqueous Extract of Crocus sativus L. Stigma and Petal in Rats. Sci. Inf. Database 2004, 3, 29–35. [Google Scholar]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest Treatments of Fresh Produce. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef]
- Ruffo Roberto, S.; Youssef, K.; Hashim, A.F.; Ippolito, A. Nanomaterials as Alternative Control Means against Postharvest Diseases in Fruit Crops. Nanomaterials 2019, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Amini, J.; Saba, M.K.; Karimi, K.; Pertot, I. Preharvest and Postharvest Application of Garlic and Rosemary Essential Oils for Controlling Anthracnose and Quality Assessment of Strawberry Fruit During Cold Storage. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of Plant Extracts and Essential Oils in Fighting against Postharvest Fruit Pathogens and Extending Fruit Shelf Life: A Review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, Plant and Animal-Derived Compounds as Alternatives to Conventional Fungicides for the >control of Postharvest Diseases of Fresh Horticultural Produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-Positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Kaveh, H. Effect of Saffron Petal Extract on Retention Quality of Fresh-Cut Watermelon Cubes. Saffron Agron. Technol. Eff. 2016, 4, 301–311. [Google Scholar] [CrossRef]
- Wilson, R.H.; Smith, A.C.; Kacuráková, M.; Saunders, P.K.; Wellner, N.; Waldron, K.W. The Mechanical Properties and Molecular Dynamics of Plant Cell Wall Polysaccharides Studied by Fourier-Transform Infrared Spectroscopy. Plant Physiol. 2000, 124, 397–406. [Google Scholar] [CrossRef]
- Lahlali, R.; Song, T.; Chu, M.; Karunakaran, C.; Yu, F.; Wei, Y.; Peng, G. Synchrotron-Based Spectroscopy and Imaging Reveal Changes in the Cell-Wall Composition of Barley Leaves in Defence Responses to Blumeria Graminis f. Sp. Tritici. Can. J. Plant Pathol. 2019, 41, 457–467. [Google Scholar] [CrossRef]
- Naumann, A.; Heine, G.; Rauber, R. Efficient Discrimination of Oat and Pea Roots by Cluster Analysis of Fourier Transform Infrared (FTIR) Spectra. Field Crops Res. 2010, 119, 78–84. [Google Scholar] [CrossRef]
- Lee, F.Y.; Htar, T.T.; Akowuah, G.A. ATR-FTIR and Spectrometric Methods for the Assay of Crocin in Commercial Saffron Spices (Crocus savitus L.). Int. J. Food Prop. 2015, 18, 1773–1783. [Google Scholar] [CrossRef]
- Kumar, S.; Lahlali, R.; Liu, X.; Karunakaran, C. Infrared Spectroscopy Combined with Imaging: A New Developing Analytical Tool in Health and Plant Science. Appl. Spectrosc. Rev. 2016, 51, 466–483. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Salas-Méndez, E.D.J.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.L.V.; Sáenz-Galindo, A.; González-Morales, S.; Flores-López, M.L.; Villarreal-Quintanilla, J.A.; Peña-Ramos, F.M.; et al. Antifungal Activity in Vitro of Ethanol and Aqueous Extracts of Leaves and Branches of Flourensia Spp. against Postharvest Fungi. Ind. Crops Prod. 2017, 107, 499–508. [Google Scholar] [CrossRef]
