Antibacterial Activity of Syzygium aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. Gas Chromatography-Mass Spectrometric Analysis
2.2. Antibacterial Activity In Vitro
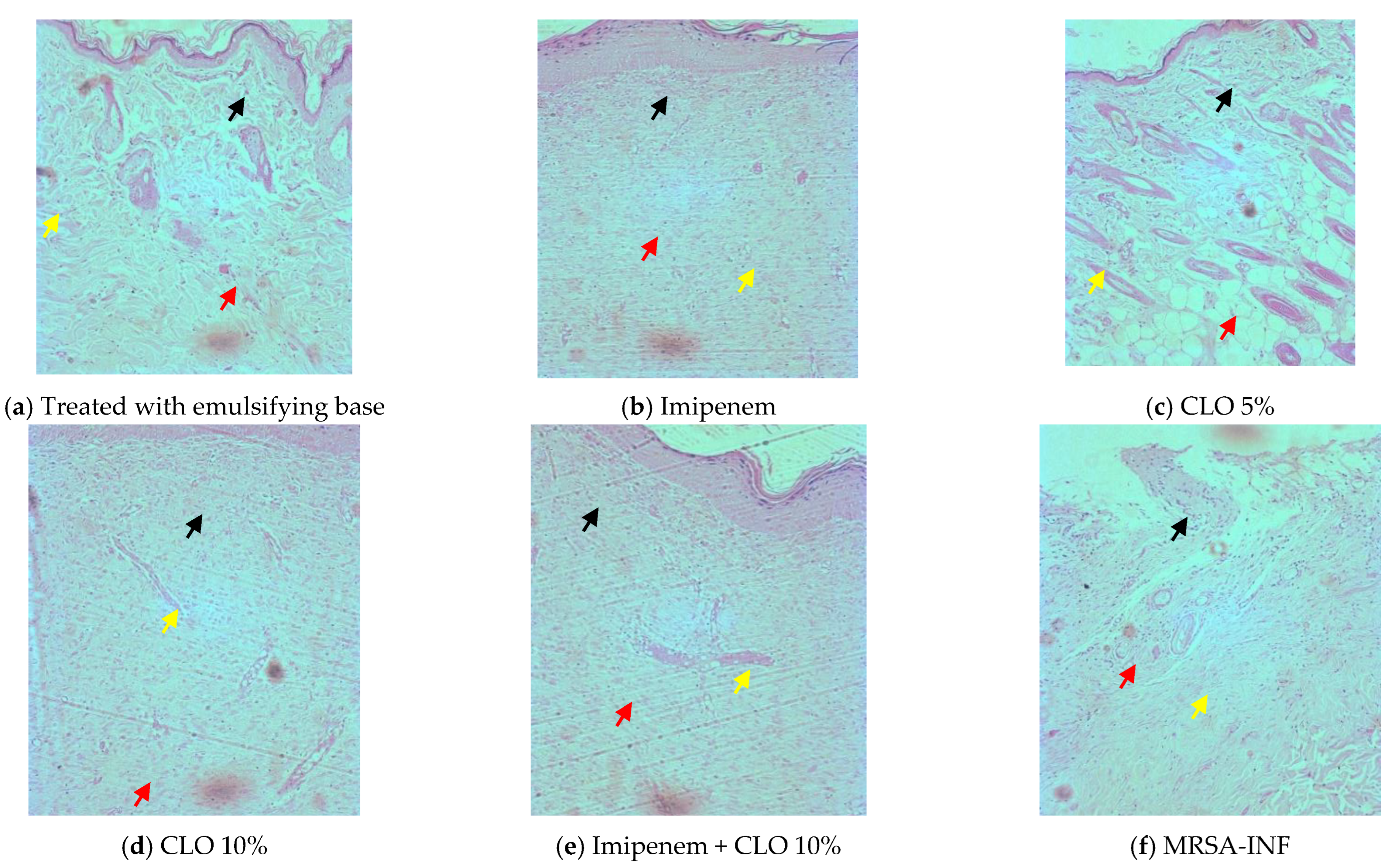
2.3. Wound-Healing and Skin Irritation Study
3. Discussion
4. Materials and Methods
4.1. GC-MS Analysis of Clove Oil
4.2. Antibiotic Sensitivity Test
4.3. Antibacterial Assay (Well Diffusion Assay)
4.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.5. Interaction of Clove Oil with Imipenem against MRSA
4.6. Wound-Healing Activity in Rats (Excision Wound Model)
4.7. Skin Irritation Test
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díez-Pascual, A.M. Antibacterial Action of Nanoparticle Loaded Nanocomposites Based on Graphene and Its Derivatives: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 3563. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital Acquired Infections. Cambridge Handb. Psychol. Health Med. Second Ed. 2022, 736–738. [Google Scholar] [CrossRef]
- Sannathimmappa, M.B.; Nambiar, V.; Aravindakshan, R. Antibiotics at the crossroads-Do we have any therapeutic alternatives to control the emergence and spread of antimicrobial resistance? J. Educ. Health Promot. 2021, 10, 438. [Google Scholar] [CrossRef]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- Mohd Israfi, N.A.; Mohd Ali, M.I.A.; Manickam, S.; Sun, X.; Goh, B.H.; Tang, S.Y.; Ismail, N.; Abdull Razis, A.F.; Ch’ng, S.E.; Chan, K.W. Essential oils and plant extracts for tropical fruits protection: From farm to table. Front. Plant Sci. 2022, 13, 3644. [Google Scholar] [CrossRef]
- Oliveira, T.A.S.; Santiago, M.B.; Santos, V.H.P.; Silva, E.O.; Martins, C.H.G.; Crotti, A.E.M. Antibacterial Activity of Essential Oils against Oral Pathogens. Chem. Biodivers. 2022, 19, e202200097. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.M.; Tayebwa, D.S.; Shaheen, H.M.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick. Borne. Dis. 2019, 10, 949–958. [Google Scholar] [CrossRef]
- Kumar Pandey, V.; Shams, R.; Singh, R.; Dar, A.H.; Pandiselvam, R.; Rusu, A.V.; Trif, M. A comprehensive review on clove (Caryophyllus aromaticus L.) essential oil and its significance in the formulation of edible coatings for potential food applications. Front. Nutr. 2022, 9, 2114. [Google Scholar] [CrossRef]
- Charoonratana, T. Clove (Syzygium aromaticum) oleoresins. Chem. Funct. Appl. 2022, 49–65. [Google Scholar] [CrossRef]
- Astuti, R.I.; Listyowati, S.; Wahyuni, W.T. Life span extension of model yeast Saccharomyces cerevisiae upon ethanol derived-clover bud extract treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 299, 012059. [Google Scholar] [CrossRef]
- Kennewell, T.; Mashtoub, S.; Howarth, G.; Cowin, A.; Kopecki, Z. Antimicrobial and healing-promoting properties of animal and plant oils for the treatment of infected wounds. Wound Pract. Res. 2019, 27, 175–183. [Google Scholar] [CrossRef]
- Senatore, F.; Soria, E.U.; Soria, R.U.; Porta, G.D.; Taddeo, R.; De Feo, V. Essential Oil of Eremocharis triradiata (Wolff.) Johnston (Apiaceae) Growing Wild in Perú. Flavour Fragr. J. 1997, 12, 257–259. [Google Scholar] [CrossRef]
- Hoskovec, M.; Grygarová, D.; Cvačka, J.; Streinz, L.; Zima, J.; Verevkin, S.P.; Koutek, B. Determining the vapour pressures of plant volatiles from gas chromatographic retention data. J. Chromatogr. A 2005, 1083, 161–172. [Google Scholar] [CrossRef]
- Bouzouita, N.; Kachouri, F.; Hamdi, M.; Chaabouni, M.M. Antimicrobial activity of essential oils from Tunisian aromatic plants. Flavour Fragr. J. 2003, 18, 380–383. [Google Scholar] [CrossRef]
