The Aging Process: A Metabolomics Perspective
Abstract
1. Introduction
2. Results
2.1. Metabolomics Data
2.2. Participant Characteristics
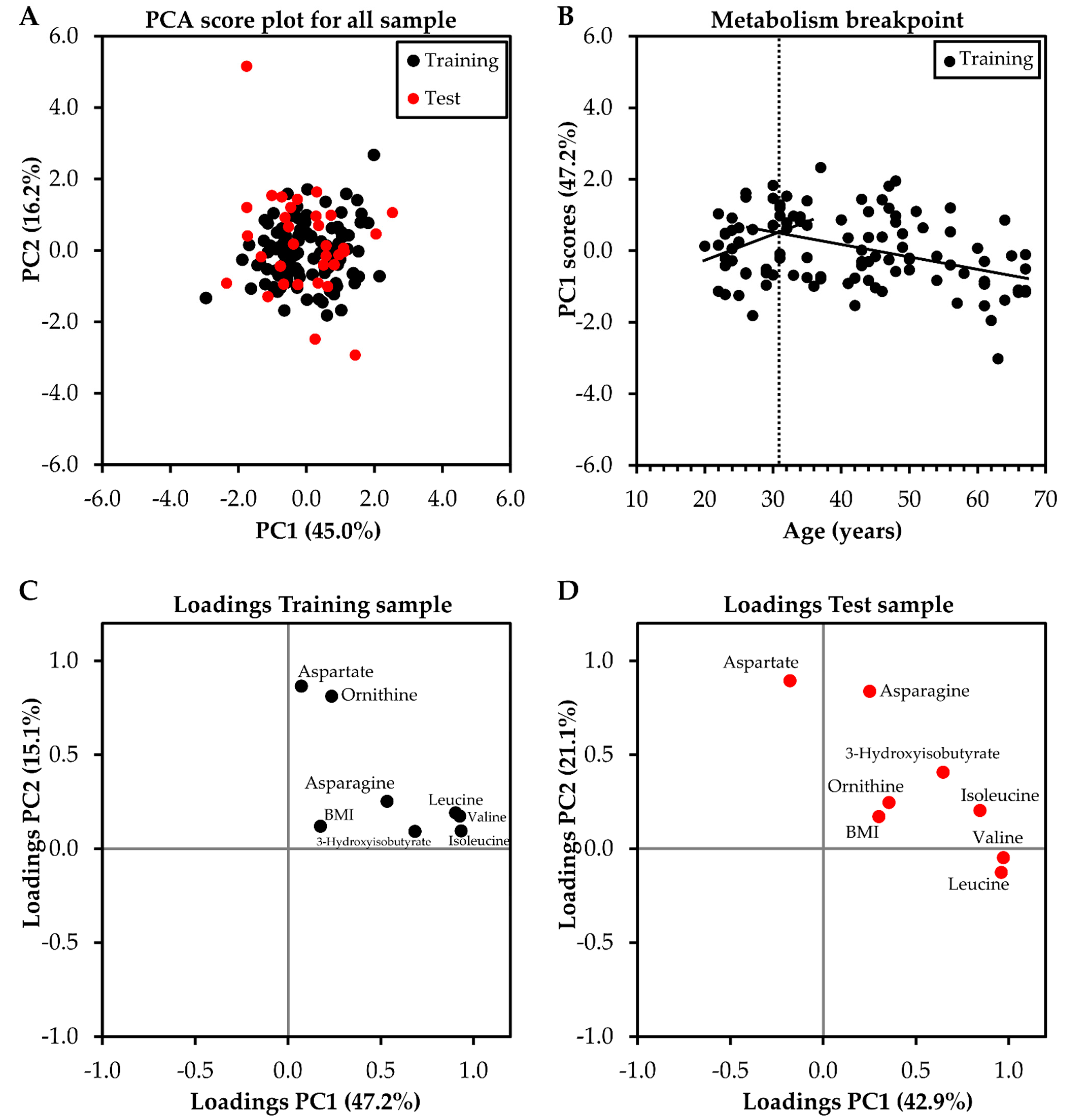
2.3. Association of Physical and Clinical Characteristics of the Participants, and Metabolomic Profile with Age
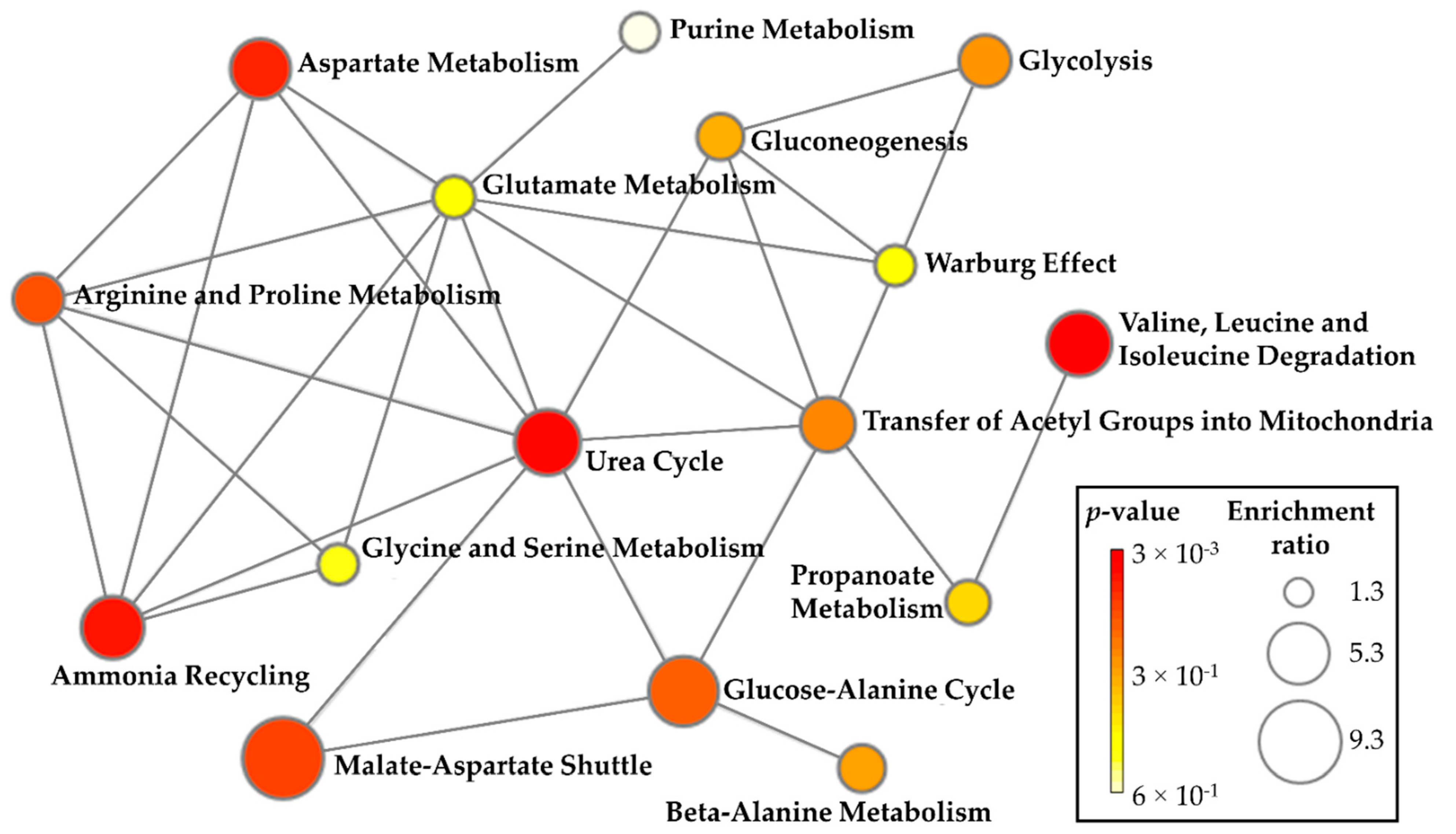
2.4. Metabolite Set Enrichment Analysis
2.5. Summary of Key Metabolites Associated with Aging
2.6. Identifying the Breakpoint in Metabolism Related to Aging
3. Discussion
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Blood Sample Collection
4.3. Clinical Markers
4.4. 1H NMR-Based Metabolomics
4.5. LC-HRMS-Based Metabolomics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adav, S.S.; Wang, Y. Metabolomics Signatures of Aging: Recent Advances. Aging Dis. 2021, 12, 646–661. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging Europe PMC Funders Group. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging Insights into the Metabolic Alterations in Aging Using Metabolomics. Metabolites 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic Control of Longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Victoria, B.; Nunez Lopez, Y.O.; Masternak, M.M. MicroRNAs and the Metabolic Hallmarks of Aging. Mol. Cell. Endocrinol. 2017, 455, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune-Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a “Good” Look at Free Radicals in the Aging Process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef]
- Panyard, D.J.; Yu, B.; Snyder, M.P. The Metabolomics of Human Aging: Advances, Challenges, and Opportunities. Sci. Adv. 2022, 8, eadd6155. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and Metabolic Dysfunction in Ageing and Age-Related Diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Orkaby, A.R. Moving beyond Chronological Age: Frailty as an Important Risk Factor for Cardiovascular Disease. Eur. Heart J. 2021, 42, 3866–3868. [Google Scholar] [CrossRef]