- Nicosia, M.G.L.D.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of Postharvest Fungal Rots on Citrus Fruit and Sweet Cherries Using a Pomegranate Peel Extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Gholamnezhad, J. Effect of Plant Extracts on Activity of Some Defense Enzymes of Apple Fruit in Interaction with Botrytis Cinerea. J. Integr. Agric. 2019, 18, 115–123. [Google Scholar] [CrossRef]
- López-Anchondo, A.N.; de la López-Cruz, D.; Gutiérrez-Reyes, E.; Castañeda-Ramírez, J.C.; de la Fuente-Salcido, N.M. Antifungal Activity In Vitro and In Vivo of Mesquite Extract (Prosopis Glandulosa) Against Phytopathogenic Fungi. Indian J. Microbiol. 2021, 61, 85–90. [Google Scholar] [CrossRef]
- Snyder, A.B.; Sweeney, C.F.; Rodriguez-Saona, L.E.; Giusti, M.M. Rapid Authentication of Concord Juice Concentration in a Grape Juice Blend Using Fourier-Transform Infrared Spectroscopy and Chemometric Analysis. Food Chem. 2014, 147, 295–301. [Google Scholar] [CrossRef]
- Balabin, R.M.; Smirnov, S.V. Melamine Detection by Mid-and near-Infrared (MIR/NIR) Spectroscopy: A Quick and Sensitive Method for Dairy Products Analysis Including Liquid Milk, Infant Formula, and Milk Powder. Talanta 2011, 85, 562–568. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of Fungal Chitosan Incorporated with Pomegranate Peel Extract as Edible Coating for Microbiological, Chemical and Sensorial Quality Enhancement of Nile Tilapia Fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Pastrana-Castro, L.; Rua-Rodríguez, M.L.; Alvarez-Pérez, O.B.; Rodríguez-Herrera, R.; Aguilar, C.N. Experimental Protocol for the Recovery and Evaluation of Bioactive Compounds of Tarbush against Postharvest Fruit Fungi. Food Chem. 2016, 198, 62–67. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the Antioxidant Activity of Total Flavonoids Extract from Persimmon (Diospyros kaki L.) Leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef]
- Termentzi, A.; Kokkalou, E. LC-DAD-MS (ESI+) Analysis and Antioxidant Capacity of Crocus Sativus Petal Extracts. Planta Med. 2008, 74, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Fazeli-Nasab, B.; Rahnama, M.; Mazarei, A. Correlation between Antioxidant Activity and Antibacterial Activity of Nine Medicinal Plant Extracts. J. Maz. Univ. Med. Sci. 2017, 27, 63–78. [Google Scholar]
- Goli, S.A.H.; Mokhtari, F.; Rahimmalek, M. Phenolic Compounds and Antioxidant Activity from Saffron (Crocus sativus, L.) Petal. J. Agric. Sci. 2012, 4, 175. [Google Scholar]
- Gómez, A.C.; Lyons, T.; Mamat, U.; Yero, D.; Bravo, M.; Daura, X.; Elshafee, O.; Brunke, S.; Gahan, C.G.M.; O’Driscoll, M. Synthesis and Evaluation of Novel Furanones as Biofilm Inhibitors in Opportunistic Human Pathogens. Eur. J. Med. Chem. 2022, 242, 114678. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Givskov, M. Pharmacological Inhibition of Quorum Sensing for the Treatment of Chronic Bacterial Infections. J. Clin. Investig. 2003, 112, 1300–1307. [Google Scholar] [CrossRef]
