Aminoclay Nanoparticles Induce Anti-Inflammatory Dendritic Cells to Attenuate LPS-Elicited Pro-Inflammatory Immune Responses
Abstract
1. Introduction
2. Results
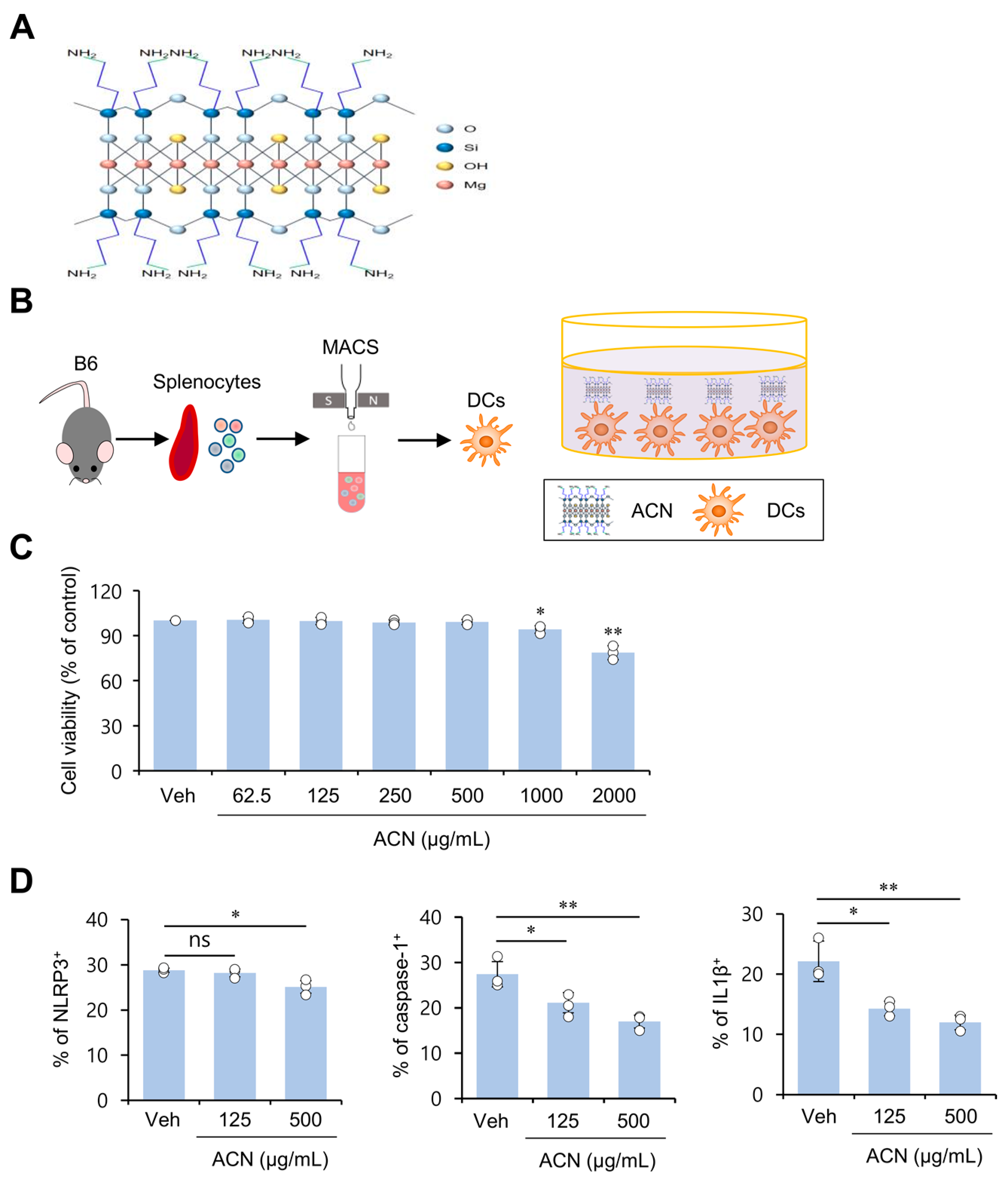
2.1. ACN Treatment Attenuates Basal Levels of Immunogenicity of Resting DCs In Vitro
2.2. ACN Treatment Down-Regulates Basal AKT/mTOR/HIF1α Signaling in DCs in a MyD88-Independent Manner In Vitro
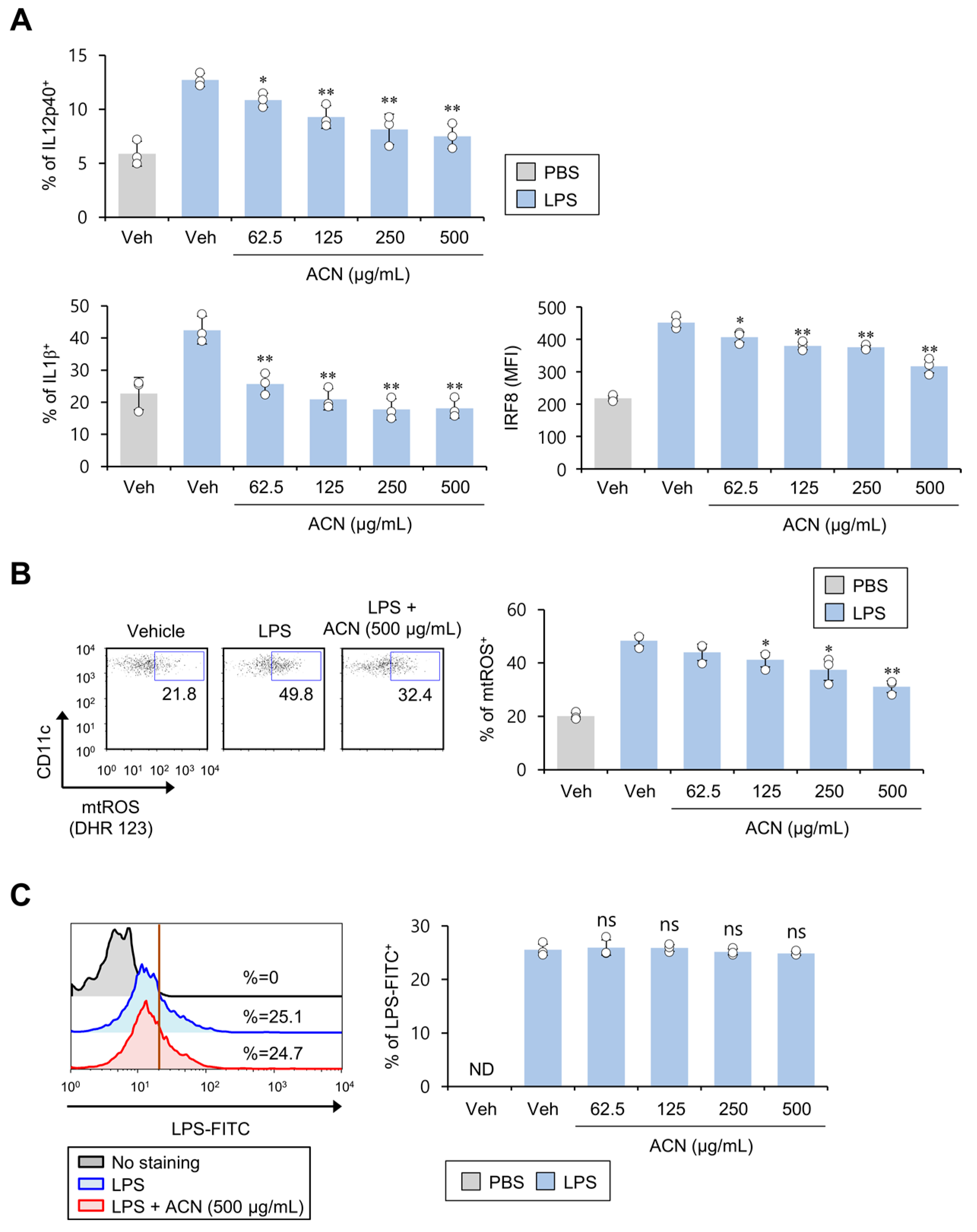
2.3. ACN Treatment Attenuates the Pro-Inflammatory Response of DCs in Response to In Vitro LPS Treatment
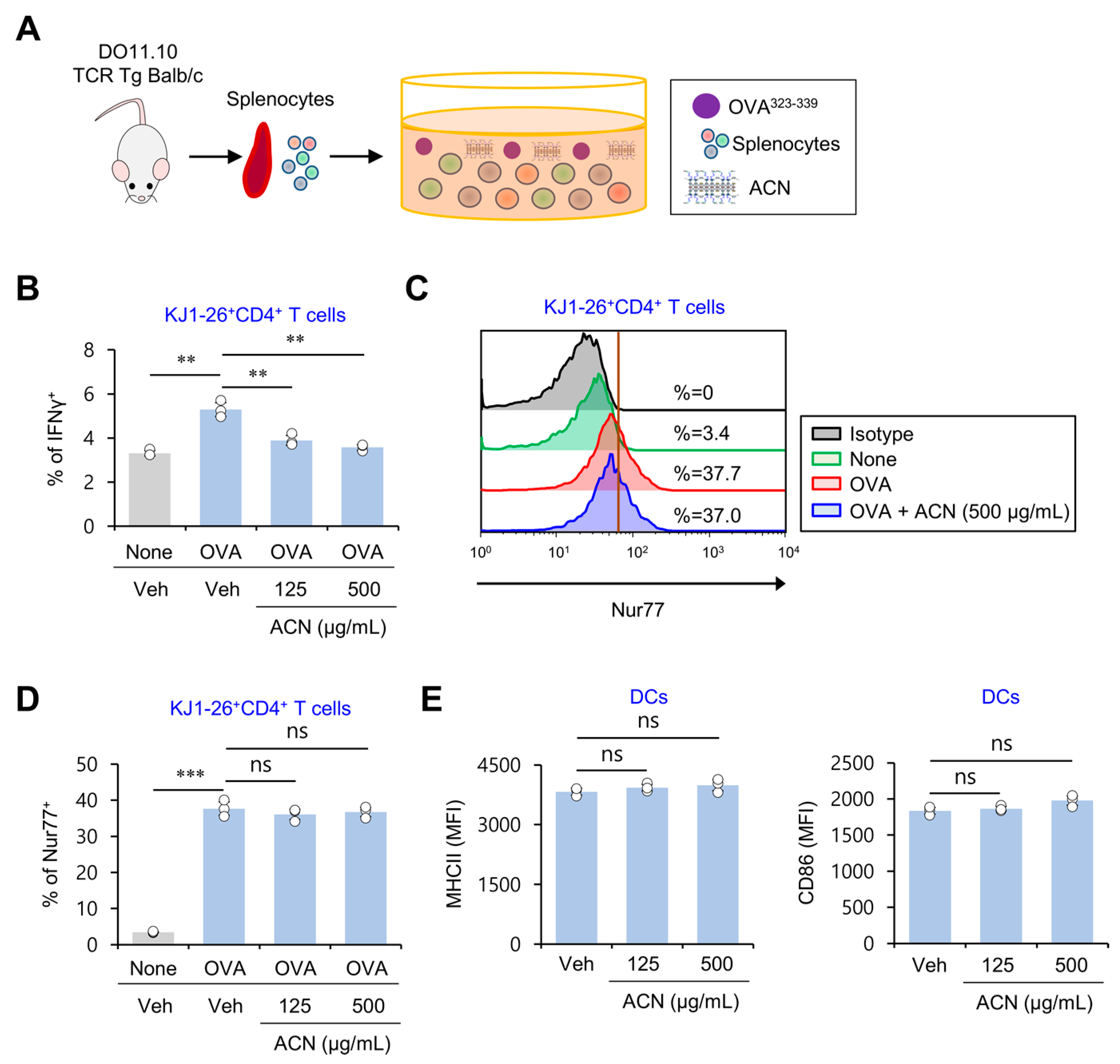
2.4. ACN Treatment Inhibits Antigen-Specific Th1 Polarization Independently of TCR Signaling
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Mice and Reagents
4.3. Preparation of ACNs
4.4. Cell Isolation and Culture
4.5. Isolation of Peritoneal Macrophages
4.6. Flow Cytometry
4.7. Intracellular Cytokine Staining
4.8. Determination of Mitochondrial ROS
4.9. Phosphoflow Analysis of Protein Phosphorylation Levels
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, H.-K.; Lee, Y.-C.; Lee, M.-Y.; Patil, A.J.; Shin, H.-J. Magnesium and Calcium Organophyllosilicates: Synthesis and In vitro Cytotoxicity Study. ACS Appl. Mater. Interfaces 2011, 3, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-K.; Yang, L.; Choi, S.-K.; Shin, H.-J. 3-aminopropyl functionalized magnesium phyllosilicate as an organoclay based drug carrier for improving the bioavailability of flurbiprofen. Int. J. Nanomed. 2013, 8, 4147–4155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, L.; Shao, Y.; Han, H.K. Improved pH-dependent drug release and oral exposure of telmisartan, a poorly soluble drug through the formation of drug-aminoclay complex. Int. J. Pharm. 2014, 471, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Song, J.G.; Lee, S.H.; Han, H.-K. Development of an M cell targeted nanocomposite system for effective oral protein delivery: Preparation, in vitro and in vivo characterization. J. Nanobiotechnol. 