Behavior of PNIPAM Microgels in Different Organic Solvents
Abstract
1. Introduction
2. Results and Discussion
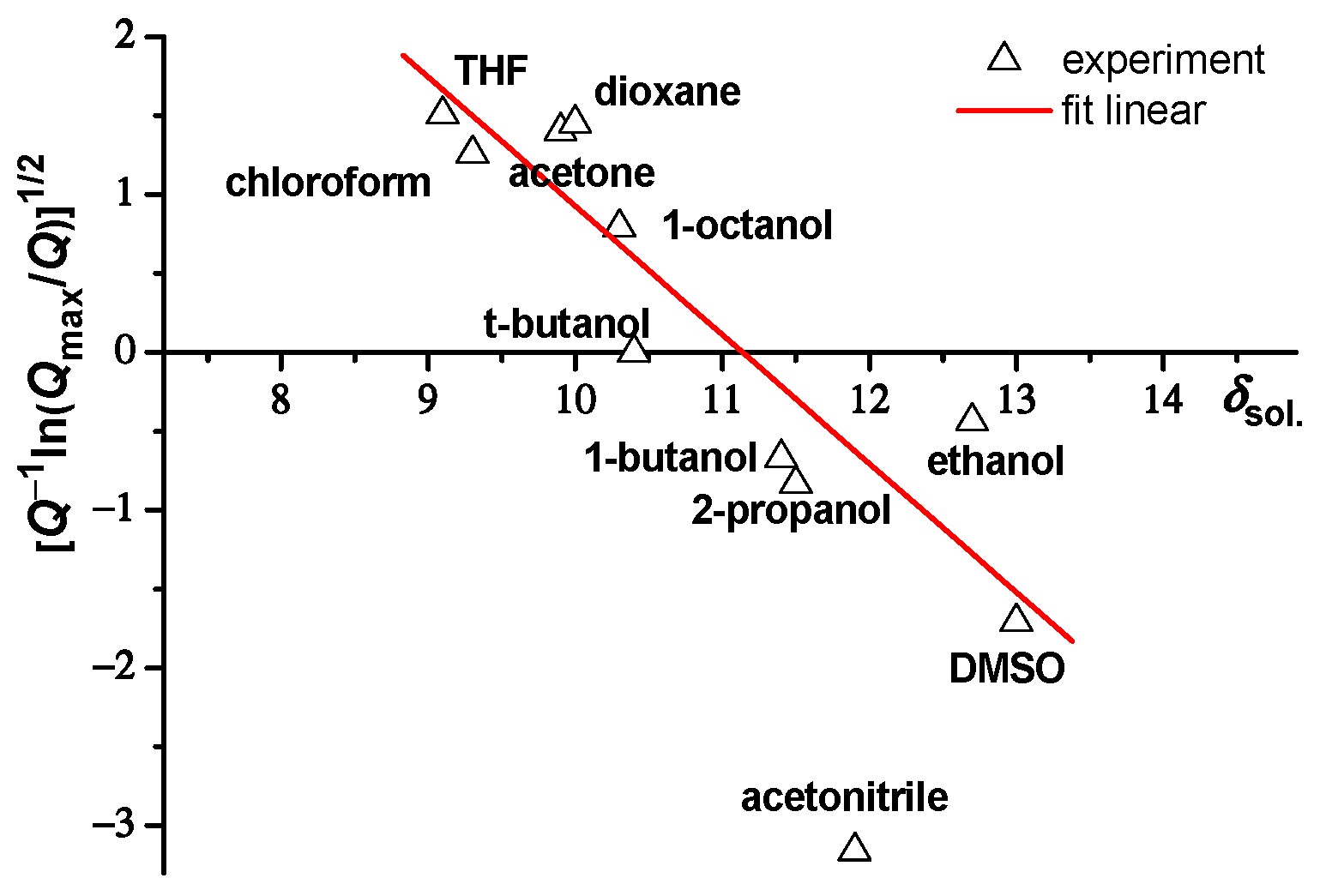
2.1. Swelling of Microgels in Different Solvents
| Name | δsol., cal1/2 cm−3/2 | Rh, nm | Rg, nm | Rg/Rh | Rh/Rh(H2O) |
|---|---|---|---|---|---|
| tetrahydrofuran | 9.1 | 273 ± 6 | 161 ± 16 | 0.59 ± 0.05 | 0.89 |
| chloroform | 9.3 | 293 ± 7 | 148 ± 14 | 0.51 ± 0.05 | 0.95 |
| acetone | 9.9 | 281 ± 7 | 163 ± 16 | 0.58 ± 0.05 | 0.91 |
| dioxane | 10.0 | 277 ± 6 | 193 ± 19 | 0.70 ± 0.06 | 0.90 |
| 1-octanol | 10.3 | 345 ± 9 | 164 ± 16 | 0.48 ± 0.05 | 1.12 |
| t-butanol | 10.4 | 462 ± 11 | 156 ± 15 | 0.34 ± 0.04 | 1.50 |
| 1-butanol | 11.4 | 362 ± 9 | 163 ± 16 | 0.45 ± 0.05 | 1.18 |
| 2-propanol | 11.5 | 337 ± 9 | 153 ± 15 | 0.45 ± 0.05 | 1.09 |
| acetonitrile | 11.9 | 196 ± 5 | 173 ± 17 | 0.88 ± 0.06 | 0.64 |
| ethanol | 12.7 | 401 ± 10 | 159 ± 15 | 0.40 ± 0.04 | 1.30 |
| dimethyl sulfoxide | 13 | 259 ± 5 | 143 ± 14 | 0.55 ± 0.05 | 0.84 |
| water | 23.4 | 308 ± 8 | 156 ± 15 | 0.51 ± 0.05 | 1 |
| mineral oil | 7.1 | precipitates | |||
| hexane | 7.3 | precipitates | |||
| decane | 7.7 | precipitates | |||
| tetradecane | 7.9 | precipitates | |||
| cyclohexane | 8.2 | precipitates | |||
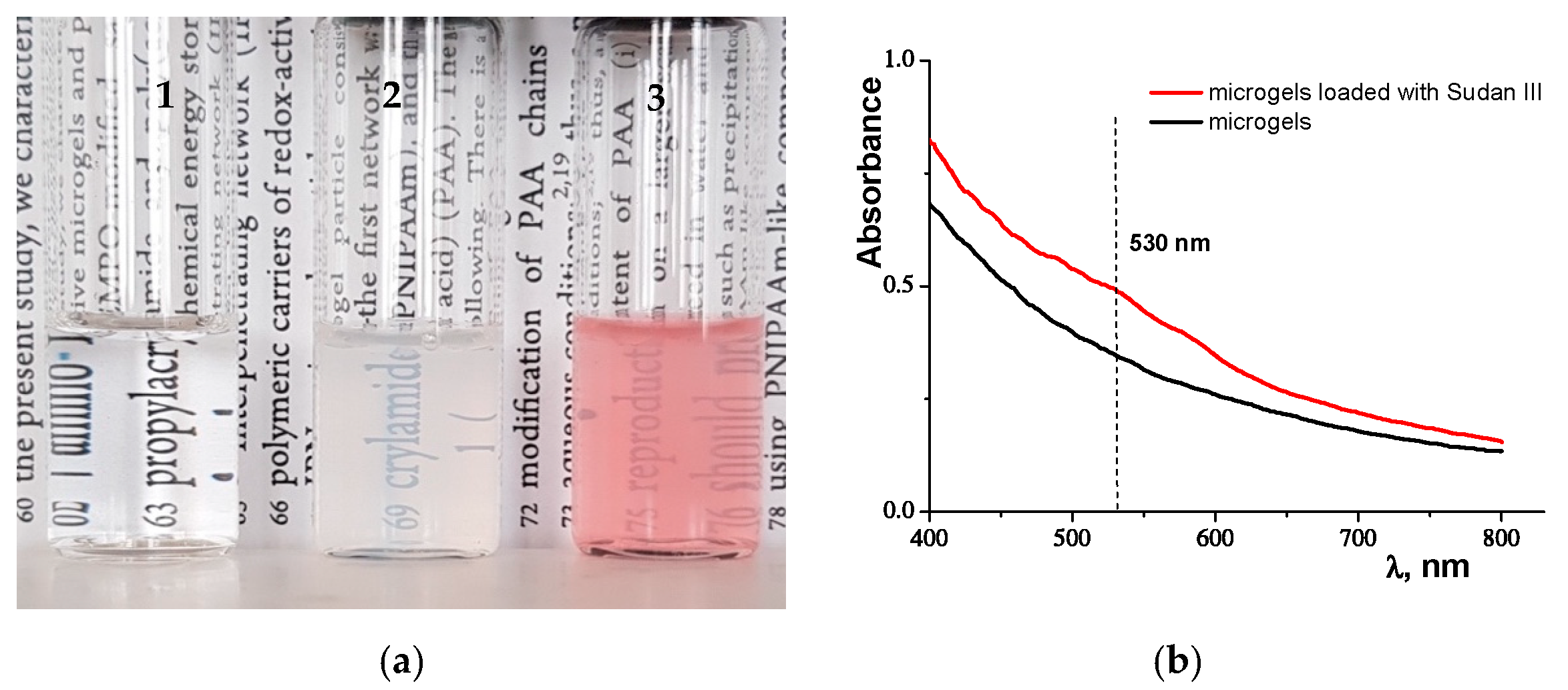
2.2. Investigation of the Water Solution of PNIPAM Microgels Loaded with Sudan III Dye
2.2.1. UV-Vis Spectrophotometry
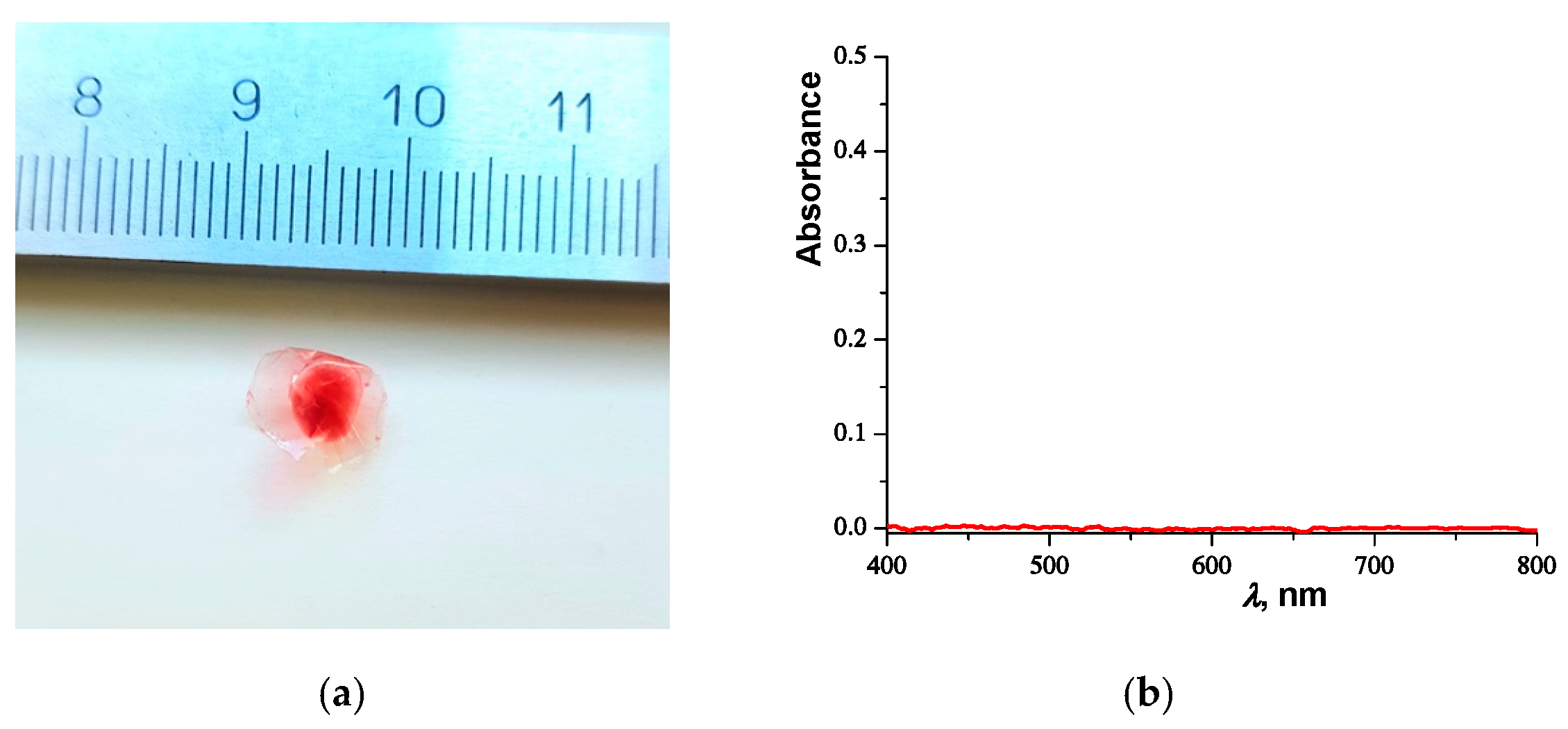
2.2.2. Ultracentrifugation
2.2.3. Determination of Sizes of PNIPAM Microgels Loaded with Sudan III Dye
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Microgels
3.3. Preparation of Solvent Solutions of Microgels
3.4. Loading of Microgels with Sudan III Dye
3.5. Dynamic and Static Light Scattering Methods
3.6. Spectrophotometry
3.7. Ultracentrifugation of Microgel Water Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pelton, R.; Hoare, T. Microgels and their synthesis: An introduction. In Microgel Suspensions; Fernandez-Nieves, A., Wyss, H.M., Mattsson, J., Weitz, D.A., Eds.; WILEY-VCH Verlag & Co. KGaA: Weinheim, Germany, 2011; p. 3. [Google Scholar]
