ZnO Nanostructures Doped with Various Chloride Ion Concentrations for Efficient Photocatalytic Degradation of Methylene Blue in Alkaline and Acidic Media
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystalline, Morphology and Composition Studied of Cl−-Doped ZnO Nanostructures
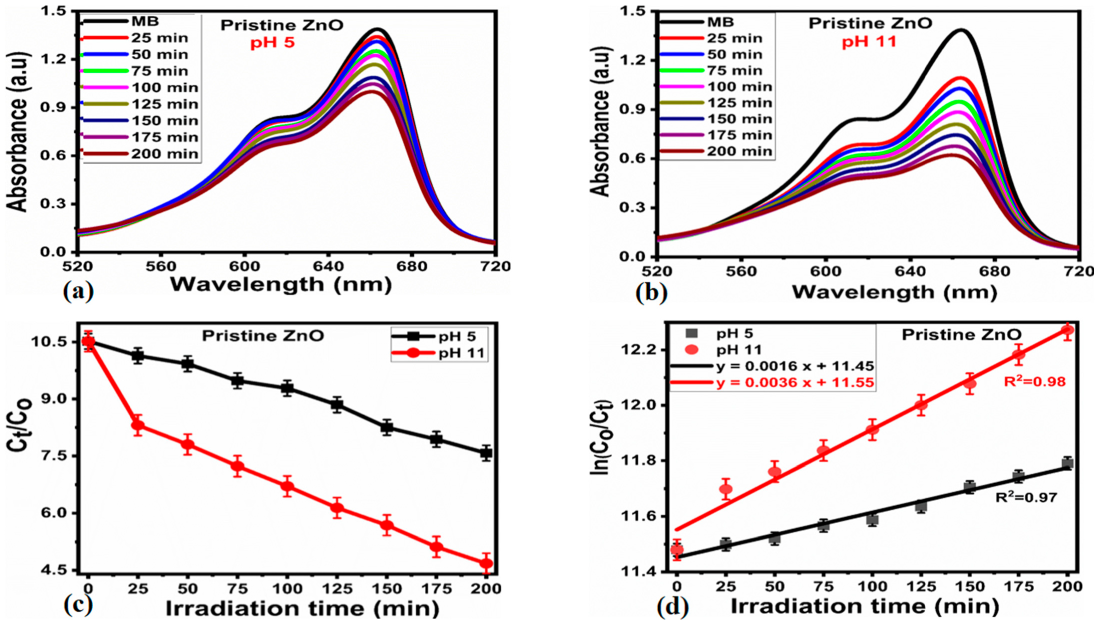
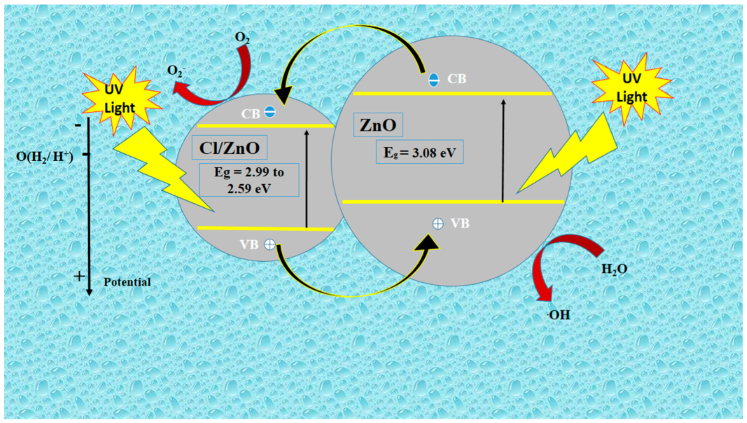
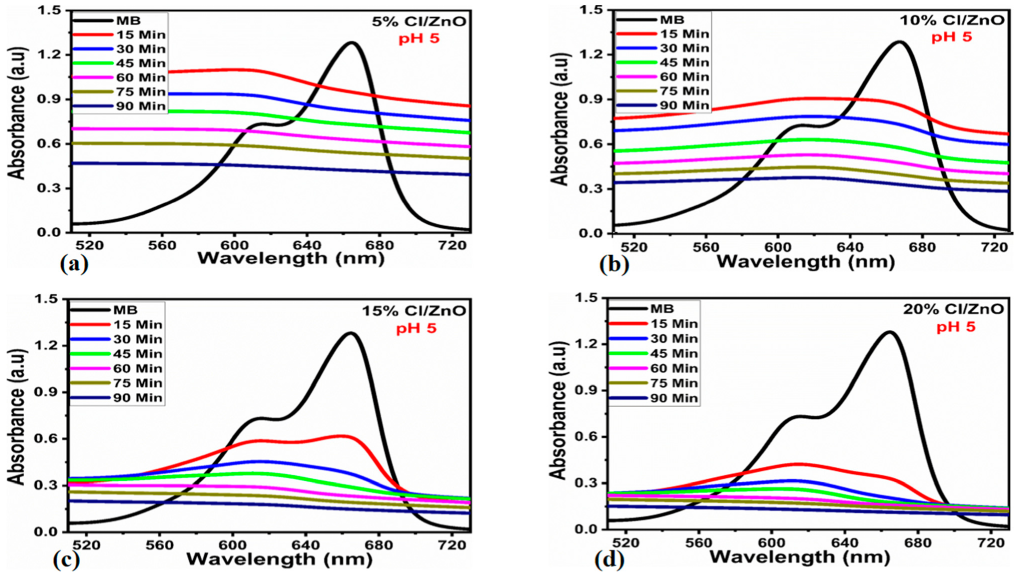
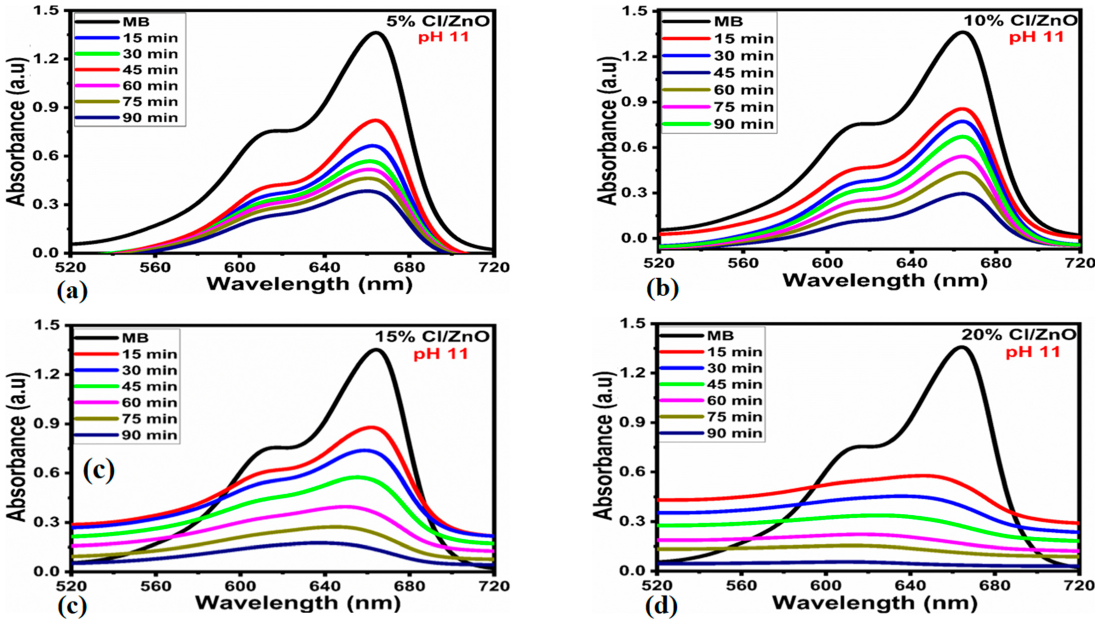
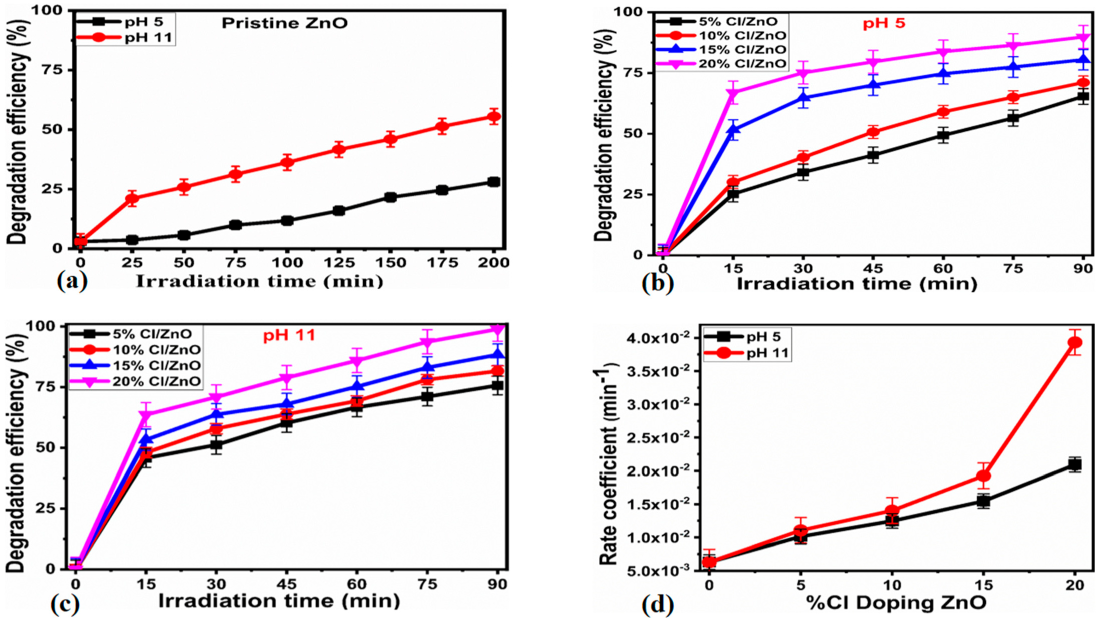
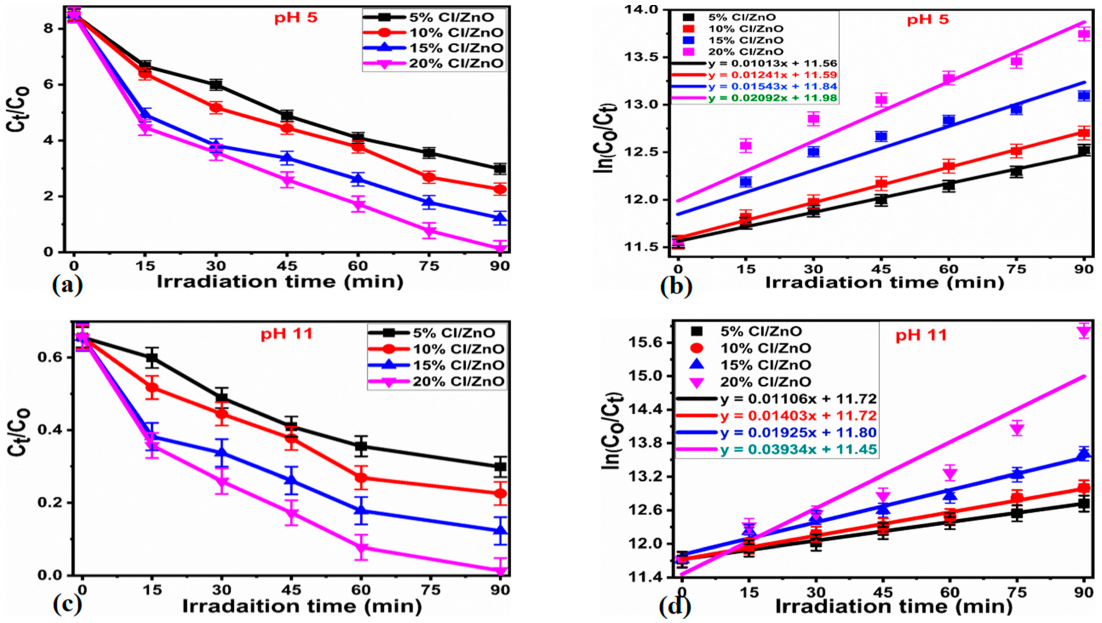
2.2. Photodegradation Activity of Cl−-doped ZnO Nanostructures
3. Materials and Methods
3.1. Used Chemicals
3.2. Preparation of Various Cl−-Doped ZnO Nanostructures by Solvothermal Method
3.3. The Evaluation of Photodegradation Effectiveness of Various Cl−-Doped ZnO Nanostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chikkanna, M.M.; Neelagund, S.E.; Rajashekarappa, K.K. Green synthesis of zinc oxide nanoparticles (ZnO NPs) and their biological activity. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Çiçek, F.; Özer, D.; Özer, A.; Özer, A. Low cost removal of reactive dyes using wheat bran. J. Hazard. Mater. 2007, 146, 408. [Google Scholar] [CrossRef] [PubMed]
- Aboamera, N.M.; Mohamed, A.; Salama, A.; Osman, T.A.; Khattab, A. An effective removal of organic dyes using surface functionalized cellulose acetate/graphene oxide composite nanofibers. Cellulose 2018, 25, 4155. [Google Scholar] [CrossRef]
- Mohamed, A.B.; Eleuch, H. Generation and robustness of bipartite non-classical correlations in two nonlinear microcavities coupled by an optical fiber. JOSA B 2018, 35, 47. [Google Scholar] [CrossRef]
- Tolia, J.; Chakraborty, M.; Murthy, Z.V. Photoluminescence of photocatalytic degradaded malachite green dye by using Mn-doped ZnS. In Proceedings of the 2011 International Conference on Biology, Environment and Chemistry ICBEE, Dubai, United Arab Emirates, 28–30 December 2011; Volume 24, pp. 489–495. [Google Scholar]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195. [Google Scholar] [CrossRef]
- Baruah, S.K.; Pal, S.; Dutta, J. Nanostructured zinc oxide for water treatment. Nanosci. Nanotechnol.-Asia 2012, 2, 90. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: Intermediates and degradation pathways. Appl. Catal. B Environ. 2003, 42, 319. [Google Scholar] [CrossRef]
- Farouq, R. Investigation of the kinetics and optimization of photocatalytic degradation of methylene blue. J. Chin. Chem. Soc. 2018, 65, 1333. [Google Scholar] [CrossRef]