- Hertel, J.; Friedrich, N.; Wittfeld, K.; Pietzner, M.; Budde, K.; Van der Auwera, S.; Lohmann, T.; Teumer, A.; Völzke, H.; Nauck, M.; et al. Measuring Biological Age via Metabonomics: The Metabolic Age Score. J. Proteome Res. 2016, 15, 400–410. [Google Scholar] [CrossRef]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Murabito, J.M.; Rhee, E.P.; Ho, J.E.; Jacques, P.F.; Ghorbani, A.; Magnusson, M.; Souza, A.L.; et al. Distinct Metabolomic Signatures Are Associated with Longevity in Humans. Nat. Commun. 2015, 6, 6791. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, H.; Kameda, M.; Yanagida, M. Whole Blood Metabolomics in Aging Research. Int. J. Mol. Sci. 2021, 22, 175. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The Apogee of the Omics Trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. NMR Metabolomics: A Look Ahead. J. Magn. Reson. 2019, 306, 155–161. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics--the Link between Genotypes and Phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Prosser, G.A.; Larrouy-Maumus, G.; de Carvalho, L.P.S. Metabolomic Strategies for the Identification of New Enzyme Functions and Metabolic Pathways. EMBO Rep. 2014, 15, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Darst, B.F.; Koscik, R.L.; Hogan, K.J.; Johnson, S.C.; Engelman, C.D. Longitudinal Plasma Metabolomics of Aging and Sex. Aging 2019, 11, 1262–1282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.C.; Parker, K.; Aguirre, B.F.; Nemkov, T.G.; D’Alessandro, A.; Johnson, S.A.; Seals, D.R.; Martens, C.R. The Plasma Metabolome as a Predictor of Biological Aging in Humans. GeroScience 2019, 41, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, M.; Li, Q. (1)H NMR-Based Metabolomics Reveals the Intrinsic Interaction of Age, Plasma Signature Metabolites, and Nutrient Intake in the Longevity Population in Guangxi, China. Nutrients 2022, 14, 2539. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, I.; Scherer, M.; Beguelin, F.; DaSilva, L.; Mari, D.; Salvioli, S.; Martin, F.-P.J.; Capri, M.; Bucci, L.; Ostan, R.; et al. Serum Profiling of Healthy Aging Identifies Phospho- and Sphingolipid Species as Markers of Human Longevity. Aging 2014, 6, 9–25. [Google Scholar] [CrossRef]
