A Comparative Study of Antimicrobial and Antioxidant Activities of Plant Essential Oils and Extracts as Candidate Ingredients for Edible Coatings to Control Decay in ‘Wonderful’ Pomegranate
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Essential Oils
2.2. In Vitro Antifungal Properties
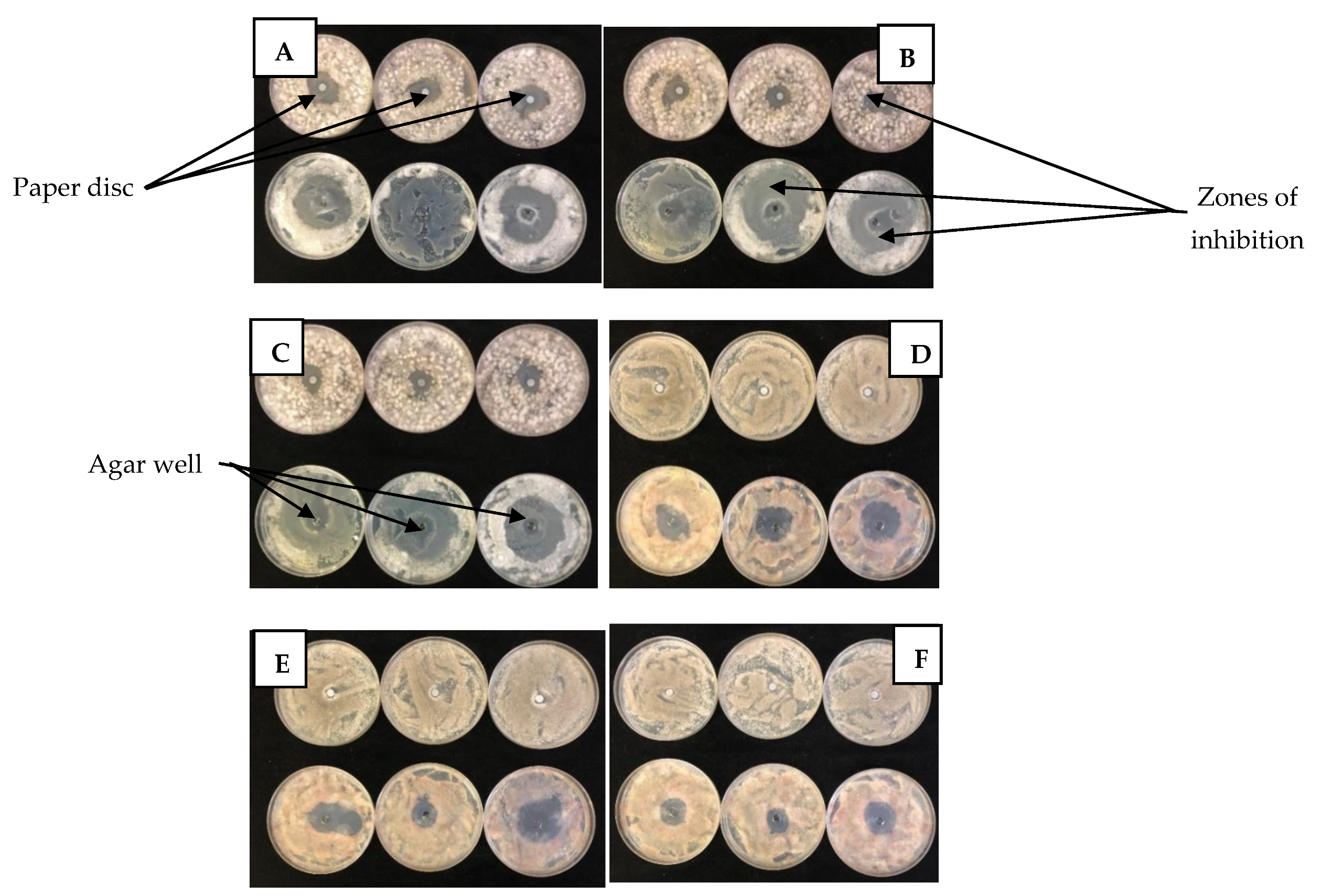
2.2.1. Disc and Well Diffusion Tests
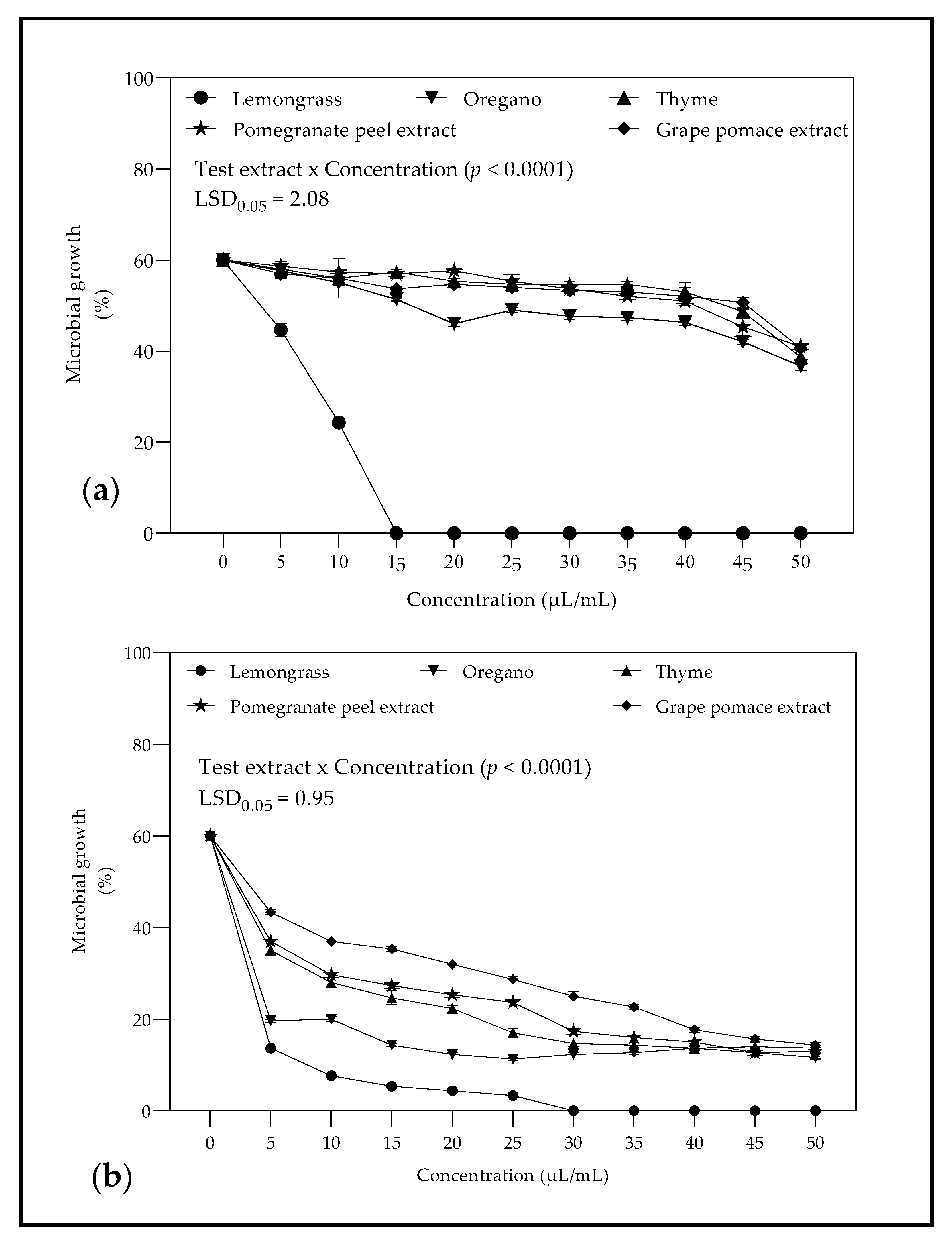
2.2.2. Minimum Inhibitory Concentration against Botrytis cinerea and Penicillium spp.
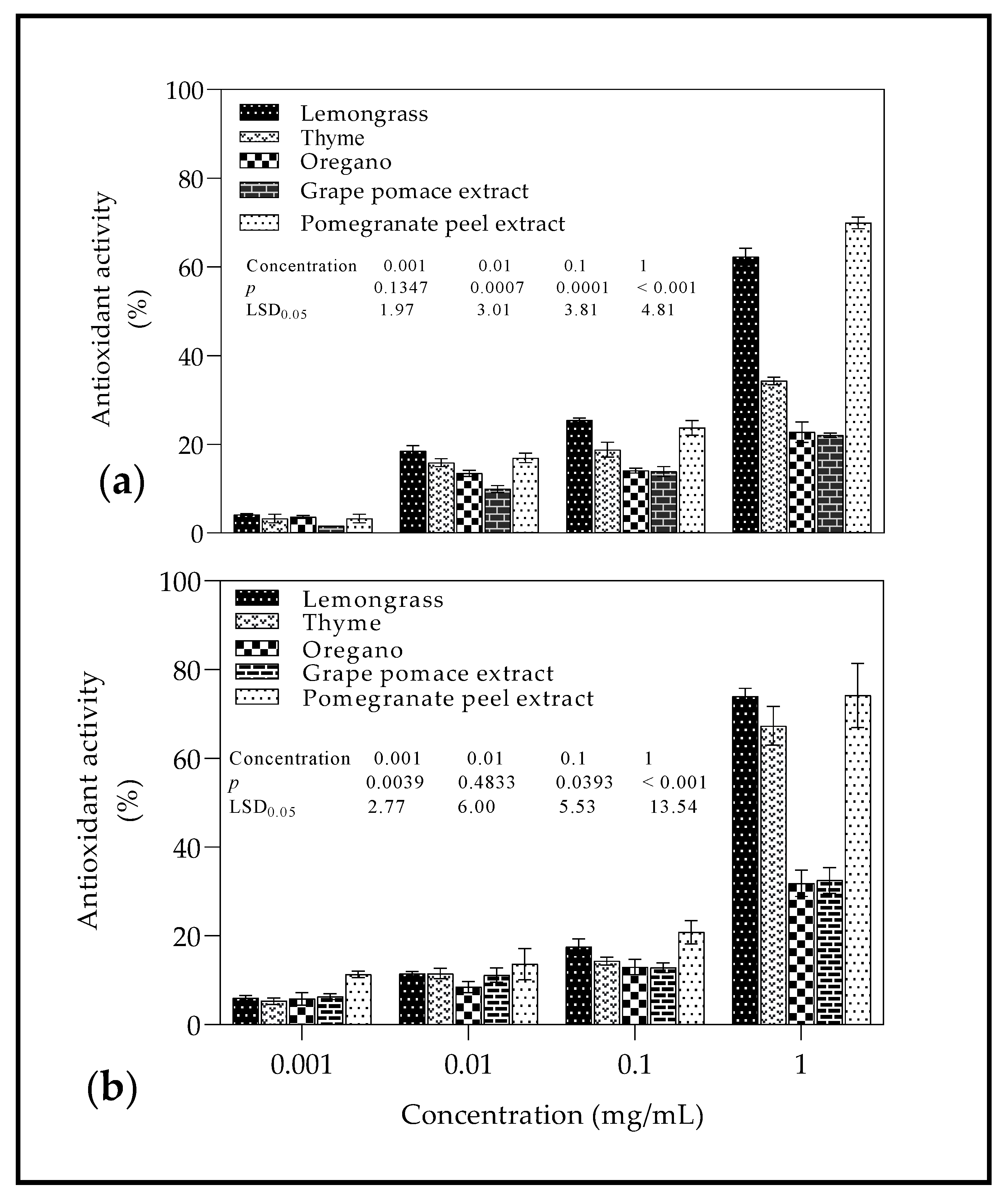
2.3. Radical Scavenging Activity
2.4. In Vitro and In Vivo Investigations with Edible Coatings
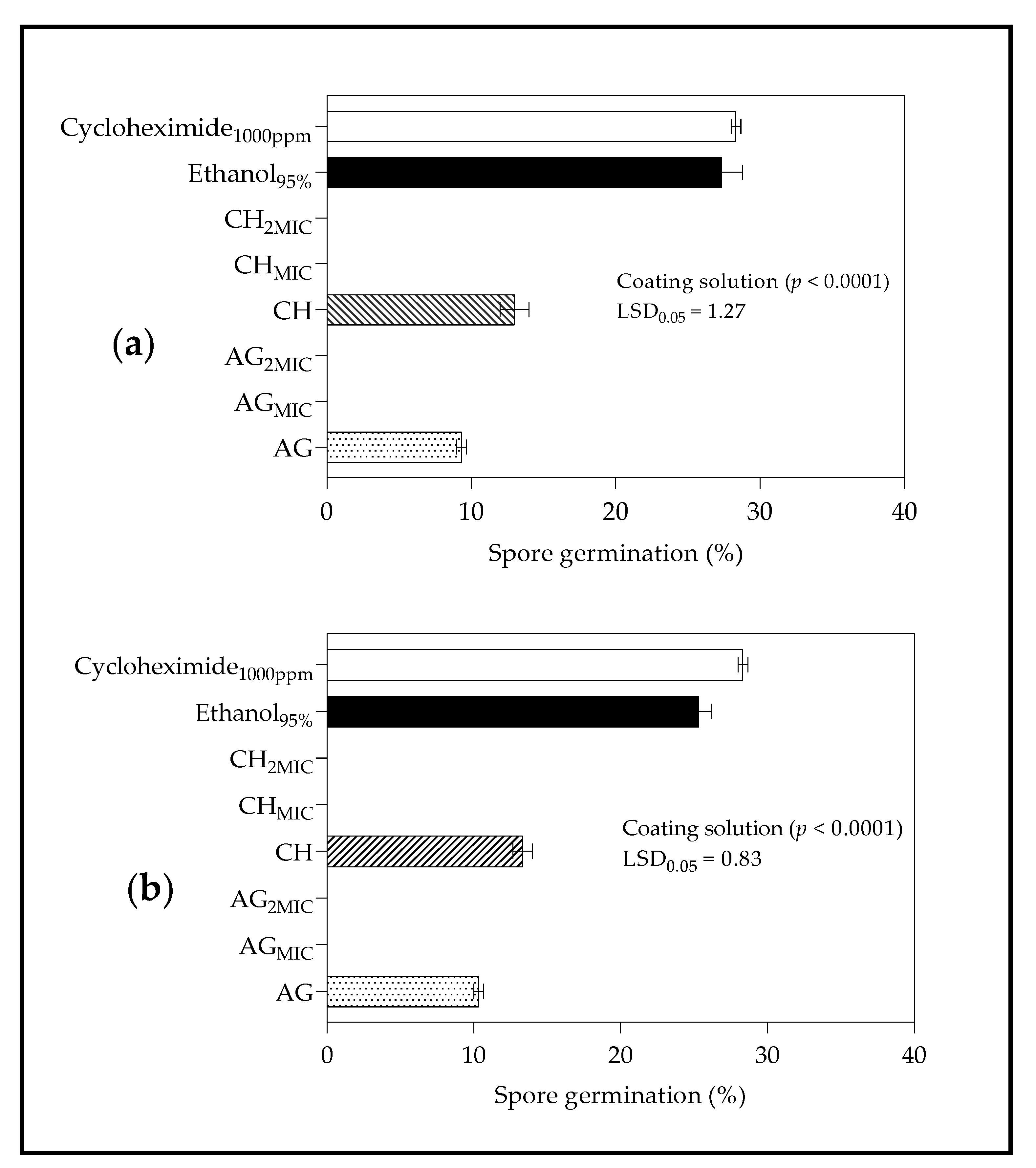
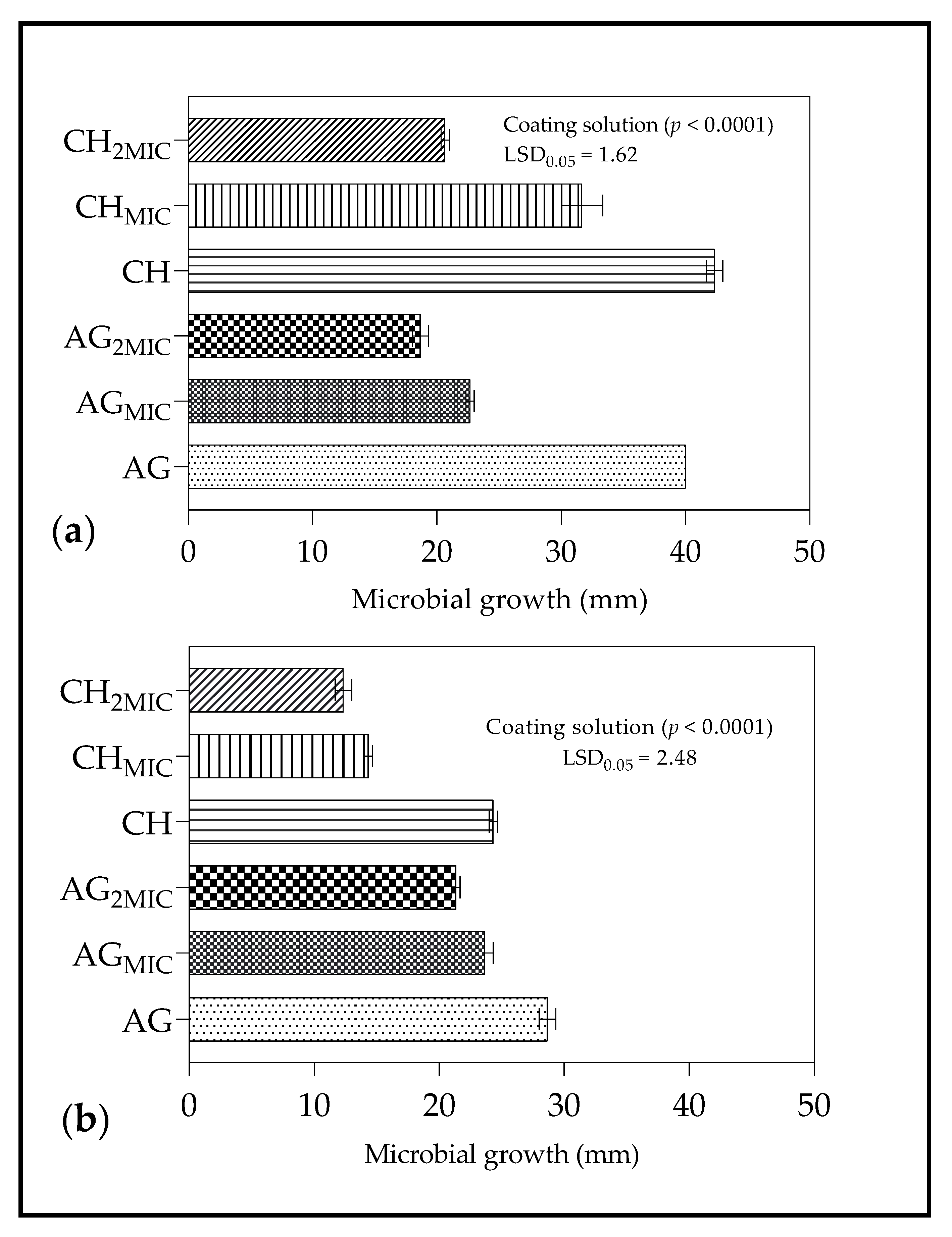
2.4.1. Spore Germination
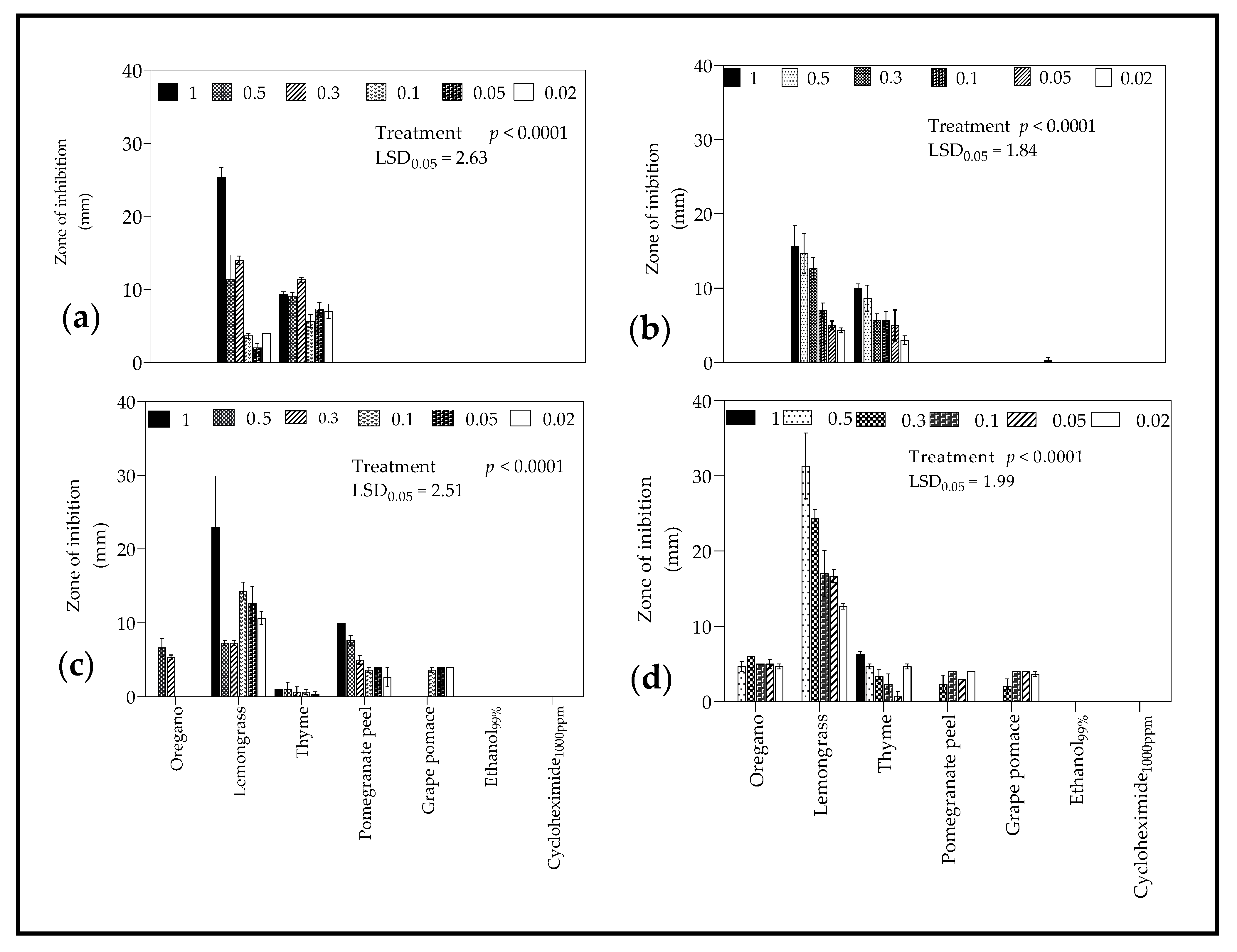
2.4.2. Zone of Inhibition
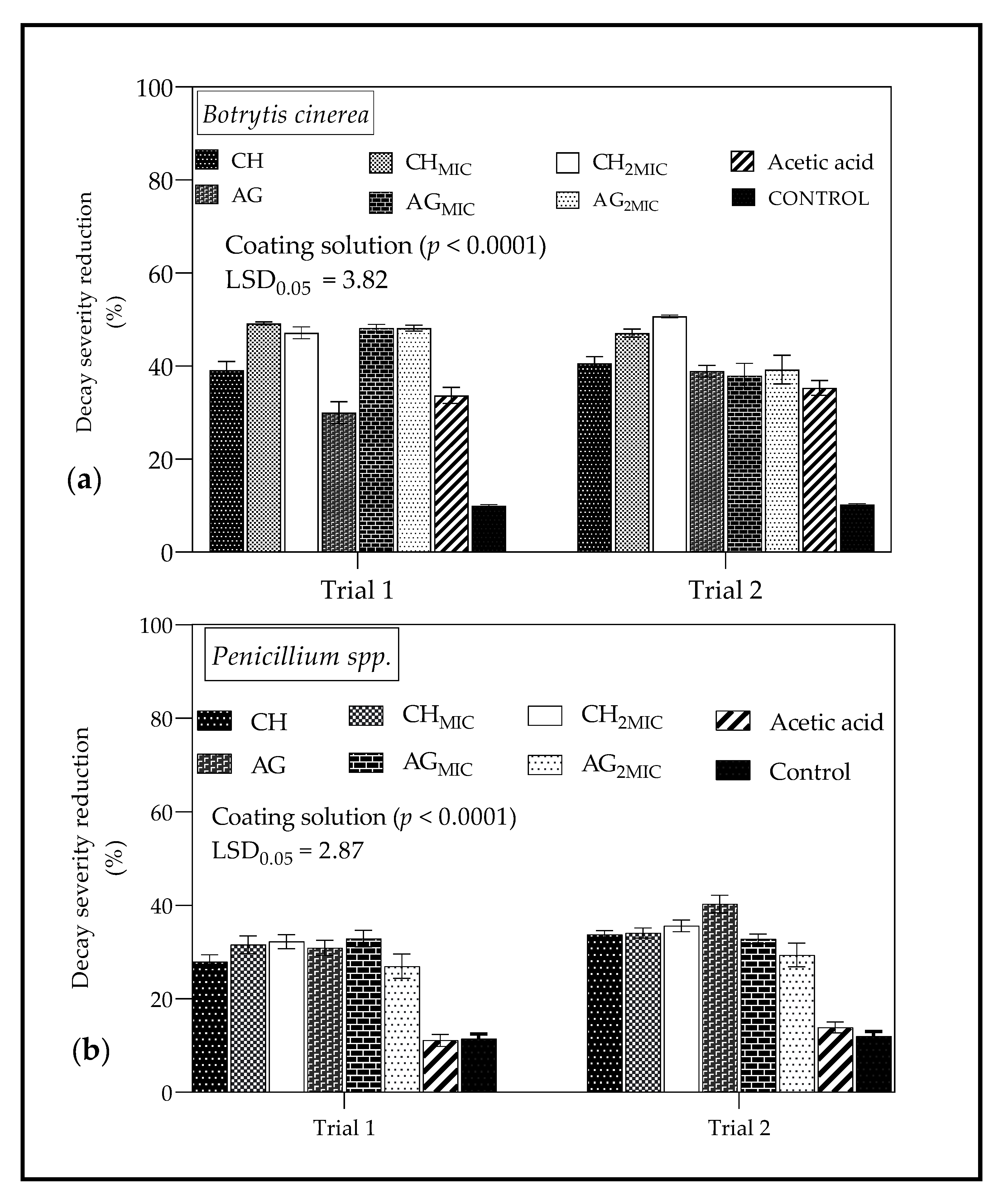
2.4.3. In Vivo Antimicrobial Properties of Chitosan and Sodium Alginate Based Coatings from Lemongrass Oil
3. Discussion
3.1. Composition of Essential Oils
3.2. In Vitro Antifungal Properties
3.2.1. Disc and Well Diffusion Test
3.2.2. Minimum Inhibitory Concentration against B. cinerea and Penicillium spp.
3.3. Radical Scavenging Activity
3.4. In Vitro and In Vivo Investigations with Edible Coatings
3.4.1. Spore Germination
3.4.2. Zone of Inhibition
3.4.3. In Vivo Antimicrobial Properties of Chitosan and Sodium Alginate Based Coatings from Lemongrass Oil
4. Materials and Methods
4.1. Fruit Supply
4.2. Preparation of Plant Extracts
4.2.1. Pomegranate Peel Extract
4.2.2. Grape Pomace Extract
4.3. Targeted Microorganisms
4.4. Gas Chromatography-Mass Spectrometry Analysis of Essential Oils
4.5. In Vitro Antifungal Properties
4.5.1. Paper Disc Diffusion Test
4.5.2. Agar Well Diffusion Test
4.5.3. Determination of Minimum Inhibitory Concentration
4.6. Radical Scavenging Activity
4.6.1. 2,2-Diphenyl-1-picrylhydrazyl Assay
4.6.2. 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulfonic Acid Assay
4.7. In Vitro and In Vivo Investigations with Edible Coatings
4.7.1. Preparation of Coating Solutions
4.7.2. Spore Germination
4.7.3. Zone of Inhibition
4.7.4. In Vivo Antimicrobial Properties of Chitosan and Sodium Alginate Based Coatings from Lemongrass Oil
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Antunes, M.D.; Miguel, M.G.; Neves, A. Sustainable postharvest handling of horticultural products. WSEAS Trans. Environ. Dev. 2007, 3, 111–116. [Google Scholar]
- Buck, J.W.; Walcott, R.R.; Beuchat, L.R. Recent trends in microbiological safety of fruits and vegetables. Plant Health Prog. 2003, 4, 25. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, E.; Abdalla, N.A.; Taha, H.S.; Fári, M. Postharvest management of fruits and vegetables storage. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed., Eds.; Springer: Cham, Switzerland, 2014; Volume 15, pp. 65–152. [Google Scholar]
