Bimetallic TiO2 Nanoparticles for Lignin-Based Model Compounds Valorization by Integrating an Optocatalytic Flow-Microreactor
Abstract
1. Introduction
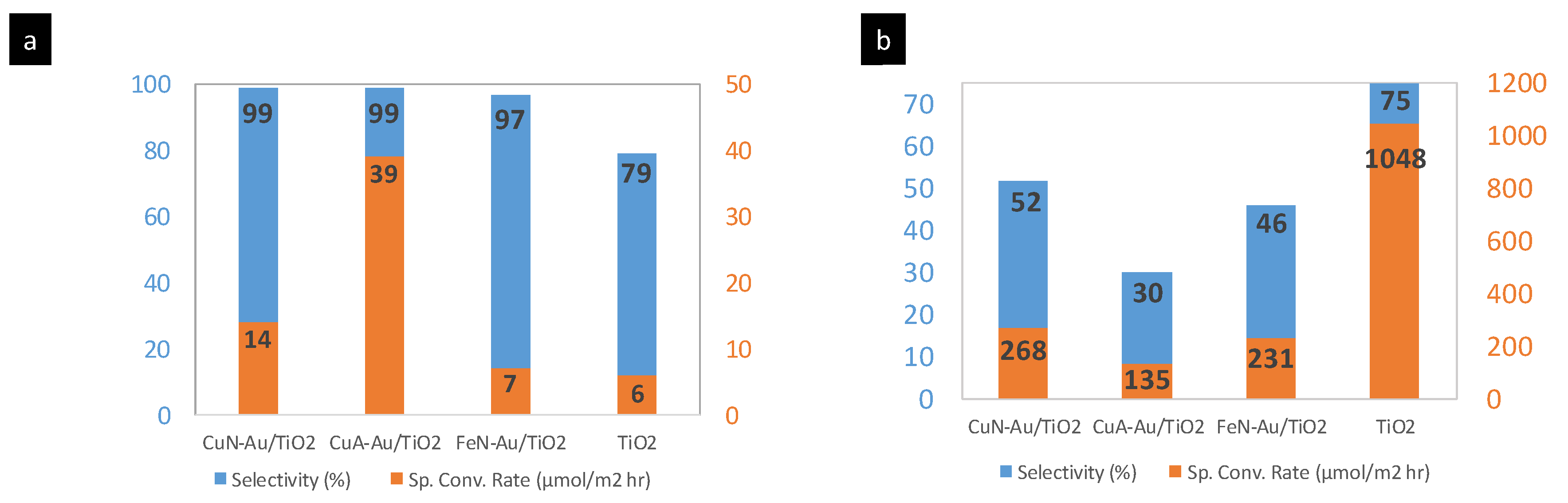
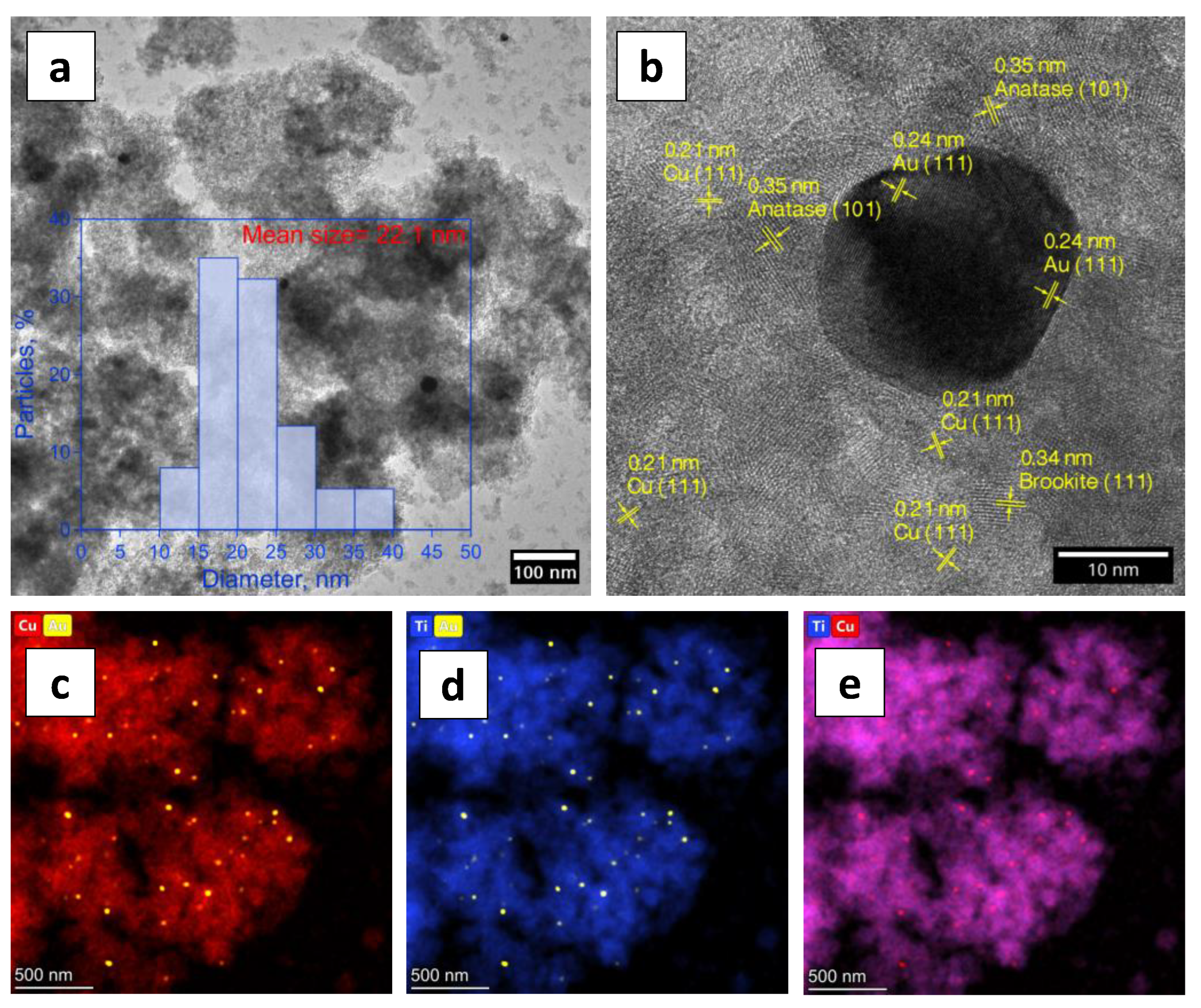
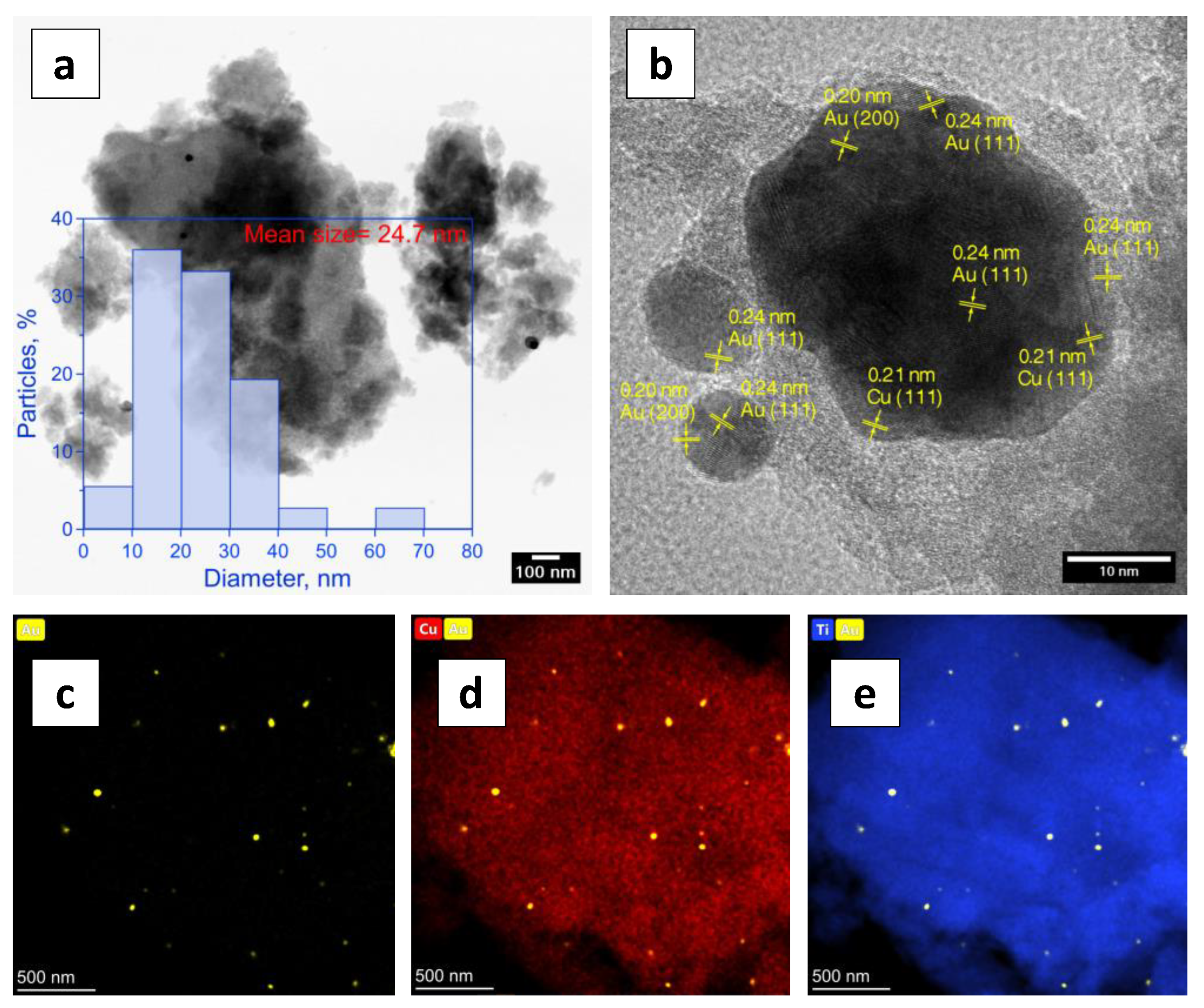
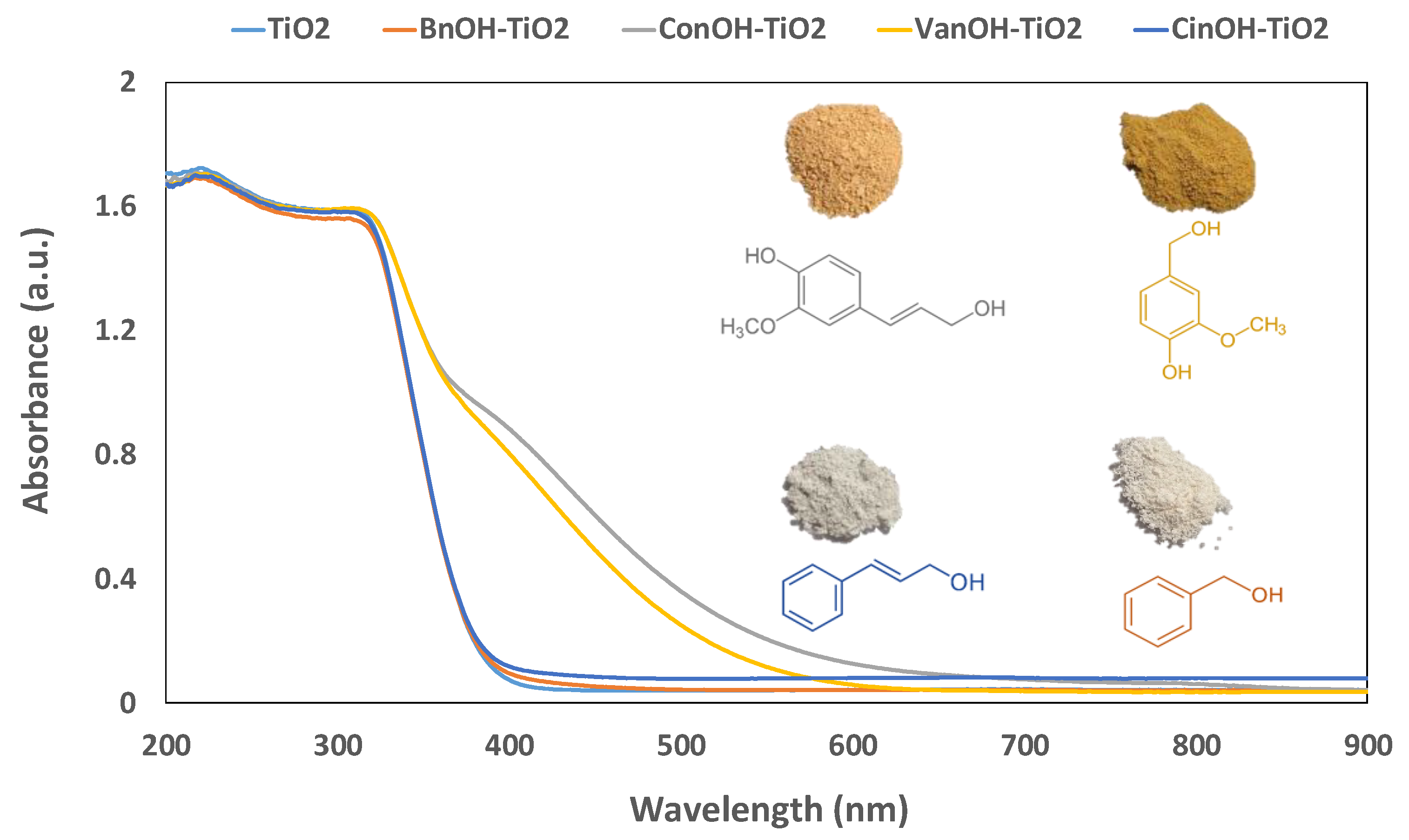
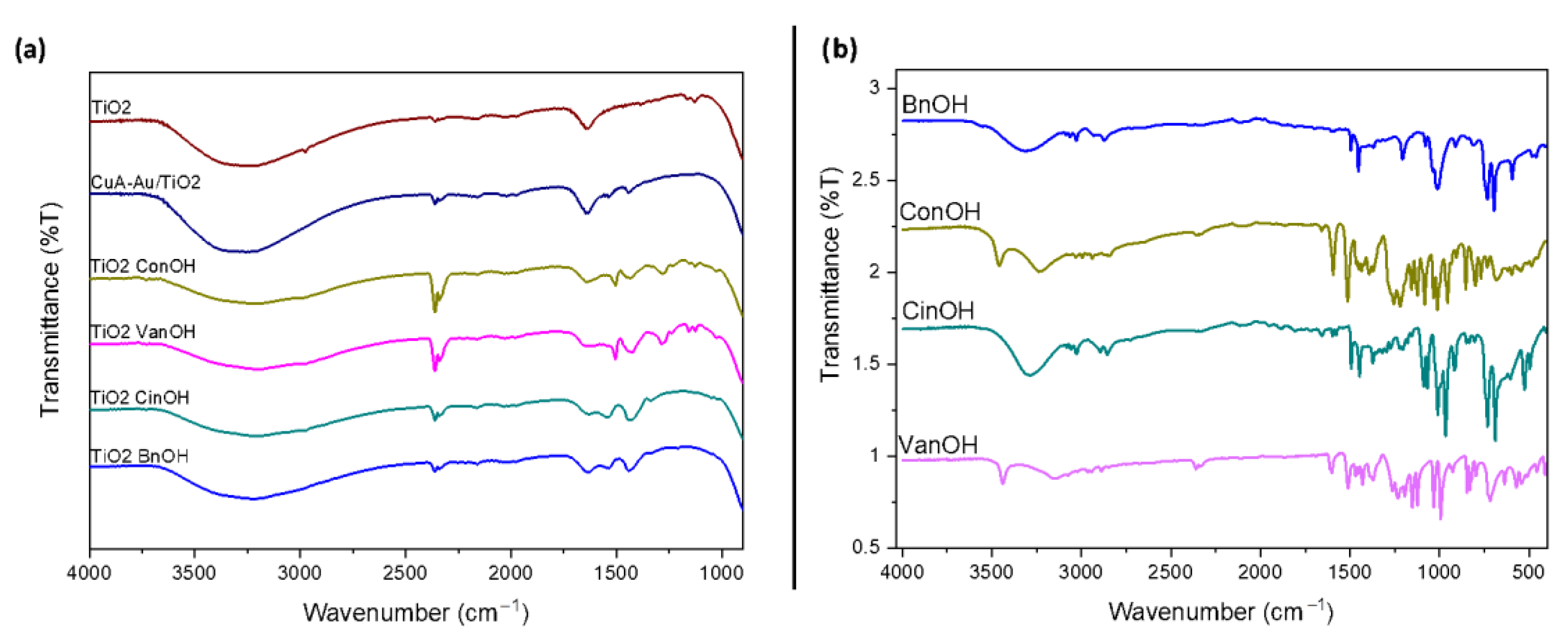
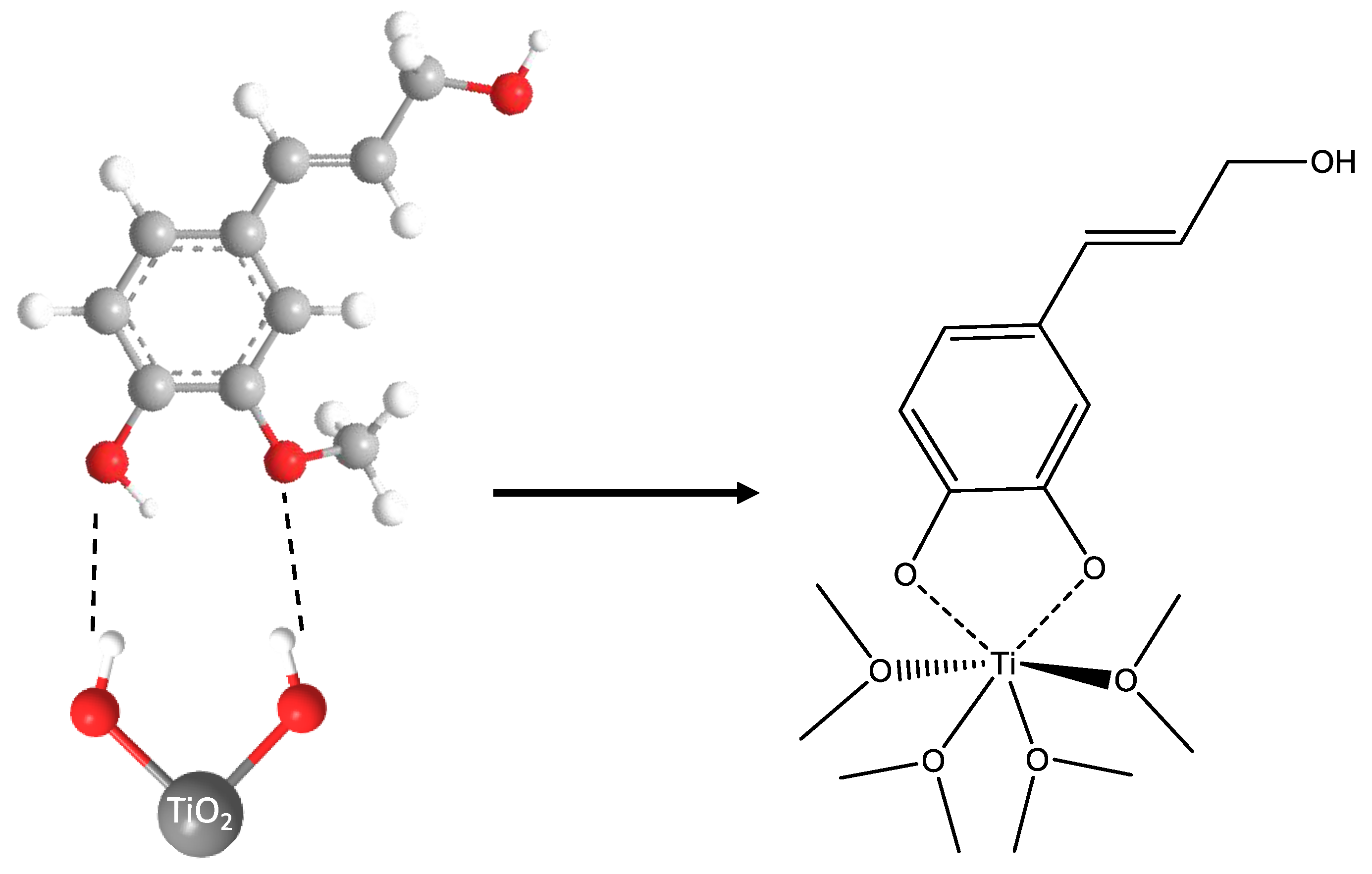
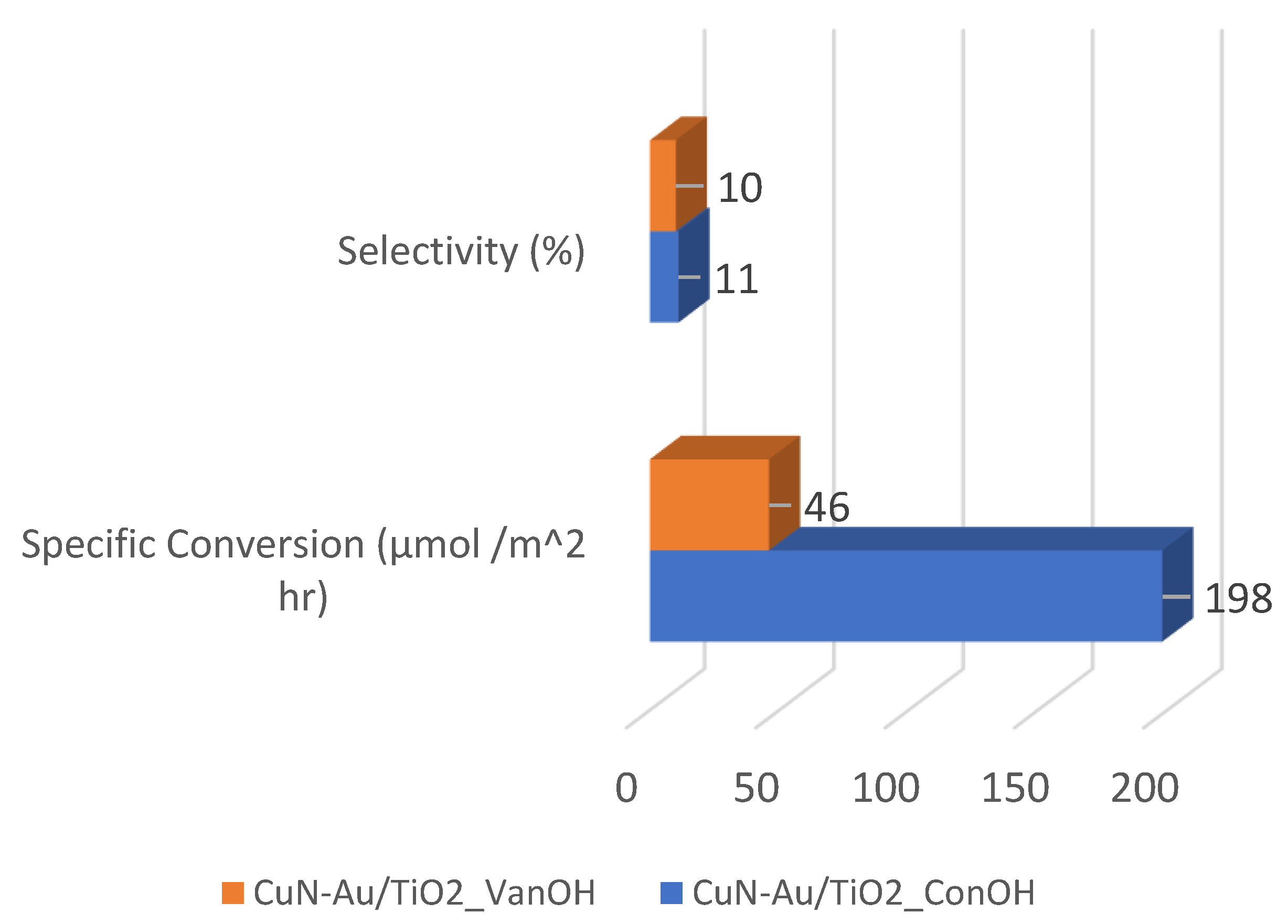
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
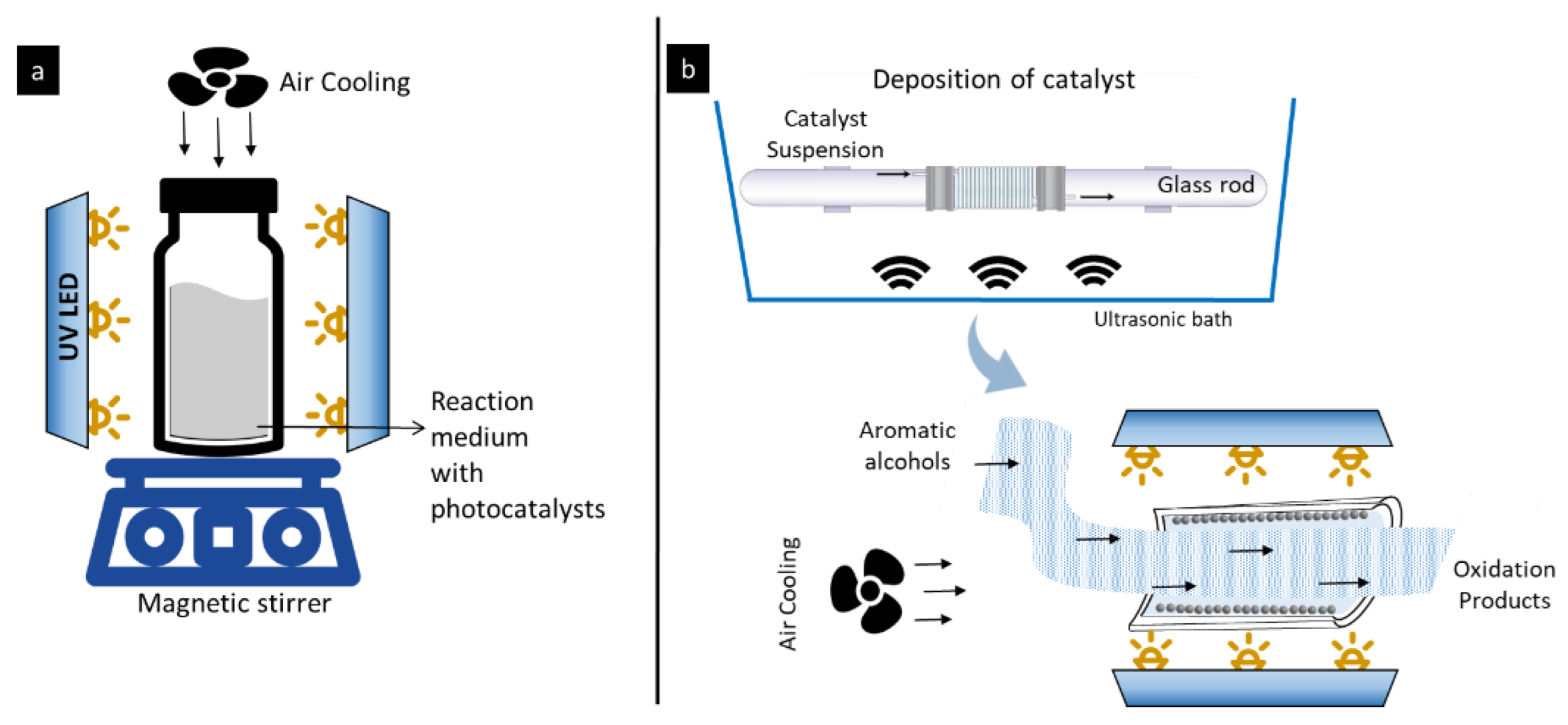
3.3. Microreactor Preparation
3.4. Catalytic Performance Test
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.V.N.; Kim, S.Y.; Van Le, Q. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Carabin, A.; Drogui, P.; Robert, D. Journal of the Taiwan Institute of Chemical Engineers Photo-degradation of carbamazepine using TiO2 suspended photocatalysts. J. Taiwan Inst. Chem. Eng. 2015, 54, 109–117. [Google Scholar] [CrossRef]
- Kashale, A.A.; Rasal, A.S.; Kamble, G.P.; Ingole, V.H.; Dwivedi, P.K.; Rajoba, S.J.; Jadhav, L.D.; Ling, Y.; Chang, J.; Ghule, A.V. Biosynthesized Co-doped TiO2 nanoparticles based anode for lithium-ion battery application and investigating the in fl uence of dopant concentrations on its performance. Compos. Part B 2019, 167, 44–50. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- El Mragui, A.; Daou, I.; Zegaoui, O. Influence of the preparation method and ZnO/(ZnO + TiO2) weight ratio on the physicochemical and photocatalytic properties of ZnO-TiO2 nanomaterials. Catal. Today 2019, 321, 41–51. [Google Scholar] [CrossRef]
