Palm Raceme as a Promising Biomass Precursor for Activated Carbon to Promote Lipase Activity with the Aid of Eutectic Solvents
Abstract
1. Introduction
2. Results
2.1. Effect of Activated Carbon on Lipase Activity
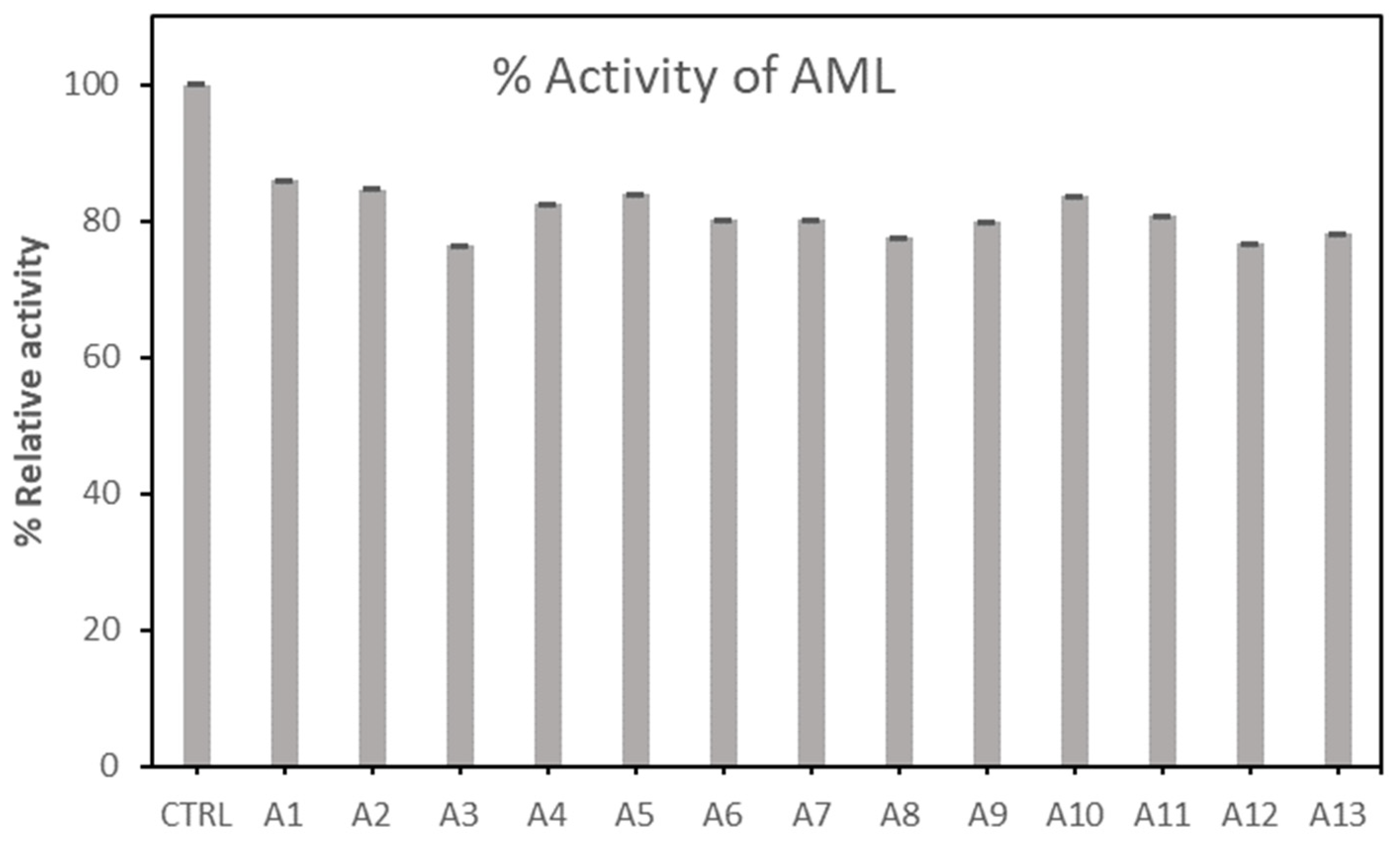
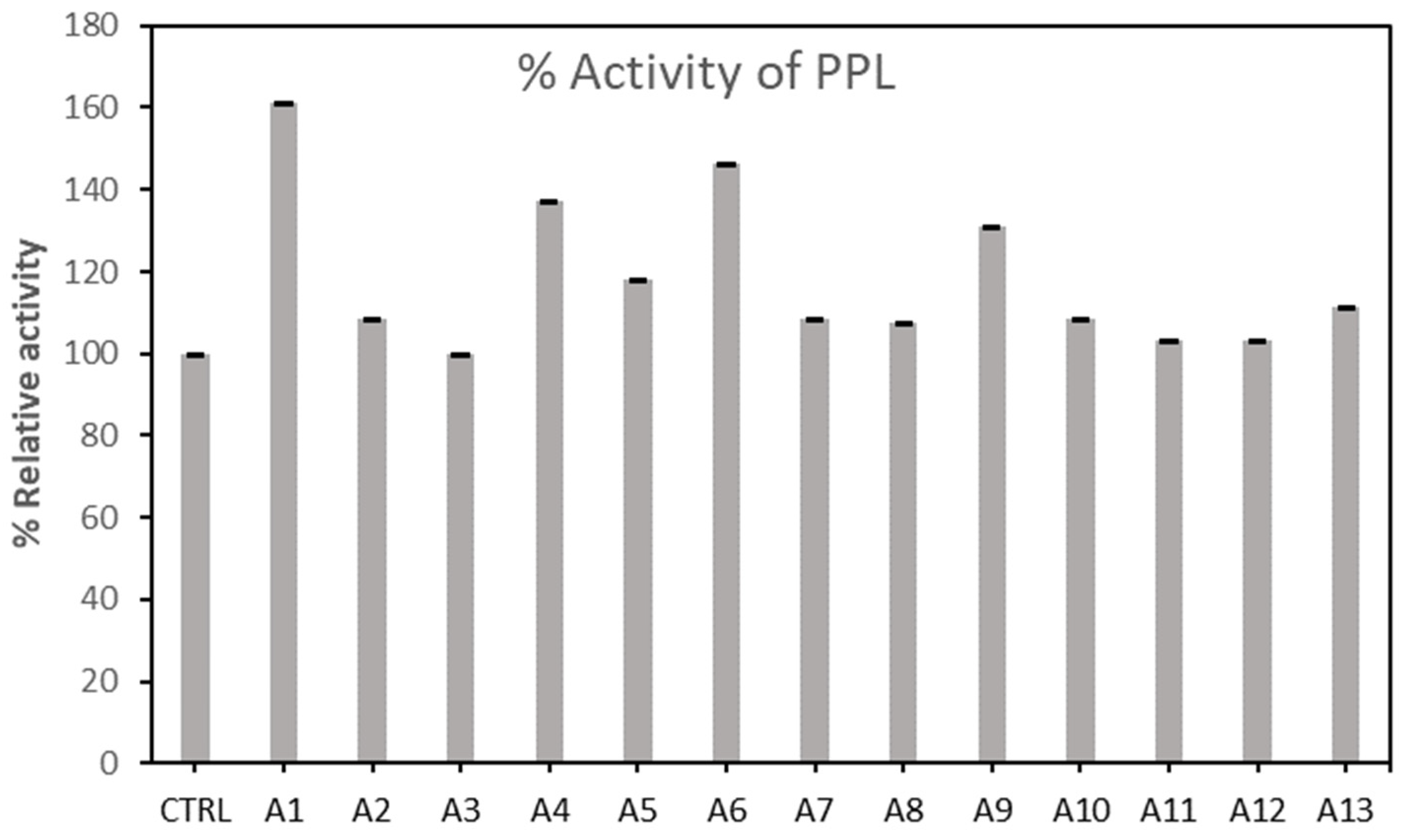
2.1.1. Screening of Lipases Immobilization in Different Activated Carbon
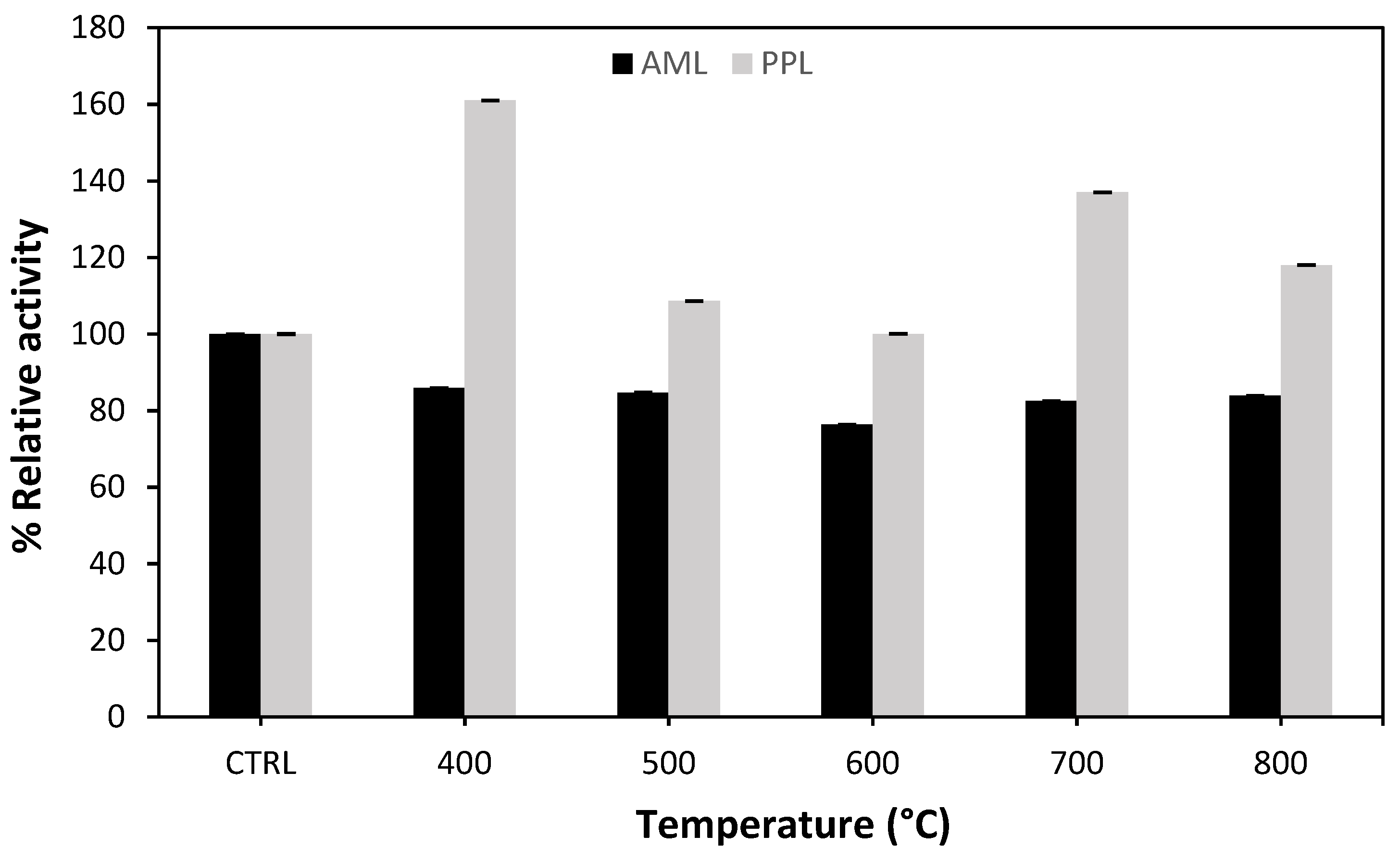
2.1.2. Carbonization Temperature Effect
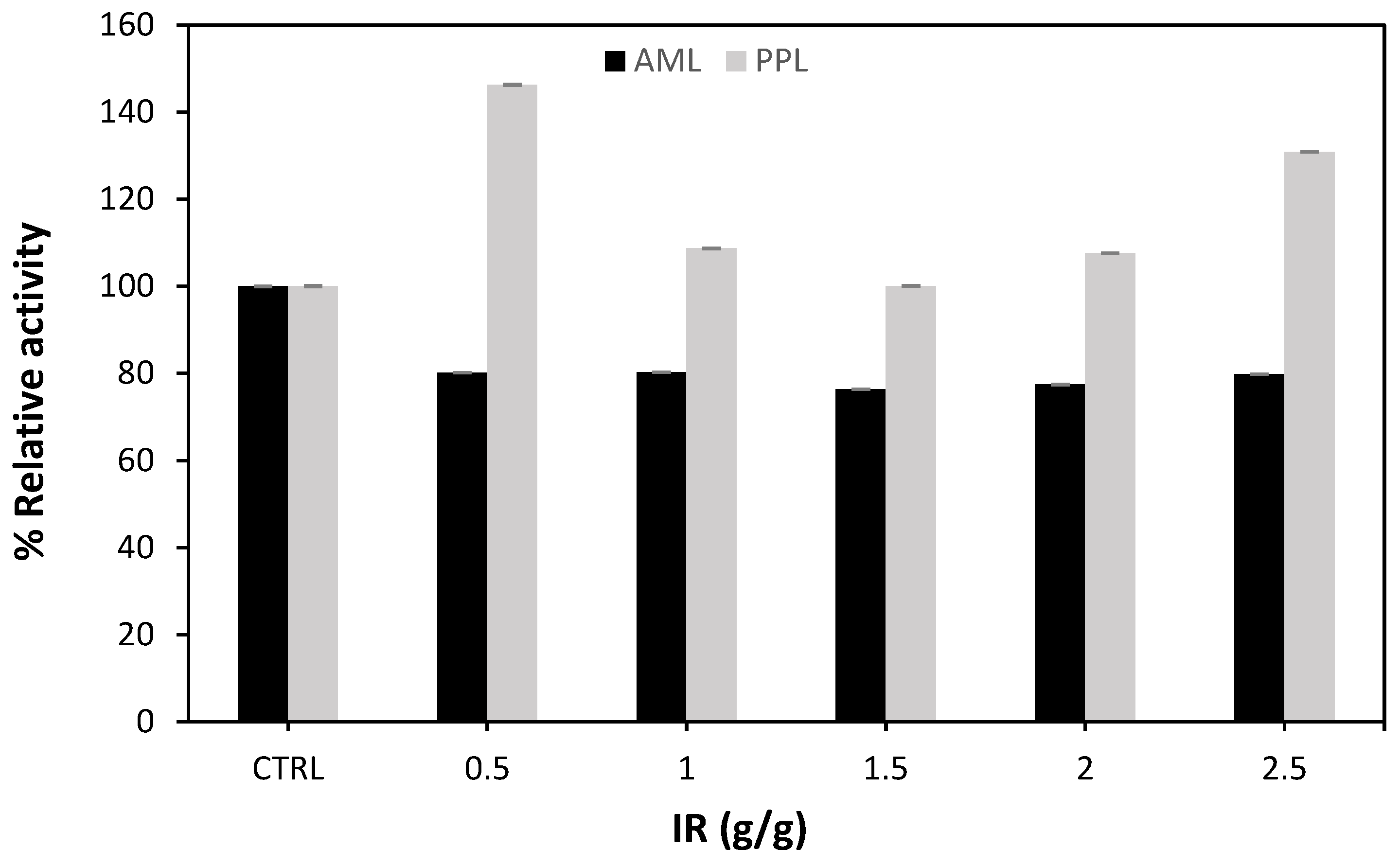
2.1.3. Impregnation Ratio Effect
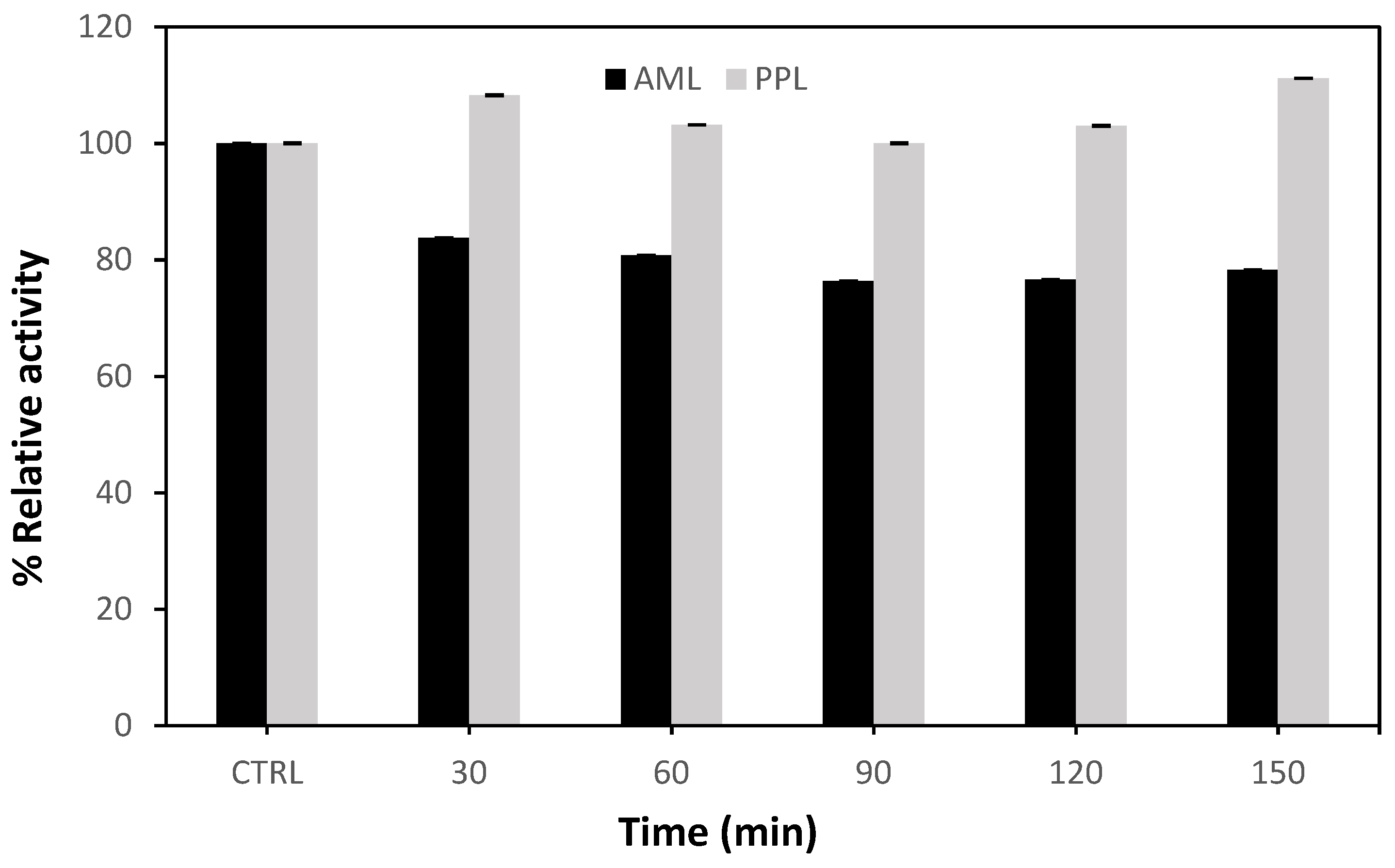
2.1.4. Effect of Carbonization Time
2.2. Activated Carbon and DESs Effect on the Activity of Lipases
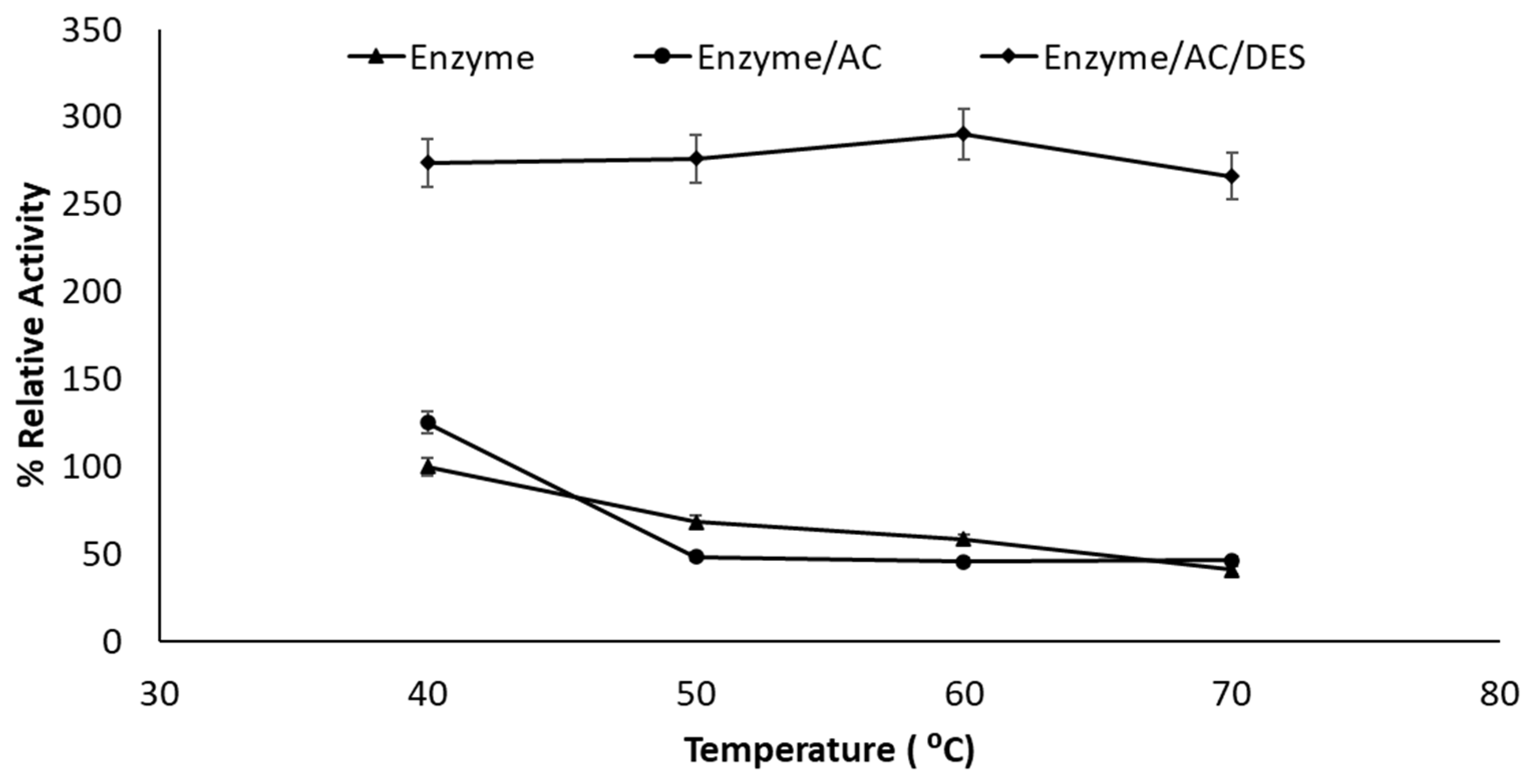
2.2.1. Effect of Reaction Temperature
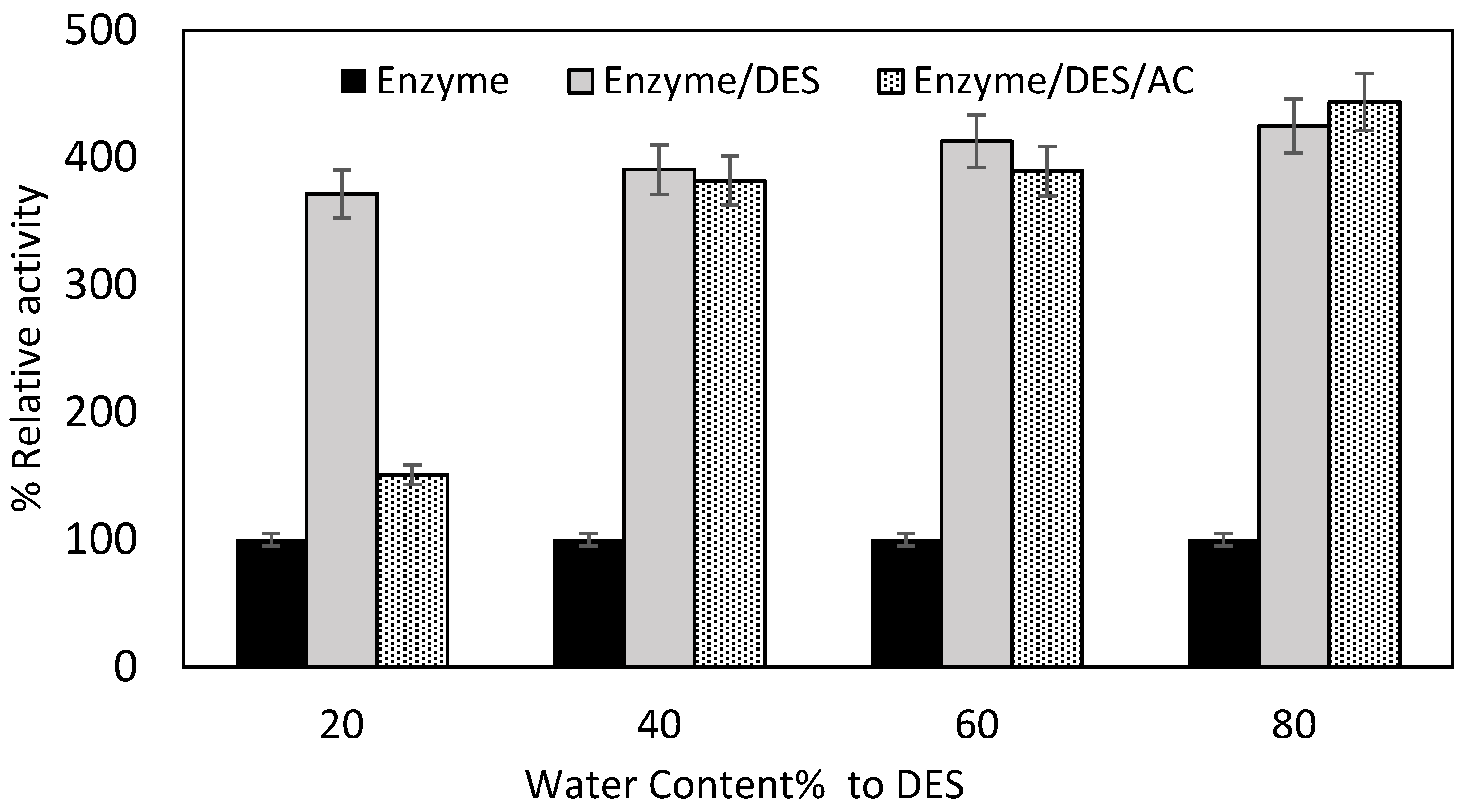
2.2.2. Impact of Water Content
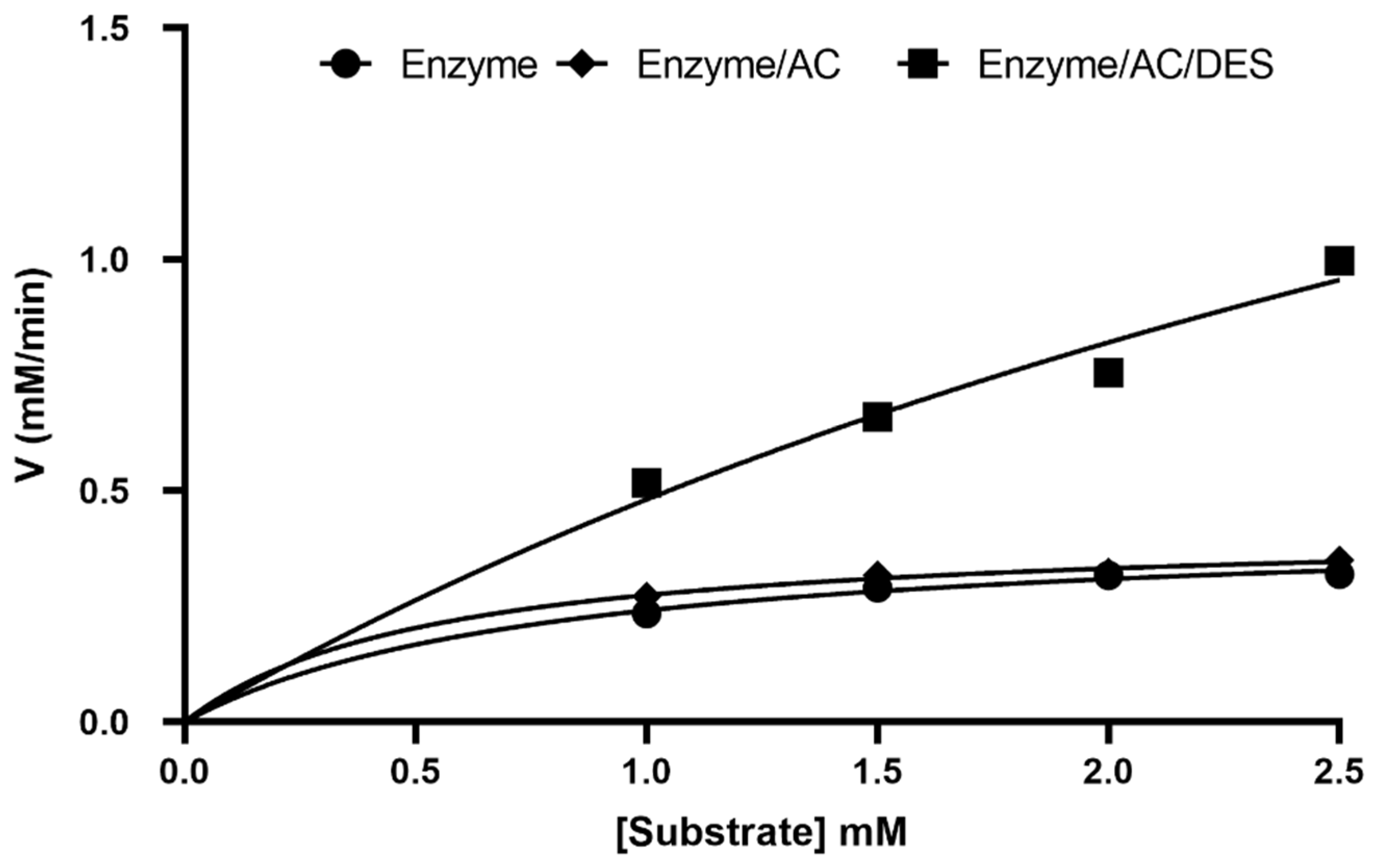
2.2.3. Kinetics Parameters
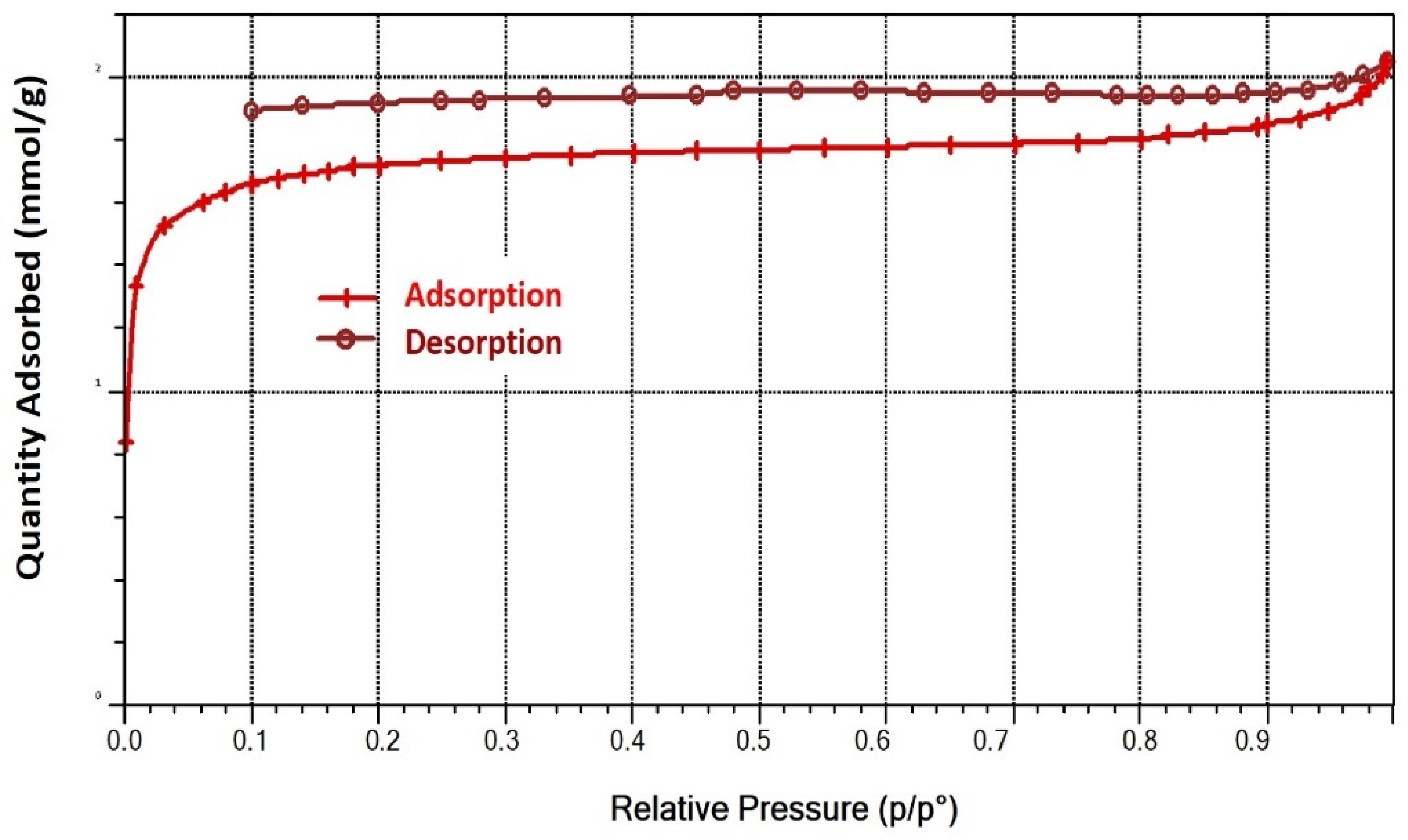
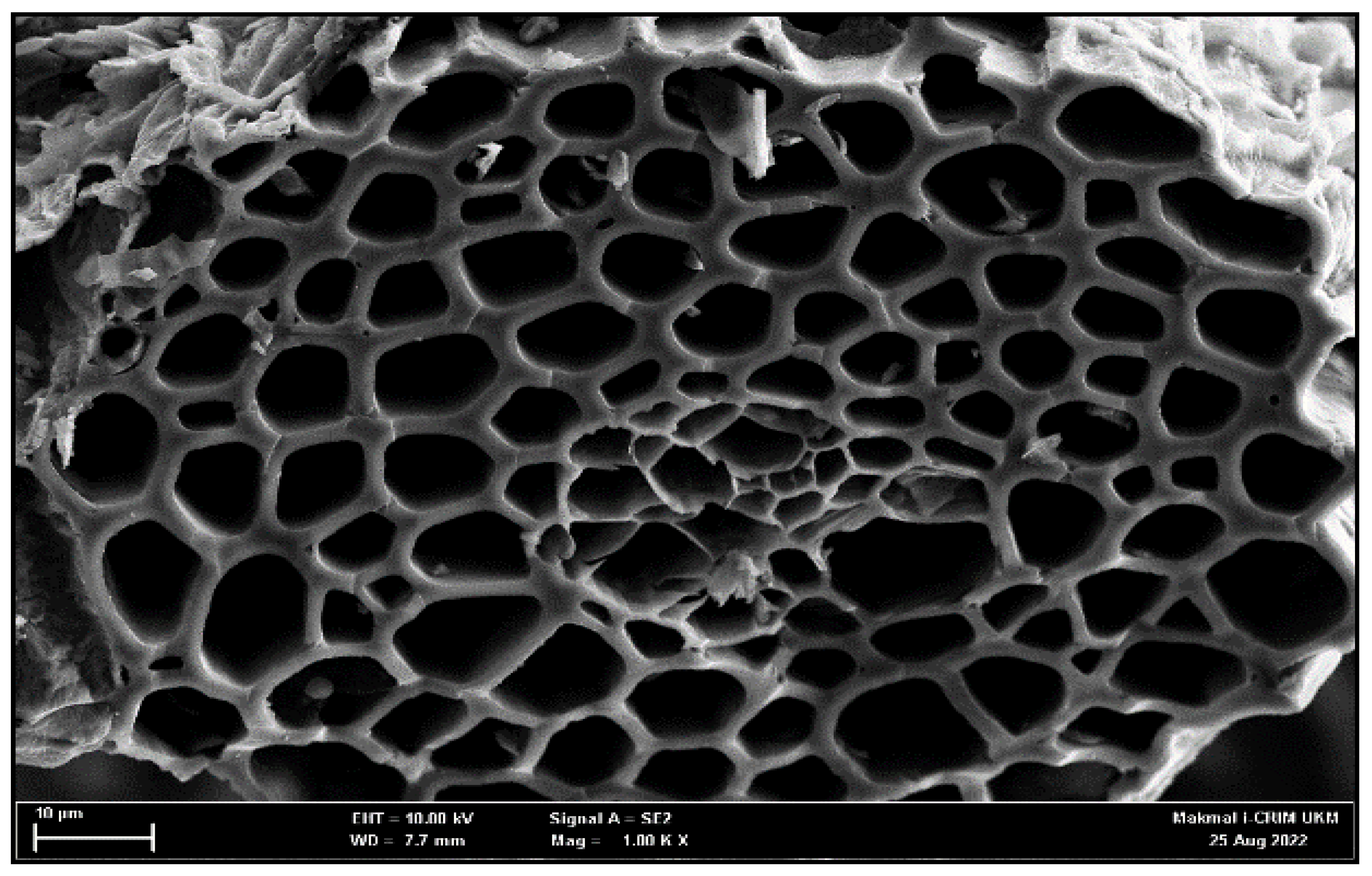
2.3. Morphology of Activated Carbon
3. Materials and Methods
3.1. Chemical and Biochemical Materials
3.2. Preparation of Activated Carbon (AC)
3.3. Preparation of DESs
3.4. Immobilization of Lipases
3.5. Assay for Lipase Activity
3.6. Screening of Lipases Activities with Different Activated Carbon
3.7. Statistical Analyses
3.8. Morphology of Activated Carbon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakar, D.A.; Anandarajah, G. Sustainability of Bioenergy in Malaysia with Reference to Palm Oil Biomass: Adopting Principles Governing Bioenergy Policy in the UK; Al-Kayiem, H.H., Brebbia, C.A., Zubir, S.S., Eds.; WIT