- Menni, C.; Kastenmüller, G.; Petersen, A.K.; Bell, J.T.; Psatha, M.; Tsai, P.-C.; Gieger, C.; Schulz, H.; Erte, I.; John, S.; et al. Metabolomic Markers Reveal Novel Pathways of Ageing and Early Development in Human Populations. Int. J. Epidemiol. 2013, 42, 1111–1119. [Google Scholar] [CrossRef]
- Yu, Z.; Zhai, G.; Singmann, P.; He, Y.; Xu, T.; Prehn, C.; Römisch-Margl, W.; Lattka, E.; Gieger, C.; Soranzo, N.; et al. Human Serum Metabolic Profiles Are Age Dependent. Aging Cell 2012, 11, 960–967. [Google Scholar] [CrossRef]
- Hartmann, A.; Hartmann, C.; Secci, R.; Hermann, A.; Fuellen, G.; Walter, M. Ranking Biomarkers of Aging by Citation Profiling and Effort Scoring. Front. Genet. 2021, 12, 686320. [Google Scholar] [CrossRef]
- Markovič, R.; Grubelnik, V.; Vošner, H.B.; Kokol, P.; Završnik, M.; Janša, K.; Zupet, M.; Završnik, J.; Marhl, M. Age-Related Changes in Lipid and Glucose Levels Associated with Drug Use and Mortality: An Observational Study. J. Pers. Med. 2022, 12, 280. [Google Scholar] [CrossRef]
- Feng, L.; Nian, S.; Tong, Z.; Zhu, Y.; Li, Y.; Zhang, C.; Bai, X.; Luo, X.; Wu, M.; Yan, Z. Age-Related Trends in Lipid Levels: A Large-Scale Cross-Sectional Study of the General Chinese Population. BMJ Open 2020, 10, e034226. [Google Scholar] [CrossRef]
- Barr, E.L.M.; Zimmet, P.Z.; Welborn, T.A.; Jolley, D.; Magliano, D.J.; Dunstan, D.W.; Cameron, A.J.; Dwyer, T.; Taylor, H.R.; Tonkin, A.M.; et al. Risk of Cardiovascular and All-Cause Mortality in Individuals with Diabetes Mellitus, Impaired Fasting Glucose, and Impaired Glucose Tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007, 116, 151–157. [Google Scholar] [CrossRef]
- Orozco-Beltran, D.; Gil-Guillen, V.F.; Redon, J.; Martin-Moreno, J.M.; Pallares-Carratala, V.; Navarro-Perez, J.; Valls-Roca, F.; Sanchis-Domenech, C.; Fernandez-Gimenez, A.; Perez-Navarro, A.; et al. Lipid Profile, Cardiovascular Disease and Mortality in a Mediterranean High-Risk Population: The ESCARVAL-RISK Study. PLoS ONE 2017, 12, e0186196. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Tsai, M.