- Gupta, A.K.; Pal, A.; Sahoo, C. Photocatalytic degradation of a mixture of Crystal Violet (Basic Violet 3) and Methyl Red dye in aqueous suspensions using Ag C doped TiO2. Dye Pigment. 2006, 69, 224–232. [Google Scholar] [CrossRef]
- Justyna, Ł.; Paszkiewicz-gawron, M. Visible-Light Photocatalytic Activity of Ionic Liquid TiO2 Spheres: Effect of the Ionic Liquid’s Anion Structure. ChemCatChem 2017, 9, 4377–4388. [Google Scholar]
- Khan, A.; Goepel, M.; Kubas, A.; Łomot, D.; Lisowski, W. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran by Visible Light-Driven Photocatalysis over In Situ Substrate-Sensitized Titania. ChemSusChem 2021, 14, 1351–1362. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Saccomanni, A.; Altomare, M.; Selli, E. Photocatalytic activity of NH4F-doped TiO2 modified by noble metal nanoparticle deposition. Photochem. Photobiol. Sci. 2013, 12, 595–601. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, J.; Chen, M.; Shen, K.; Zhang, D.; Zhang, J.; Zhang, G.; Liu, Q. Boosted visible-light photodegradation of methylene blue by V and Co co-doped TiO2. Materials 2018, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.R.; Lisovytskiy, D.; Colmenares, J.C. Flow photomicroreactor coated with monometal containing TiO2 using sonication: A versatile tool for visible light oxidation. Catal. Commun. 2022, 162, 106375. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Luís Pinto da Silva, L.; Zegaoui, O.; da Silva, J.C.G.E. Synthesis of fe-and co-doped TiO2 with improved photocatalytic activity under visible irradiation toward carbamazepine degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef] [PubMed]
