Abstract
Biodiesel is one of the important biofuels as an alternative to petroleum-based diesel fuels. In the current study, enzymatic transesterification reaction was carried out for the production of biodiesel from waste cooking oil (WCO) and experimental conditions were optimized, in order to reach maximum biodiesel yield. Bacillus stearothermophilus and Staphylococcus aureus lipase enzymes were individually immobilized on CaCO3 to be used as environmentally friendly catalysts for biodiesel production. The immobilized lipases exhibited better stability than free ones and were almost fully active after 60 days of storage at 4 °C. A significant biodiesel yield of 97.66 ± 0.57% was achieved without any pre-treatment and at 1:6 oil/methanol molar ratio, 1% of the enzyme mixture (a 1:1 ratio mixture of both lipase), 1% water content, after 24 h at 55 °C reaction temperature. The biocatalysts retained 93% of their initial activities after six cycles. The fuel and chemical properties such as the cloud point, viscosity at 40 °C and density at 15 °C of the produced biodiesel complied with international specifications (EN 14214) and, therefore, were comparable to those of other diesels/biodiesels. Interestingly, the resulting biodiesel revealed a linolenic methyl ester content of 0.55 ± 0.02% and an ester content of 97.7 ± 0.21% which is in good agreement with EN14214 requirements. Overall, using mixed CaCO3-immobilized lipases to obtain an environmentally friendly biodiesel from WCO is a promising and effective alternative for biodiesel production catalysis.
1. Introduction
One of the fundamental building blocks for a nation’s economic and social development is energy. Currently, non-renewable sources supply the majority, approximately 78%, of the world’s energy needs [1]. These resources, now limited in supply, are being consumed swiftly. Immense amounts of carbon dioxide (CO2) and greenhouse gas emissions (GHGs) from different anthropogenic activities continue to trigger environmental pollution, environmental deterioration and global warming [2,3]. As a result, lowering these emissions is essential, making it imperative to seek alternative non-fossil fuel-based energy systems that are environmentally safe, inexpensive, sustainable, and renewable [1]. For example, switching to biodiesel from petroleum-based fuels could be a feasible way to reduce CO2 and other GHG emissions. Biodiesel is a sustainable, clean-burning liquid fuel that can be produced by trans-esterifying biomass sources, including used cooking oil. Because biodiesel made from ordinary foods is more expensive than regular diesel, it is critical to concentrate on looking for cheap and easily accessible food sources in order to address this issue [4]. Waste materials such as used cooking oil are viable alternatives for the manufacturing of reasonably priced and ecologically friendly biodiesel [5]. Globally, more than 15 million tons of cooking oil are generated annually; using it to produce biodiesel would lower the environmental pollution issues associated with its disposal. The production of biodiesel fuel from waste cooling oils (WCOs) is accomplished by utilizing the lipase enzymes [6]. Lipases are the most significant class of biocatalysts, which catalyze the hydrolysis of the glycerol esters in long-chain triglycerides [4,5,6,7]. They react with the triacylglycerol’s carboxyl ester linkages, releasing fatty acids and glycerol as well as the reverse reaction, synthesis of esters from fatty acids and alcohol [7].
The rapid advancement of enzyme technology has drawn much attention to microbial lipases. These latter are more valuable than those derived from plants or animals because of the wide range of catalytic activities they can perform, the ease of genetically manipulating them, their high yield production, the lack of seasonal variations, the regular supply, and the extremely high growth rates of microorganisms in economically advantageous media. Several bacterial strains, including Pseudomonas alcaligenes, P. aeruginosa, P. fragi, P. fluorescens BJ-10, Bacillus subtilis, and B. nealsonii S2MT, as well as various fungi, including Penicillium expansum, P. chrysogenum, and Trichoderma, are known to produce in large quantities [7].
The main obstacle to using lipase as a biocatalyst to produce biodiesel fuel is its production cost. This issue could be solved by using immobilized enzymes. Large surface area, low cost, re-useability, good chemicals, mechanical and thermal stability and insolubility are all important qualities of solid supports utilized for immobilization [8]. Additionally, immobilization makes it simple to separate the enzyme from the final product, which reduces or completely eliminates protein contamination [9,10]. The most practical and economical methods of immobilizing enzymes continue to be adsorption onto solid supports or entrapment in bio-polymer matrixes, such as calcium alginate and carbonate. It has been proposed that immobilized lipase improves mass transfer by dispersing the enzyme over a large surface area and preventing enzyme particles from aggregating [11]. Calcium carbonate (CaCO3), as an immobilized phase, has the advantage of being non-toxic and unreactive to chemicals [12,13]. The benefit of using such an immobilization approach is that the enzyme does not chemically interact with the polymer, perhaps preventing denaturation. In our previous study, CaCO3 was successfully used to immobilize Bacillus stearothermophilus (B. stearothermophilus) and Staphylococcus aureus (S. aureus) lipases [14,15]; however, it has not been applied in biodiesel synthesis so far. Interestingly, some studies demonstrated that the use of a combination of lipases significantly increase the yield biodiesel production [16,17]. Therefore, the current study aimed to investigate the effects of single and mixed CaCO3-immobilized lipases on biodiesel production. After optimization of experimental conditions, the characteristics of the WCO biodiesel obtained by using CaCO3-immobilized lipase enzymes were evaluated and compared with the international specifications.
2. Results and Discussion
WCO is a hazardous waste produced at significant levels all over the world. However, discarding WCO into various ecosystems, such as water and soil, may cause dangerous environmental consequences. In addition, given its energy content, misconduct of this hazardous waste could also be considered as a loss of resources. Therefore, looking for eco-friendly and cost-effective alternative routes to manage and valorize WCO, for instance conversion into biodiesel by enzymatic transesterification, has been extensively sought and has encouraged researchers to emphasis on emerging renewable and clean energy to overwhelmed fossil fuel supplies depletion and the global warming phenomena.