- Mahmood, U.; Kaul, V.K.; Acharya, R. Volatile constituents of Capillipedium parviflorum. Phytochemistry 2004, 65, 2163–2166. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, T.; Yang, B.; Liu, X.; Duan, G. Development of gas chromatography–mass spectrometry with microwave distillation and simultaneous solid-phase microextraction for rapid determination of volatile constituents in ginger. J. Pharm. Biomed. Anal. 2007, 43, 24–31. [Google Scholar] [CrossRef]
- De Feo, V.; Della Porta, G.; Soria, E.U.; Soria, R.U.; Senatore, F. Composition of the essential oil of Tagetes filifolia Lag. Flavour Fragr. J. 1998, 13, 145–147. [Google Scholar] [CrossRef]
- Shatar, S. Essential Oil of Ferula ferulaoides from Western Mongolia. Chem. Nat. Compd. 2005, 41, 607–608. [Google Scholar] [CrossRef]
- Nagalakshmi, M.A.H.; Thangadurai, D.; Anuradha, T.; Pullaiah, T. Essential oil constituents of Melia dubia, a wild relative of Azadirachta indica growing in the Eastern Ghats of Peninsular India. Flavour Fragr. J. 2001, 16, 241–244. [Google Scholar] [CrossRef]
- Özel, M.Z.; Göǧüş, F.; Lewis, A.C. Comparison of direct thermal desorption with water distillation and superheated water extraction for the analysis of volatile components of Rosa damascena Mill. using GCxGC-TOF/MS. Anal. Chim. Acta 2006, 566, 172–177. [Google Scholar] [CrossRef]
- Adams, R.P.; Morris, J.A.; Pandey, R.N.; Schwarzbach, A.E. Cryptic speciation between Juniperus deltoides and Juniperus oxycedrus (Cupressaceae) in the Mediterranean. Biochem. Syst. Ecol. 2005, 33, 771–787. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Oboh, G.; Akinbola, I.A.; Ademosun, A.O.; Sanni, D.M.; Odubanjo, O.V.; Olasehinde, T.A.; Oyeleye, S.I. Essential Oil from Clove Bud (Eugenia aromatica Kuntze) Inhibit Key Enzymes Relevant to the Management of Type-2 Diabetes and Some Pro-oxidant Induced Lipid Peroxidation in Rats Pancreas in vitro. J. Oleo Sci. 2015, 64, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Alfikri, F.N.; Pujiarti, R.; Wibisono, M.G.; Hardiyanto, E.B. Yield, Quality, and Antioxidant Activity of Clove (Syzygium aromaticum L.) Bud Oil at the Different Phenological Stages in Young and Mature Trees. Scientifica 2020, 2020, 9701701. [Google Scholar] [CrossRef] [PubMed]
- SOULSBY, J. The new ointment bases of the British Pharmocopoeia, 1948. Br. J. Dermatol. Syph. 1949, 61, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, Anti-MRSA Activity and Toxicity of Essential Oils from Cymbopogon Species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef]
- Caron, J.P.; Bolin, C.A.; Hauptman, J.G.; Johnston, K.A. Minimum inhibitory concentration and postantibiotic effect of amikacin for equine isolates of methicillin-resistant Staphylococcus aureus in vitro. Vet. Surg. 2009, 38, 664–669. [Google Scholar] [CrossRef]
- Jamil, S.; Saad, U.; Hafiz, S. Can amoxicillin clavulanate be used for treating MRSA? J. Pharmacol. Med. Chem. 2017, 1, 21–23. [Google Scholar]
- Foxley, M.A.; Friedline, A.W.; Jensen, J.M.; Nimmo, S.L.; Scull, E.M.; King, J.B.; Strange, S.; Xiao, M.T.; Smith, B.E.; Thomas, K.J.; et al. Efficacy of Ampicillin Against Methicillin-Resistant Staphylococcus aureus Restored Through Synergy with Branched Poly(ethylenimine). J. Antibiot. 2016, 69, 871. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Almutairi, M.; Alm, R.A.; Lahiri, S.D.; Martin, M.S.; Chen, A.; Ambler, J.E. Ceftaroline efficacy against high-MIC clinical Staphylococcus aureus isolates in an in vitro hollow-fibre infection model. J. Antimicrob. Chemother. 2017, 72, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Namysl, E.; Galinski, J.; Szponar, M.; Piechowicz, L. WRAZLIWOSC BAKTERII IZOLOWANYCH Z MATERIALU KLINICZNEGO NA CYPROFLOKSACYNE. Med. Dosw. Mikrobiol. 1995, 47, 5–9. [Google Scholar] [PubMed]