- Opara, I.K.; Fawole, O.A.; Kelly, C.; Opara, U.L. Quantification of on-farm pomegranate fruit postharvest losses and waste, and implications on sustainability indicators: South African case study. Sustainability 2021, 13, 5187. [Google Scholar] [CrossRef]
- Opara, I.K.; Fawole, O.A.; Opara, U.L. Postharvest losses of pomegranate fruit at the packhouse and implications for sustainability indicators. Sustainability 2021, 13, 5168. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Opara, U.L. Major diseases of pomegranate (Punica granatum L.), their causes and management—A review. Sci. Hortic 2016, 211, 126–139. [Google Scholar] [CrossRef]
- Moss, M.O. Fungi, quality and safety issues in fresh fruits and vegetables. J. Appl. Microb. 2008, 104, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; di Francesco, A.; Bertolini, P. Control of fruit postharvest diseases: Old issues and innovative approaches. Stewart Postharvest Rev. 2014, 10, 1–4. [Google Scholar]
- Förster, H.; Driever, G.F.; Thompson, D.C.; Adaskaveg, J.E. Postharvest decay management for stone fruit crops in California using the ‘reduced-risk’ fungicides fludioxonil and fenhexamid. Plant Dis. 2007, 91, 209–215. [Google Scholar] [CrossRef][Green Version]
- Russouw, A.; Meitz-Hopkins, J.; Den Breeyen, A.; Lennox, C. Postharvest applications of fludioxonil and pyrimethanil to control Phlyctema vagabunda on apple in South Africa. Cr. Prot. 2021, 141, 105451. [Google Scholar] [CrossRef]
- Lutz, M.C.; Sosa, M.C.; Colodner, A.D. Effect of pre and postharvest application of fungicides on postharvest decay of ‘Bosc’ pear caused by Alternaria—Cladosporium complex in North Patagonia, Argentina. Sci. Hortic. 2017, 225, 810–817. [Google Scholar] [CrossRef]
- Rajwana, I.; Amin, M.; Khan, A.; Saeed, M. Effect of combined application of fungicides and hot water quarantine treatment on postharvest diseases and quality of mango fruit. Pak. J. Bot 2011, 43, 65–73. [Google Scholar]
- Atukuri, J.; Fawole, O.A.; Opara, U.L. Effect of exogenous fludioxonil postharvest treatment on physiological response, physico-chemical, textural, phytochemical and sensory characteristics of pomegranate fruit. J. Food Meas. Charact. 2017, 11, 1081–1093. [Google Scholar] [CrossRef]
- Opara, U.L.; Atukuri, J.; Fawole, O.A. Application of physical and chemical postharvest treatments to enhance storage and shelf life of pomegranate fruit-A review. Sci. Hortic. 2015, 197, 41–49. [Google Scholar] [CrossRef]