2021, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-A.; Lee, Y.-C.; Lee, J.-Y.; Shin, H.-J.; Han, H.-K.; Kim, G.-J. A simple bacterial transformation method using magnesium- and calcium-aminoclays. J. Microbiol. Methods 2013, 95, 97–101. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, S.-J.; Han, H.-K.; Lim, S.-J. Aminoclay as a highly effective cationic vehicle for enhancing adenovirus-mediated gene transfer through nanobiohybrid complex formation. Acta Biomater. 2017, 49, 521–530. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, D.H.; Park, H.J.; Ma, H.W.; Park, I.S.; Son, M.; Ro, S.Y.; Hong, S.; Han, H.K.; Lim, S.J.; et al. Nanocomposites-based targeted oral drug delivery systems with infliximab in a murine colitis model. J. Nanobiotechnol. 2020, 18, 133. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Ashour, D.; Arampatzi, P.; Pavlovic, V.; Förstner, K.U.; Kaisho, T.; Beilhack, A.; Erhard, F.; Lutz, M.B. IL-12 from endogenous cDC1, and not vaccine DC, is required for Th1 induction. JCI Insight 2020, 5, e135143. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.W.; Park, S.H.; Van Kaer, L.; Hong, S. Selective Expansion of Double-Negative iNKT Cells Inhibits the Development of Atopic Dermatitis in Valpha14 TCR Transgenic NC/Nga Mice by Increasing Memory-Type CD8(+) T and Regulatory CD4(+) T Cells. J. Investig. Dermatol. 2021, 141, 1512–1521. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Franchi, L.; Nunez, G. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 2013, 190, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.J.; Everts, B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015, 15, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, S.; Gotoh, K.; Nakashima, Y.; Setoyama, D.; Takata, Y.; Ohga, S.; Kang, D. Mitochondrial Reactive Oxygen Species Are Essential for the Development of Psoriatic Inflammation. Front. Immunol. 2021, 12, 714897. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Pang, E.S.; Patton, T.; O’Keeffe, M. Dendritic cell subsets. Semin. Cell Dev. Biol. 2018, 84, 11–21. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Yang, G.; Van Kaer, L. Therapeutic Targeting of Immune Cell Autophagy in Multiple Sclerosis: Russian Roulette or Silver Bullet? Front. Immunol. 2021, 12, 724108. [Google Scholar] [CrossRef]
- Ernst, O.; Glucksam-Galnoy, Y.; Athamna, M.; Ben-Dror, I.; Ben-Arosh, H.; Levy-Rimler, G.; Fraser, I.D.C.; Zor, T. The cAMP Pathway Amplifies Early MyD88-Dependent and Type I Interferon-Independent LPS-Induced Interleukin-10 Expression in Mouse Macrophages. Mediat. Inflamm. 2019, 2019, 3451461. [Google Scholar] [CrossRef]
- Luda, K.M.; Joeris, T.; Persson, E.K.; Rivollier, A.; Demiri, M.; Sitnik, K.M.; Pool, L.; Holm, J.B.; Melo-Gonzalez, F.; Richter, L.; et al. IRF8 Transcription-Factor-Dependent Classical Dendritic Cells Are Essential for Intestinal T Cell Homeostasis. Immunity 2016, 44, 860–874. [Google Scholar] [CrossRef]
- Cho, E.J.; Doh, K.-O.; Park, J.; Hyun, H.; Wilson, E.M.; Snyder, P.W.; Tsifansky, M.D.; Yeo, Y. Zwitterionic chitosan for the systemic treatment of sepsis. Sci. Rep. 2016, 6, 29739. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, H.J.; Pei, Y.; Yeo, Y.; Hong, S. Topical application of zwitterionic chitosan suppresses neutrophil-mediated acute skin inflammation. Int. J. Biol. Macromol. 2020, 158, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of Monocytes, Macrophages, and Dendritic Cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.; Hughes, J. Macrophages and dendritic cells: What is the difference? Kidney Int. 2008, 74, 5–7. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, X.-R.; Cai, L.; Zhao, X.; Dai, Z.; Wu, G.; Li, X. Putrescine mitigates intestinal atrophy through suppressing inflammatory response in weanling piglets. J. Anim. Sci. Biotechnol. 2019, 10, 69. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, H.Y. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 2012, 19, 31. [Google Scholar] [CrossRef]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine Inhibits Proinflammatory Cytokine Synthesis in Human Mononuclear Cells: A Counterregulatory Mechanism that Restrains the Immune Response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef]
- Li, G.; Ding, H.; Yu, X.; Meng, Y.; Li, J.; Guo, Q.; Zhou, H.; Shen, N. Spermidine Suppresses Inflammatory DC Function by Activating the FOXO3 Pathway and Counteracts Autoimmunity. iScience 2020, 23, 100807. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Rakshit, M.; Chua, H.M.; Darwitan, A.; Nguyen, L.T.H.; Muktabar, A.; Venkatraman, S.; Ng, K.W. Liposome interaction with macrophages and foam cells for atherosclerosis treatment: Effects of size, surface charge and lipid composition. Nanotechnology 2021, 32, 505105. [Google Scholar] [CrossRef]
- Filion, M.C.; Phillips, N.C. Anti-inflammatory activity of cationic lipids. Br. J. Pharmacol. 1997, 122, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, T.C.; Park, Y.H.; Lee, S.W.; Jeon, J.; Park, S.H.; Van Kaer, L.; Hong, S. Repeated alpha-GalCer Administration Induces a Type 2 Cytokine-Biased iNKT Cell Response and Exacerbates Atopic Skin Inflammation in Valpha14(Tg) NC/Nga Mice. Biomedicines 2021, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.; Jeon, J.; Park, Y.; Kim, T.-C.; Jeon, S.; Seong, R.; Van Kaer, L.; Hong, S. Ubiquitous Overexpression of Chromatin Remodeling Factor SRG3 Exacerbates Atopic Dermatitis in NC/Nga Mice by Enhancing Th2 Immune Responses. Int. J. Mol. Sci. 2021, 22, 1553. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; Van Kaer, L.; Hong, S. Roles and therapeutic potential of CD1d-Restricted NKT cells in inflammatory skin diseases. Front. Immunol. 2022, 13, 979370. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.W.; Hong, S. Regulation of Allergic Immune Responses by Microbial Metabolites. Immune Netw. 2018, 18, e15. [Google Scholar] [CrossRef]
