Melatonin Inhibits EMT in Bladder Cancer by Targeting Autophagy
Abstract
1. Introduction
2. Results
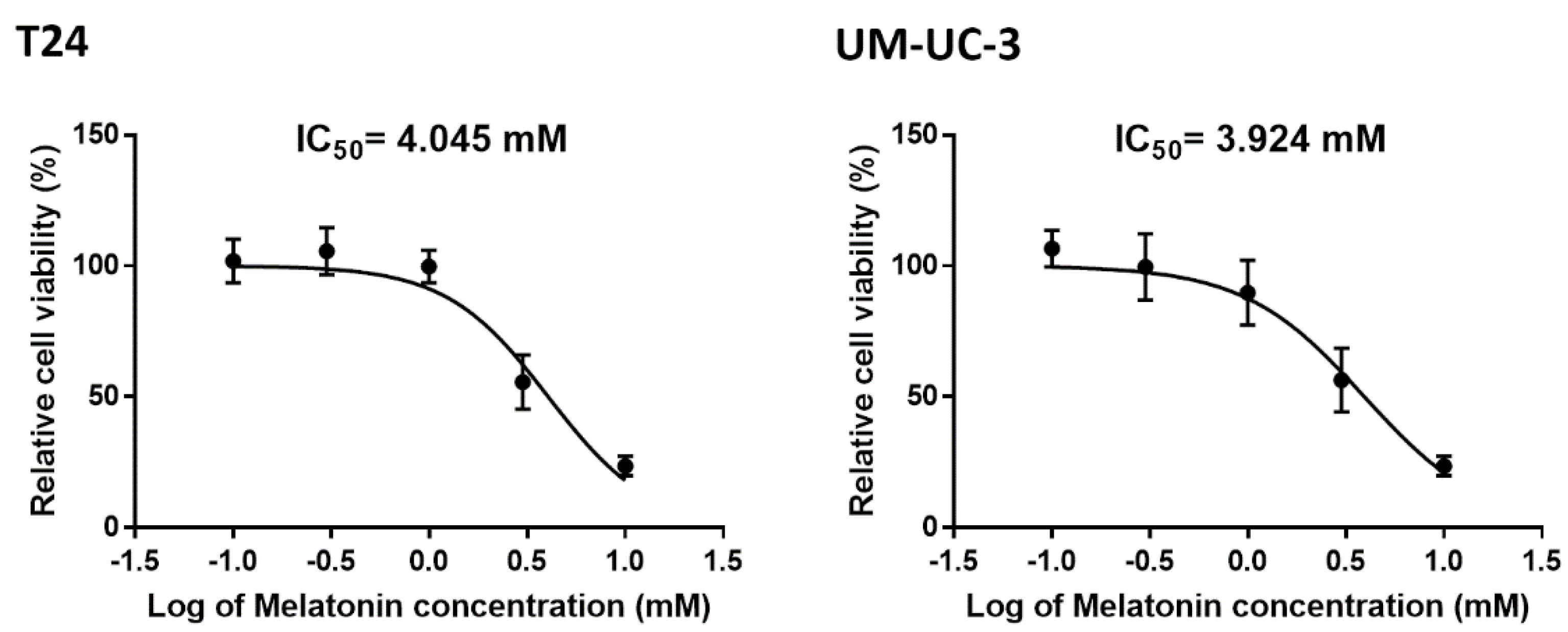
2.1. Cell Viability Test in BCa Cells Treated with Melatonin
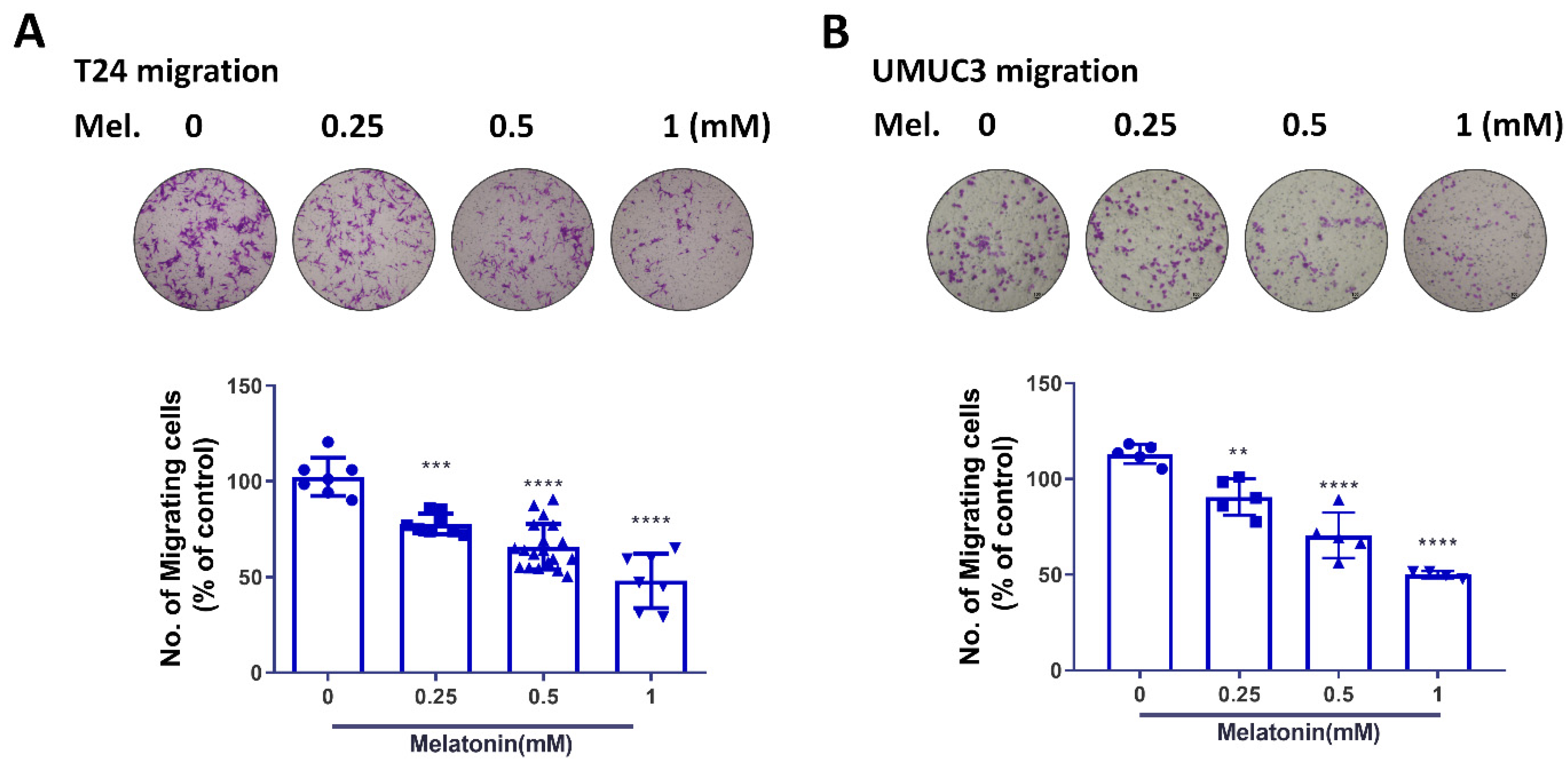
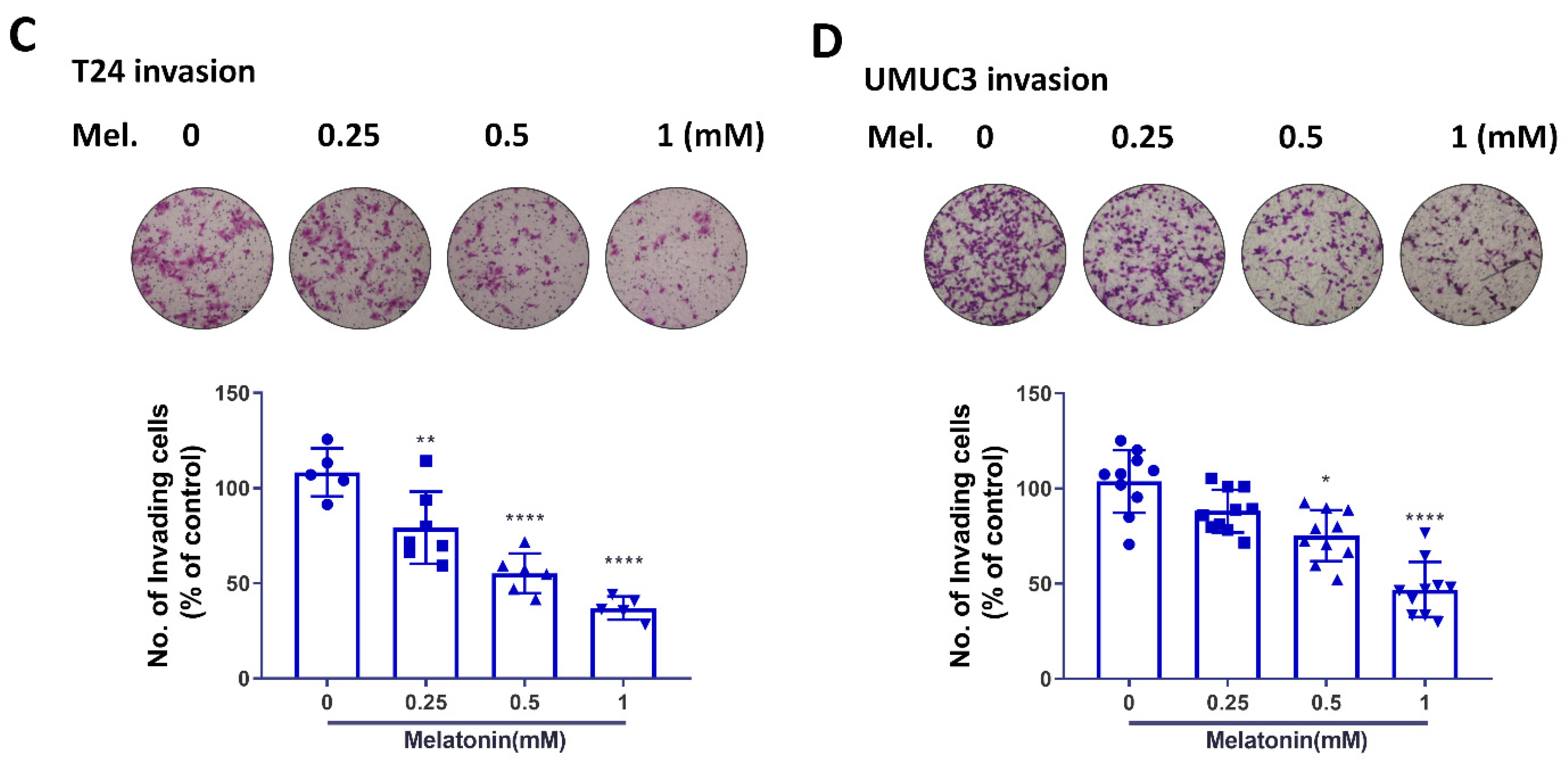
2.2. Melatonin Suppresses BCa Cell Migratory Potential
2.3. Melatonin Inhibits EMT and Thus Contributes to Antimigratory Effects
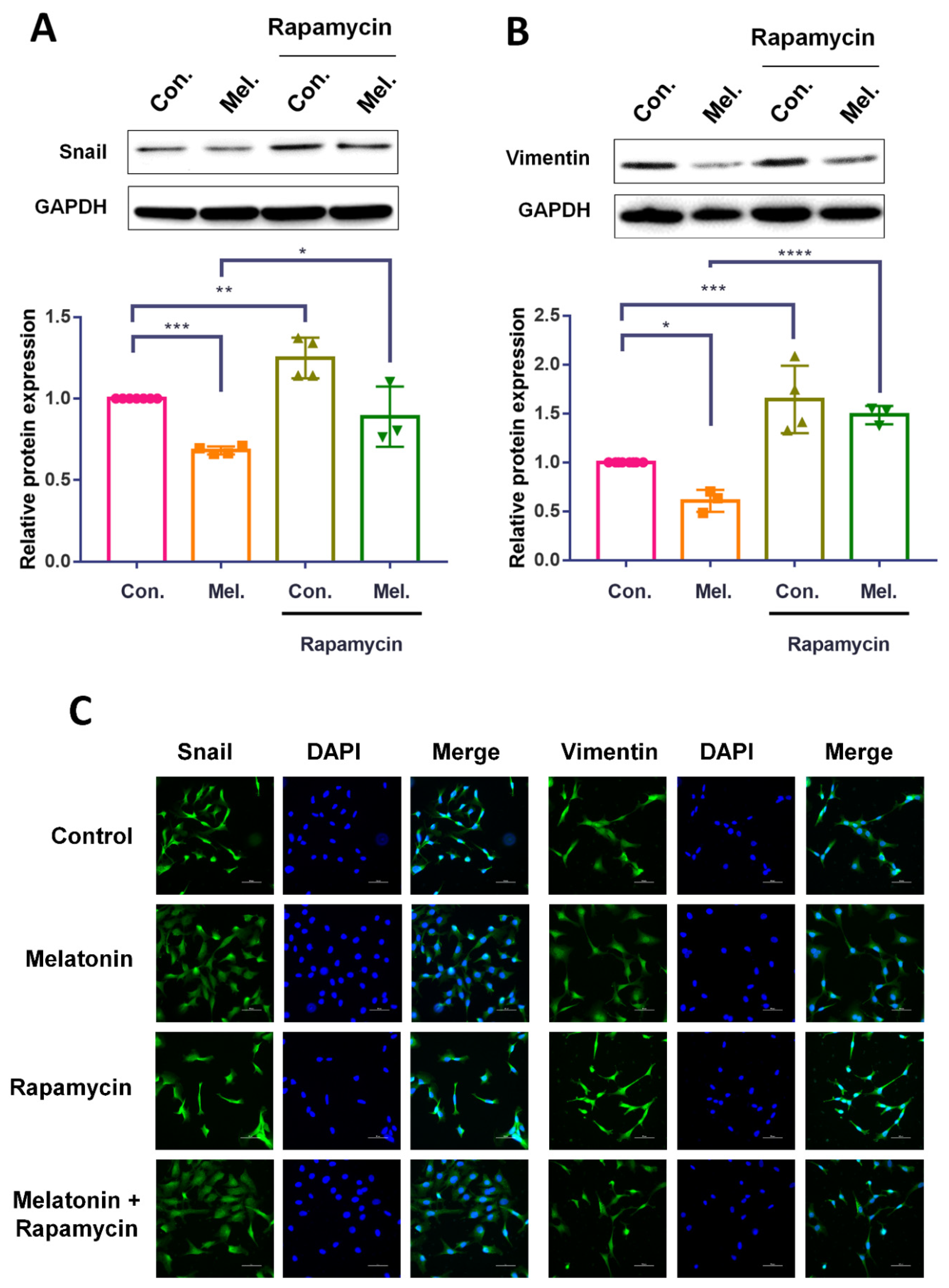
2.4. Melatonin Reduces Autophagy and Inhibits the Migratory Capacity of BCa Cells
2.5. Intravesical Instillation of Melatonin in an Orthotopic Mouse Model of BCa
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assays
4.3. Migration Assay
4.4. Immunofluorescence Staining
4.5. Western Blot Analysis
4.6. Quantitative Real-Time PCR
4.7. Orthotopic BCa Animal Model
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hung, C.F.; Yang, C.K.; Ou, Y.C. Urologiczz cancer in Taiwan. Jpn. J. Clin. Oncol. 2016, 46, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–465; discussion 475–467. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.W.; Newgreen, D.F.; Tarin, D. Carcinoma invasion and metastasis: A role for epithelial-mesenchymal transition? Cancer Res. 2005, 65, 5991–5991; discussion 5995. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Chin, V.L.; Lim, C.L. Epithelial-mesenchymal plasticity-engaging stemness in an interplay of phenotypes. Stem Cell Investig. 2019, 6, 25. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- McConkey, D.J.; Choi, W.; Marquis, L.; Martin, F.; Williams, M.B.; Shah, J.; Svatek, R.; Das, A.; Adam, L.; Kamat, A.; et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metast. Rev. 2009, 28, 335–344. [Google Scholar] [CrossRef]