- Rafaie, H.A.; Nor, R.M.; Azmina, M.S.; Ramlia, N.I.T.; Mohamed, R. Decoration of ZnO microstructures with Ag nanoparticles enhanced the catalytic photodegradation of methylene blue dye. J. Environ. Chem. Eng. 2017, 5, 3963. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833. [Google Scholar] [CrossRef] [PubMed]
- Kiwaan, H.A.; Atwee, T.M.; Azab, E.A.; El-Bindary, A.A. Efficient photocatalytic degradation of Acid Red 57 using synthesized ZnO nanowires. J. Chin. Chem. Soc. 2019, 66, 89. [Google Scholar] [CrossRef]
- Wang, J.; Cao, J.; Fang, B.; Lu, P.; Deng, S.; Wang, H. Synthesis and characterization of multipod, flower-like, and shuttle-like ZnO frameworks in ionic liquids. Mater. Lett. 2005, 11, 1405. [Google Scholar] [CrossRef]
- Shafi, M.A.; Bouich, A.; Fradi, K.; Guaita, J.M.; Khan, L.; Mari, B. 2022. Effect of deposition cycles on the properties of ZnO thin films deposited by spin coating method for CZTS-based solar cells. Optik 2022, 258, 168854. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium dioxide: From engineering to applications. Catalysts 2019, 2, 191. [Google Scholar] [CrossRef]
- Whang, T.J.; Hsieh, M.T.; Chen, H.H. Visible-light photocatalytic degradation of methylene blue with laser-induced Ag/ZnO nanoparticles. Appl. Surf. Sci. 2012, 7, 2796. [Google Scholar] [CrossRef]
- El-Kemary, M.; Abdel-Moneam, Y.; Madkour, M.; El-Mehasseb, I. Enhanced photocatalytic degradation of Safranin-O by heterogeneous nanoparticles for environmental applications. J. Lumin. 2011, 131, 570. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, H.Y.; Park, S.J. Ag-ZnO photocatalyst anchored on carbon nanofibers: Synthesis, characterization, and photocatalytic activities. Synth. Met. 2016, 220, 533. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Mohan, A.C.; Renjanadevi, B. Preparation of zinc oxide nanoparticles and its characterization using scanning electron microscopy (SEM) and X-ray diffraction (XRD). Procedia Technol. 2016, 24, 761–766. [Google Scholar] [CrossRef]
- Fradi, K.; Bouich, A.; Slimi, B.; Chtourou, R. Towards improving the optoelectronics properties of MAPbI3 (1− x) B3x/ZnO heterojunction by bromine doping. Optik 2022, 249, 168283. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem. 2016, 19, 211. [Google Scholar] [CrossRef]
- Bekkari, R.; Boyer, D.; Mahiou, R.; Jaber, B. Influence of the sol gel synthesis parameters on the photoluminescence properties of ZnO nanoparticles. Mater. Sci. Semicond. Process. 2017, 71, 181. [Google Scholar] [CrossRef]