The main factors affecting the transesterification process are oil to alcohol molar ratio, type and amount of the biocatalyst, incubation temperature, reaction time, free fatty acids (FFA) content and water in substrate oil as well as the stirring speed during the chemical reaction. Table 1 summarized the main characteristics of WCO including water and free fatty acid contents, saponification, iodine and acid values. The WCO was not suitable for transesterification using an alkali catalyst because the acid content was notably high (0.85 ± 0.02%).

Table 1.
Physicochemical characterization of waste cooking oil (WCO). Values are the averages of triplicate determinations ± standard deviation (SD).
Furthermore, an FFA content of more than 3% results in the formation of soap and water [18]. However, if enzymatic catalysts are used, transesterification is not affected by a high FFA in the raw materials [19]. Recorded data showed that the water content of the WCO was 0.09 ± 0.002%, and levels below 0.5% are known to be suitable for biodiesel production [20,21]. Moreover, the WCO saponification value (190 ± 3 mg KOH/g) was consistent with previously reported ranges (188–207 mg KOH/g) (Table 1) [22]. Therefore, based on its studied properties, the WCO obtained after a simulated cooking of olive oil was suitable for biodiesel production using enzymatic transesterification.
Thanks to its low-cost, methanol, the highly reactive alcohol, is commonly used for biodiesel production by transesterification reaction which requires 3 mol of alcohol per 1 mol of triacylglycerol to produce 3 mol of fatty acid methyl ester (FAME) and 1 mol of glycerol. Since it is highly recommended for base catalyst reactions to use an excess methanol: oil ratio [22], the effect of several oil/methanol molar ratios (ranging from 3:1 to 18:1) on the biodiesel yield, produced by using individual immobilized lipase, was investigated. Obtained data are illustrated in Figure 1 and clearly showed that the highest biodiesel yield values of 90 ± 2.64% and 88.66 ± 2.3% were achieved using a ratio of 6:1 by Bacillus and Staphylococcus lipases, respectively (Figure 1).
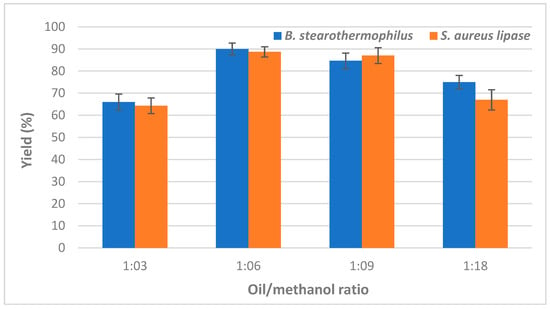
Figure 1.
Effect of oil to methanol molar ratio on CaCO3-immobilized-lipase catalyzed transesterification of waste cooking oil (WCO) for biodiesel synthesis. Experimental conditions were: reaction temperature 45 °C, reaction time 24 h, water content 5%, lipase loading 1%, agitation speed 150 rpm. Experiments were performed in triplicate and error bars indicate mean ± standard deviation.
Indeed, esterification and transesterification are reversible processes that occur simultaneously, and excess methanol is used to produce esters [23,24]. However, the lower biodiesel yields recorded for both lipases as the oil:methanol molar ratio further increases could be attributed to the denaturation and inactivation of the enzyme exposed to higher methanol concentrations [25]. Moreover, the amount of methanol has been previously reported to depend on the catalyst and substrate used [26]. For further experiments, a molar ratio of 1:6 was used.
Although most biodiesel is produced using only one lipase, some reports have demonstrated that enzyme mixtures increase biodiesel yields as the specificity of the lipase depends on the enzyme source and the substrate [27]. In the current study, B. stearothermophilus and S. aureus lipases were first individually immobilized on CaCO3, and the effects of immobilized single and mixed lipases were investigated at a 1% enzyme loading (Table 2).

Table 2.
Effect of CaCO3-immobilized individual and mixed lipases on the transesterification of waste cooking oil (WCO) under the following experimental conditions: enzyme loading 1% wt, 1:6 oil to methanol molar ratio, 5% water content, a 45 °C temperature reaction, a 24 h reaction time and 150 rpm agitation. Values are the mean of the three replicates ± SD.
One can see that the lowest biodiesel yields were recorded when single immobilized S. aureus (88.66 ± 2.3%) or B. stearothermophilus (90 ± 2.6%) lipases were used. However, the highest biodiesel yield (92.66 ± 1.15%) was obtained when 1% of mixed immobilized B. stearothermophilus and S. aureus (1:1) were used. The use of mixed lipases at ratios different from 1:1 also showed lower yields (89–90%) (Table 2). This finding clearly showed that using immobilized lipase mixtures for biodiesel production was remarkably more efficient than using individual immobilized lipases suggesting that co-immobilization could be a promising alternative to optimize biodiesel production. In fact, rapid conversions could be achieved by co-immobilization of the specific enzymes within the pathway because of the localized high levels of the generated intermediates. Interestingly, the apparent lag phase is notably reduced in the presence of more enzymes in the pathway [28] which could be attributed to the synergistic activity of enzymes to break down the complex composition of WCO [29]. In the current study, the biodiesel production yield (92.66 ± 1.15%) was remarkably higher than that obtained from co-immobilization of C. antarctica and R. miehei lipases (78.4%) [30], whereas Binhayeeding et al. [16] reported a higher biodiesel production yield (96.5%) from WCO using a mixture of polyhydroxybutyrate-immobilized C. rugosa and R. miehei lipases (1:1) [16]. Similarly, the highest FAME conversion (97.2%) was obtained using a mixture of C. antarctica and P. cepacia lipases at a 25:75 ratio and microalga lipids as a substrate [27]. To our knowledge, this is the first report of biodiesel production from WCO using a combination of B. stearothermophilus and S. aureus lipases immobilized on CaCO3 particles at a ratio of 1:1 (50% of each enzyme) and resulting in the highest biodiesel yields. For further experiments, this ratio (1:1) was therefore selected.