- Hodille, E.; Badiou, C.; Bouveyron, C.; Bes, M.; Tristan, A.; Vandenesch, F.; Lina, G.; Dumitrescu, O. Clindamycin suppresses virulence expression in inducible clindamycin-resistant Staphylococcus aureus strains 11 Medical and Health Sciences 1108 Medical Microbiology. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 1–6. [Google Scholar] [CrossRef]
- Yildiz, Ö.; Çoban, A.Y.; Şener, A.G.; Coşkuner, S.A.; Bayramoğlu, G.; Güdücüoğlu, H.; Özyurt, M.; Tatman-Otkun, M.; Karabiber, N.; Özkütük, N.; et al. Antimicrobial susceptibility and resistance mechanisms of methicillin resistant Staphylococcus aureus isolated from 12 Hospitals in Turkey. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 1–6. [Google Scholar] [CrossRef]
- Lemaître, N.; Sougakoff, W.; Masmoudi, A.; Fievet, M.H.; Bismuth, R.; Jarlier, V. Characterization of Gentamicin-Susceptible Strains of Methicillin-Resistant Staphylococcus aureus Involved in Nosocomial Spread. J. Clin. Microbiol. 1998, 36, 81. [Google Scholar] [CrossRef]
- Su, Y.L.; Hong, W.F.; Sutherland, C.; DeRyke, A.C.; Nicolau, D.P. Antibacterial effects of moxifloxacin and levofloxacin simulating epithelial lining fluid concentrations against community-acquired methicillin-resistant Staphylococcus aureus. Drugs R. D. 2007, 8, 69–77. [Google Scholar] [CrossRef]
- Conceição, T.; Coelho, C.; de Lencastre, H.; Aires-de-Sousa, M. Frequent occurrence of oxacillin-susceptible mecA-positive Staphylococcus aureus (OS-MRSA) strains in two African countries. J. Antimicrob. Chemother. 2015, 70, 3200–3204. [Google Scholar] [CrossRef]
- Trzcinski, K.; Cooper, B.S.; Hryniewicz, W.; Dowson, C.G. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 45, 763–770. [Google Scholar] [CrossRef]
- Harris, T.M.; Bowen, A.C.; Holt, D.C.; Sarovich, D.S.; Stevens, K.; Currie, B.J.; Howden, B.P.; Carapetis, J.R.; Giffard, P.M.; Tong, S.Y.C. Investigation of trimethoprim/sulfamethoxazole resistance in an emerging sequence type 5 methicillin-resistant Staphylococcus aureus clone reveals discrepant resistance reporting. Clin. Microbiol. Infect. 2018, 24, 1027–1029. [Google Scholar] [CrossRef]
- Rosarior, V.L.; Lim, P.S.; Wong, W.K.; Yue, C.S.; Yam, H.C.; Tan, S.A. Antioxidant-rich Clove Extract, A Strong Antimicrobial Agent against Urinary Tract Infections-causing Bacteria in vitro. Trop. Life Sci. Res. 2021, 32, 45. [Google Scholar] [CrossRef]
- Wongsawan, K.; Chaisri, W.; Tangtrongsup, S.; Mektrirat, R. Bactericidal Effect of Clove Oil against Multidrug-Resistant Streptococcus suis Isolated from Human Patients and Slaughtered Pigs. Pathogens 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, M.; İrem Omurtag Korkmaz, B. The anti-campylobacter activity of eugenol and its potential for poultry meat safety: A review. Food Chem. 2022, 394, 133519. [Google Scholar] [CrossRef] [PubMed]
- Taleuzzaman, M.; Jain, P.; Verma, R.; Iqbal, Z.; Mirza, M.A. Eugenol as a Potential Drug Candidate: A Review. Curr. Top. Med. Chem. 2021, 21, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.L.; Yang, S.K.; Osman, M.A.; Yuswan, M.H.; Loh, J.Y.; Lim, W.M.; Lim, S.H.E.; Lai, K.S. Antibacterial Activity and Mode of Action of β-caryophyllene on Bacillus cereus. Polish J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Cheng Kao, C.; Matsunami, H.; Mescher, A. Beta-caryophyllene enhances wound healing through multiple routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Rahimi, V.; Askari, V.R. A mechanistic review on immunomodulatory effects of selective type two cannabinoid receptor β-caryophyllene. Biofactors 2022, 48, 857–882. [Google Scholar] [CrossRef]