- Wallerand, H.; Robert, G.; Pasticier, G.; Ravaud, A.; Ballanger, P.; Reiter, R.E.; Ferriere, J.M. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol. Oncol. 2010, 28, 473–479. [Google Scholar] [CrossRef]
- Lum, J.J.; Bauer, D.E.; Kong, M.; Harris, M.H.; Li, C.; Lindsten, T.; Thompson, C.B. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005, 120, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, M.T.; Ryan, K.M. The role of autophagy in tumour development and cancer therapy. Expert Rev. Mol. Med. 2009, 11, e36. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 1845–1846. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Kanzawa, T.; Sawaya, R.; Kondo, S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 2005, 5, 726–734. [Google Scholar] [CrossRef]
- Klionsky, D.J. Coming soon to a journal near you—The updated guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2014, 10, 1691. [Google Scholar] [CrossRef][Green Version]
- Klionsky, D.J. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007, 8, 931–937. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62, e12370. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Zheng, X.; Du, X. Therapeutic strategies of melatonin in cancer patients: A systematic review and meta-analysis. OncoTargets Ther. 2018, 11, 7895–7908. [Google Scholar] [CrossRef]
- Cutando, A.; Lopez-Valverde, A.; Arias-Santiago, S.; De Vicente, J.; De Diego, R.G. Role of melatonin in cancer treatment. Anticancer Res. 2012, 32, 2747–2753. [Google Scholar]
- Bruck, R.; Aeed, H.; Avni, Y.; Shirin, H.; Matas, Z.; Shahmurov, M.; Avinoach, I.; Zozulya, G.; Weizman, N.; Hochman, A. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J. Hepatol. 2004, 40, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grunert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.L.; Bhat-Nakshatri, P.; Clare, S.E.; Morimiya, A.; Badve, S.; Nakshatri, H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene 2007, 26, 711–724. [Google Scholar] [CrossRef]
- Chao, C.C.; Chen, P.C.; Chiou, P.C.; Hsu, C.J.; Liu, P.I.; Yang, Y.C.; Reiter, R.J.; Yang, S.F.; Tang, C.H. Melatonin suppresses lung cancer metastasis by inhibition of epithelial-mesenchymal transition through targeting to Twist. Clin. Sci. 2019, 133, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Dikic, I. Autophagy Captures the Nobel Prize. Cell 2016, 167, 1433–1435. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.F.; Wen, S.I.; Yang, S.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I. Inhibition of High Basal Level of Autophagy Induces Apoptosis in Human Bladder Cancer Cells. J. Urol. 2016, 195, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef]
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef]