Press: Billerica, MA, USA, 2015; Volume 206. [Google Scholar]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural Waste Material as Potential Adsorbent for Sequestering Heavy Metal Ions from Aqueous Solutions—A Review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.A.; Yusup, S. Production of Palm Kernel Shell-Based Activated Carbon by Direct Physical Activation for Carbon Dioxide Adsorption. Environ. Sci. Pollut. Res. 2019, 26, 33732–33746. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste Materials for Activated Carbon Preparation and Its Use in Aqueous-Phase Treatment: A Review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Sathiavelu, A.; Mythili, S. Mini Review on Nanoimmobilization of Lipase and Cellulase for Biofuel Production. Biofuels 2020, 11, 191–200. [Google Scholar] [CrossRef]
- Arsalan, A.; Younus, H. Enzymes and Nanoparticles: Modulation of Enzymatic Activity via Nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of High Surface Area Activated Carbon from Coconut Shells Using Microwave Heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef]
- Atkinson, J.D.; Rood, M.J. Preparing Microporous Carbon from Solid Organic Salt Precursors Using in Situ Templating and a Fixed-Bed Reactor. Microporous Mesoporous Mater. 2012, 160, 174–181. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P. Preparation of High-Surface-Area Activated Carbons from Coconut Shell. Microporous Mesoporous Mater. 1999, 27, 11–18. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A Review on Utilization of Wood Biomass as a Sustainable Precursor for Activated Carbon Production and Application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Baysal, M.; Bilge, K.; Yılmaz, B.; Papila, M.; Yürüm, Y. Preparation of High Surface Area Activated Carbon from Waste-Biomass of Sunflower Piths: Kinetics and Equilibrium Studies on the Dye Removal. J. Environ. Chem. Eng. 2018, 6, 1702–1713. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-Based Deep Eutectic Solvents: Physical Properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Evaluation of Toxicity and Biodegradability for Cholinium-Based Deep Eutectic Solvents. RSC Adv. 2015, 5, 83636–83647. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Frisch, G.; Ryder, K.S.; Silva, A.F. The Effect of Additives on Zinc Electrodeposition from Deep Eutectic Solvents. Electrochim. Acta 2011, 56, 5272–5279. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S. Densities and Viscosities of (Choline Chloride+ Urea) Deep Eutectic Solvent and Its Aqueous Mixtures in the Temperature Range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Hayyan, A.; Hizaddin, H.F.; Abed, K.M.; Mjalli, F.S.; Hashim, M.A.; Abo-Hamad, A.; Saleh, J.; Aljohani, A.S.M.; Alharbi, Y.M.; Alhumaydhi, F.A.; et al. Encapsulated Deep Eutectic Solvent for Esterification of Free Fatty Acid. Biomass Convers. Biorefinery 2021, 12, 3725–3735. [Google Scholar] [CrossRef]
- Hayyan, A.; Rashid, S.N.; Hayyan, M.; Zulkifliy, M.Y.; Hashim, M.A.; Osman, N.A. Synthesis of Novel Eutectic Catalyst for the Esterification of Crude Palm Oil Mixed with Sludge Palm Oil. J. Oil Palm Res. 2017, 29, 373–379. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep Eutectic Solvents: Recent Advances in Fabrication Approaches and Pharmaceutical Applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef]