The effects of enzyme loading on biodiesel production from WCOs were also investigated and obtained data are shown in Figure 2. The amount of CaCO3-immobilized lipase ranged from 1 to 4%. B. stearothermophilus and S. aureus lipases were mixed at a 1:1 ratio, while other factors including methanol:oil ratio (6:1), total reaction time (24 h), water content (5%), stirring speed (150 rpm) and temperature (45 °C) were kept constant. After 24 h, the highest biodiesel yield (92.66 ± 1.15%) was observed at a 1% enzyme loading (Figure 2).
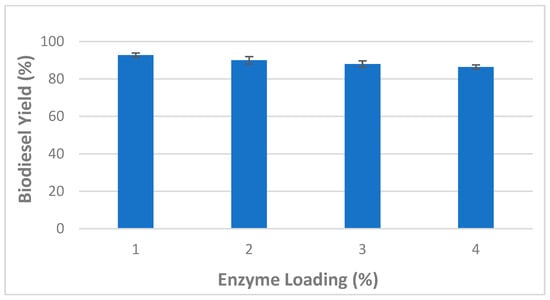
Figure 2.
Effect of mixed CaCO3-immobilized lipases loading on the transesterification process of waste cooking oil (WCO) for biodiesel synthesis. Experimental conditions were: methanol/oil molar ratio: 6:1, reaction temperature: 45 °C, reaction time 24 h, water content 5%, agitation speed 150 rpm. Experiments were performed in triplicate and error bars indicate mean ± standard deviation.
However, the gradual decrease of the biodiesel yield observed above this value (>1%) could be explained by the interference of enzymes, at higher levels, with the active sites of substrates causing their aggregation [31]. Therefore, a 1% CaCO3-immobilized lipase loading rate was found optimum for biodiesel production under the experimental conditions of the current study.
It is well established that the conversion of triglycerides to biodiesel is also influenced by the water level which can affect enzyme activity equilibrium value [32,33].
Consequently, adequate water content is necessary to maintain a high enzyme activity while using organic solvents. Accordingly, the effect of different water contents (varying from 0 to 20%) on the transesterification of WCO was also investigated using CaCO3-immobilized lipases (at a methanol/oil molar ratio of 6:1, 1% of the enzyme mixture, after 24 h and at a temperature of 45 °C) (Figure 3).
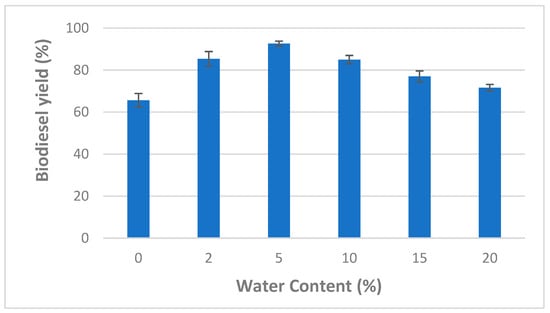
Figure 3.
Effect of water content on mixed CaCO3-immobilized-lipase catalyzed transesterification process of waste cooking oil (WCO) for biodiesel synthesis. Experimental conditions were: methanol/oil molar ratio: 6:1, lipase loading 1%, reaction temperature: 45 °C, reaction time 24 h, agitation speed 150 rpm. Experiments were performed in triplicate and error bars indicate mean ± standard deviation.
One can see from Figure 3 that the highest biodiesel yield (92.66 ± 1.15%) was observed at a water concentration of 5%. However, at higher water content values (>5%), the biodiesel production decreased markedly. Similarly, previous studies reported that maximal lipase activity is obtained at a water content ranging from 2 to 5% [16,34,35]. In fact, lipase enzymes are able to act at the interface between the aqueous and the organic phase. Therefore, enzyme activity could be affected by increasing water contents which, in turn, could increase the interfacial area [36,37]. To optimize biodiesel production by CaCO3 immobilized lipases, water content of 5% was used for further experiments.
Furthermore, the effect of incubation temperature ranging from 45 to 70 °C was also investigated and obtained results are illustrated in Figure 4. It was observed that there was a significant increase in the conversion of the triglycerides to methyl esters as incubation temperature increased showing the maximum average conversion yield (97.33 + 1.15%) at an incubation temperature of 55 °C. Above 55 °C, biodiesel production yield markedly decreased due to the protein denaturation and reduced catalytic activity [38] (Figure 4).
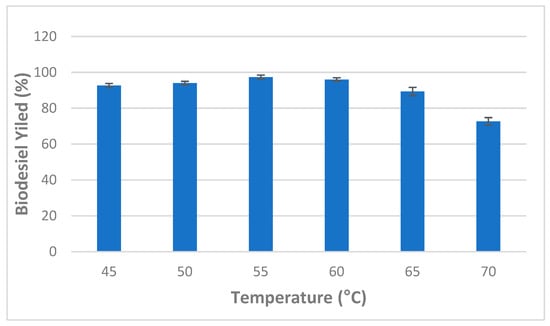
Figure 4.
Effect of incubation temperature on mixed CaCO3-immobilized-lipase catalyzed transesterification process of waste cooking oil (WCO) for biodiesel synthesis. Experimental conditions were: methanol/oil molar ratio: 6:1, lipase loading 1%, water content 5%, reaction time 24 h, agitation speed 150 rpm. Experiments were performed in triplicate and error bars indicate mean ± standard deviation.
Binhayeeding et al. [16] reported that 45 °C was found to be the optimum temperature for the production of biodiesel using PHB-immobilized mixed C. rugosa and R. miehei lipases [16]. Similarly, Tran et al. [24] found that biodiesel production using C. vulgaris ESP-31 lipase decreased when temperature values increased up to 50 °C. In this study, subsequent experiments were carried out at 55 °C [24].