- Mahdavi, R.; Talesh, S.S. The effect of ultrasonic irradiation on the structure, morphology and photocatalytic performance of ZnO nanoparticles by sol-gel method. Ultrason. Sonochemistry 2017, 39, 504. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K.; Babu, R.R.; Sethuraman, K.; Babu, M. Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl. Surf. Sci. 2017, 418, 138. [Google Scholar] [CrossRef]
- Ghoderao, K.P.; Jamble, S.N.; Kale, R.B. Influence of pH on hydrothermally derived ZnO nanostructures. Optik 2018, 156, 758. [Google Scholar] [CrossRef]
- Bazazi, S.; Arsalani, N.; Khataee, A.; Tabrizi, A.G. Comparison of ball milling-hydrothermal and hydrothermal methods for synthesis of ZnO nanostructures and evaluation of their photocatalytic performance. J. Ind. Eng. Chem. 2018, 62, 265. [Google Scholar] [CrossRef]
- Kumar, S.; Sahare, P.D.; Kumar, S. Optimization of the CVD parameters for ZnO nanorods growth: Its photoluminescence and field emission properties. Mater. Res. Bull. 2018, 105, 237. [Google Scholar] [CrossRef]
- Narin, P.O.; Kutlu, E.; Atmaca, G.; Atilgan, A.B.; Yildiz, A.; Lisesivdin, S.B. Structural and optical properties of hexagonal ZnO nanostructures grown by ultrasonic spray CVD. Optik 2018, 168, 86. [Google Scholar] [CrossRef]
- Jeena, V.; Robinson, R.S. Convenient photooxidation of alcohols using dye sensitised zinc oxide in combination with silver nitrate and TEMPO. Chem. Commun. 2012, 48, 299. [Google Scholar] [CrossRef]
- Huang, L.; Fan, H. Room-temperature solid state synthesis of ZnO/α-Fe2O3 hierarchical nanostructures and their enhanced gas-sensing properties. Sens. Actuators B Chem. 2012, 171, 1257. [Google Scholar] [CrossRef]
- Zhang, L.W.; Cheng, H.Y.; Zong, R.L.; Zhu, Y.F. Significant Visible Photoactivity and Antiphotocorrosion Performance of CdS Photocatalysts after Monolayer Polyaniline Hybridization. J. Phys. Chem. C 2009, 113, 2368. [Google Scholar] [CrossRef]
- Vasei, H.V.; Masoudpanah, S.M.; Adeli, M.; Aboutalebi, M.R. Solution combustion synthesis of ZnO powders using CTAB as fuel. Ceram. Int. 2018, 44, 7741. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M.; Alaei, S. Influence of propylene glycol/nitrates ratio on microwave-assisted combustion synthesis of CuO-ZnO-Al2O3 nanocatalyst: Structural and catalytic properties toward hydrogen production from methanol. Mater. Res. Bull. 2018, 102, 142. [Google Scholar] [CrossRef]