- Plamer, F.A.; Richtering, W. Functional microgels and microgel systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and microgels: From model colloids to applications, recent developments, and future trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.F.; Izak-Nau, E.; Braun, S.; Pich, A.; Richtering, W.; Göstl, R. Microgels react to force: Mechanical properties, syntheses, and force-activated functions. Chem. Soc. Rev. 2022, 51, 2939–2956. [Google Scholar] [CrossRef] [PubMed]
- Anakhov, M.; Gumerov, R.A.; Potemkin, I.I. Stimuli-responsive aqueous microgels: Properties and applications. Mendeleev Commun. 2020, 30, 555–562. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Stimuli-responsive microgels and microgel-based systems: Advances in the exploitation of microgel colloidal properties and their interfacial activity. Polymers 2018, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Oberdisse, J.; Hellweg, T. Recent advances in stimuli-responsive core-shell microgel particles: Synthesis, characterisation, and applications. Colloid Polym. Sci. 2020, 298, 921–935. [Google Scholar] [CrossRef]
- Saha, P.; Santu, M.; Emondts, M.; Roth, H.; Rahimi, K.; Grosskurth, J.; Ganduly, R.; Wessling, M.; Singha, N.; Pich, A. Stimuli-responsive zwitterionic core–shell microgels for antifouling surface coatings. Appl. Mater. Interfaces 2020, 12, 58223–58238. [Google Scholar] [CrossRef]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA hydrogels and microgels for biosensing and biomedical applications. Adv. Mater. 2019, 32, 1806538. [Google Scholar] [CrossRef]
- Shu, T.; Hu, L.; Hunter, H.; Balasuriya, N.; Fang, C.; Zhang, Q.; Serpe, M.J. Multi-responsive micro/nanogels for optical sensing. Adv. Phys. X 2022, 7, 2043185. [Google Scholar] [CrossRef]
- Su, Y.; Gu, L.; Zhang, Z.; Chang, C.; Li, J.; McClements, J.D.; Yang, Y. Encapsulation and release of egg white protein in alginate microgels: Impact of pH and thermal treatment. Int. Food Res. J. 2019, 120, 305–311. [Google Scholar] [CrossRef]
- Bowen, J.J.; Rose, M.A.; Morin, S.A. Surface molding of multi-stimuli-responsive microgels actuators. MRS Bull. 2021, 46, 337–344. [Google Scholar] [CrossRef]