-C.; Yeh, T.-L.; Hsu, L.-Y.; Hwang, L.-C.; Chien, K.-L. Association of Baseline as Well as Change in Lipid Levels with the Risk of Cardiovascular Diseases and All-Cause Deaths. Sci. Rep. 2021, 11, 7381. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.S.; Rezende, L.F.M.; Laurindo, F.R.M. Analysing the Impact of Modifiable Risk Factors on Cardiovascular Disease Mortality in Brazil. PLoS ONE 2022, 17, e0269549. [Google Scholar] [CrossRef] [PubMed]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual Variability in Human Blood Metabolites Identifies Age-Related Differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched Chain Amino Acids, Aging and Age-Related Health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.C. Amino Acids in the Regulation of Aging and Aging-Related Diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Zierle-Ghosh, A.; Jan, A. Physiology, Body Mass Index. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Macek, P.; Terek-Derszniak, M.; Biskup, M.; Krol, H.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. Assessment of Age-Induced Changes in Body Fat Percentage and Bmi Aided by Bayesian Modelling: A Cross-Sectional Cohort Study in Middle-Aged and Older Adults. Clin. Interv. Aging 2020, 15, 2301–2311. [Google Scholar] [CrossRef]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2020, 10, 861. [Google Scholar] [CrossRef]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A Branched-Chain Amino Acid Metabolite Drives Vascular Fatty Acid Transport and Causes Insulin Resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Ribeiro, R.; Senior, A.; Hsu, B.; Hirani, V.; Blyth, F.M.; Waite, L.M.; Simpson, S.J.; Naganathan, V.; Cumming, R.G.; et al. Branched Chain Amino Acids, Cardiometabolic Risk Factors and Outcomes in Older Men: The Concord Health and Ageing in Men Project. J. Gerontol. A. Biol. Sci. Med. Sci. 2020, 75, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Merriam, G.R.; Kargi, A.Y. Growth Hormone in Aging. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279163/ (accessed on 27 September 2022).