- Baghal-Sadriforoush, S.; Bagheri, M.; Abdi Rad, I.; Sotoodehnejadnematalahi, F. Melatonin Sensitizes OVCAR-3 Cells to Cisplatin through Suppression of PI3K/Akt Pathway. Cell Mol. Biol. 2022, 68, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Nyamsambuu, A.; Khan, M.A.; Zhou, X.; Chen, H.C. Molecular mechanism of inhibitory effects of melatonin on prostate cancer cell proliferation, migration and invasion. PLoS ONE 2022, 17, e0261341. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Anarkooli, M.; Dabirian, S.; Zendedel, A.; Moladoust, H.; Bahadori, M.H. Effects of melatonin on the toxicity and proliferation of human anaplastic thyroid cancer cell line. Acta Histochem. 2021, 123, 151700. [Google Scholar] [CrossRef] [PubMed]
- Laothong, U.; Hiraku, Y.; Oikawa, S.; Intuyod, K.; Murata, M.; Pinlaor, S. Melatonin induces apoptosis in cholangiocarcinoma cell lines by activating the reactive oxygen species-mediated mitochondrial pathway. Oncol. Rep. 2015, 33, 1443–1449. [Google Scholar] [CrossRef]
- Lopes, J.R.; Maschio, L.B.; Jardim-Perassi, B.V.; Moschetta, M.G.; Ferreira, L.C.; Martins, G.R.; Gelaleti, G.B.; De Campos Zuccari, D.A. Evaluation of melatonin treatment in primary culture of canine mammary tumors. Oncol. Rep. 2015, 33, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Emamgholipour, S.; Hossein-Nezhad, A.; Ansari, M. Can Melatonin Act as an Antioxidant in Hydrogen Peroxide-Induced Oxidative Stress Model in Human Peripheral Blood Mononuclear Cells? Biochem. Res. Int. 2016, 2016, 5857940. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gong, X.; Zhu, X.; Xue, D.; Liu, Y.; Wang, P. Rab27A overexpression promotes bladder cancer proliferation and chemoresistance through regulation of NF-kappaB signaling. Oncotarget 2017, 8, 75272–75283. [Google Scholar] [CrossRef]