- Lefroy, K.S.; Murray, B.S.; Ries, M.E. Advances in the use of microgels as emulsion stabilisers and as a strategy for cellulose functionalisation. Cellulose 2021, 28, 647–670. [Google Scholar] [CrossRef]
- Fan, T.-F.; Hwang, Y.; Ibrahim, M.S.; Ferracii, G.; Cho, N.-J. Influence of chemical and physical change of pollen microgels on swelling/deswelling behavior. Macromol. Rapid Commun. 2020, 41, 2000155. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Mergel, O.; van der Mei, H.C.; Busscher, H.J.; van Rijn, P. Inhibiting bacterial adhesion by mechanically modulated microgel coatings. Biomacromolecules 2019, 20, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Kurey, T.; Witte, J.; Pipich, V.; Feoktystov, A.; Koutsioubas, A.; Vezhlev, E.; Frielinghaus, H.; von Klitzing, R.; Wellert, S.; Holderer, O. Influence of the cross-linker content on adsorbed functionalised microgel coatings. Polymer 2019, 169, 29–35. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Komarova, G.A.; Vyshivannaya, O.V.; Nasimova, I.R.; Kuvarina, A.E.; Sadykova, V.S. Antiseptic materials on the base of polymer interpenetrating networks microgels and benzalkonium chloride. Int. J. Mol. Sci. 2022, 23, 4394. [Google Scholar] [CrossRef]
- Rey, M.; Fernandez-Rodriguez, M.A.; Karg, M.; Isa, L.; Vogel, N. Poly-N-isopropylacrylamide nanogels and microgels at fluid interfaces. Acc. Chem. Res. 2020, 53, 414–424. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Retama, J.R.; Laurenti, M.; Barbeo, A.F.; Cabarcos, E.L. Structure and polymer dynamics within PNIPAM-based microgel particles. Adv. Colloid Interface Sci. 2014, 205, 113–123. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y. PNIPAM microgels for biomedical applications: From dispersed particles to 3D assemblies. Soft Matter. 2011, 7, 6375–6384. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Yang, Y.; Jimenez-Guerra, M.; del Castillo-Santaella, T.; Ramos, J.; Martin-Molina, A. Complexation of dna with thermoresponsive charged microgels: Role of swelling state and electrostatics. Gels 2022, 8, 184. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Vyshivannaya, O.V.; Nasimova, I.R. “Smart” IPN microgels with different network structures: Self-crosslinked vs conventionally crosslinked. Polymer 2019, 176, 127–134. [Google Scholar] [CrossRef]