- Lönn-Stensrud, J.; Landin, M.A.; Benneche, T.; Petersen, F.C.; Scheie, A.A. Furanones, Potential Agents for Preventing Staphylococcus Epidermidis Biofilm Infections? J. Antimicrob. Chemother. 2009, 63, 309–316. [Google Scholar] [CrossRef]
- Han, Y.; Hou, S.; Simon, K.A.; Ren, D.; Luk, Y.Y. Identifying the Important Structural Elements of Brominated Furanones for Inhibiting Biofilm Formation by Escherichia Coli. Bioorg. Med. Chem. Lett. 2008, 18, 1006–1010. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernandez, J.; Lopez-Ibanez, S.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Plant Phytochemicals in Food Preservation: Antifungal Bioactivity: A Review. J. Food Prot. 2020, 83, 163–171. [Google Scholar] [CrossRef]
- Nandhavathy, G.; Dharini, V.; Babu, P.A.; Nambiar, R.B.; Selvam, S.P.; Sadiku, E.R.; Kumar, M.M. Determination of Antifungal Activities of Essential Oils Incorporated-Pomegranate Peel Fibers Reinforced-Polyvinyl Alcohol Biocomposite Film against Mango Postharvest Pathogens. Mater. Today Proc. 2021, 38, 923–927. [Google Scholar] [CrossRef]
- Xin, Z.; OuYang, Q.; Wan, C.; Che, J.; Li, L.; Chen, J.; Tao, N. Isolation of Antofine from Cynanchum Atratum BUNGE (Asclepiadaceae) and Its Antifungal Activity against Penicillium Digitatum. Postharvest Biol. Technol. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Ma, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S.; Chen, T. Honokiol Suppresses Mycelial Growth and Reduces Virulence of Botrytis Cinerea by Inducing Autophagic Activities and Apoptosis. Food Microbiol. 2020, 88, 103411. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053. [Google Scholar] [CrossRef]
- Rubio-Moraga, Á.; Gómez-Gómez, L.; Trapero, A.; Castro-Díaz, N.; Ahrazem, O. Saffron Corm as a Natural Source of Fungicides: The Role of Saponins in the Underground. Ind. Crops Prod. 2013, 49, 915–921. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Yang, Q.; Apaliya, M.T. Recent Developments in the Enhancement of Some Postharvest Biocontrol Agents with Unconventional Chemicals Compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Vázquez-González, Y.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. Characterization and Antifungal Activity of Jackfruit (Artocarpus heterophyllus Lam.) Leaf Extract Obtained Using Conventional and Emerging Technologies. Food Chem. 2020, 330, 127211. [Google Scholar] [CrossRef]
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-Senescence Compounds: A Potential Nutraceutical Approach to Healthy Aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Askarne, L.; Ezrari, S.; El Ghadaroui, L.; Tahiri, A.; Hrustić, J.; Amiri, S. Efficacy Assessment of Pomegranate Peel Aqueous Extract for Brown Rot (Monilinia spp.) Disease Control. Physiol. Mol. Plant Pathol. 2020, 110, 101482. [Google Scholar] [CrossRef]
- Ghanbari, J.; Khajoei-Nejad, G.; van Ruth, S.M.; Aghighi, S. The Possibility for Improvement of Flowering, Corm Properties, Bioactive Compounds, and Antioxidant Activity in Saffron (Crocus Sativus L.) by Different Nutritional Regimes. Ind. Crops Prod. 2019, 135, 301–310. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Rosas-Romero, A.; Saavedra, G.; Murcia, M.A.; Jiménez, A.M.; Codina, C. Investigation of Bolivian Plant Extracts for Their Radical Scavenging Activity and Antioxidant Activity. Life Sci. 2003, 73, 1667–1681. [Google Scholar] [CrossRef]