- Anandan, S.; Miyauchi, M. Ce-doped ZnO (Ce x Zn 1− x O) becomes an efficient visible-light-sensitive photocatalyst by co-catalyst (Cu 2+) grafting. Phys. Chem. Chem. Phys. 2011, 33, 14937. [Google Scholar] [CrossRef]
- Chen, H.; Wen, W.; Wang, Q.; Hanson, J.C.; Muckerman, J.T.; Fujita, E.; Frenkel, A.I.; Rodriguez, J.A. Preparation of (Ga1− x Zn x)(N1− x O x) Photocatalysts from the Reaction of NH3 with Ga2O3/ZnO and ZnGa2O4: In Situ Time-Resolved XRD and XAFS Studies. J. Phys. Chem. C 2009, 113, 3650. [Google Scholar] [CrossRef]
- Patil, A.B.; Patil KRa, A.B.; Pardeshi, S.K. Ecofriendly synthesis and solar photocatalytic activity of S-doped ZnO. J. Hazard. Mater. 2010, 183, 315. [Google Scholar] [CrossRef]
- Chen, L.C.; Tu, Y.J.; Wang, Y.S.; Kan, R.S.; Huang, C.M. Characterization and photoreactivity of N-, S-, and C-doped ZnO under UV and visible light illumination. J. Photochem. Photobiol. A Chem. 2008, 199, 170. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Yu, J.; Xiang, Q. Improved visible-light photocatalytic activity of porous carbon self-doped ZnO nanosheet-assembled flowers. CrystEngComm. 2011, 13, 2533. [Google Scholar] [CrossRef]
- Shao, D.; Gao, J.; Xin, G.; Wang, Y.; Li, L.; Shi, J.; Sawyer, S. Cl-Doped ZnO Nanowire Arrays on 3D Graphene Foam with Highly Efficient Field Emission and Photocatalytic Properties. Small 2015, 11, 4785. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kang, K.M.; Lee, H.S.; Park, H.H. Non-laminated growth of chlorine-doped zinc oxide films by atomic layer deposition at low temperatures. J. Mater. Chem. C 2015, 3, 8336–8343. [Google Scholar] [CrossRef]
- Peng, X.; Li, J.; Liu, X.; Yi, L.; Cai, P.; Wen, Z. Cl-doped carbon nitride nanostrips for remarkably improving visible-light photocatalytic hydrogen production. Int. J. Hydrogen Energy 2021, 46, 28591. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. TiO2@ carbon core/shell nanofibers: Controllable preparation and enhanced visible photocatalytic properties. Nanoscale 2011, 3, 2943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Sun, Y.; Liu, Y. Core/shell nanofibers of TiO 2@ carbon embedded by Ag nanoparticles with enhanced visible photocatalytic activity. J. Mater. Chem. 2011, 21, 7746. [Google Scholar] [CrossRef]
- Liu, S.; Weng, B.; Tang, Z.R.; Xu, Y.J. Constructing one-dimensional silver nanowire doped reduced graphene oxide integrated with CdS nanowire network hybrid structures toward artificial photosynthesis. Nanoscale 2015, 7, 861. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.; Suganthi, A.; Rajarajan, M.; Sara, S.A. Photocatalytic activity of AgI sensitized ZnO nanoparticles under visible light irradiation. Powder Technol. 2012, 224, 331. [Google Scholar] [CrossRef]