- Hoare, T.; Pelton, R. Engineering glucose swelling responses in poly(N-isopropylacrylamide)-based microgels. Macromolecules 2007, 40, 670–678. [Google Scholar] [CrossRef]
- Hu, L.; Serpe, M.J. Color-tunable etalons assembled from poly (N-isopropylacrylamide) based microgels. Polymers 2012, 4, 134–149. [Google Scholar] [CrossRef]
- Ancia, C.; Lapeyre, V.; Gosse, I. Designed glucose-responsive microgels with selective shrinking behavior. Langmuir 2011, 27, 12693–12701. [Google Scholar]
- Wu, W.T.; Zhou, T.; Aiello, M.; Zhou, S.Q. Polymeric assemblies and nanoparticles with stimuli-responsive fluorescence emission characteristics. Chem. Mater. 2009, 21, 4905–4913. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Rudyak, V.Y.; Li, X.; Shibayama, M.; Peters, G.S.; Vyshivannaya, O.V.; Nasimova, I.R.; Chertovich, A.V. Microphase separation of stimuli-responsive interpenetrating network microgels investigated by scattering methods. J. Colloid Interface Sci. 2021, 597, 297–305. [Google Scholar] [CrossRef]
- Rudyak, V.Y.; Kozhunova, E.Y.; Chertovich, A.V. Simulation of interpenetrating networks microgel synthesis. Soft Matter 2020, 16, 4858–4865. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Ruzicka, B. Swelling behavior in multi-responsive microgels. Colloids Surfaces A Physicochem. Eng. Asp. 2017, A532, 389–396. [Google Scholar] [CrossRef]
- Islam, M.R.; Xie, S.; Huang, D.; Smyth, K.; Serpe, M.J. Poly (N-Isopropylacrylamide) microgel-based optical devices for humidity sensing. Anal. Chem. Acta 2015, 898, 101–108. [Google Scholar] [CrossRef]
- Kim, J.; Serpe, M.J.; Lyon, L.A. Hydrogel microparticles as dynamically tunable microlenses. J. Am. Chem. 2004, 126, 9512–9513. [Google Scholar] [CrossRef]
- Gawlitza, K.; Georgieva, R.; Tavraz, N.; Keller, J.; von Klitzing, R. Immobilization of water-soluble HRP within poly-n-isopropylacrylamide microgel particles for use in organic media. Langmuir 2013, 29, 16002–16009. [Google Scholar] [CrossRef] [PubMed]