Moreover, several studies demonstrated that high biodiesel production yields might be reached by immobilized enzymes using an optimized agitation speed. In this regard, the effect of various agitation speeds, ranging from 100 to 300 rpm, on biodiesel production was also evaluated and obtained data are shown in Figure 5. As we can see, the highest percentage yield of biodiesel (97.66 ± 0.57%) was attained using an agitation speed of 200 rpm (Figure 5).
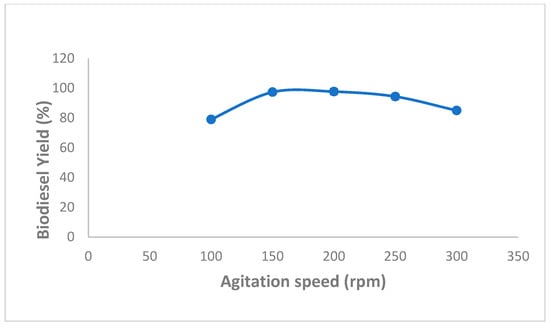
Figure 5.
Effect of speed agitation on mixed CaCO3-immobilized-lipase catalyzed transesterification process of waste cooking oil (WCO) for biodiesel synthesis. Experimental conditions were: methanol/oil molar ratio: 6:1, lipase loading 1%, water content 5%, reaction temperature 55 °C, reaction time 24 h. Experiments were performed in triplicate and error bars indicate mean ± standard deviation.
This finding could be explained by the fact that an increased agitation speed might reduce mass transfer resistance between oil and methanol and increase the reaction rate [24,39,40]. However, at an agitation speed above 200 rpm, biodiesel yields were significantly reduced, which could most likely be attributed to the agitation-associated mechanical damage to the immobilized enzymes.
Based on the importance of the reaction time as one of the most important factors that greatly influence the reaction equilibrium, biodiesel production from WCO using CaCO3-mixed immobilized B. stearothermophilus and S. aureus lipases was followed over a reaction time of 0–48 h. Results presented in Figure 6 clearly showed that as the reaction time increased, the biodiesel production yield increased to reach a maximum of 97.66 ± 0.57% after a 24 h reaction period (Figure 6). In contrast, after 24 h, The remarkable decrease in production rates was probably due to the substrate lack. Interestingly, at 1% enzyme loading, biodiesel yields reached average values up to 56 ± 1% and 67.66 ± 2.55% after 3 h and 6 h, respectively, indicating a fast kinetics of CaCO3-mixed immobilized B. stearothermophilus and S. aureurs lipases (Figure 6).
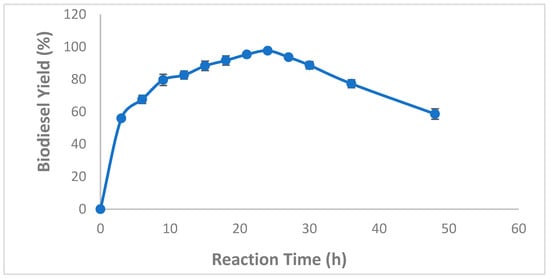
Figure 6.
Effect of reaction time on mixed CaCO3-immobilized-lipase catalyzed transesterification process of waste cooking oil (WCO) for biodiesel synthesis. Experimental conditions were: methanol/oil molar ratio: 6:1, lipase loading 1%, water content 5%, reaction temperature 55 °C, agitation speed 150 rpm. Experiments were performed in triplicate and error bars indicate mean ± standard deviation.
Our finding collaborated with previous findings demonstrating that the optimum reaction time generally ranges from 24–48 h depending on the raw material used and the enzyme source [16,18,41]. It is worthy to note that short reaction times offer several advantages such lower energy consumption, thereby facilitating large-scale production.
The use of immobilized lipases as biocatalysts displays great potential to ameliorate the environmental sustainability and efficiency of several industrial processes, in particular biodiesel production industry. Interestingly, Zhang et al. [42] reported that CaCO3 adsorption technique exhibits exceptional reusable ability and mechanical rigidity for many cycles of the catalyzed reaction [42]. Thus, the reusability of immobilized lipase is of a particular importance to ascertain the feasibility of industrial-scale enzymatic biodiesel production [43]. The reusability of immobilized lipase mixture was therefore tested over 14 reaction cycles (Figure 7). First, 91 ± 2.64% of the original lipase activity was maintained even after 6 cycles, then approximately 55% of the initial lipase activity was recorded after 10 cycles indicating a promising potential reusability of the lipase mixture under the optimized experimental conditions, probably due to the enzyme interaction with the support enhancing its stability. However, after 12 cycles, the enzyme activity diminished remarkably likely due to methanol-induced conformational change and/or inactivation of lipase after numerous reaction cycles and the de-sorption, rupture of chemical bonds or erosion of the support material during the washing and recovery processes [44,45] (Figure 7).
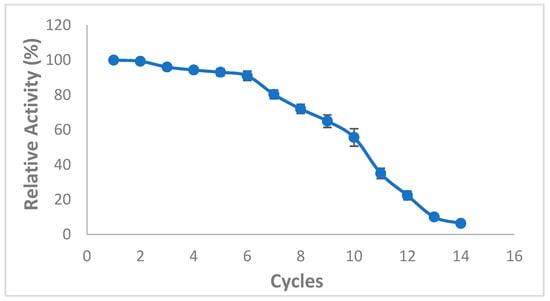
Figure 7.
Effect of mixed CaCO3-immobilized-lipases reuse on the transesterification of waste cooking oil (WCO) for biodiesel synthesis. The following experimental conditions were used: methanol/oil molar ratio: 6:1, lipase loading 1%, water content 5%, reaction temperature 55 °C, reaction time 24 h, agitation speed 200 rpm. 100% is the biodiesel yield of the first reaction, while the successive reaction yields were calculated accordingly. Experiments were performed in triplicate and error bars indicate mean ± standard deviation.