- El Mragui, A.; Zegaoui, O.; Daou, I.; Esteves da Silva, J.C.G. Preparation, characterization, and photocatalytic activity under UV and visible light of Co, Mn, and Ni mono-doped and (P,Mo) and (P,W) co-doped TiO2 nanoparticles: A comparative study. Environ. Sci. Pollut. Res. 2021, 28, 25130–25145. [Google Scholar] [CrossRef] [PubMed]
- Gołąbiewska, A.; Lisowski, W.; Jarek, M.; Nowaczyk, G.; Michalska, M.; Jurga, S.; Zaleska-Medynska, A. The effect of metals content on the photocatalytic activity of TiO2 modified by Pt/Au bimetallic nanoparticles prepared by sol-gel method. Mol. Catal. 2017, 442, 154–163. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, Y.; Tang, Z.; Jiang, H. Metal—Organic Framework Supported Gold Nanoparticles as a Highly Active Heterogeneous Catalyst for Aerobic Oxidation of Alcohols. J. Phys. Chem. C 2010, 114, 13362–13369. [Google Scholar] [CrossRef]
- Mullen, G.M.; Zhang, L.; Evans, E.J.; Yan, T.; Henkelman, G.; Mullins, C.B. Oxygen and Hydroxyl Species Induce Multiple Reaction Pathways for the Partial Oxidation of Allyl Alcohol on Gold. J. Am. Chem. Soc. 2014, 136, 6489–6498. [Google Scholar] [CrossRef]
- Jiang, T.; Jia, C.; Zhang, L.; He, S.; Sang, Y.; Li, H.; Li, Y.; Xu, X.; Liu, H. Gold and gold–palladium alloy nanoparticles on heterostructured TiO2 nanobelts as plasmonic photocatalysts for benzyl alcohol oxidation. Nanoscale 2015, 7, 209–217. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future w. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Kavitha, R.; Kumar, S.G. Review on bimetallic-deposited TiO2: Preparation methods, charge carrier transfer pathways and photocatalytic applications. Chem. Pap. 2020, 74, 717–756. [Google Scholar] [CrossRef]