- Gautam, K.; Singh, I.; Bhatnagar, P.K.; Peta, K.R. Role of Cl doping on the growth and relaxation dynamics of ZnO nanorods synthesized by hydrothermal method. Chem. Phys. Lett. 2016, 662, 196. [Google Scholar] [CrossRef]
- Jiamprasertboon, A.; Dixon, S.C.; Sathasivam, S.; Powell, M.J.; Lu, Y.; Siritanon, T.; Carmalt, C.J. Low-cost one-step fabrication of highly conductive ZnO: Cl transparent thin films with tunable photocatalytic properties via aerosol-assisted chemical vapor deposition. ACS Appl. Electron. Mater. 2019, 1, 1408. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, J.; Lin, X.; Wu, W.; Li, W.; Yang, B.; Shi, M. Quality-Improved GaN Epitaxial Layers Grown on Striped Patterned Sapphire Substrates Ablated by Femtosecond Laser. Appl. Sci. 2018, 10, 1842. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, L.T.; Duong, A.T.; Nguyen, B.D.; Quang Hai, N.; Chu, V.H.; Nguyen, T.D.; Bach, L.G. Preparation, characterization and photocatalytic activity of La-doped zinc oxide nanoparticles. Materials 2019, 8, 1195. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Shah, A.A.; Almani, K.F.; Tahira, A.; Chalangar, S.E.; dad Chandio, A.; Nur, O.; Willander, M.; Ibupoto, Z.H. Efficient photo catalysts based on silver doped ZnO nanorods for the photo degradation of methyl orange. Ceram. Int. 2019, 45, 23289. [Google Scholar] [CrossRef]
- Payra, S.; Ganeshan, S.K.; Challagulla, S.; Roy, S. A correlation story of syntheses of ZnO and their influence on photocatalysis. Adv. Powder Technol. 2020, 31, 510–520. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M.B. Hexagonal ZnO nanorods assembled flowers for photocatalytic dye degradation: Growth, structural and optical properties. Superlattices Microstruct. 2013, 64, 495. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Niaei, A.; Rasoulifard, M.H.; Khataee, A.R. Immobilization of ‘TiO2 nanopowder on glass beads for the photocatalytic decolorization of an azo dye CI Direct Red 23. J. Environ. Sci. Health Part A 2005, 40, 1605. [Google Scholar] [CrossRef]
- Alahiane, S.; Qourzal, S.; Ouardi, M.; Abaamrane, A.; Assabbane, A. Factors Influencing the Photocatalytic Degradation of Reactive Yellow 145 by TiO2-Coated Non-Woven Fibers. Am. J. Anal. Chem. 2014, 5, 445. [Google Scholar] [CrossRef]