It is well documented that the long-term storage of immobilized lipase enzymes for future use is among the most important factor for their industrial applications which is considered a significant advantage of immobilized lipases over free ones [14,16,46]. In the current report, both CaCO3-immobilized B. stearothermophilus and S. aureus lipases exhibited an enhanced storage capacity compared to free enzymes. Additionally, both mixed and single immobilized lipases displayed comparable storage capacities. Both immobilized lipases were almost fully active after storage for 60 days at 4 °C and then lost up to 47% activity after 150 days of storage (Figure 8). However, free enzymes retained only ~50% of their initial activities after 90 days and almost full activity was lost after 150 days of storage. Interestingly, even stored at 25 °C, both CaCO3-immobilized enzymes were almost fully active after 20 days compared to free lipases retaining only about 50% of their respective original activities.
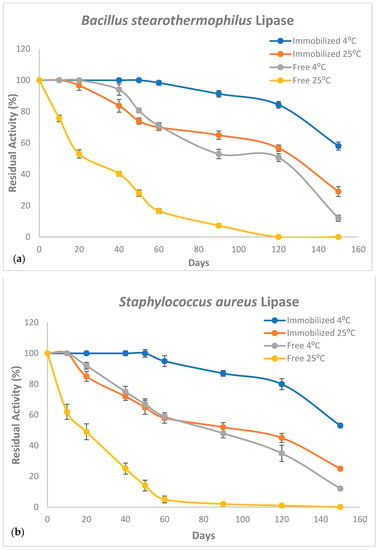
Figure 8.
Activity retention rates (%) of CaCO3-immobilized and free and B. stearothermophilus (a) and S. aureus (b) lipases at 25 °C and 4 °C. Measurements were performed in triplicate and error bars indicate mean ± standard deviation.
GC-MS analyses of derivatized biodiesel, derivatized waste cooking oil and olive oil were carried out to check the efficacy of the biodiesel synthesis. Hence, FAMEs composition of the produced biodiesel was determined by comparing with the retention times of derivatized WCOs and of a pure FAME standard (C8–C24 standard, Sigma Aldrich, St. Louis, MO, USA). As shown in Table 3, before cooking simulation, the olive oil contained 15 fatty acids, including: myristic, pentadedecylic, palmitic, margaric, stearic, arachidic, behenic, lignoceric, palmitoleic, oleic, Cis-vaccenic, gondoic, erucic, linoleic, and linolenic acids. However, novel compounds, generated by the cooking process (caprylic acid, capric acid, lauric acid, octadecanoic acid, stearic acid allyl 9.10 epoxy, octadecanoic acid 15.16 epoxy, and 14-methylhexadecanoic acid) were observed after cooking simulation.

Table 3.
Fatty acid compositions (%) of olive oil from Sigma Aldrich, waste cooking oil (WCO), and produced biodiesel with of 1% of immobilized lipase mixture concentration.
One can notice, in particular, the palmitoleic acid decrease against the appearance of 14-methylhexadecanoic acid together with the increase in palmitic acid (Table 3). Moreover, methylation of stearic acid likely resulted in formation of octadecanoic acid 15.16 epoxy and octadecanoic acid 9.10 epoxy probably generated from epoxidation of linolenic and linoleic acid which happens during cooking process. On the other hand, the ability of the biocatalyst to convert the oil fatty acids into methyl esters could be clearly shown from the comparison between the GC-MS analyses of derivatized biodiesel and WCOs (Table 3).
It is also worthy to note that a reduced catalytic activity was recorded as fatty acid chains length increases. The quantities of both octadecanoic acid 15.16 epoxy and octadecanoic acid 9.10 epoxy rise just seemingly, as they now weigh on an esterified smaller fraction of the initial oil. Ultimately, the difference between 100% and the calculated biodiesel yields, after 24 h, is attributed to the existence of unconverted acids, which quantity is more pronounced the longer the chains to be converted. Biodiesel produced from waste cooking oil using mixed CaCO3-immobilized B. stearothermophilus and S. aureus, after 24 h of synthesis, showed an amount of 0.55 ± 0.02% of linolenic methyl ester, which is in line with the EN14214. Likewise, ester content was calculated according to the modified protocol of EN14214, was found to be equal to 97.7 ± 0.21% which is in good agreement with the EN14214 as well. Furthermore, the determined iodine value (65.3 ± 1.15 g iodine/100 g) using EN 14214 was also found to be in line with the European standard. Due to the variety, complexity, and origin of the raw material, it is more essential, in this case, to validate the feasibility of WCOs oil biodiesel as fuel through comparison with the standard which is typically a key aspect. Interestingly, in the present study, the biodiesel conversions yield (97.66 ± 0.57%) is significantly high and comparable to previous reports on various oils [16,47,48,49]. However, a pre-treatment of the cooking oil may be required to improve the conversion yield and to respect the standard as well. Table 4 showing the recorded data of biodiesel characterization clearly demonstrates the feasibility of WCOs biodiesel as fuel.

Table 4.
Characterization of the biodiesel produced from waste cooking oil (WCO).
3. Materials and Methods
3.1. Physicochemical Properties of WCOs
WCOs were obtained after a simulated cooking of olive oil (Sigma Aldrich-O1514, analytical grade) for 5 h at 180 °C [51]. Before the biodiesel production, WCOs physicochemical properties including: water and free fatty acid contents, acid, iodine and saponification values were determined and analyzed in accordance with EN14214. All experiments were carried out in triplicate and mean ± standard deviation (SD) values were shown in Table 1.