- Durndell, L.J.; Parlett, C.M.A.; Hondow, N.S.; Wilsona, K.; Lee, A.F. Tunable Pt nanocatalysts for the aerobic selox of cinnamyl alcohol. Nanoscale 2013, 123, 5412–5419. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.R.; Colmenares-Quintero, R.F.; Quintero, J.C.C. Designing microflowreactors for photocatalysis using sonochemistry: A systematic review article. Molecules 2019, 24, 3315. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Jiang, M.; Cheng, D.; Chen, F. Continuous flow technology-a tool for safer oxidation chemistry. React. Chem. Eng. 2022, 7, 490–550. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Yu, X.; Yu, J.; Cheng, B.; Jaroniec, M. Synthesis of Hierarchical Flower-like AlOOH and TiO2/AlOOH Superstructures and their Enhanced Photocatalytic Properties. J. Phys. Chem. C 2009, 113, 17527–17535. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Dash, T.; Saran, S.; Bhandari, S.; Kumar, U. Self-organized copper impregnation and doping in TiO2 with enhanced photocatalytic conversion of H2O and CO2 to fuel. Int. J. Hydrogen Energy 2018, 43, 19468–19480. [Google Scholar] [CrossRef]
- Kubiak, A.; Bielan, Z.; Kubacka, M.; Gabała, E.; Zgoła-Grześkowiak, A.; Janczarek, M.; Zalas, M.; Zielińska-Jurek, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Microwave-assisted synthesis of a TiO2-CuO heterojunction with enhanced photocatalytic activity against tetracycline. Appl. Surf. Sci. 2020, 520, 146344. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, J.; Gan, Y.; Liu, L.; Ju, M. Environmental Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl. Catal. B Environ. 2016, 191, 94–105. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, J.; Wang, J.; Wang, R.; Liu, G.; Qi, Y.; Xu, W.; Luo, G.; Xu, M. The effect of different morphology of fluoride-mediated TiO2 based on Ostwald ripening on photocatalytic activity. Colloids Surf. A 2021, 610, 125702. [Google Scholar] [CrossRef]