- Errampalli, D.; Brubacher, N.R.; DeEll, J.R. Sensitivity of Penicillium expansum to diphenylamine and thiabendazole and postharvest control of blue mold with fludioxonil in ‘McIntosh’ apples. Postharvest Biol. Technol. 2006, 39, 101–107. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A–Review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Teksur, P.K. Alternative technologies to control postharvest diseases of pomegranate. Stewart Postharvest Rev. 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Mari, M.; Bertolini, P.; Pratella, G.C. Non-conventional methods for the control of postharvest pear diseases. J. Appl. Microb. 2003, 94, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Cr. Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The use of essential oils for postharvest decay control. A–Review. Flavour Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Saei-Dehkordi, S.S.; Tajik, H.; Moradi, M.; Khalighi-Sigaroodi, F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 2010, 48, 1562–1567. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems. A–Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microb. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L.; et al. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Sendra, E. Essential oils in foods: From ancient times to the 21st century. Foods 2016, 5, 43. [Google Scholar] [CrossRef]
- Ali, A.; Wee Pheng, T.; Mustafa, M.A. Application of lemongrass oil in vapour phase for the effective control of anthracnose of ‘Sekaki’ papaya. J. Appl. Microb. 2015, 118, 1456–1464. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Ali, A.; Hei, G.K.; Keat, Y.W. Efficacy of ginger oil and extract combined with gum arabic on anthracnose and quality of papaya fruit during cold storage. J. Food Sci. Technol. 2016, 53, 1435–1444. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Baz, A.F.; Salem, M.F.; El-Hadary, M.H. Potential applications of pomegranate peel extract for the control of citrus green mould. J. Plant Dis. Prot. 2009, 116, 252–256. [Google Scholar] [CrossRef]
- Mendoza, L.; Navarro, F.; Melo, R.; Báez, F.; Cotoras, M. Characterization of polyphenol profile of extracts obtained from grape pomace and synergistic effect of these extracts and fungicides against Botrytis Cinerea. J. Chil. Chem. Soc. 2019, 64, 4607–4609. [Google Scholar] [CrossRef]
- Nicosia, M.G.L.D.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Adams, A.; Chonga, J.; Thomas, B. Effect of Ginger extracts and storage temperature on shelf life of ‘Kent’ mango fruits. Int. J. Postharvest Technol. Innov. 2016, 4, 75–82. [Google Scholar]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess. Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Application of gum arabic and methyl cellulose coatings enriched with thyme oil to maintain quality and extend shelf life of ‘Acco’ pomegranate arils. Plants 2020, 9, 1690. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during postharvest storage. Cr. Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Mohamed, M.T.M.; Siddiqui, Y.; Zahid, N. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol. Technol. 2011, 62, 71–76. [Google Scholar] [CrossRef]