- Yang, S.J.; Han, S.H.; Lee, A.R.; Jun, J.H.; Son, M.W.; Oh, S.H.; Kim, J.; Paik, S.Y. Evaluation of antimicrobial effects of commercial mouthwashes utilized in South Korea. BMB Rep. 2015, 48, 42. [Google Scholar] [CrossRef]
- Hamad Al-Mijalli, S.; Elsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of Volatile Compounds of Mentha piperita and Lavandula multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evid. Based. Complement. Alternat. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Jang, H.I.; Rhee, K.J.; Eom, Y. Bin Antibacterial and antibiofilm effects of α-humulene against Bacteroides fragilis. Can. J. Microbiol. 2020, 66, 389–399. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Wu, J.; Wu, Y.; Wang, Y.; Hu, X.; Wang, X. Berberine Damages the Cell Surface of Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 621. [Google Scholar] [CrossRef]
- Sardari, K.; Kakhki, E.G.; Mohri, M. Evaluation of wound contraction and epithelialization after subcutaneous administration of Theranekron® in cows. Comp. Clin. Pathol. 2006, 16, 197–200. [Google Scholar] [CrossRef]
- El-Tookhy, O.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological Evaluation of Experimentally Induced Critical Size Defect Skin Wounds Using Exosomal Solution of Mesenchymal Stem Cells Derived Microvesicles. Int. J. stem cells 2017, 10, 144–153. [Google Scholar] [CrossRef]
- Van De Vyver, M.; Boodhoo, K.; Frazier, T.; Hamel, K.; Kopcewicz, M.; Levi, B.; Maartens, M.; Machcinska, S.; Nunez, J.; Pagani, C.; et al. Histology Scoring System for Murine Cutaneous Wounds. Stem Cells Dev. 2021, 30, 1141–1152. [Google Scholar] [CrossRef]
- Banerjee, K.; Madhyastha, H.; Sandur, V.R.; Manikandanath, N.T.; Thiagarajan, N.; Thiagarajan, P. Anti-inflammatory and wound healing potential of a clove oil emulsion. Colloids Surf. B Biointerfaces 2020, 193, 111102. [Google Scholar] [CrossRef]
- Dimic, D.S.; Kaluderovic, G.N.; Avdovic, E.H.; Milenkovic, D.A.; Živanovic, M.N.; Potocnák, I.; Samolová, E.; Dimitrijevic, M.S.; Saso, L.; Markovic, Z.S.; et al. Synthesis, Crystallographic, Quantum Chemical, Antitumor, and Molecular Docking/Dynamic Studies of 4-Hydroxycoumarin-Neurotransmitter Derivatives. Int. J. Mol. Sci. 2022, 23, 1001. [Google Scholar] [CrossRef]
- Kováts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fankam, A.G.; Kuete, V.; Voukeng, I.K.; Kuiate, J.R.; Pages, J.M. Antibacterial activities of selected Cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement. Altern. Med. 2011, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnopharmacol. 2020, 262, 113135. [Google Scholar] [CrossRef]
- Nayeem, N.; Mohammed Basheeruddin Asdaq, S.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Mohzari, Y.; Alrashed, A.A.; Alotaibi, N.; Aalhathal, A.S.; Alharbi, M.A.; et al. Wound healing potential of Dodonaea viscosa extract formulation in experimental animals. J. King Saud Univ.-Sci. 2021, 33, 101476. [Google Scholar] [CrossRef]
- Kolhe, S.S.; Shinde, K.; Jori, R.; Gadhave, M.V.; Jadhav, S.L.; Gaikwad, D.D. Evaluation of polyherbal ointment for wound healing activity in Wistar rats. J. Drug Deliv. Ther. 2018, 8, 26–31. [Google Scholar] [CrossRef]
- Anesthesia (Guideline)|Vertebrate Animal Research. Available online: https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia (accessed on 13 February 2022).