- Hayyan, A.; Yeow, A.T.H.; Abed, K.M.; Basirun, W.J.; Kiat, L.B.; Saleh, J.; Han, G.W.; Min, P.C.; Aljohani, A.S.M.; Zulkifli, M.Y. The Development of New Homogenous and Heterogeneous Catalytic Processes for the Treatment of Low Grade Palm Oil. J. Mol. Liq. 2021, 344, 117574. [Google Scholar] [CrossRef]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic Liquids and Eutectic Mixtures as Solvent and Template in Synthesis of Zeolite Analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef]
- Abed, K.; Hayyan, A.; Hizaddin, H.F.; Hashim, M.A.; Basirun, W.J. Functionalized nanotubes. In Graphene, Nanotubes and Quantum Dots-Based Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 421–444. [Google Scholar]
- Hou, Y.; Li, Z.; Ren, S.; Wu, W. Separation of Toluene from Toluene/Alkane Mixtures with Phosphonium Salt Based Deep Eutectic Solvents. Fuel Process. Technol. 2015, 135, 99–104. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Requejo, P.F.; Kroon, M.C. Aliphatic–Aromatic Separation Using Deep Eutectic Solvents as Extracting Agents. Ind. Eng. Chem. Res. 2015, 54, 11404–11412. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Hayyan, M.; Hayyan, A.; Basirun, W.J.; Salleh, H.M.; Mirghani, M.E.S. A Grand Avenue to Integrate Deep Eutectic Solvents into Biomass Processing. Biomass Bioenergy 2020, 137, 105550. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at Interfaces: A Review. Adv. Colloid Interface Sci. 2009, 147, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-M.; Dong, C. Recent Advances in Nano-Carrier Immobilized Enzymes and Their Applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on Surface Modification of Nanocarriers to Overcome Diffusion Limitations: An Enzyme Immobilization Aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar]
- Elias, N.; Wahab, R.A.; Chandren, S.; Razak, F.I.A.; Jamalis, J. Effect of Operative Variables and Kinetic Study of Butyl Butyrate Synthesis by Candida Rugosa Lipase Activated by Chitosan-Reinforced Nanocellulose Derived from Raw Oil Palm Leaves. Enzyme Microb. Technol. 2019, 130, 109367. [Google Scholar] [CrossRef]
- Putra, S.S.S.; Basirun, W.J.; Elgharbawy, A.A.M.; Hayyan, A.; Hayyan, M.; Mohammed, M.A. Nanocellulose and Natural Deep Eutectic Solvent as Potential Biocatalyst System toward Enzyme Immobilization. Mol. Catal. 2022, 528, 112422. [Google Scholar] [CrossRef]
- Tsai, W. Study of Activated Carbon Adsorption and Catalyst Combustion of VOCs; National Taiwan University: Taipei, Taiwan, 1994. [Google Scholar]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous Activated Carbon Prepared from NaOH Activation of Rattan (Lacosperma secundiflorum) Hydrochar for Methylene Blue Removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Hayyan, A.; Hayyan, M.; Rashid, S.N.; Nor, M.R.M.; Zulkifli, M.Y.; Alias, Y.; Mirghani, M.E.S. Shedding Light on Lipase Stability in Natural Deep Eutectic Solvents. Chem. Biochem. Eng. Q. 2018, 32, 359–370. [Google Scholar] [CrossRef]