- Tju, H.; Shabrany, H.; Taufik, A.; Saleh, R. Degradation of methylene blue (MB) using ZnO/CeO2/nanographene platelets (NGP) photocatalyst: Effect of various concentration of NGP. InAIP Conf. Proc. 2017, 1862, 030037. [Google Scholar]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520. [Google Scholar] [CrossRef]
- Sunitha, S.; Rao, A.N.; Abraham, L.S.; Dhayalan, E.; Thirugnanasambandam, R.; Kumar, V.G. Enhanced bactericidal effect of silver nanoparticles synthesized using marine brown macro algae. J. Chem. Pharma. Res. 2015, 7, 191. [Google Scholar]
- Kazeminezhad, I.; Sadollahkhani, A. Influence of pH on the photocatalytic activity of ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 5, 4206. [Google Scholar] [CrossRef]
- Dodd, A.C.; McKinley, A.J.; Saunders, M.; Tsuzuki, T. Effect of particle size on the photocatalytic activity of nanoparticulate zinc oxide. J. Nanopart. Res. 2006, 1, 43. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Chanthai, S.; Mahachai, R.; Oh, W.C. Sonocatalytic performance of ZnO/graphene/TiO2 nanocomposite for degradation of dye pollutants (methylene blue, texbrite BAC-L, texbrite BBU-L and texbrite NFW-L) under ultrasonic irradiation. Dye. Pigment. 2016, 134, 487. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Efficient degradation of methylene blue dye over highly reactive Cu doped strontium titanate (SrTiO3) nanoparticles photocatalyst under visible light. J. Nanosci. Nanotechnol. 2012, 9, 7181. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, M.J.; Mahdi, M.A.; Hassan, J.J. Influence of pH on the photocatalytic activity of ZnO nanorods. Mater. Int. 2020, 2, 64. [Google Scholar]
- Bhatti, M.A.; Almaani, K.F.; Shah, A.A.; Tahira, A.; Chandio, A.D.; Mugheri, A.Q.; Ibupoto, Z.H. Low Temperature Aqueous Chemical Growth Method for the Doping of W into ZnO Nanostructures and Their Photocatalytic Role in the Degradration of Methylene Blue. J. Clust. Sci. 2021, 1, 1445–1456. [Google Scholar] [CrossRef]
- Chanu, L.A.; Singh, W.J.; Singh, K.J.; Devi, K.N. Effect of operational parameters on the photocatalytic degradation of Methylene blue dye solution using manganese doped ZnO nanoparticles. Results Phys. 2019, 12, 1230. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Abedini, A.; Bahmanrokh, G.; Navasery, M.; Vaziri, P. Visible light-induced degradation of methylene blue in the presence of photocatalytic ZnS and CdS nanoparticles. Int. J. Mol. Sci. 2012, 13, 12242. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Haghighi, M.; Aghamohammadi, S. Sono-precipitation fabrication of ZnO over modified SAPO-34 zeotype for effective degradation of methylene blue pollutant under simulated solar light illumination. Process Saf. Environ. Prot. 2022, 165, 307. [Google Scholar] [CrossRef]