- Stravopodis, D.J.; Karkoulis, P.K.; Konstantakou, E.G.; Melachroinou, S.; Lampidonis, A.D.; Anastasiou, D.; Kachrilas, S.; Messini-Nikolaki, N.; Papassideri, I.S.; Aravantinos, G.; et al. Grade-dependent effects on cell cycle progression and apoptosis in response to doxorubicin in human bladder cancer cell lines. Int. J. Oncol. 2009, 34, 137–160. [Google Scholar]
- Reiter, R.J. Mechanisms of cancer inhibition by melatonin. J. Pineal Res. 2004, 37, 213–214. [Google Scholar] [CrossRef]
- Cos, S.; Fernandez, R.; Guezmes, A.; Sanchez-Barcelo, E.J. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998, 58, 4383–4390. [Google Scholar]
- Wu, S.M.; Lin, W.Y.; Shen, C.C.; Pan, H.C.; Keh-Bin, W.; Chen, Y.C.; Jan, Y.J.; Lai, D.W.; Tang, S.C.; Tien, H.R.; et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPbeta and NFkappaB cleavage. J. Pineal Res. 2016, 60, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, K.B.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; Tang, C.H.; Yang, J.S.; Hsu, Y.M.; Peng, S.F.; Chung, J.G. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-kappaB signaling pathways. J. Food Biochem. 2019, 43, e12902. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Tsai, C.W.; Yang, J.S.; Hsu, Y.M.; Shih, L.C.; Chiu, H.Y.; Bau, D.T.; Tsai, F.J. Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 207–212. [Google Scholar] [CrossRef]