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.O.; Pichard, C. Age-Related Differences in Fat-Free Mass, Skeletal Muscle, Body Cell Mass and Fat Mass between 18 and 94 Years. Eur. J. Clin. Nutr. 2001, 55, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Fleg, J.L.; Morrell, C.H.; Bos, A.G.; Brant, L.J.; Talbot, L.A.; Wright, J.G.; Lakatta, E.G. Accelerated Longitudinal Decline of Aerobic Capacity in Healthy Older Adults. Circulation 2005, 112, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Kouchiwa, T.; Wada, K.; Uchiyama, M.; Kasezawa, N.; Niisato, M.; Murakami, H.; Fukuyama, K.; Yokogoshi, H. Age-Related Changes in Serum Amino Acids Concentrations in Healthy Individuals. Clin. Chem. Lab. Med. 2012, 50, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, H.T.; Oja, S.S.; Kemppainen, K.; Seppä, J.M.; Mero, A.A. Serum Amino Acid Concentrations in Aging Men and Women. Amino Acids 2003, 24, 413–421. [Google Scholar] [CrossRef]
- Yaku, K.; Okabe, K.; Nakagawa, T. NAD Metabolism: Implications in Aging and Longevity. Ageing Res. Rev. 2018, 47, 1–17. [Google Scholar] [CrossRef]
- Ramos-Chávez, L.A.; Roldán-Roldán, G.; García-Juárez, B.; González-Esquivel, D.; Pérez de la Cruz, G.; Pineda, B.; Ramírez-Ortega, D.; García Muñoz, I.; Jiménez Herrera, B.; Ríos, C.; et al. Low Serum Tryptophan Levels as an Indicator of Global Cognitive Performance in Nondemented Women over 50 Years of Age. Oxid. Med. Cell. Longev. 2018, 2018, 8604718. [Google Scholar] [CrossRef]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Brüning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of Glutamine and Interlinked Asparagine Metabolism in Vessel Formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine Promotes Cancer Cell Proliferation through Use as an Amino Acid Exchange Factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef]
- Chak, C.M.; Lacruz, M.E.; Adam, J.; Brandmaier, S.; Covic, M.; Huang, J.; Meisinger, C.; Tiller, D.; Prehn, C.; Adamski, J.; et al. Ageing Investigation Using Two-Time-Point Metabolomics Data from KORA and CARLA Studies. Metabolites 2019, 9, 44. [Google Scholar] [CrossRef]
- Nelson, D.; Cox, M. Lehninger Principles of Biochemistry; W.H. Freeman and Company: New York, NY, USA, 2013. [Google Scholar]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef]
- Musch, W.; Verfaillie, L.; Decaux, G. Age-Related Increase in Plasma Urea Level and Decrease in Fractional Urea Excretion: Clinical Application in the Syndrome of Inappropriate Secretion of Antidiuretic Hormone. Clin. J. Am. Soc. Nephrol. 2006, 1, 909–914. [Google Scholar] [CrossRef]
- Ivanovski, I.; Ješić, M.; Ivanovski, A.; Garavelli, L.; Ivanovski, P. Metabolically Based Liver Damage Pathophysiology in Patients with Urea Cycle Disorders—A New Hypothesis. World J. Gastroenterol. 2017, 23, 7930–7938. [Google Scholar] [CrossRef] [PubMed]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite Patterns Predicting Sex and Age in Participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) Study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Caterino, M.; Sotgiu, G.; Ruoppolo, M.; Franconi, F.; Campesi, I. Sex Differences in the Human Metabolome. Biol. Sex Differ. 2022, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- De Maria, B.; Bari, V.; Cairo, B.; Vaini, E.; Martins de Abreu, R.; Perseguini, N.M.; Milan-Mattos, J.; Rehder-Santos, P.; Minatel, V.; Catai, A.M.; et al. Cardiac Baroreflex Hysteresis Is One of the Determinants of the Heart Period Variability Asymmetry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R539–R551. [Google Scholar] [CrossRef]