- Loke, J.Y.; Zaki, R.M.; Setiabudi, H.D. Photocatalytic degradation of methylene blue using ZnO supported on wood waste-derived activated carbon (ZnO/AC). Mater. Today Proc. 2022, 57, 1315. [Google Scholar] [CrossRef]
- Shubha, J.P.; Adil, S.F.; Khan, M.; Hatshan, M.R.; Khan, A. Facile fabrication of a ZnO/Eu2O3/NiO-Based ternary heterostructure nanophotocatalyst and its application for the degradation of methylene blue. ACS Omega 2021, 6, 3866. [Google Scholar] [CrossRef] [PubMed]
- Haounati, R.; El Guerdaoui, A.; Ouachtak, H.; El Haouti, R.; Bouddouch, A.; Hafid, N.; Bakiz, B.; Santos, D.M.F.; Taha, M.L.; Jada, A.; et al. Design of direct Z-scheme superb magnetic nanocomposite photocatalyst Fe3O4/Ag3PO4@ Sep for hazardous dye degradation. Sep. Purif. Technol. 2021, 277, 119399. [Google Scholar] [CrossRef]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Addi, A.A.; Saleh, T.A. A comparative study of photocatalytic degradation of Rhodamine B using natural-based zeolite composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]

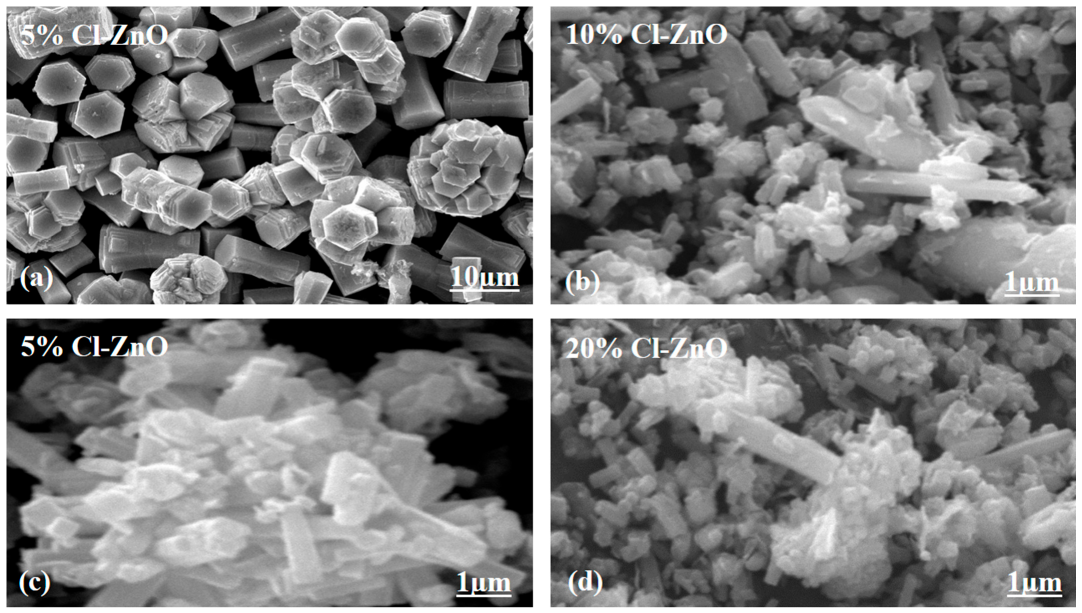
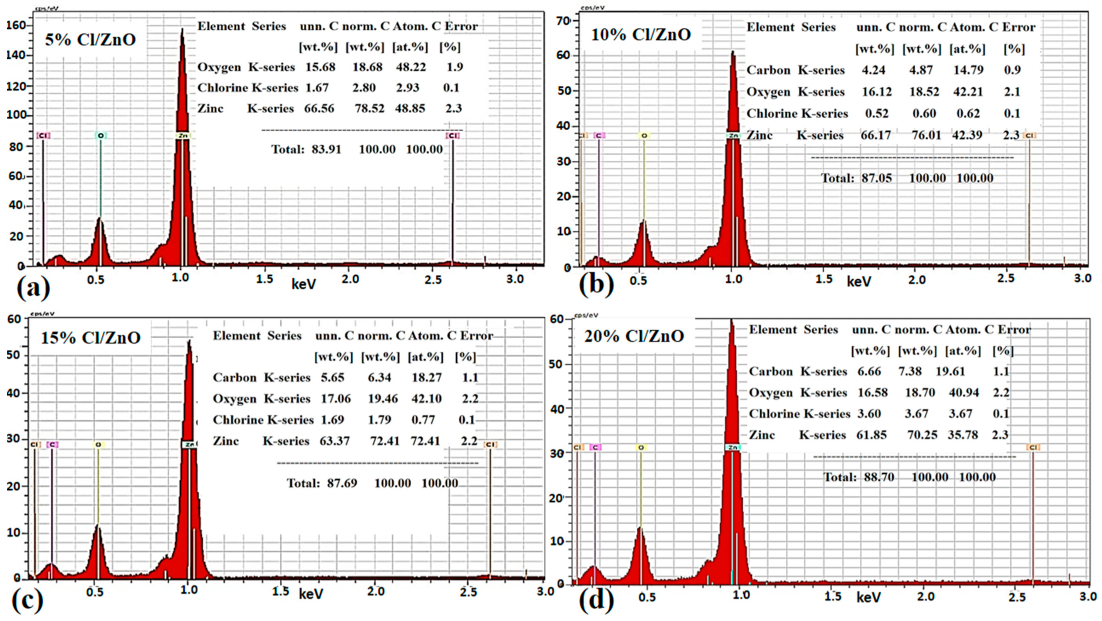
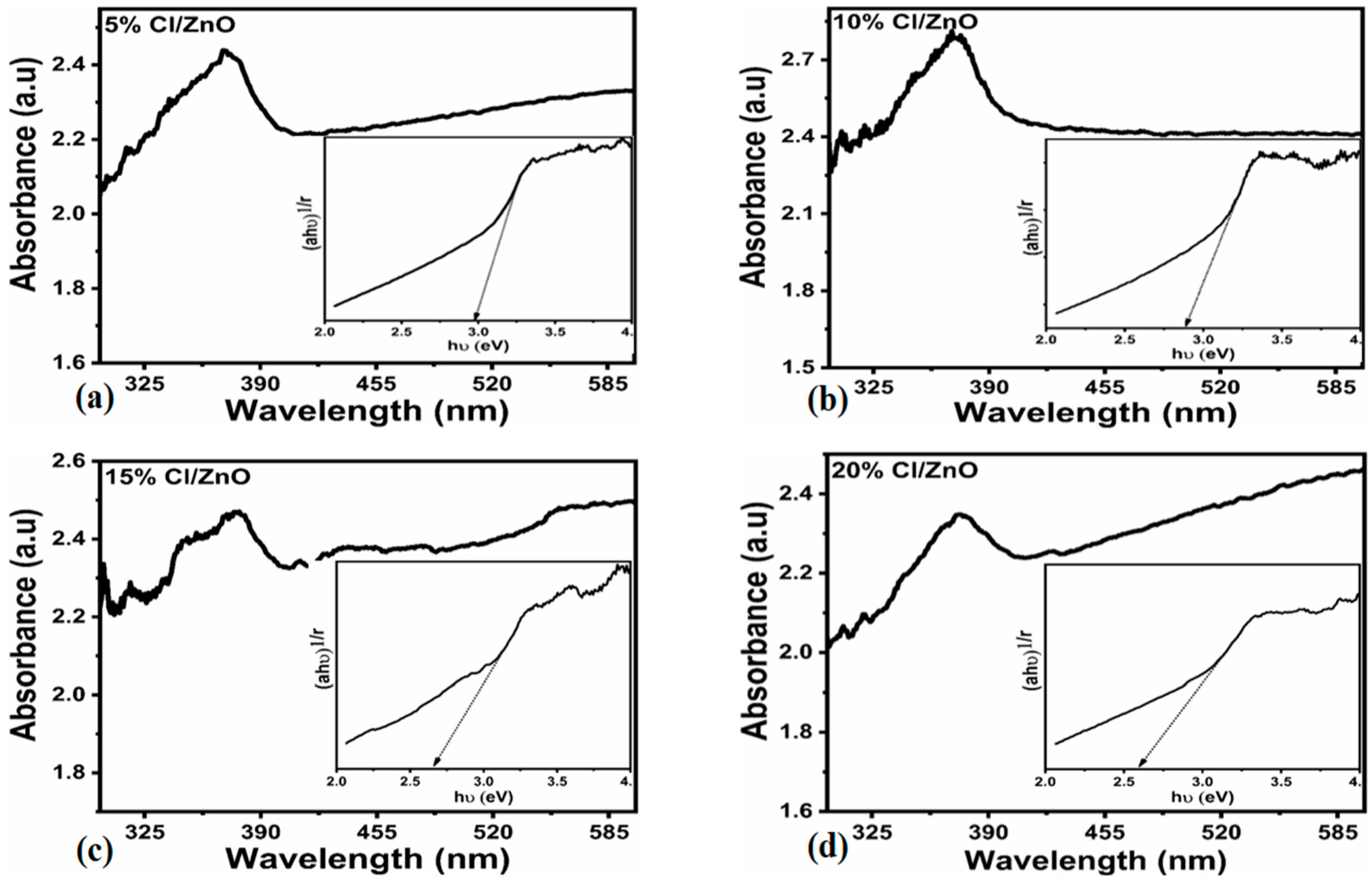


| Samples | FWHM (Degree) | Crystalline Size (nm) | a (Å) | c (Å) | c/a (Å) | d-Spacing (nm) | Volume (nm)3 |
|---|---|---|---|---|---|---|---|
| Pristine ZnO | 0.388 | 30.23 ± 0.04 | 1.86 | 3.22 | 1.73 | 0.80 | 9.66 |
| 5%Cl/ZnO | 0.376 | 27.23 ± 0.03 | 1.88 | 3.26 | 1.73 | 0.81 | 10.02 |
| 10%Cl/ZnO | 0.381 | 25.27 ± 0.02 | 1.89 | 3.28 | 1.73 | 0.82 | 10.27 |
| 15%Cl/ZnO | 0.425 | 20.54 ± 0.05 | 1.92 | 3.33 | 1.73 | 0.83 | 10.66 |
| 20%Cl/ZnO | 0.542 | 13.66 ± 0.01 | 2.00 | 3.47 | 1.73 | 0.86 | 12.06 |
| Samples | Band Gap (eV) | pH 5 | pH 11 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Deg. Eff. (%) | R2 | Rate constant (min−1) | Time (min) | Deg. Eff. (%) | R2 | Rate Constant (min−1) | ||
| Pristine ZnO | 3.08 | 200 | 57.56 | 0.99 | 0.004 | 200 | 75.78 | 0.97 | 0.006 |
| 5%Cl/ZnO | 2.99 | 90 | 64.47 | 0.98 | 0.010 | 90 | 75.20 | 0.97 | 0.012 |
| 10%Cl/ZnO | 2.88 | 90 | 71.35 | 0.97 | 0.014 | 90 | 81.19 | 0.98 | 0.015 |
| 15%Cl/ZnO | 2.66 | 90 | 80.03 | 0.95 | 0.018 | 90 | 88.67 | 0.97 | 0.018 |
| 20%Cl/ZnO | 2.59 | 90 | 89.91 | 0.93 | 0.021 | 90 | 99.45 | 0.91 | 0.041 |