- Ballesteros, A.; Plou, F.J.; Iborra, J.L.; Halling, P.J. Engineering Stability of Enzymes in Systems with Organic Solvents. In Stability and Stabilization of Biocatalysts, Proceedings of an International Symposium Organized Under Auspices of the Working Party on Applied Biocatalysis of the European Federation of Biotechnology, the University of Cordoba, Spain, and the Spanish Soc, Cordoba, Spain, 19–22 April 1998; Elsevier Science Limited: New York, NY, USA, 1998; Volume 15, p. 355. [Google Scholar]
- Shehata, M.; Unlu, A.; Sezerman, U.; Timucin, E. Lipase and Water in a Deep Eutectic Solvent: Molecular Dynamics and Experimental Studies of the Effects of Water-In-Deep Eutectic Solvents on Lipase Stability. J. Phys. Chem. B 2020, 124, 8801–8810. [Google Scholar] [CrossRef]
- Fogarty, A.C.; Laage, D. Water Dynamics in Protein Hydration Shells: The Molecular Origins of the Dynamical Perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. Deep Eutectic Solvents: Synthesis, Application, and Focus on Lipase-catalyzed Reactions. Eur. J. Lipid Sci. Technol. 2013, 115, 379–385. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Wu, B.-P.; Wen, Q.; Yang, T.-X.; Yang, Z. Deep Eutectic Solvents Can Be Viable Enzyme Activators and Stabilizers. J. Chem. Technol. Biotechnol. 2014, 89, 1975–1981. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Putra, S.S.S.; Hayyan, M.; Hayyan, A.; Basirun, W.J.; Mirghani, M.E.S.; Majrashi, A.A.; Nawawi, W.M.F.W.; Alias, Y.; Salleh, H.M. Polyamidoamine Dendrimers: Favorable Polymeric Nanomaterials for Lipase Activation. Mater. Today Commun. 2020, 25, 101492. [Google Scholar] [CrossRef]