- San-Miguel, B.; Crespo, I.; Sanchez, D.I.; Gonzalez-Fernandez, B.; Ortiz de Urbina, J.J.; Tunon, M.J.; Gonzalez-Gallego, J. Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride-induced fibrosis. J. Pineal Res. 2015, 59, 151–162. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Zhang, K.; Zhao, J.; Liu, H.; Ma, X.; Guo, J.; Wang, J.; Lu, W. Melatonin inhibits autophagy in TM3 cells via AKT/FOXO1 pathway. Mol. Biol. Rep. 2022, 49, 2925–2932. [Google Scholar] [CrossRef]
- Kang, J.W.; Cho, H.I.; Lee, S.M. Melatonin inhibits mTOR-dependent autophagy during liver ischemia/reperfusion. Cell Physiol. Biochem. 2014, 33, 23–36. [Google Scholar] [CrossRef]
- Chen, H.T.; Liu, H.; Mao, M.J.; Tan, Y.; Mo, X.Q.; Meng, X.J.; Cao, M.T.; Zhong, C.Y.; Liu, Y.; Shan, H.; et al. Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol. Cancer 2019, 18, 101. [Google Scholar] [CrossRef]
- Gugnoni, M.; Sancisi, V.; Manzotti, G.; Gandolfi, G.; Ciarrocchi, A. Autophagy and epithelial-mesenchymal transition: An intricate interplay in cancer. Cell Death Dis. 2016, 7, e2520. [Google Scholar] [CrossRef]
- Colella, B.; Faienza, F.; Di Bartolomeo, S. EMT Regulation by Autophagy: A New Perspective in Glioblastoma Biology. Cancers 2019, 11, 312. [Google Scholar] [CrossRef]
- Scarpato, K.R.; Morgans, A.K.; Moses, K.A. Optimal management of muscle-invasive bladder cancer—A review. Res. Rep. Urol. 2015, 7, 143–151. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. Role and Therapeutic Potential of Melatonin in Various Type of Cancers. OncoTargets Ther. 2021, 14, 2019–2052. [Google Scholar] [CrossRef]