- Kim, I.H.; Lee, H.; Kim, J.E.; Song, K.B.; Lee, Y.S.; Chung, D.S.; Min, S.C. Plum coatings of lemongrass oil-incorporating carnauba wax-based nanoemulsion. J. Food Sci. 2013, 78, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce Penicillium digitatum postharvest decay of oranges. Food Microb. 2018, 74, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction techniques, pharmaceutical and therapeutic potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of alginate-based edible coatings enriched with essential oils constituents on Arbutus unedo L. fresh fruit storage. Postharvest Biol. Technol. 2015, 100, 226–233. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green tea and grape seed extracts–Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Martins, J.T.; Teixeira, J.A.; Vicente, A.A. Seed extracts of Gleditsia triacanthos: Functional properties evaluation and incorporation into galactomannan films. Food Res. Int. 2010, 43, 2031–2038. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microb. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Demo, M.; Oliva, M.D.L.M.; López, M.L.; Zunino, M.P.; Zygadlo, J.A. Antimicrobial activity of essential oils obtained from aromatic plants of Argentina. Pharm. Biol. 2005, 43, 129–134. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microb. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of strawberries with an antifungal edible coating using peony extracts in chitosan. Food Bioprocess. Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538. [Google Scholar] [CrossRef]
- Faleiro, M.L. The mode of antibacterial action of essential oils. Sci. Microb. Path. Commun. Curr. Res. Technol. Adv. 2011, 3, 1143–1156. [Google Scholar]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microb. 2012, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Munhuweyi, K.; Caleb, O.J.; Lennox, C.L.; van Reenen, A.J.; Opara, U.L. In vitro and in vivo antifungal activity of chitosan-essential oils against pomegranate fruit pathogens. Postharvest Biol. Technol. 2017, 129, 9–22. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar] [PubMed]
- Grigore, A.; Paraschiv, I.; Colceru-Mihul, S.; Bubueanu, C.; Draghici, E.; Ichim, M. Chemical composition and antioxidant activity of Thymus vulgaris L. volatile oil obtained by two different methods. Rom. Biotechnol. Lett. 2010, 15, 5436–5443. [Google Scholar]
- Tavakoli, S.; Yassa, N.; Delnavazi, M.R.; Akhbari, M.; Hadjiakhoondi, A.; Hajimehdipoor, H.; Khalighi-Sigaroodi, F.; Hajiaghaee, R. Chemical composition and biological activities of the essential oils from different parts of Ferulago trifida Boiss. J. Essent. Oil Res. 2017, 29, 407–419. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Grosso, N.R.; Lante, A.; Lomolino, G.; Zygadlo, J.A.; Nepote, V. Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and Lippia turbinata essential oils. Int. J. Food Sci. Technol. 2013, 48, 642–649. [Google Scholar] [CrossRef]