- Mukherjee, P.K.; Verpoorte, R.; Suresh, B. Evaluation of in-vivo wound healing activity of Hypericum patulum (Family: Hypericaceae) leaf extract on different wound model in rats. J. Ethnopharmacol. 2000, 70, 315–321. [Google Scholar] [CrossRef]
- Fayez, M.S.; Hakim, T.A.; Agwa, M.M.; Abdelmoteleb, M.; Aly, R.G.; Montaser, N.N.; Abdelsattar, A.S.; Rezk, N.; El-Shibiny, A. Topically Applied Bacteriophage to Control Multi-Drug Resistant Klebsiella pneumoniae Infected Wound in a Rat Model. Antibiotics 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Ekom, S.E.; Tamokou, J.D.D.; Kuete, V. Methanol extract from the seeds of Persea americana displays antibacterial and wound healing activities in rat model. J. Ethnopharmacol. 2022, 282, 114573. [Google Scholar] [CrossRef]
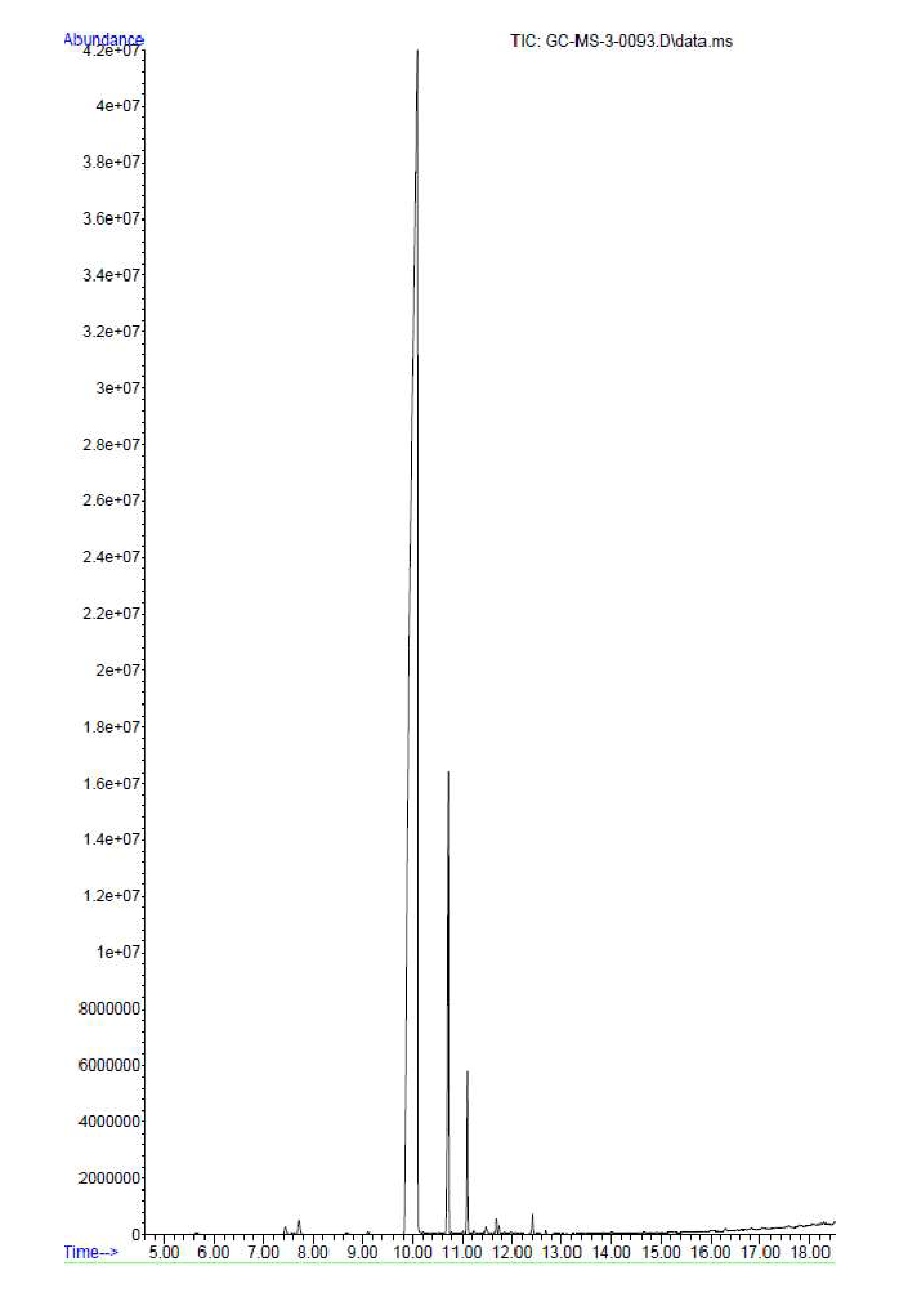
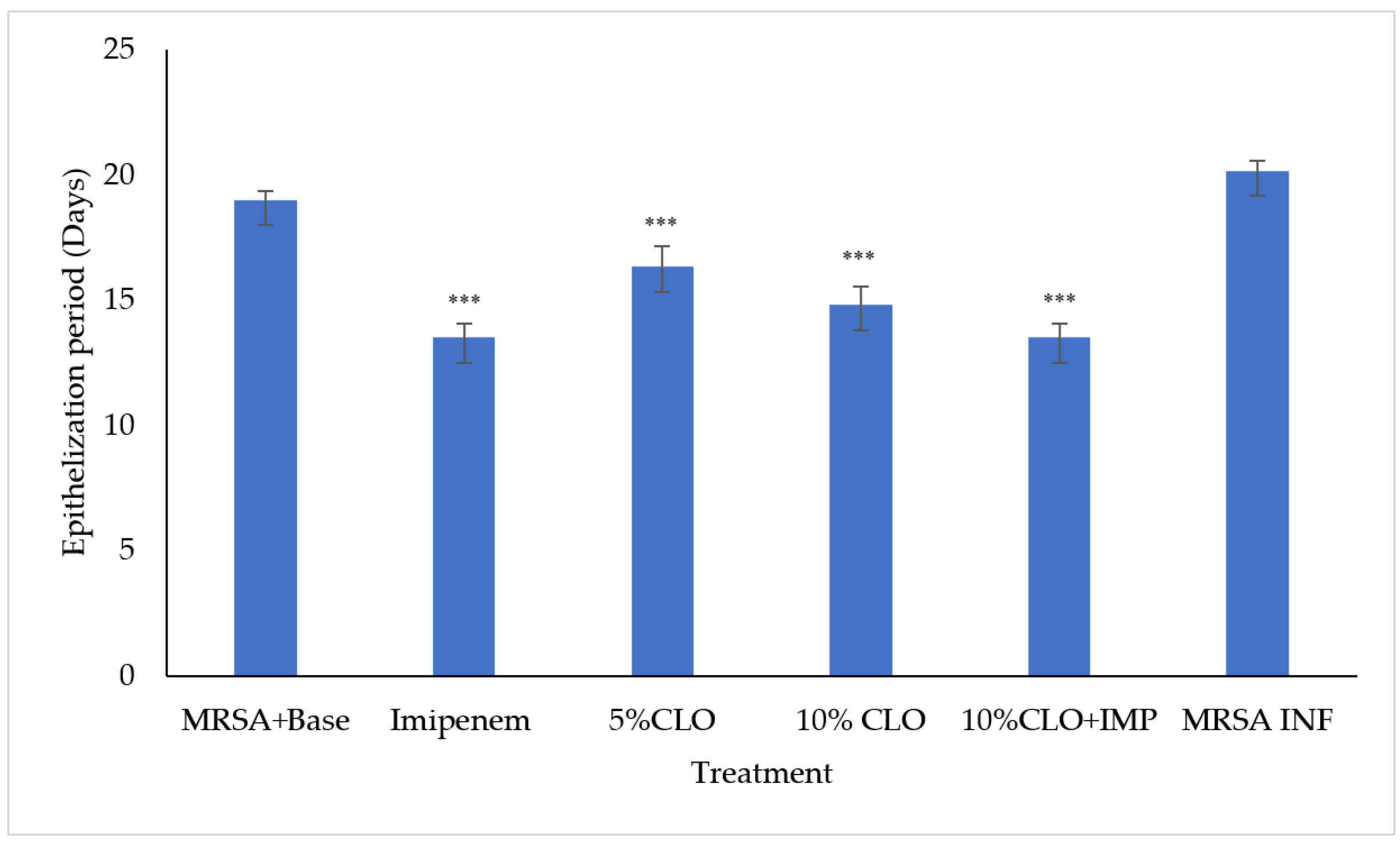
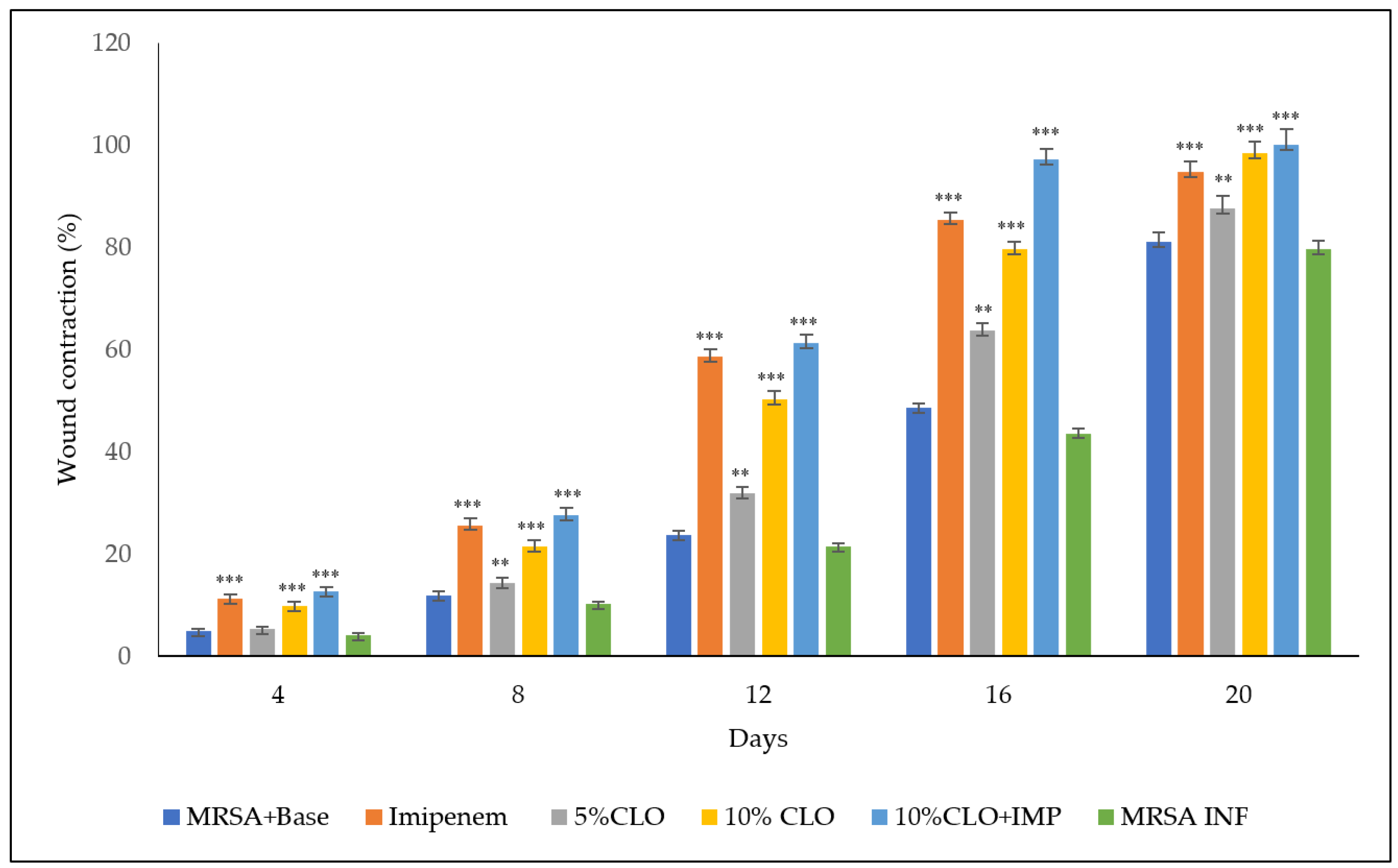
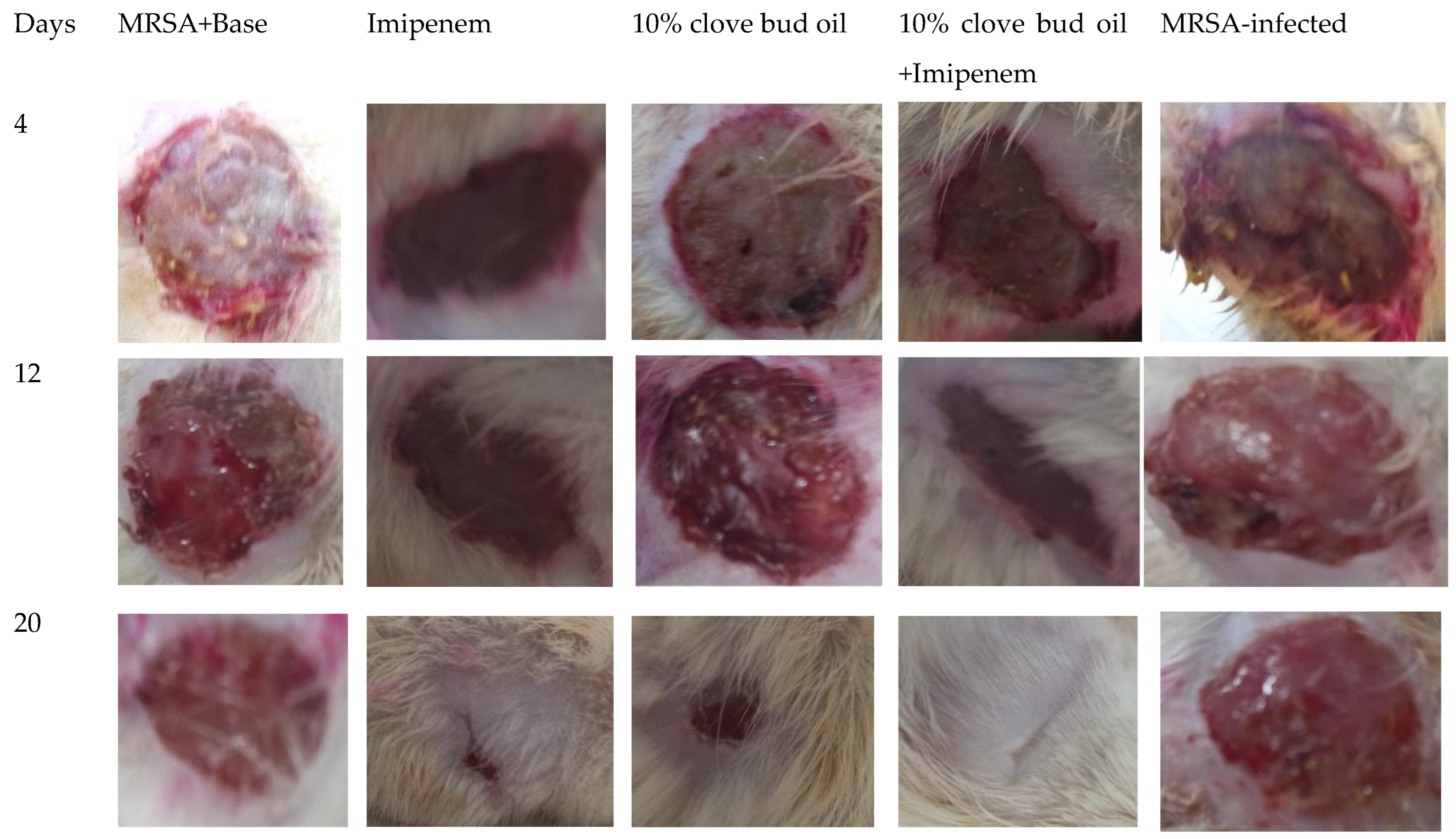
| Peak | Name | Retention Time | Area (%) | RI cal | RI lit | Reference |