- Nogawa, T.; Isobe, T.; Matsushita, S.; Nakajima, A. Preparation and visible-light photocatalytic activity of Au- and Cu-modi fi ed TiO2 powders. Mater. Lett. 2012, 82, 174–177. [Google Scholar] [CrossRef]
- Monga, A.; Bathla, A.; Pal, B. A Cu-Au bimetallic co-catalysis for the improved photocatalytic activity of TiO2 under visible light radiation. Sol. Energy 2017, 155, 1403–1410. [Google Scholar] [CrossRef]
- Li, N.; Geng, D.; Zhou, J. Ag and Cu Nanoparticles Synergistically Enhance Photocatalytic—CO2 Reduction Activity of B Phase—TiO2. Catal. Lett. 2022, 152, 124–138. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 46401. [Google Scholar] [CrossRef] [PubMed]
- Go, A.; Zieli, A.; Zaleska, A. Characterization of TiO2 Modified with Bimetallic Ag/Au Nanoparticles Obtained in Microemulsion System Characterization of TiO2 Modified with Bimetallic Ag/Au Nanoparticles Obtained in Microemulsion System. J. Adv. Oxid. Technol. 2012, 15, 71. [Google Scholar]
- Liu, S.; Xu, Y. Photo-induced transformation process at gold clusters- semiconductor interface: Implications for the complexity of gold clusters-based photocatalysis. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, L.; Wu, X.; Tian, Y.; Zhou, X.; Xu, S.; Xie, Z. Size and Shape Effect of Gold Nanoparticles in “ Far-Field ” Surface Plasmon Resonance. Part. Part. Syst. Charact. 2019, 1800077, 1–8. [Google Scholar] [CrossRef]
- Goł, A.; Malankowska, A.; Jarek, M.; Lisowski, W.; Nowaczyk, G.; Jurga, S.; Zaleska-medynska, A. Environmental The effect of gold shape and size on the properties and visible. Appl. Catal. B Environ. 2016, 196, 27–40. [Google Scholar]