- Naveed, R.; Hussain, I.; Tawab, A.; Tariq, M.; Rahman, M.; Hameed, S.; Mahmood, M.S.; Siddique, A.B.; Iqbal, M. Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria. BMC Compl. Altern. Med. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Uddhav, B.; Sivagurunathan, S. Antibiotic susceptibility testing: A review on current practices. Int. J. Pharm. 2016, 6, 11–17. [Google Scholar]
- Tzortzakis, N.G.; Economakis, C.D. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innov. Food Sci. Emerg. Technol. 2007, 8, 253–258. [Google Scholar] [CrossRef]
- Guynot, M.E.; Ramos, A.J.; Setó, L.; Purroy, P.; Sanchis, V.; Marín, S. Antifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products. J. Appl. Microbiol. 2003, 94, 893–899. [Google Scholar] [CrossRef]
- Siripornvisal, S.; Rungprom, W.; Sawatdikarn, S. Antifungal activity of essential oils derived from some medicinal plants against grey mould (Botrytis cinerea). Asian J. Food Agro Ind. 2009, 2, 229–233. [Google Scholar]
- Reang, S.P.; Mishra, J.P.; Prasad, R. In vitro antifungal activities of five plant essential oils against Botrytis cinerea causing gray mold of orange. J. Pharmacogn. Phytochem. 2020, 9, 1046–1048. [Google Scholar]
- Saikia, D.; Khanuja, S.P.; Kahol, A.P.; Gupta, S.C.; Kumar, S. Comparative antifungal activity of essential oils and constituents from three distinct genotype of Cymbopogon spp. Curr. Sci. 2001, 80, 1264–1266. [Google Scholar]
- Ma-In, K.; H-Kittikun, A.; Phongpaichit, S. Application of plant essential oils in prevention of fungal growth on Para rubber wood. Eur. J. Wood Prod. 2014, 72, 413–416. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Compl. Altern. Med. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Narsaiah, K.; Borah, A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010, 85, 155–1559. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.W.; Yin, Z.Q.; Wei, Q.; Jia, R.Y.; Zhou, L.J.; Xu, J.; Song, X.; Zhou, Y.; Du, Y.H.; et al. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014, 7, 1721–1727. [Google Scholar] [PubMed]
- Kulišić, T.; Dragović-Uzelac, V.; Miloš, M. Antioxidant activity of aqueous tea infusions prepared from oregano, thyme and wild thyme. Food Technol. Biotechnol. 2006, 44, 485–492. [Google Scholar]
- Siddique, A.B.; Mizanur Rahman, S.M.; Hossain, M.A. Chemical composition of essential oil by different extraction methods and fatty acid analysis of the leaves of Stevia Rebaudiana Bertoni. Arab J. Chem. 2016, 9, 1185–1189. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut ‘Fuji’ apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Sigge, G.O.; Opara, U.L. Investigating the effects of crab shell chitosan on fungal mycelial growth and postharvest quality attributes of pomegranate whole fruit and arils. Sci. Hortic. 2017, 220, 78–89. [Google Scholar] [CrossRef]