- Scherzinger, C.; Schwarz, A.; Bardow, A.; Leonhard, K.; Richtering, W. Cononsolvency of poly-N-isopropyl acrylamide (PNIPAM): Microgels versus linear chains and macrogels. Curr. Opin. Colloid Interface Sci. 2014, 19, 84–94. [Google Scholar] [CrossRef]
- Yagi, Y.; Inomata, H.; Saito, S. Solubility parameter of an N-isopropylacrylamide gel. Macromolecules 1992, 25, 2997–2998. [Google Scholar] [CrossRef]
- Martinez, M.V.; Molina, M.; Barbero, C.A. Poly(N-isopropylacrylamide) cross-linked gels as intrinsic amphiphilic materials: Swelling properties used to build novel interphases. J. Phys. Chem. B 2018, 122, 9038–9048. [Google Scholar] [CrossRef] [PubMed]
- Bonham, J.A.; Faers, M.A.; van Duijneveldt, J.S. Non-aqueous microgel particles: Synthesis, properties and applications. Soft Matter 2014, 10, 9384–9398. [Google Scholar] [CrossRef] [PubMed]
- Amantea, B.E.; Piazza, R.D.; Chacon, J.R.V.; Santos, C.C.; Costa, T.P.; Rocha, C.O.; Brandt, J.V.; Godoi, D.R.M.; Jafelicci, M., Jr.; Mardues, R.F.C. Esterification influence in thermosensitive behavior of copolymers PNIPAm-co-PAA and PNVCL-co-PAA in magnetic nanoparticles surface. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 18–26. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Fujimoto, K.; Mizuhara, Y. Hydrogel microspheres III. Temperature-dependent adsorption of proteins on poly-N-isopropylacrylamide hydrogel microspheres. Colloid Polym. Sci. 1992, 270, 53–57. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Li, X.; Islam, M.R.; Wei, M.; Serpe, M.J. Light switchable optical materials from azobenzene crosslinked poly(N-isopropylacrylamide)-based microgels. J. Mater. Chem. C 2014, 2, 6961–6965. [Google Scholar] [CrossRef]
- Shen, L.; Pich, A.; Fava, D.; Wang, M.; Kumar, S.; Wu, C.; Scholes, G.D.; Winnik, M.A. Loading quantum dots into thermo-responsive microgels by reversible transfer from organic solvents to water. J. Mater. Chem. 2008, 18, 763–770. [Google Scholar] [CrossRef]