3.2. Production and Immobilization of Bacterial Lipases
B. stearothermophilus and S. aureus lipases were produced and partially purified by two steps (precipitation with 65% ammonium sulfate followed by thermal treatment at 70 °C for 15 min) as previously described [52,53]. Then, both enzymes were immobilized on CaCO3 according to Rosu et al. [54] as reported in our previous works [14,15,54]. Briefly, 4500 U or 3500 U of lipase solutions from B. stearothermophilus and S. aureus, respectively, was first mixed with 1 g of the support and then kept under stirring at 4 °C for 1 h. The enzyme activity was measured in the supernatant obtained after a centrifugation (5 min at 8000 rpm). Finally, the enzyme solution filtrate was washed with double-distilled water three times and dried at room temperature for 8 h in vacuum desiccators. The resulting immobilized B. stearothermophilus and S. aureus lipases had high immobilization yields (~95%) and protein loading capacity (2.75 mg/g of immobilizing agent). Measurement of the immobilized lipase activities were carried out using a pH-stat and olive oil emulsion as a substrate under appropriate standard conditions of pH and temperature [55]. All experiments were carried out in triplicate.
3.3. Optimization of Biodiesel Synthesis Process from WCO
Optimization of enzymatic biodiesel production was carried out in a 250 mL screw-capped flask. The effects of mixed and individual immobilized lipases were first examined by mixing WCO (10 g) and enzyme solution (1% wt; oil basis) while fixing the other parameters. The process was carried out for 24 h at 150 rpm, a 45 °C temperature, a methanol to oil molar ratio of 6:1 and 5% water content. After that, methanol to oil molar ratio (3:1–18:1), enzyme loading (1–4% wt), water content (0–5%), incubation temperature (45–70 °C), reaction time (1–48 h) and mixing speed (100–300 rpm) were varied. At the end of each batch, the obtained product was separated by centrifugation (20 min, 8500 rpm) and left for 1 to 2 h in a separating funnel till the resulting biodiesel was totally separated and situated in the upper layer. Next, collected biodiesel was washed twice with 20 mL hot deionized water, followed by oven-drying for 24 h at 105 °C [56].
The biodiesel yield (oil conversion to methyl ester) was determined as the ratio of the ester mass divided by the oil mass (%). The analysis of the fatty acids methyl esters (FAME) produced was performed by a gas chromatography–mass spectrometry (GC–MS) using a QP2010 Ultra chromatograph (Shimadzu, Japan) equipped with a model QP2010 mass spectrometer quadrupole detector. A Rxi-5Sil MS column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness). GC-MS configuration: initial temperature 120 °C for 4 min, rate 1, 6.5 °C/min to 170 °C, rate 2, 2.75 °C/min to 250 °C for 9 min. Injector and detector temperatures were set 250 °C and 230 °C, respectively. Helium was used as the carrier gas. Composition of FAMEs obtained from WCOs derivatization using Methanol:BF3 method [57] was compared with the biodiesel produced and a known concentration FAME mixture. EN14214 was used to evaluate methyl ester content in the produced biodiesel [58]. Measurements were performed in triplicate.
3.4. Reuse of Immobilized Lipases
Immobilized enzymes’ reusability was assessed by recovering them after each transesterification reaction and utilizing them for subsequent cycles under the same conditions. The immobilized lipase was filtered at the end of each cycle, using a paper filter, then washed twice with ultrapure water and hexane and finally dried for 24 h in a desiccator prior to use. After that, both enzyme and the substrate were used again for 14 cycles under optimal experimental conditions. A total of 100% is the biodiesel yield of the first reaction, while the successive reaction yields were calculated accordingly.
3.5. Storage Stability
The stabilities of the immobilized mixed and individual lipases were determined by calculating the residual activity over a period of 150 days. Both free and immobilized (in wet form) enzymes were stored at 4 °C or 25 °C in a 0.2 M Tris-HCL buffer (pH 8).
4. Conclusions
Enzymatic catalysts have recently received increased attention in biodiesel production industry by employing immobilized lipases as biocatalysts. Herein, biodiesel production was effectively improved using a mixture of CaCO3-immobilized B. stearothermophilus and S. aureus lipases through the enzymatic transesterification of WCO. The best biodiesel production yield (97.66 ± 0.57%) was attained under the optimal experimental conditions: a methanol: oil ratio of 6:1, enzyme loading 1% (50% of each lipase), 1% water, reaction temperature of 55 °C for 24 h under continuous stirring speed of 200 rpm. Similarly, to previous studies, higher biodiesel yields were achieved by using mixture of immobilized stearothermophilus and S. aureus lipases than either of the lipases alone. Moreover, the characteristics of the biodiesel described in the current study were comparable to those of commercial biodiesel and in agreement with the international requirements (EN 14214). Collectively, our data demonstrated that CaCO3-immobilized lipases mixtures are extremely versatile, require slight amounts of biocatalyst, process a rapid production time, accelerate substrates’ conversion into products, and could be easily recycled. The interesting characteristics of enzymes described is this study as well as their biodiesel production yields open the door to using them as biocatalysts to synthesize other biodiesel such as ethyl esters or a mixture of methyl and ethyl esters as described by Yussof et al. [59] and other high value-added products using other residual materials in a context of circular bioeconomy.
Author Contributions
Conceptualization, A.B.B. and M.A.; methodology, M.A. and I.B.A.; software, A.B.B. and M.G.A.; validation, I.B.A., M.A. and A.B.B.; formal analysis, A.B.B., H.H. and I.B.A.; investigation, I.B.A. and H.H.; resources, M.A. and M.G.A.; data curation, A.B.B. and M.A.; writing—original draft preparation, A.B.B. and M.G.A.; writing—review and editing, M.A., I.B.A. and H.H.; visualization, A.B.B. and H.H.; supervision, A.B.B.; project administration, H.H. and M.G.A.; funding acquisition, M.A. and M.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the main text.