- Sharples, G.P.; Williams, S.T. Fine structure of spore germination in actinomycetes. Microbiology 1976, 96, 323–332. [Google Scholar] [CrossRef]
- Hernández-Lauzardo, A.N. Current status of action mode and effect of chitosan against phytopathogens fungi. Afr. J. Microbiol. Res. 2011, 5, 4243–4247. [Google Scholar]
- Acharya, C.; Panda, C.R.; Bhaskara, P.K.; Sasmal, A.; Shekhar, S.; Sen, A.K. Physicochemical and antimicrobial properties of sodium alginate/gelatin-based silver nanoformulations. Polym. Bull. 2017, 74, 689–706. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Adzahan, N.M. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Citrus and lemongrass essential oils inhibit Botrytis cinerea on ‘Golden Delicious’, ‘Pink Lady’ and ‘Granny Smith’ apples. J. Pl. Dis. Prot. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Rojas-Graü, M.A.; Mosqueda-Melgar, J.; Martín-Belloso, O. Comparative study on essential oils incorporated into an alginate-based edible coating to assure the safety and quality of fresh-cut ‘Fuji’ apples. J. Food Prot. 2008, 71, 1150–1561. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Compl. Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Yañez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind. Crop. Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Sigge, G.O.; Opara, U.L. In vitro effects of crab shell chitosan against mycelial growth of Botrytis sp., Penicillium sp. and Pilidiella granati. Act. Hortic. 2016, 1144, 403–407. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Mahajan, P.V.; Opara, U.L. Response of pomegranate arils (cv. Wonderful) to low oxygen stress under active modified atmosphere condition. J. Sci. Food Agric. 2018, 99, 1088–1097. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Mahajan, P.V.; Manley, M.; Mokwena, L.; Tredoux, A.G.J. Effect of modified atmosphere packaging and storage temperature on volatile composition and postharvest life of minimally-processed pomegranate arils (cvs. ‘Acco’ and ‘Herskawitz’). Postharvest Biol. Technol. 2013, 79, 54–61. [Google Scholar] [CrossRef]
- Thanissery, R.; Kathariou, S.; Smith, D.P. Rosemary oil, clove oil, and a mix of thyme-orange essential oils inhibit Salmonella and Campylobacterin vitro. J. Appl. Poult. Res. 2014, 23, 221–227. [Google Scholar] [CrossRef]
- Er, Y.; Sivri, N.; Mirik, M. Antimicrobial activity of essential oil against rhizobium (Agrobacterium) vitis using agar well and disc diffusion method. Bacteriol. J. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P.; Saavedra-Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A. Antioxidant activity of the essential oil and its major terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2018, 13, 875–880. [Google Scholar]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Adebayo, O.; Bélanger, A.; Khanizadeh, S. Variable inhibitory activities of essential oils of three Monarda species on the growth of Botrytis Cinerea. Can. J. Plant Sci. 2013, 93, 987–995. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan-tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