- Montoto, E.C.; Nagarjuna, G.; Hui, J.; Burdess, M.; Sekerak, N.M.; Hernandez-Burgos, K.; Wei, T.-S.; Kneer, M.; Grolman, J.; Cheng, K.J.; et al. Redox active colloids as discrete energy storage carriers. J. Am. Chem. Soc. 2016, 138, 13230–13237. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Gvozdik, N.A.; Motyakin, M.V.; Vyshivannanya, O.V.; Stevenson, K.J.; Itkis, D.M.; Chertovich, A.V. Redox-active aqueous microgels for energy storage applications. J. Phys. Chem. Lett. 2020, 11, 10561–10565. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Bae, J.; Zhao, F.; Yu, G. Functional hydrogels for next-generation batteries and supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar]
- Zhang, J.; Zhang, M.; Tang, K.; Verpoort, F.; Sun, T. Polymer-based stimuli-responsive recyclable catalytic systems for organic synthesis. Small 2014, 10, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Horobin, R.W. Dis-, tris- and polyazo dyes. In Conn’s Biological Stains: A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine; Horobin, R.W., Kiernan, J.A., Eds.; Taylor & Trancis: London, UK; New York, NY, USA, 2002; p. 126. [Google Scholar]
- Herbst, W.; Hunger, K. Industrial Dyes: Chemistry, Properties, Applications, 3rd ed.; Hunger, K., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; p. 633. [Google Scholar]
- Barselo, D.; Kostianoy, A.G. Biodegradation of azo dyes. In The Handbook of Environmental Chemistry; Atacag Erkurt, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 9, pp. 133–150. [Google Scholar]
- Huggins, C.M.; Pimentel, G.C.; Shoolery, J.N. Proton magnetic resonance studies of chloroform in solution: Evidence for hydrogen bonding. J. Chem. Phys. 1955, 23, 1244–1247. [Google Scholar] [CrossRef]
- Destribats, M.; Lapeyre, V.; Sellier, E.; Leal-Calderon, F.; Schmitt, V.; Ravaine, V. Water-in-oil emulsions stabilized by water-dispersible poly(N-isopropylacrylamide) microgels: Understanding anti-Finkle behavior. Langmuir 2011, 27, 14096–14107. [Google Scholar] [CrossRef]
- Gumerov, R.A.; Rudyak, V.Y.; Gavrilov, A.A.; Chertovich, A.V.; Potemkin, I.I. Effect of network topology and crosslinker reactivity on microgel structure and ordering at liquid-liquid interface. Soft Matter 2022, 18, 3738–3747. [Google Scholar] [CrossRef]
- Barton, A.F.M. Solubility parameters. Chem. Rev. 1975, 75, 731–753. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; WILEY-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2011; p. 78. [Google Scholar]