| Name of Material | Light Source | Dye | pH | Catalyst Dose | Time | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO | UV light | MB | 11 | - | 240 min | 81% | [66] |
| W-ZnO | UV light | MB | 11 | 20 mg | 90 min | 89% | [67] |
| ZnO | UV light | MB | 11 | 0.05 g | 240 min | 81% | [53] |
| Mn-ZnO | UV light | MB | 05 | 0.30 g/L | 120 min | 41% | [68] |
| WO3/SiO2 | UV light | MB | 11 | 0.1 g/L | 120 min | 91% | [69] |
| ZnS:CdS | UV light | MB | 10 | 0.1 g/L | 360 | 73% | [70] |
| ZnO/SAPO-34 (AC-U)-U | Sunlight | MB | 6 | 1 g/L | 150 min | 95.7% | [71] |
| Ag/ZnO | UV light | MB | 8 | 3 g/L | 180 min | 100% | [72] |
|
ZnO/Eu2O3/NiO
| UV light | MB | 10 | 0.03 g/L | 150 min | 98 | [73] |
| Fe3PO4/Ag3PO4@Sep | Sunlight | MG | 7 | 1 g/L | 16 min | 99 | [74] |
| Zeo-ZnO | UV light | RhB | - | 100 mg | 80 min | 81% | [75] |
| Cl−-doped ZnO | UV light | MB | 05 | 0.1 g/L | 90 min | 89.91% | Present Work |
| Cl−-doped ZnO | UV light | MB | 11 | 0.1 g/L | 90 min | 99.45% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshgari, R.A.; Ujjan, Z.A.; Shah, A.A.; Bhatti, M.A.; Tahira, A.; Shaikh, N.M.; Kumar, S.; Ibupoto, M.H.; Elhawary, A.; Nafady, A.; et al. ZnO Nanostructures Doped with Various Chloride Ion Concentrations for Efficient Photocatalytic Degradation of Methylene Blue in Alkaline and Acidic Media. Molecules 2022, 27, 8726. https://doi.org/10.3390/molecules27248726
Alshgari RA, Ujjan ZA, Shah AA, Bhatti MA, Tahira A, Shaikh NM, Kumar S, Ibupoto MH, Elhawary A, Nafady A, et al. ZnO Nanostructures Doped with Various Chloride Ion Concentrations for Efficient Photocatalytic Degradation of Methylene Blue in Alkaline and Acidic Media. Molecules. 2022; 27(24):8726. https://doi.org/10.3390/molecules27248726
Chicago/Turabian StyleAlshgari, Razan A., Zaheer Ahmed Ujjan, Aqeel Ahmed Shah, Muhammad Ali Bhatti, Aneela Tahira, Nek Muhammad Shaikh, Susheel Kumar, Mazhar Hussain Ibupoto, Amal Elhawary, Ayman Nafady, and et al. 2022. "ZnO Nanostructures Doped with Various Chloride Ion Concentrations for Efficient Photocatalytic Degradation of Methylene Blue in Alkaline and Acidic Media" Molecules 27, no. 24: 8726. https://doi.org/10.3390/molecules27248726
APA StyleAlshgari, R. A., Ujjan, Z. A., Shah, A. A., Bhatti, M. A., Tahira, A., Shaikh, N. M., Kumar, S., Ibupoto, M. H., Elhawary, A., Nafady, A., Vigolo, B., & Ibhupoto, Z. H. (2022). ZnO Nanostructures Doped with Various Chloride Ion Concentrations for Efficient Photocatalytic Degradation of Methylene Blue in Alkaline and Acidic Media. Molecules, 27(24), 8726. https://doi.org/10.3390/molecules27248726