Acknowledgments
The authors extend their appreciation to the researchers’ Supporting Project number (RSP-2021/237), King Saud University, Riyadh, Saudi Arabia, for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Erchamo, Y.S.; Mamo, T.T.; Workneh, G.A.; Mekonnen, Y.S. Improved biodiesel production from waste cooking oil with mixed methanol–ethanol using enhanced eggshell-derived CaO nano-catalyst. Sci. Rep. 2021, 11, 6708. [Google Scholar] [CrossRef] [PubMed]
- Banković–Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of nano CaO–based catalysts. Renew. Sustain. Energy Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Yang, X.-X.; Wang, Y.-T.; Yang, Y.-T.; Feng, E.-Z.; Luo, J.; Zhang, F.; Yang, W.-J.; Bao, G.-R. Catalytic transesterification to biodiesel at room temperature over several solid bases. Energy Convers. Manag. 2018, 164, 112–121. [Google Scholar] [CrossRef]
- Arachchige, U. Production of Biodiesel from Waste Cooking Oil in Laboratory Scale: A Review. Int. J. Sci. Eng. Sci. 2021, 5, 28–34. [Google Scholar]
- Moecke, E.H.S.; Feller, R.; dos Santos, H.A.; Machado, M.D.M.; Cubas, A.L.V.; Dutra, A.; Santos, L.L.V.; Soares, S.R. Biodiesel production from waste cooking oil for use as fuel in artisanal fishing boats: Integrating environmental, economic and social aspects. J. Clean. Prod. 2016, 135, 679–688. [Google Scholar] [CrossRef]
- Rahadianti, E.S.; Yerizam, Y.; Martha, M. Biodiesel Production from Waste Cooking Oil. Indones. J. Fundam. Appl. Chem. 2018, 3, 77–82. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Narwal, S.; Gupta, R. Biodiesel production by transesterification using immobilized lipase. Biotechnol. Lett. 2013, 35, 479–490. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Che Marzuki, N.H.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35 Pt 6, 1583–1587. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti, R.H., Jr.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Ghamgui, H.; Miled, N.; Karra-Chaâbouni, M.; Gargouri, Y. Immobilization studies and biochemical properties of free and immobilized Rhizopus oryzae lipase onto CaCO3: A comparative study. Biochem. Eng. J. 2007, 37, 34–41. [Google Scholar] [CrossRef]
- Lee, C.H.; Jin, E.S.; Lee, J.H.; Hwang, E.T. Immobilization and Stabilization of Enzyme in Biomineralized Calcium Carbonate Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 553591. [Google Scholar] [CrossRef]
- Ben Bacha, A.; Abid, I.; Nehdi, I.; Horchani, H. Hydrolysis of oils in the Wadi Hanifah River in Saudi Arabia by free and immobilized Staphylococcus aureus ALA1 lipase. Environ. Prog. Sustain. Energy 2019, 38, e13000. [Google Scholar] [CrossRef]
- Ben Bacha, A.; Alonazi, M.; Alanazi, H.; Alharbi, M.G.; Jallouli, R.; Karray, A. Biochemical Study of Bacillus stearothermophilus Immobilized Lipase for Oily Wastewater Treatment. Processes 2022, 10, 2220. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of biodiesel production using waste cooking oil and applying single and mixed immobilised lipases on polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Kuepethkaew, S.; Sangkharak, K.; Benjakul, S.; Klomklao, S. Optimized synthesis of biodiesel using lipase from Pacific white shrimp (Litopenaeus vannamei) hepatopancreas. Renew. Energy 2017, 104, 139–147. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Hassan, F.-U.; Arshad, M.; Khan, M.; Ahmad, M.; Sultana, S. Base catalyzed transesterification of sunflower oil biodiesel. Afr. J. Biotechnol. 2010, 9, 8630–8635. [Google Scholar]
- Asadi, M.; Hooper, J.F.; Lupton, D.W. Biodiesel synthesis using integrated acid and base catalysis in continuous flow. Tetrahedron 2016, 72, 3729–3733. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Cocchi, S. A Pilot-Scale Study of Waste Vegetable Oil Transesterification with Alkaline and Acidic Catalysts. Energy Procedia 2014, 45, 198–206. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Surendhar, A.; Thirumarimurugan, M.; Kannadasan, T. Recent Strategy of Biodiesel Production from Waste Cooking Oil and Process Influencing Parameters: A Review. J. Energy 2013, 2013, 926392. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Alam, M.Z.; Jami, M.S. Enzymatic biodiesel production from sludge palm oil (SPO) using locally produced Candida cylindracea lipase. Afr. J. Biotechnol. 2013, 12, 4966–4974. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Effect of solvents and oil content on direct transesterification of wet oil-bearing microalgal biomass of Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized lipase as the biocatalyst. Bioresour. Technol. 2013, 135, 213–221. [Google Scholar] [CrossRef]
- Yücel, Y. Biodiesel production from pomace oil by using lipase immobilized onto olive pomace. Bioresour. Technol. 2011, 102, 3977–3980. [Google Scholar] [CrossRef] [PubMed]
- Anjai, P.A.; Singh, V.B.N. Biodiesel production by esterification of free fatty acid over sulfated zirconia. Renew. Energy 2013, 51, 227–233. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Biodiesel Production (FAEEs) by Heterogeneous Combi-Lipase Biocatalysts Using Wet Extracted Lipids from Microalgae. Catalysts 2019, 9, 296. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, H.-Y.; Suh, Y.J.; Han, S.O.; Park, C.; Kim, S.W. Process development using co-immobilized lipases for biodiesel production. Int. Proc. Chem. Biol. Environ. Eng. 2012, 28, 171–174. [Google Scholar]
- Ramos, M.D.; Miranda, L.P.; Fernandez-Lafuente, R.; Kopp, W.; Tardioli, P.W. Improving the Yields and Reaction Rate in the Ethanolysis of Soybean Oil by Using Mixtures of Lipase CLEAs. Molecules 2019, 24, 4392. [Google Scholar] [CrossRef]