|---|---|---|---|---|---|---|
| 1. | Cyclohexanone, 5-methyl-2-(1-methylethyl)-, trans- | 7.431 | 0.13 | 1114 | 1124 | [13] |
| 2. | * Levomenthol | 7.709 | 0.28 | 1123 | 1143.95 | [14] |
| 3. | * Phenol, 4-(2-propenyl)- | 8.664 | 0.05 | 1151 | -- | |
| 4. | * Eugenol | 10.097 | 72.54 | 1388 | 1378 | [15] |
| 5. | Caryophyllene | 10.731 | 5.16 | 1405 | 1392.14 | [14] |
| 6. | Humulene (α- Caryophyllene) | 11.097 | 1.55 | 1415 | 1428 | [16] |
| 7. | Bicyclosesquiphellandrene | 11.219 | 0.06 | 1519 | 1521 | [17] |
| 8. | α-Farnesene | 11.475 | 0.13 | 1525 | 1522 | [18] |
| 9. | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- | 11.686 | 0.25 | 1531 | 1528 | [19] |
| 10. | Caryophyllene oxide | 12.408 | 0.22 | 1538 | 1537 | [20] |
| 11. | * 1-Decanol, 2-hexyl | 15.163 | 0.09 | 1492 | 1504 | [21] |
| 12. | Heptadecane | 15.741 | 0.15 | 1719 | -- | |
| 13. | Hexatriacontyl pentafluoropropionate | 17.308 | 0.50 | 1962 | -- | |
| 14. | Heptadecane, 2,6,10,15-tetramethyl- | 17.563 | 0.59 | 2168 | -- | |