- Duan, Z.; Huang, Y.; Zhang, D.; Chen, S. Electrospinning Fabricating Au/TiO2 Network-like Nanofibers as Visible Light Activated Photocatalyst. Sci. Rep. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Anwar, D.I.; Mulyadi, D. Synthesis of Fe-TiO2 Composite as a Photocatalyst for Degradation of Methylene Blue. Procedia Chem. 2015, 17, 49–54. [Google Scholar] [CrossRef]
- Wang, Z.; De Soto, L.S.; Me, C.; Casale, S.; Delannoy, L. A selective and stable Fe/TiO2 catalyst for selective hydrogenation of butadiene in alkene-rich stream. ChemComm 2021, 57, 7031–7034. [Google Scholar] [CrossRef]
- Kruanetr, S.; Wanchanthuek, R. Studies on preparation and characterization of Fe/TiO2 catalyst in photocatalysis applications Studies on preparation and characterization of Fe/TiO2 catalyst in photocatalysis applications. Mater. Res. Express 2017, 4, 76507. [Google Scholar] [CrossRef]
- Bulasara, V.K.; Uppaluri, R.; Purkait, M.K. Effect of Ultrasound on the Performance of Nickel Hydrazine Electroless Plating Baths Effect of Ultrasound on the Performance of Nickel Hydrazine Electroless Plating Baths. Mater. Manuf. Process. 2012, 6914, 201–206. [Google Scholar] [CrossRef]
- Wu, G.; Brett, G.L.; Cao, E.; Constantinou, A.; Ellis, P.; Kuhn, S.; Hutchings, G.J.; Bethell, D.; Gavriilidis, A. Catalysis Science & Technology Oxidation of cinnamyl alcohol using bimetallic continuous flow packed bed microreactor. Catal. Sci. Technol. 2016, 6, 4749–4758. [Google Scholar]