- Shahedi, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; As’Habi, M.A. Co-immobilization of Rhizomucor miehei lipase and Candida antarctica lipase B and optimization of biocatalytic biodiesel production from palm oil using response surface methodology. Renew. Energy 2019, 141, 847–857. [Google Scholar] [CrossRef]
- Kumari, A.; Mahapatra, P.; Garlapati, V.K.; Banerjee, R. Enzymatic transesterification of Jatropha oil. Biotechnol. Biofuels 2009, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.-D.; Sun, L.; Xu, M.; Zhou, W.-G.; Liang, X.-H. Solid superacid catalyzed fatty acid methyl esters production from acid oil. Appl. Energy 2010, 87, 2369–2373. [Google Scholar] [CrossRef]
- Kaieda, M.; Samukawa, T.; Kondo, A.; Fukuda, H. Effect of Methanol and water contents on production of biodiesel fuel from plant oil catalyzed by various lipases in a solvent-free system. J. Biosci. Bioeng. 2001, 91, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Li, G.-L.; Fan, Y.-L.; Yan, Y.-J. Enhancing biodiesel production via a synergic effect between immobilized Rhizopus oryzae lipase and Novozym 435. Fuel Process. Technol. 2015, 137, 298–304. [Google Scholar] [CrossRef]
- Atadashi, I.; Aroua, M.; Aziz, A.A.; Sulaiman, N. The effects of water on biodiesel production and refining technologies: A review. Renew. Sustain. Energy Rev. 2012, 16, 3456–3470. [Google Scholar] [CrossRef]
- Yang, W.; He, Y.; Xu, L.; Zhang, H.; Yan, Y. A new extracellular thermo-solvent-stable lipase from Burkholderia ubonensis SL-4: Identification, characterization and application for biodiesel production. J. Mol. Catal. B Enzym. 2016, 126, 76–89. [Google Scholar] [CrossRef]
- Maceiras, R.; Vega, M.; Costa, C.; Ramos, P.; Márquez, M. Effect of methanol content on enzymatic production of biodiesel from waste frying oil. Fuel 2009, 88, 2130–2134. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Y. Production of biodiesel catalyzed by immobilized Pseudomonas cepacia lipase from Sapium sebiferum oil in micro-aqueous phase. Appl. Energy 2010, 87, 3148–3154. [Google Scholar] [CrossRef]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef]
- Raita, M.; Champreda, V.; Laosiripojana, N. Biocatalytic ethanolysis of palm oil for biodiesel production using microcrystalline lipase in tert-butanol system. Process Biochem. 2010, 45, 829–834. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Yuwen, L.-X.; Peng, L.-J. Parameters Affecting the Performance of Immobilized Enzyme. J. Chem. 2013, 2013, 946248. [Google Scholar] [CrossRef]
- Gharat, N.; Rathod, V.K. Ultrasound assisted enzyme catalyzed transesterification of waste cooking oil with dimethyl carbonate. Ultrason. Sonochemistry 2013, 20, 900–905. [Google Scholar] [CrossRef] [PubMed]
- El-Batal, A.I.; Farrag, A.A.; Elsayed, M.A.; El-Khawaga, A.M. Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles. Bioengineering 2016, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, J. Immobilized lipase on magnetic chitosan microspheres for transesterification of soybean oil. Biomass- Bioenergy 2012, 36, 373–380. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Biodiesel production from waste cooking oil. Green Process. Synth. 2019, 8, 828–836. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Li, D.; Yang, B.; Wang, Y. One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol. 2017, 235, 18–24. [Google Scholar] [CrossRef]
- Rushang, M.J.; Michael, J.P. Flow properties of biodiesel fuel blends at low temperatures. Fuel 2007, 86, 143–151. [Google Scholar]
- Brenes, M.; García, A.; Dobarganes, M.C.; Velasco, J.; Romero, C. Influence of Thermal Treatments Simulating Cooking Processes on the Polyphenol Content in Virgin Olive Oil. J. Agric. Food Chem. 2002, 50, 5962–5967. [Google Scholar] [CrossRef]
- Ben Bacha, A.; Moubayed, N.M.S.; Abid, I. Thermostable, alkaline and detergent-tolerant lipase from a newly isolated thermophilic Bacillus stearothermophilus. Indian J. Biochem. Biophys. 2015, 52, 179–188. [Google Scholar] [PubMed]
- Ben Bacha, A.; Moubayed, N.M.; Al-Assaf, A. An organic solvent-stable lipase from a newly isolated Staphylococcus aureus ALA1 strain with potential for use as an industrial biocatalyst. Biotechnol. Appl. Biochem. 2016, 63, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Rosu, R.; Uozaki, Y.; Iwasaki, Y.; Yamane, T. Repeated use of immobilized lipase for monoacylglycerol production by solid-phase glycerolysis of olive oil. J. Am. Oil Chem. Soc. 1997, 74, 445–450. [Google Scholar] [CrossRef]
- Rathelot, J.; Julien, R.; Bosc-Bierne, I.; Gargouri, Y.; Canioni, P.; Sarda, L. Horse pancreatic lipase. Interaction with colipase from various species. Biochimie 1981, 63, 227–234. [Google Scholar] [CrossRef]
- Zheng, S.; Kates, M.; Dubé, M.; McLean, D. Acid-catalyzed production of biodiesel from waste frying oil. Biomass- Bioenergy 2006, 30, 267–272. [Google Scholar] [CrossRef]
- Hadaruga, D.I.; Hădărugă, N.; Hermenean, A.; Riviş, A.; Paslaru, V.; Codina, G. Bionanomaterials: Thermal stability of the oleic acid / α- and β-cyclodextrin complexes. Rev. Chim. 2008, 59, 994–998. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Highly active and stable Fe3O4/Au nanoparticles supporting lipase catalyst for biodiesel production from waste tomato. Appl. Surf. Sci. 2019, 474, 135–146. [Google Scholar] [CrossRef]
- Yusoff, M.F.M.; Xu, X.; Guo, Z. Comparison of fatty acid methyl and ethyl esters as biodiesel base stock: A review on processing and production requirements. J. Am. Oil Chem. Siciety 2014, 91, 525–531. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).