| Compound | Compound | RT | Relative Abundance (%) | ||
|---|---|---|---|---|---|
| Lemongrass | Thyme | Oregano | |||
| Sesquiterpenes | α-farnesene | 21.05 | 0.08 ± 0.01 j | - | 0.39 ± 0.28 h–j |
| α-ylangene | 13.96 | 0.08 ± 0.01 j | - | - | |
| Caryophyllene oxide | 22.04 | 0.06 ± 0.01 hi | - | 2.75 ± 0.07 e | |
| γ-Cadinene | 17.80 | 0.08 ± 0.01 j | - | 0.07 ± 0.01 j | |
| Germacrene | 17.51 | 0.07 ± 0.01 j | - | - | |
| α-Cubebene | 14.15 | 0.16 ± 0.01 j | - | 0.3657 ± 0.06 g–j | |
| Myrcene | 7.22 | 0.29 ± 0.01 hij | 0.09 ± 0.00 ij | ||
| α-Humulene | 16.91 | - | - | 0.5888 ± 0.02 gh | |
| Trans-Caryophyllene | 15.98 | 0.23 ± 0.06 j | - | 60.84 ± 0.56 a | |
| Terpinolene | 17.16 | - | - | 0.06 ± 0.01 j | |
| Cis-Caryophyllene | 15.80 | 0.45 ± 0.01 hi | - | 0.52 ± 0.05 e | |
| Monoterpene | 1,8-cineole | 8.31 | 0.61 ± 0.04 h | 2.69 ± 0.01 d | 17.53 ± 0.08 b |
| α-pinene | 3.77 | 0.15 ± 0.01 ij | 1.24 ± 0.01 g | 4.1845 ± 0.06 cd | |
| Camphene | 4.63 | 0.27 ± 0.00 ij | 0.13 ± 0.03 ij | 0.075 ± 0.01 j | |
| Limonene | 8.08 | 16.59 ± 0.41 c | 1.62 ± 0.01 f | 2.21 ± 0.08 d | |
| Sabinene | 6.05 | 0.05 ± 0.00 j | - | 0.3680 ± 0.02 g–j | |
| Linalyl acetate | 15.07 | - | 0.34 ± 0.01 hij | - | |
| Cymene | 9.65 | 0.17 ± 0.01 ij | - | 0.1727± 0.05 ij | |
| Terpinene | 9.16 | 1.22 ± 0.03 g | - | ||
| α-thujone | 3.86 | - | 0.10 ± 0.01 ij | ||
| Delta-3-Carene | 6.78 | - | 0.16 ± 0.01 ij | 0.2673 ± 0.00 g–j | |
| Exobornyl acetate | 15.56 | - | 1.56 ± 0.03 fg | ||
| γ-Terpinene | 9.15 | - | 3.26 ± 0.01 c | 0.499 ± 0.00 j | |
| Terpinen-4-ol | 15.78 | - | 0.32 ± 0.01 hij | ||
| Camphor | 14.33 | - | 2.85 ± 0.01 d | - | |
| β-Pinene | 5.69 | 0.15 ± 0.00 j | 0.59 ± 0.01 h | 3.9591 ± 0.04 d | |
| Sesquiterpenoid | Isocaryophyllene | 15.44 | - | - | 0.4734 ± 0.03 ghi |
| Alpha-bourbonene | 14.57 | - | - | 0.08± 0.00 g–j | |
| Monoterpenoid | Citronellal | 13.73 | 0.09 ± 0.01 ij | - | - |
| Citronellol | 18.13 | 5.03 ± 0.04 e | - | - | |
| α-citral | 17.73 | 27.45 ± 0.10 a | - | - | |
| Geranyl acetate | 18.03 | 1.00 ± 0.05 g | 0.04 ± 0.01 j | - | |
| Myrtanal | 12.65 | 0.06 ± 0.01 j | - | ||
| Nerol | 18.59 | 0.32 ± 0.11 hij | 0.06 ± 0.01 j | ||
| α-terpineol | 17.17 | 8.32 ± 0.36 d | - | 0.08 ± 0.00 j | |
| β-citral | 17.00 | 27.43 ± 0.25 b | - | ||
| Beta-ocimene | 8.95 | 0.06 ± 0.01 j | - | ||
| Thymol | 23.36 | - | 33.31 ± 0.12 b | 4.5477 ± 0.06 c | |
| Isocineole | 7.58 | - | 0.09 ± 0.01 ij | ||
| Para cymene | 9.76 | - | 43.26 ± 0.56 a | ||
| β-Phellandrene | 7.21 | - | - | 0.22 ± 0.04 hij | |