| 15. | 1-Docosene | 17.774 | 0.051 | 2179 | 2190 | [22] |
| 16. | * 2-(octadecyloxy)ethanol | 18.285 | 1.00 | 1284 | -- |
| Antibacterial Agent | Zone of Inhibition (mm) |
|---|---|
| Clove oil (20 µL/mL) | 10 |
| Clove oil (40 µL/mL) | 13 |
| Imipenem (4 µL/mL) | 22 |
| Imipenem (4 µL/mL) + Clove oil (40 µL/mL) | 22 |
| Clove Oil (µL/mL) | Visible Growth (MIC) | Growth on Mannitol Salt Agar (MBC) |
|---|---|---|
| 20 | – | – |
| 10 | – | – |
| 5 | – | – |
| 2.5 | – | + |
| 1.25 | ++ | + |
| 0.62 | ++ | + |
| 0.31 | ++ | ++ |
| Treatment | Bacterial Load (Log10 CFU/g of Tissue) |
|---|---|
| MRSA+base | 10.34 ± 0.49 |
| MRSA-infected + Imipenem | 4.11 ± 0.48 *** |
| MRSA-infected + 5% CLO | 5.89 ± 0.47 *** |
| MRSA-infected + 10% CLO | 3.95 ± 0.33 *** |
| MRSA-infected + imipenem + 10% clove oil | 3.1 ± 0.32 *** |
| MRSA-infected wounds | 11.07 ± 0.41 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.K.; Alqasmi, M.H.; Alrouji, M.; Kuriri, F.A.; Almuhanna, Y.; Joseph, B.; Asad, M. Antibacterial Activity of Syzygium aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 8551. https://doi.org/10.3390/molecules27238551
Alanazi AK, Alqasmi MH, Alrouji M, Kuriri FA, Almuhanna Y, Joseph B, Asad M. Antibacterial Activity of Syzygium aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus aureus. Molecules. 2022; 27(23):8551. https://doi.org/10.3390/molecules27238551
Chicago/Turabian StyleAlanazi, Abdulaziz Khaleef, Mohammed Hussein Alqasmi, Mohammed Alrouji, Fahd A. Kuriri, Yasir Almuhanna, Babu Joseph, and Mohammed Asad. 2022. "Antibacterial Activity of Syzygium aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus aureus" Molecules 27, no. 23: 8551. https://doi.org/10.3390/molecules27238551
APA StyleAlanazi, A. K., Alqasmi, M. H., Alrouji, M., Kuriri, F. A., Almuhanna, Y., Joseph, B., & Asad, M. (2022). Antibacterial Activity of Syzygium aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus aureus. Molecules, 27(23), 8551. https://doi.org/10.3390/molecules27238551