- Taboada-puig, R.; Moreira, T.; Lema, J.M.; Fagerstedt, K.; Heikkinen, H. Polymerization of Coniferyl Alcohol by Mn 3 1-ediated (Enzymatic) Oxidation: Effects of H2O2 Concentration, Aqueous Organic Solvents, and pH. Biotechnol. Prog. 2018, 34, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Choi, W. A low-cost sensitizer based on a phenolic resin for charge-transfer type photocatalysts working under visible light w. ChemComm 2012, 48, 10621–10623. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, W. Visible-Light-Induced Photocatalytic Degradation of 4-Chlorophenol and Phenolic Compounds in Aqueous Suspension of Pure Titania: Demonstrating the Existence of a Surface-Complex-Mediated Path. J. Phys. Chem. B 2005, 109, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Imparato, C.; D’Errico, G.; Macyk, W.; Kobielusz, M.; Vitiello, G.; Antonio, A. Interfacial Charge Transfer Complexes in TiO2-Enediol Hybrids Synthesized by Sol-Gel. Langmuir 2022, 38, 1821–1832. [Google Scholar] [CrossRef]
- Zhang, G.; Kim, G.; Choi, W. Visible light driven photocatalysis mediated via ligand-to-metal charge transfer (LMCT): An alternative approach to solar activation of titania. Energy Environ. Sci. 2014, 7, 954. [Google Scholar] [CrossRef]
- Pradhan, S.R.; Nair, V.; Giannakoudakis, D.A.; Lisovytskiy, D.; Colmenares, J.C. Design and development of TiO2 coated microflow reactor for photocatalytic partial oxidation of benzyl alcohol. Mol. Catal. 2020, 486, 110884. [Google Scholar] [CrossRef]
- Borra, S.; Chandrasekhar, D.; Adhikary, S.; Rasala, S.; Gokulnath, S.; Maurya, R.A. Visible-Light Driven Photocascade Catalysis: Union of N,N-Dimethylanilines and α-Azidochalcones in Flow Microreactors. J. Org. Chem. 2017, 82, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
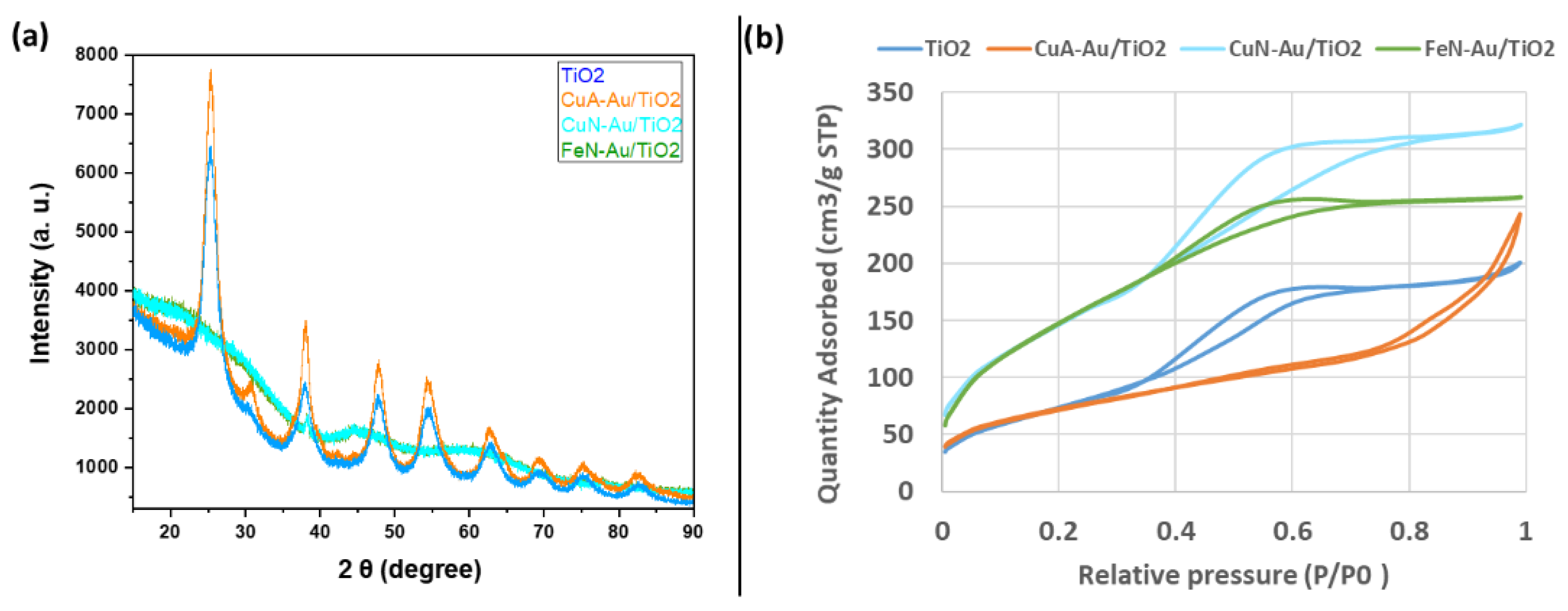
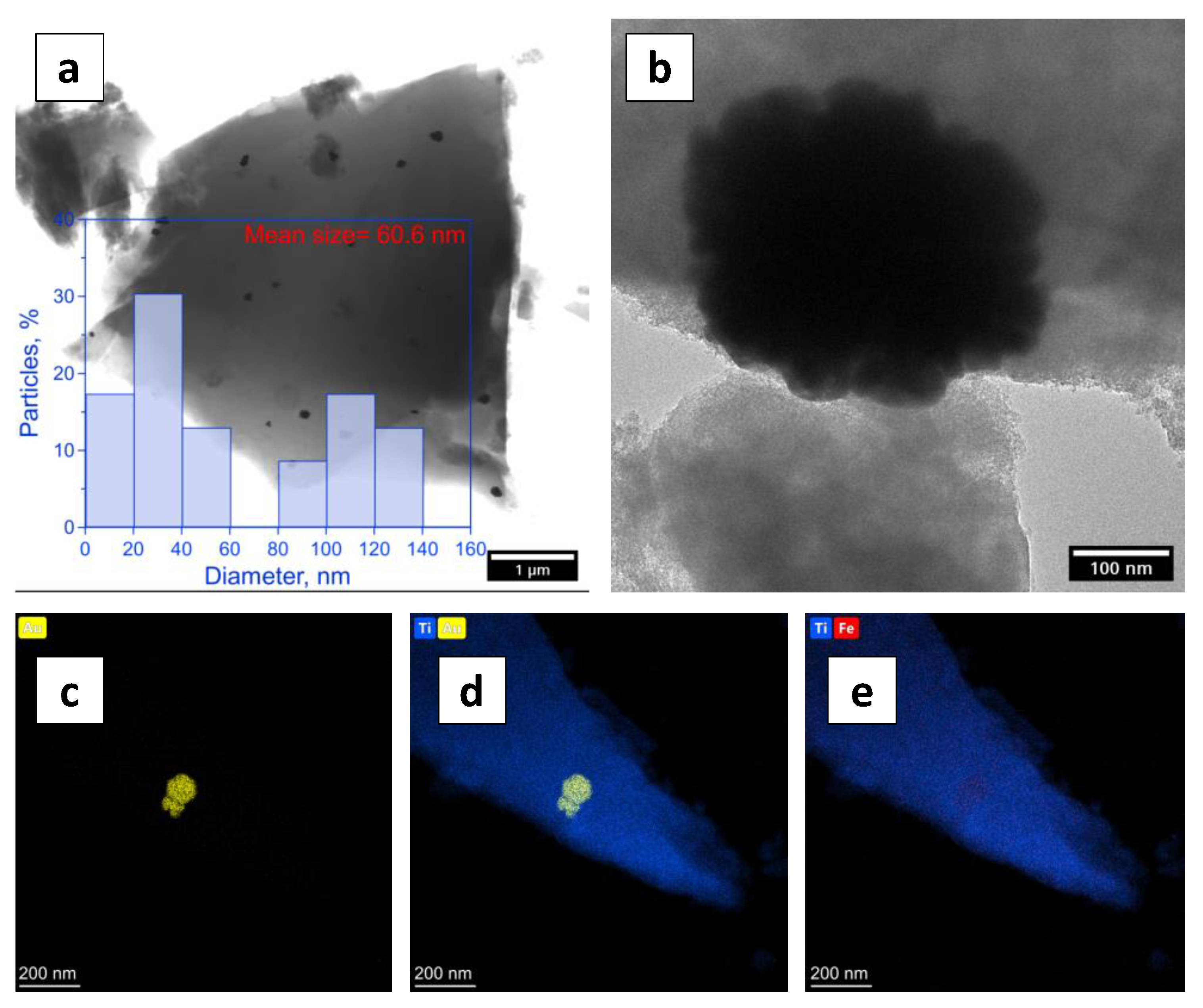
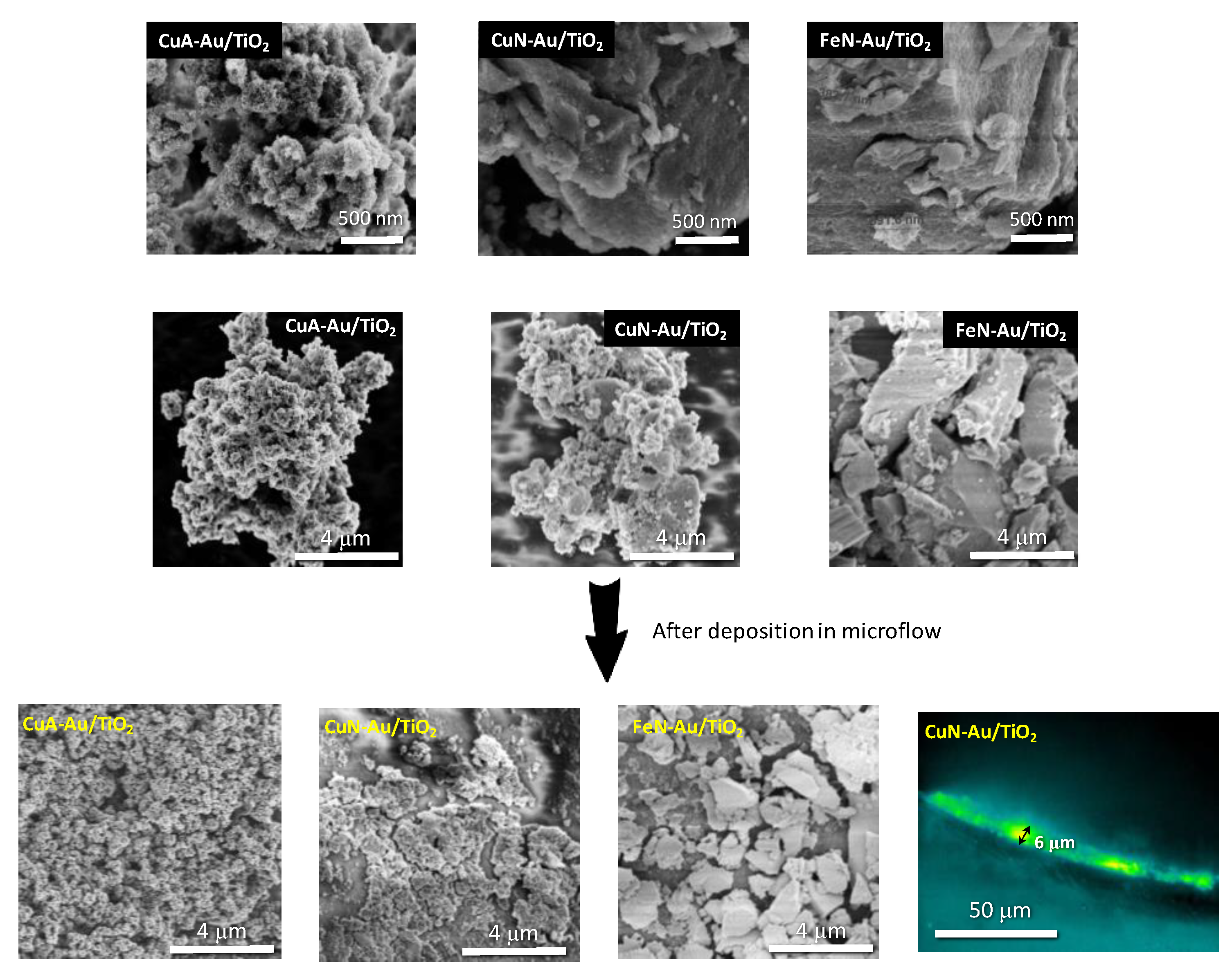