| Para-cymen-8-ol | 19.22 | - | 0.05 ± 0.01 j | ||
| Ketones | 6-methyl-5-hepten-2-one | 11.12 | 0.31 ± 0.02 hij | - | 0.066 ± 0.01 j |
| 4-Nonanone | 10.99 | 0.25 ± 0.01 j | - | - | |
| Terpene alcohol | Dihydrolinalool | 14.67 | 0.07 ± 0.01 j | 0.07 ± 0.00 ij | - |
| Linalool | 14.86 | 3.86 ± 0.02 f | 3.27 ± 0.02 c | ||
| Cis-Linalool oxide | 13.13 | - | 0.54 ± 0.03 h | ||
| Trans-Linalool oxide | 13.62 | - | 0.33 ± 0.00 hij | - | |
| Alcohols | 1-octanol | 16.70 | 0.06 ± 0.00 j | - | - |
| Geraniol | 19.24 | 3.87 ± 0.09 f | 0.13 ± 0.01 ij | - | |
| Heptan-2-ol | 16.72 | 0.16 ± 0.01 j | 0.54 ± 0.02 h | ||
| Aldehydes | Decanal | 14.10 | 0.07 ± 0.01 j | 0.44 ± 0.01 hi | - |
| Octanal | 16.70 | 0.07 ± 0.00 j | - | - | |
| Phenylpropene | Estragole | 25.18 | 0.36 ± 0.08 hij | 2.05 ± 0.07 e | 0.0873±0.01 j |
| Pr > F | |||||
| Essential oil | Lemongrass oil | Thyme | Oregano | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Fungi | Treatment | Storage Duration (Days) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 8 | 10 | ||
| CH |  |  |  |  |  |  | |
| CHMIC |  |  |  |  |  |  | |
| CH2MIC |  |  |  |  |  |  | |
| Botrytis cinerea | AG |  |  |  |  |  |  |
| AGMIC |  |  |  |  |  |  | |
| AG2MIC |  |  |  |  |  |  | |
| Penicillium sp. | CH |  |  |  |  |  |  |
| CHMIC |  |  |  |  |  |  | |
| CH2MIC |  |  |  |  |  |  | |
| AG |  |  |  |  |  |  | |
| AGMIC |  |  |  |  |  |  | |
| AG2MIC |  |  |  |  |  |  | |
| Acetic acid |  |  |  |  |  |  | |
| Control |  |  |  |  |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawhena, T.G.; Opara, U.L.; Fawole, O.A. A Comparative Study of Antimicrobial and Antioxidant Activities of Plant Essential Oils and Extracts as Candidate Ingredients for Edible Coatings to Control Decay in ‘Wonderful’ Pomegranate. Molecules 2021, 26, 3367. https://doi.org/10.3390/molecules26113367
Kawhena TG, Opara UL, Fawole OA. A Comparative Study of Antimicrobial and Antioxidant Activities of Plant Essential Oils and Extracts as Candidate Ingredients for Edible Coatings to Control Decay in ‘Wonderful’ Pomegranate. Molecules. 2021; 26(11):3367. https://doi.org/10.3390/molecules26113367
Chicago/Turabian StyleKawhena, Tatenda Gift, Umezuruike Linus Opara, and Olaniyi Amos Fawole. 2021. "A Comparative Study of Antimicrobial and Antioxidant Activities of Plant Essential Oils and Extracts as Candidate Ingredients for Edible Coatings to Control Decay in ‘Wonderful’ Pomegranate" Molecules 26, no. 11: 3367. https://doi.org/10.3390/molecules26113367
APA StyleKawhena, T. G., Opara, U. L., & Fawole, O. A. (2021). A Comparative Study of Antimicrobial and Antioxidant Activities of Plant Essential Oils and Extracts as Candidate Ingredients for Edible Coatings to Control Decay in ‘Wonderful’ Pomegranate. Molecules, 26(11), 3367. https://doi.org/10.3390/molecules26113367