- Milan-Mattos, J.C.; Anibal, F.F.; Perseguini, N.M.; Minatel, V.; Rehder-Santos, P.; Castro, C.A.; Vasilceac, F.A.; Mattiello, S.M.; Faccioli, L.H.; Catai, A.M. Effects of Natural Aging and Gender on Pro-Inflammatory Markers. Brazilian J. Med. Biol. Res. = Rev. Bras. Pesqui. Med. Biol. 2019, 52, e8392. [Google Scholar] [CrossRef]
- Wallace, M.; Hashim, Y.Z.H.-Y.; Wingfield, M.; Culliton, M.; McAuliffe, F.; Gibney, M.J.; Brennan, L. Effects of Menstrual Cycle Phase on Metabolomic Profiles in Premenopausal Women. Hum. Reprod. 2010, 25, 949–956. [Google Scholar] [CrossRef]
- Bub, A.; Kriebel, A.; Dörr, C.; Bandt, S.; Rist, M.; Roth, A.; Hummel, E.; Kulling, S.; Hoffmann, I.; Watzl, B. The Karlsruhe Metabolomics and Nutrition (KarMeN) Study: Protocol and Methods of a Cross-Sectional Study to Characterize the Metabolome of Healthy Men and Women. JMIR Res. Protoc. 2016, 5, e146. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; Ferreira, M.L.V.; de Andrade, A.L.L.; Gáspari, A.F.; de Marchi-Silva, L.; de Oliveira-Nunes, S.G.; Cavaglieri, C.R.; Ghosh, S.; Bouchard, C.; et al. Association of Skeletal Muscle and Serum Metabolites with Maximum Power Output Gains in Response to Continuous Endurance or High-Intensity Interval Training Programs: The TIMES Study—A Randomized Controlled Trial. PLoS ONE 2019, 14, e021211. [Google Scholar] [CrossRef]
- Kohl, S.M.; Klein, M.S.; Hochrein, J.; Oefner, P.J.; Spang, R.; Gronwald, W. State-of-the Art Data Normalization Methods Improve NMR-Based Metabolomic Analysis. Metabolomics 2012, 8, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.L. The Analysis of Incomplete Multivariate Data; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Benjamini, Y.; Ochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MSEA: A Web-Based Tool to Identify Biologically Meaningful Patterns in Quantitative Metabolomic Data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; Silva, L.M.; Ferreira, M.L.V.; Andrade, A.L.L.; Bernardes, C.F.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Understanding the Relationship between Intrinsic Cardiorespiratory Fitness and Serum and Skeletal Muscle Metabolomics Profile. J. Proteome Res. 2021, 20, 2397–2409. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; de Oliveira-Nunes, S.G.; de Andrade, A.L.L.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Association Between Changes in Serum and Skeletal Muscle Metabolomics Profile with Maximum Power Output Gains in Response to Different Aerobic Training Programs: The Times Study. Front. Physiol. 2021, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.M.; Peterson, B.; Schranner, D.; Tahir, U.A.; Rienmüller, T.; Deng, S.; Keyes, M.J.; Katz, D.H.; Beltran, P.M.J.; Barber, J.L.; et al. Human Plasma Proteomic Profiles Indicative of Cardiorespiratory Fitness. Nat. Metab. 2021, 3, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Crozara, L.F.; Castro, A.; De Almeida Neto, A.F.; Laroche, D.P.; Cardozo, A.C.; Gonçalves, M. Utility of Electromyographic Fatigue Threshold during Treadmill Running. Muscle Nerve 2015, 52, 1030–1039. [Google Scholar] [CrossRef]