- Williams, J.W.; Tjota, M.Y.; Clay, B.S.; Vander Lugt, B.; Bandukwala, H.S.; Hrusch, C.L.; Decker, D.C.; Blaine, K.M.; Fixsen, B.R.; Singh, H.; et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 2013, 4, 2990. [Google Scholar] [CrossRef]
- Ju, A.; Lee, S.W.; Lee, Y.E.; Han, K.-C.; Kim, J.-C.; Shin, S.C.; Park, H.J.; Kim, E.E.; Hong, S.; Jang, M. A carrier-free multiplexed gene editing system applicable for suspension cells. Biomaterials 2019, 217, 119298. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.W.; Park, Y.H.; Kim, T.C.; Van Kaer, L.; Hong, S. CD1d-independent NK1.1(+) Treg cells are IL2-inducible Foxp3(+) T cells co-expressing immunosuppressive and cytotoxic molecules. Front. Immunol. 2022, 13, 951592. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J.; Lee, S.W.; Song, J.G.; Van Kaer, L.; Cheon, J.H.; Lim, S.-J.; Han, H.-K.; Hong, S. Aminoclay Nanoparticles Induce Anti-Inflammatory Dendritic Cells to Attenuate LPS-Elicited Pro-Inflammatory Immune Responses. Molecules 2022, 27, 8743. https://doi.org/10.3390/molecules27248743
Park HJ, Lee SW, Song JG, Van Kaer L, Cheon JH, Lim S-J, Han H-K, Hong S. Aminoclay Nanoparticles Induce Anti-Inflammatory Dendritic Cells to Attenuate LPS-Elicited Pro-Inflammatory Immune Responses. Molecules. 2022; 27(24):8743. https://doi.org/10.3390/molecules27248743
Chicago/Turabian StylePark, Hyun Jung, Sung Won Lee, Jae Geun Song, Luc Van Kaer, Jae Hee Cheon, Soo-Jeong Lim, Hyo-Kyung Han, and Seokmann Hong. 2022. "Aminoclay Nanoparticles Induce Anti-Inflammatory Dendritic Cells to Attenuate LPS-Elicited Pro-Inflammatory Immune Responses" Molecules 27, no. 24: 8743. https://doi.org/10.3390/molecules27248743
APA StylePark, H. J., Lee, S. W., Song, J. G., Van Kaer, L., Cheon, J. H., Lim, S.-J., Han, H.-K., & Hong, S. (2022). Aminoclay Nanoparticles Induce Anti-Inflammatory Dendritic Cells to Attenuate LPS-Elicited Pro-Inflammatory Immune Responses. Molecules, 27(24), 8743. https://doi.org/10.3390/molecules27248743