- Naim, N.; Ennahli, N.; Hanine, H.; Lahlali, R.; Tahiri, A.; Fauconnier, M.L.; Madani, I.; Ennahli, S. ATR-FTIR Spectroscopy Combined with DNA Barcoding and GC-MS to Assess the Quality and Purity of Saffron (Crocus sativus, L.). Vib. Spectrosc. 2022, 123, 103446. [Google Scholar] [CrossRef]
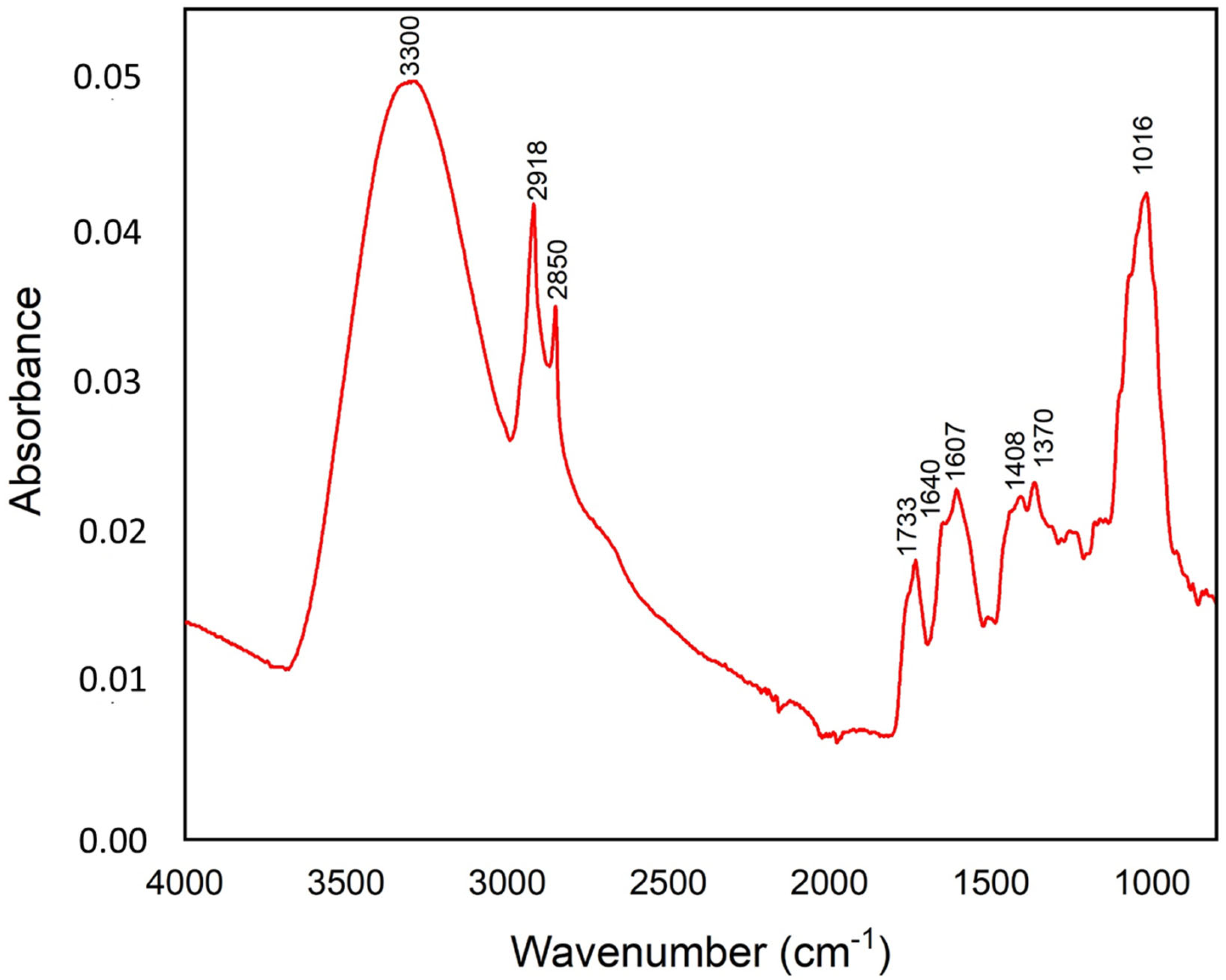
| Wavenumber (cm−1) | Group | Characteristics |
|---|---|---|
| 3300 | O-H str. of the hydroxyl group | Hydroxyl of the phenolic compounds |
| 2918 | C-H str. (asym) of CH2 | Aliphatic C-H from the lipid acyl chains |
| 2850 | C-H str. (sym) of CH2 from | Aliphatic C-H from the lipid acyl chains |
| 1733 | C-O stretching vibration | Carbonyl ester group (lipid) |
| 1640 | C=O and C=C stretching vibratons of cis-alkene | Carboxylic groups, hemicellulose or amide groups in proteins |
| 1607 | –C=C group of alkenes and conjugated C=O group | Aromatic group, phenolic ring, pectin ester group |
| 1408 | C-H stretching | Aromatic group |
| 1370 | CH2 scissors vibration | Xyloglucan, cellulose |
| 1016 | C-O stretchings | Pectins |
| SPE | Total Phenolic (mg GAE/g DW) | TFC (mg QE/g DW) | DPPH (IC50) (µg/mL) |
| 3.09 ± 0.012 | 0.92 ± 0.004 | 235.15 ± 2.12 |
| Compound | Cas Number | RI Lit | RI Calculated | % Peak Area |
|---|---|---|---|---|
| 2(5H)-Furanone | 497-23-4 | 951 | 920 | 92.10 |
| Limonene | 138-86-3 | 1029.5 | 1033 | 1.48 |
| Phenylethyl alcohol | 60-12-8 | 1114.9 | 1120 | 0.70 |
| 3,5,5-trimethyl-3-cyclohexen-1-one | 471-01-2 | 1429 | 1128 | 0.52 |
| 2,6,6-trimethyl-2-cyclohexene-1,4-dione | 1125-21-9 | 1142 | 1150 | 0.51 |
| Safranal | 116-26-7 | 1201 | 1204 | 3.56 |
| Carvone | 99-49-0 | 1242 | 1227 | 0.53 |
| Thymol | 89-83-8 | 1290.1 | 1293 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naim, N.; Fauconnier, M.-L.; Ennahli, N.; Tahiri, A.; Baala, M.; Madani, I.; Ennahli, S.; Lahlali, R. Chemical Composition Profiling and Antifungal Activity of Saffron Petal Extract. Molecules 2022, 27, 8742. https://doi.org/10.3390/molecules27248742
Naim N, Fauconnier M-L, Ennahli N, Tahiri A, Baala M, Madani I, Ennahli S, Lahlali R. Chemical Composition Profiling and Antifungal Activity of Saffron Petal Extract. Molecules. 2022; 27(24):8742. https://doi.org/10.3390/molecules27248742
Chicago/Turabian StyleNaim, Nadia, Marie-Laure Fauconnier, Nabil Ennahli, Abdessalem Tahiri, Mohammed Baala, Ilham Madani, Said Ennahli, and Rachid Lahlali. 2022. "Chemical Composition Profiling and Antifungal Activity of Saffron Petal Extract" Molecules 27, no. 24: 8742. https://doi.org/10.3390/molecules27248742
APA StyleNaim, N., Fauconnier, M.-L., Ennahli, N., Tahiri, A., Baala, M., Madani, I., Ennahli, S., & Lahlali, R. (2022). Chemical Composition Profiling and Antifungal Activity of Saffron Petal Extract. Molecules, 27(24), 8742. https://doi.org/10.3390/molecules27248742