- Candotti, C.T.; Loss, J.F.; de Melo, M.O.; La Torre, M.; Pasini, M.; Dutra, L.A.; de Oliveira, J.L.N.; de Oliveira, L.P. Comparing the Lactate and EMG Thresholds of Recreational Cyclists during Incremental Pedaling Exercise. Can. J. Physiol. Pharmacol. 2008, 86, 272–278. [Google Scholar] [CrossRef] [PubMed]

| Variables | Training Sample (n = 105) | Test Sample (n = 33) | p-Value # | ||
|---|---|---|---|---|---|
| Age (years) | 42.0 | (30.0–51.5) | 40.0 | (29.0–53.0) | 0.887 |
| Height (m) | 1.69 | (1.62–1.76) | 1.65 | (1.62–1.77) | 0.467 |
| Body mass (kg) | 70.4 | (63.1–80.0) | 68.4 | (58.6–80.0) | 0.485 |
| BMI (km·m−2) | 24.8 | (22.9–26.9) | 24.9 | (21.7–26.6) | 0.873 |
| Total cholesterol (mg·dL−1) | 188.0 | (165.0–204.0) | 184.0 | (163.0–203.0) | 0.454 |
| HDL (mg·dL−1) | 52.0 | (43.5–63.0) | 57.0 | (44.0–66.5) | 0.455 |
| LDL (mg·dL−1) | 113.0 | (93.0–130) | 103.0 | (90.5–120.0) | 0.177 |
| VLDL (mg·dL−1) | 19.0 | (14.0–24.5) | 15.0 | (13.5–28.0) | 0.974 |
| Triacylglyceride (mg·dL−1) | 93.0 | (69.0–122.5) | 77.0 | (67.5–138.5) | 0.998 |
| Uric acid (mg·dL−1) | 5.10 | (4.35–6.10) | 5.20 | (4.3–6.40) | 0.851 |
| Creatinine (mg·dL−1) | 0.88 | (0.76–1.00) | 0.94 | (0.78–1.02) | 0.417 |
| Glucose (mg·dL−1) | 90.8 | (86.0–94.0) | 94.0 | (86.5–97.5) | 0.110 |
| Urea (mg·dL−1) | 31.0 | (27.0–37.0) | 32.0 | (25.5–34.5) | 0.367 |
| hs-CRP (mg·dL−1) | 0.62 | (0.18–1.22) | 0.37 | (0.14–1.29) | 0.307 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, A.; Signini, É.F.; De Oliveira, J.M.; Di Medeiros Leal, M.C.B.; Rehder-Santos, P.; Millan-Mattos, J.C.; Minatel, V.; Pantoni, C.B.F.; Oliveira, R.V.; Catai, A.M.; et al. The Aging Process: A Metabolomics Perspective. Molecules 2022, 27, 8656. https://doi.org/10.3390/molecules27248656
Castro A, Signini ÉF, De Oliveira JM, Di Medeiros Leal MCB, Rehder-Santos P, Millan-Mattos JC, Minatel V, Pantoni CBF, Oliveira RV, Catai AM, et al. The Aging Process: A Metabolomics Perspective. Molecules. 2022; 27(24):8656. https://doi.org/10.3390/molecules27248656
Chicago/Turabian StyleCastro, Alex, Étore F. Signini, Juliana Magalhães De Oliveira, Maria Carolina Bezerra Di Medeiros Leal, Patrícia Rehder-Santos, Juliana C. Millan-Mattos, Vinicius Minatel, Camila B. F. Pantoni, Regina V. Oliveira, Aparecida M. Catai, and et al. 2022. "The Aging Process: A Metabolomics Perspective" Molecules 27, no. 24: 8656. https://doi.org/10.3390/molecules27248656
APA StyleCastro, A., Signini, É. F., De Oliveira, J. M., Di Medeiros Leal, M. C. B., Rehder-Santos, P., Millan-Mattos, J. C., Minatel, V., Pantoni, C. B. F., Oliveira, R. V., Catai, A. M., & Ferreira, A. G. (2022). The Aging Process: A Metabolomics Perspective. Molecules, 27(24), 8656. https://doi.org/10.3390/molecules27248656