- Ramani, K.; Karthikeyan, S.; Boopathy, R.; Kennedy, L.J.; Mandal, A.B.; Sekaran, G. Surface Functionalized Mesoporous Activated Carbon for the Immobilization of Acidic Lipase and Their Application to Hydrolysis of Waste Cooked Oil: Isotherm and Kinetic Studies. Process Biochem. 2012, 47, 435–445. [Google Scholar] [CrossRef]
- Soni, S.; Dwivedee, B.P.; Banerjee, U.C. Facile Fabrication of a Recyclable Nanobiocatalyst: Immobilization of: Burkholderia cepacia Lipase on Carbon Nanofibers for the Kinetic Resolution of a Racemic Atenolol Intermediate. RSC Adv. 2018, 8, 27763–27774. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
| ANOVA Summary | AML | PPL |
|---|---|---|
| F | 1096 | 15871 |
| p value | <0.0001 | <0.0001 |
| Significant diff. among means (p < 0.05) | Yes | Yes |
| R squared | 0.9980 | 0.9999 |
| Medium | KM (mM) | VMAX (mM min−1) | Kcat (min−1) | Kcat/KM (min mM−1) |
|---|---|---|---|---|
| Enzyme only | 0.78 ± 0.03 | 0.43 ± 0.03 | 4.01 ± 0.03 | 5.14 ± 0.01 |
| Enzyme/AC | 0.17 ± 0.02 | 0.42 ± 0.03 | 3.71 ± 0.02 | 21.82 ± 0.03 |
| Enzyme/DES/AC | 4.79 ± 0.03 | 2.80 ± 0.02 | 49.07 ± 0.01 | 10.24 ± 0.03 |
| No. | Sample | Activation Temperature/°C | Impregnation Ratio (NaOH/biomass)/(g/g) | Activation Time/min |
|---|---|---|---|---|
| 1 | A 1 | 400 | 1.5 | 90 |
| 2 | A 2 | 500 | 1.5 | 90 |
| 3 | A 3 | 600 | 1.5 | 90 |
| 4 | A 4 | 700 | 1.5 | 90 |
| 5 | A 5 | 800 | 1.5 | 90 |
| 6 | A 6 | 600 | 0.5 | 90 |
| 7 | A 7 | 600 | 1.0 | 90 |
| 8 | A 8 | 600 | 2.0 | 30 |
| 9 | A 9 | 600 | 2.5 | 90 |