- Vaughan, C.D. Using solubility parameters in cosmetics formulation. J. Soc. Cosmet. Chem. 1985, 36, 319–333. [Google Scholar]
- Ahmad, H. Solubility parameter of acrylamide series polymers through its components and group contribution technique. J. Macromol. Sci.-Chem. 1982, A17, 585–600. [Google Scholar] [CrossRef]
- Gee, G. The interaction between rubber and liquids. IV. Factors governing the absorption of oil by rubber. Rubber Chem. Technol. 1943, 16, 818–833. [Google Scholar] [CrossRef]
- Zakerhamidi, M.S.; Seyed Ahmadian, S.M.; Kian, R. The specific and nonspecific solvatochromic behavior of Sudan dyes in different solvents. Can. J. Chem. 2015, 93, 639–647. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Schärtl, W. Selected examples of light scattering experiments. In Light Scattering from Polymer Solutions Nanoparticle Dispersions; Springer: Berlin/Heidelberg, Germany, 2007; pp. 51–175. [Google Scholar]
| Microgels | Rh, nm | Rg, nm | Rg/Rh |
|---|---|---|---|
| “Standard” at 20 °C | 308 ± 9 | 156 ± 15 | 0.51 ± 0.03 |
| Loaded with Sudan III at 20 °C | 351 ± 10 | 141 ± 14 | 0.40 ± 0.02 |
| “Standard” at 45 °C | 114 ± 5 | 90 ± 9 | 0.79 ± 0.06 |
| Loaded with Sudan III at 45 °C | 119 ± 5 | 90 ± 9 | 0.76 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarova, G.A.; Kozhunova, E.Y.; Potemkin, I.I. Behavior of PNIPAM Microgels in Different Organic Solvents. Molecules 2022, 27, 8549. https://doi.org/10.3390/molecules27238549
Komarova GA, Kozhunova EY, Potemkin II. Behavior of PNIPAM Microgels in Different Organic Solvents. Molecules. 2022; 27(23):8549. https://doi.org/10.3390/molecules27238549
Chicago/Turabian StyleKomarova, Galina A., Elena Yu. Kozhunova, and Igor I. Potemkin. 2022. "Behavior of PNIPAM Microgels in Different Organic Solvents" Molecules 27, no. 23: 8549. https://doi.org/10.3390/molecules27238549
APA StyleKomarova, G. A., Kozhunova, E. Y., & Potemkin, I. I. (2022). Behavior of PNIPAM Microgels in Different Organic Solvents. Molecules, 27(23), 8549. https://doi.org/10.3390/molecules27238549