- Lissoni, P.; Chilelli, M.; Villa, S.; Cerizza, L.; Tancini, G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: A randomized trial. J. Pineal Res. 2003, 35, 12–15. [Google Scholar] [CrossRef]
- Seely, D.; Wu, P.; Fritz, H.; Kennedy, D.A.; Tsui, T.; Seely, A.J.; Mills, E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr. Cancer Ther. 2012, 11, 293–303. [Google Scholar] [CrossRef]
- Mills, E.; Wu, P.; Seely, D.; Guyatt, G. Melatonin in the treatment of cancer: A systematic review of randomized controlled trials and meta-analysis. J. Pineal Res. 2005, 39, 360–366. [Google Scholar] [CrossRef]
- Mehrzadi, M.H.; Hosseinzadeh, A.; Juybari, K.B.; Mehrzadi, S. Melatonin and urological cancers: A new therapeutic approach. Cancer Cell Int. 2020, 20, 444. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-Y.; Tang, C.-H.; Chen, P.-C.; Lin, T.-H.; Chao, C.-C. Melatonin Inhibits EMT in Bladder Cancer by Targeting Autophagy. Molecules 2022, 27, 8649. https://doi.org/10.3390/molecules27248649
Hsiao S-Y, Tang C-H, Chen P-C, Lin T-H, Chao C-C. Melatonin Inhibits EMT in Bladder Cancer by Targeting Autophagy. Molecules. 2022; 27(24):8649. https://doi.org/10.3390/molecules27248649
Chicago/Turabian StyleHsiao, Sheng-Yen, Chih-Hsin Tang, Po-Chun Chen, Tien-Huang Lin, and Chia-Chia Chao. 2022. "Melatonin Inhibits EMT in Bladder Cancer by Targeting Autophagy" Molecules 27, no. 24: 8649. https://doi.org/10.3390/molecules27248649
APA StyleHsiao, S.-Y., Tang, C.-H., Chen, P.-C., Lin, T.-H., & Chao, C.-C. (2022). Melatonin Inhibits EMT in Bladder Cancer by Targeting Autophagy. Molecules, 27(24), 8649. https://doi.org/10.3390/molecules27248649