| 10 | A 10 | 600 | 1.5 | 30 |
| 11 | A 11 | 600 | 1.5 | 60 |
| 12 | A 12 | 600 | 1.5 | 120 |
| 13 | A 13 | 600 | 1.5 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abed, K.M.; Hayyan, A.; Elgharbawy, A.A.M.; Hizaddin, H.F.; Hashim, M.A.; Hasan, H.A.; Hamid, M.D.; Zuki, F.M.; Saleh, J.; Aldaihani, A.G. Palm Raceme as a Promising Biomass Precursor for Activated Carbon to Promote Lipase Activity with the Aid of Eutectic Solvents. Molecules 2022, 27, 8734. https://doi.org/10.3390/molecules27248734
Abed KM, Hayyan A, Elgharbawy AAM, Hizaddin HF, Hashim MA, Hasan HA, Hamid MD, Zuki FM, Saleh J, Aldaihani AG. Palm Raceme as a Promising Biomass Precursor for Activated Carbon to Promote Lipase Activity with the Aid of Eutectic Solvents. Molecules. 2022; 27(24):8734. https://doi.org/10.3390/molecules27248734
Chicago/Turabian StyleAbed, Khalid M., Adeeb Hayyan, Amal A. M. Elgharbawy, Hanee F. Hizaddin, Mohd Ali Hashim, Hassimi Abu Hasan, Mahar Diana Hamid, Fathiah M. Zuki, Jehad Saleh, and Ahmad GH Aldaihani. 2022. "Palm Raceme as a Promising Biomass Precursor for Activated Carbon to Promote Lipase Activity with the Aid of Eutectic Solvents" Molecules 27, no. 24: 8734. https://doi.org/10.3390/molecules27248734
APA StyleAbed, K. M., Hayyan, A., Elgharbawy, A. A. M., Hizaddin, H. F., Hashim, M. A., Hasan, H. A., Hamid, M. D., Zuki, F. M., Saleh, J., & Aldaihani, A. G. (2022). Palm Raceme as a Promising Biomass Precursor for Activated Carbon to Promote Lipase Activity with the Aid of Eutectic Solvents. Molecules, 27(24), 8734. https://doi.org/10.3390/molecules27248734