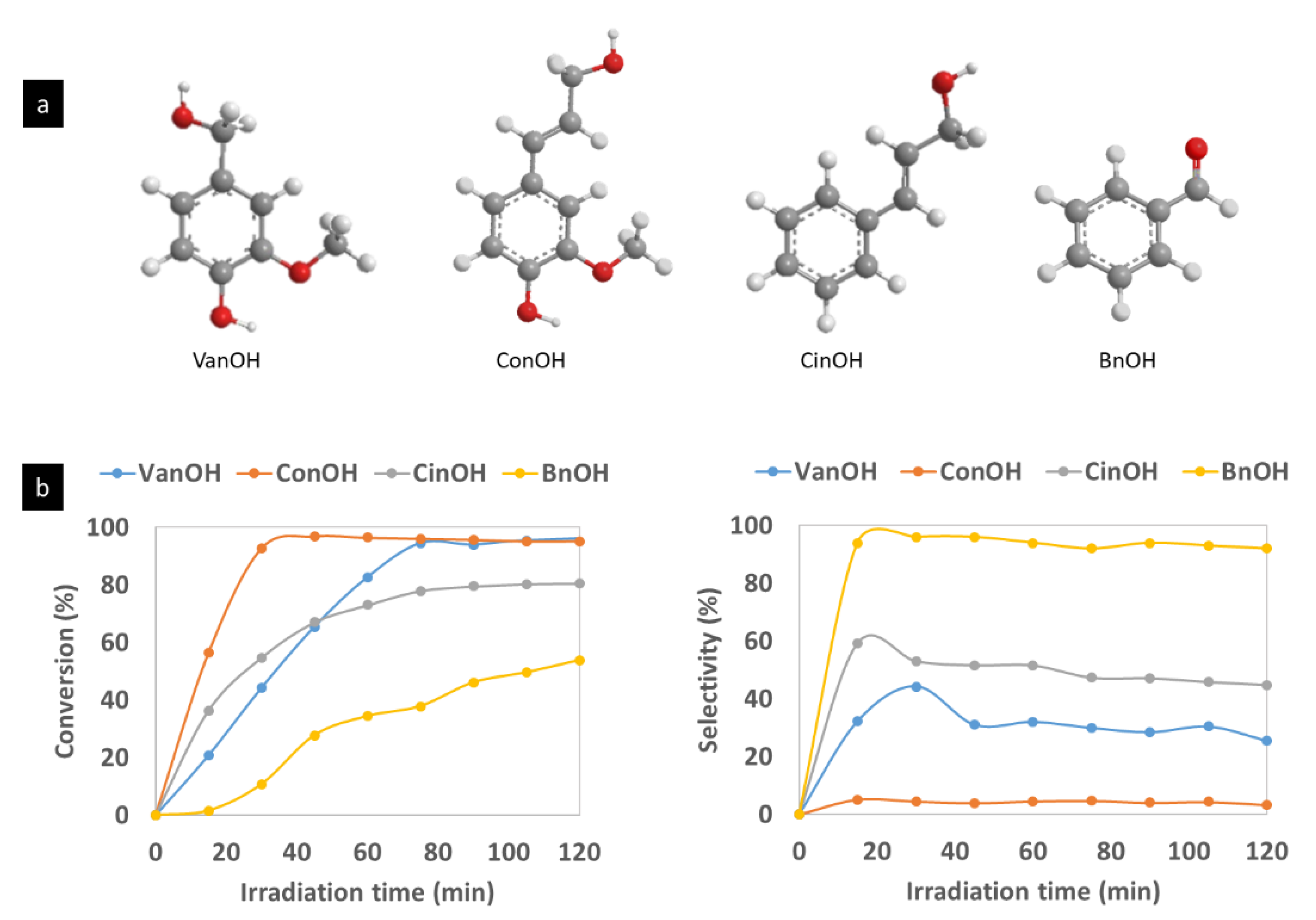
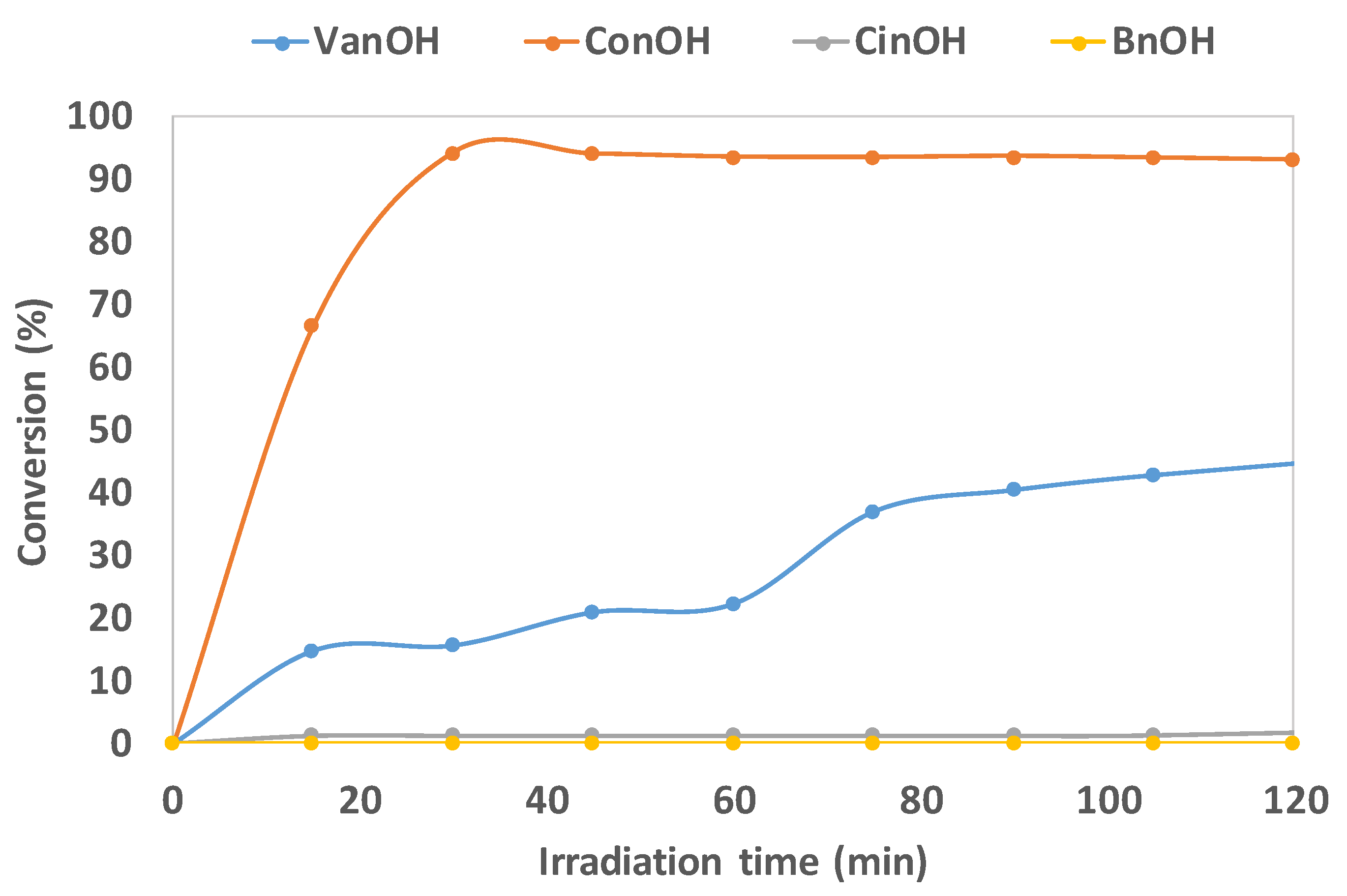
| Sample | Anatase: Brookite: Gold Crystalline Phases (%) | Specific Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (BJH) (cm3/g) | Average Crystallite Size (Dcr) [nm] (Au Phase) by XRD |
|---|---|---|---|---|---|
| TiO2 | 69:31:0 | 284 | 3.2 | 0.3 | n/a |
| CuA-Au/TiO2 | 68:31:0.44 | 251 | 6.8 | 0.3 | 11 |
| CuN-Au/TiO2 | 541 | 3.1 | 0.4 | 18 | |
| FeN-Au/TiO2 | 547 | 2.8 | 0.2 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, S.R.; Paszkiewicz-Gawron, M.; Łomot, D.; Lisovytskiy, D.; Colmenares, J.C. Bimetallic TiO2 Nanoparticles for Lignin-Based Model Compounds Valorization by Integrating an Optocatalytic Flow-Microreactor. Molecules 2022, 27, 8731. https://doi.org/10.3390/molecules27248731
Pradhan SR, Paszkiewicz-Gawron M, Łomot D, Lisovytskiy D, Colmenares JC. Bimetallic TiO2 Nanoparticles for Lignin-Based Model Compounds Valorization by Integrating an Optocatalytic Flow-Microreactor. Molecules. 2022; 27(24):8731. https://doi.org/10.3390/molecules27248731
Chicago/Turabian StylePradhan, Swaraj Rashmi, Marta Paszkiewicz-Gawron, Dariusz Łomot, Dmytro Lisovytskiy, and Juan Carlos Colmenares. 2022. "Bimetallic TiO2 Nanoparticles for Lignin-Based Model Compounds Valorization by Integrating an Optocatalytic Flow-Microreactor" Molecules 27, no. 24: 8731. https://doi.org/10.3390/molecules27248731
APA StylePradhan, S. R., Paszkiewicz-Gawron, M., Łomot, D., Lisovytskiy, D., & Colmenares, J. C. (2022). Bimetallic TiO2 Nanoparticles for Lignin-Based Model Compounds Valorization by Integrating an Optocatalytic Flow-Microreactor. Molecules, 27(24), 8731. https://doi.org/10.3390/molecules27248731







