How to Identify Roast Defects in Coffee Beans Based on the Volatile Compound Profile
Abstract
1. Introduction
2. Results
2.1. Analysis of Volatile Compounds in Green Coffee
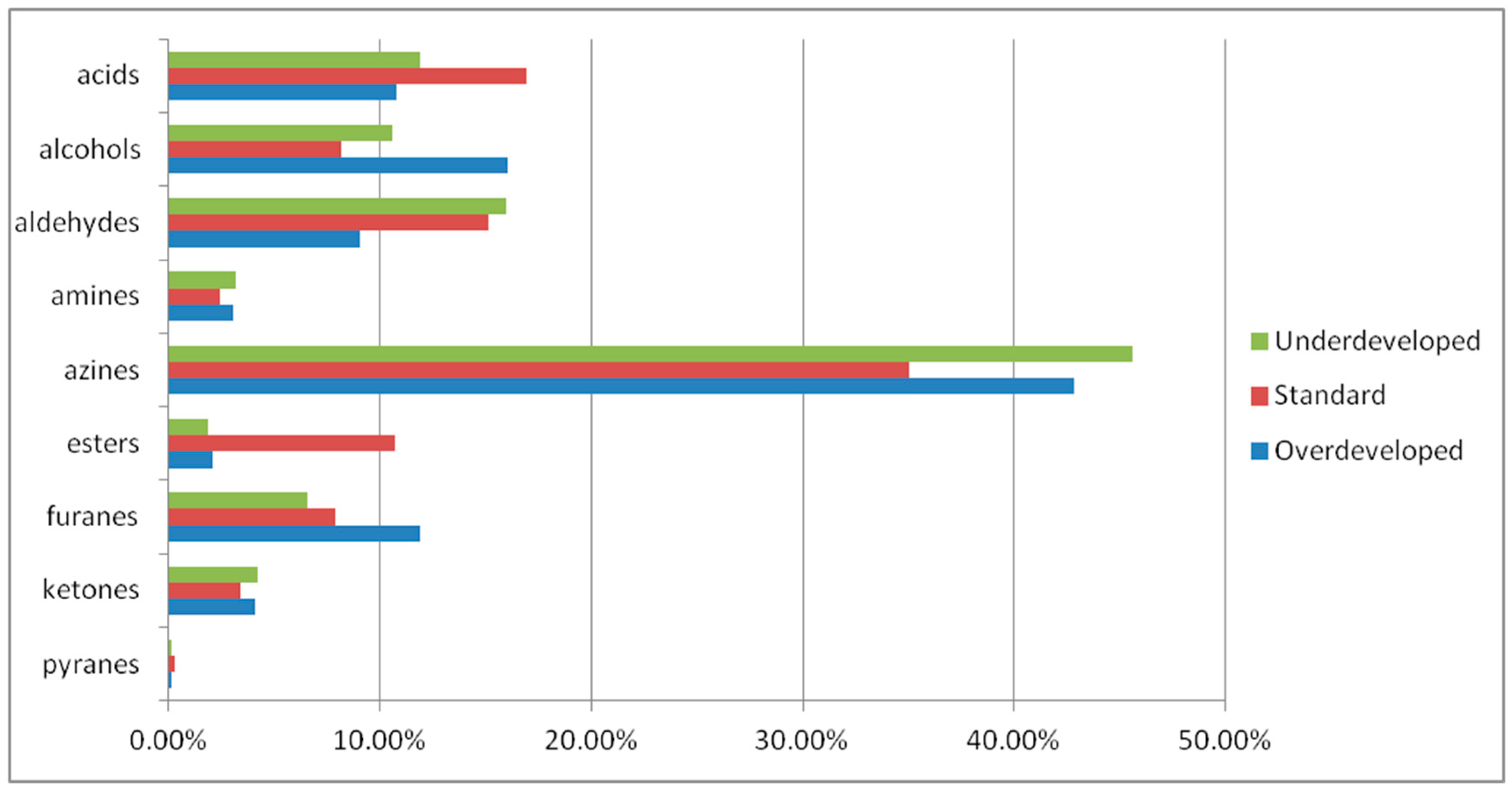
2.2. Analysis of Volatile Compounds in Roasted Coffee
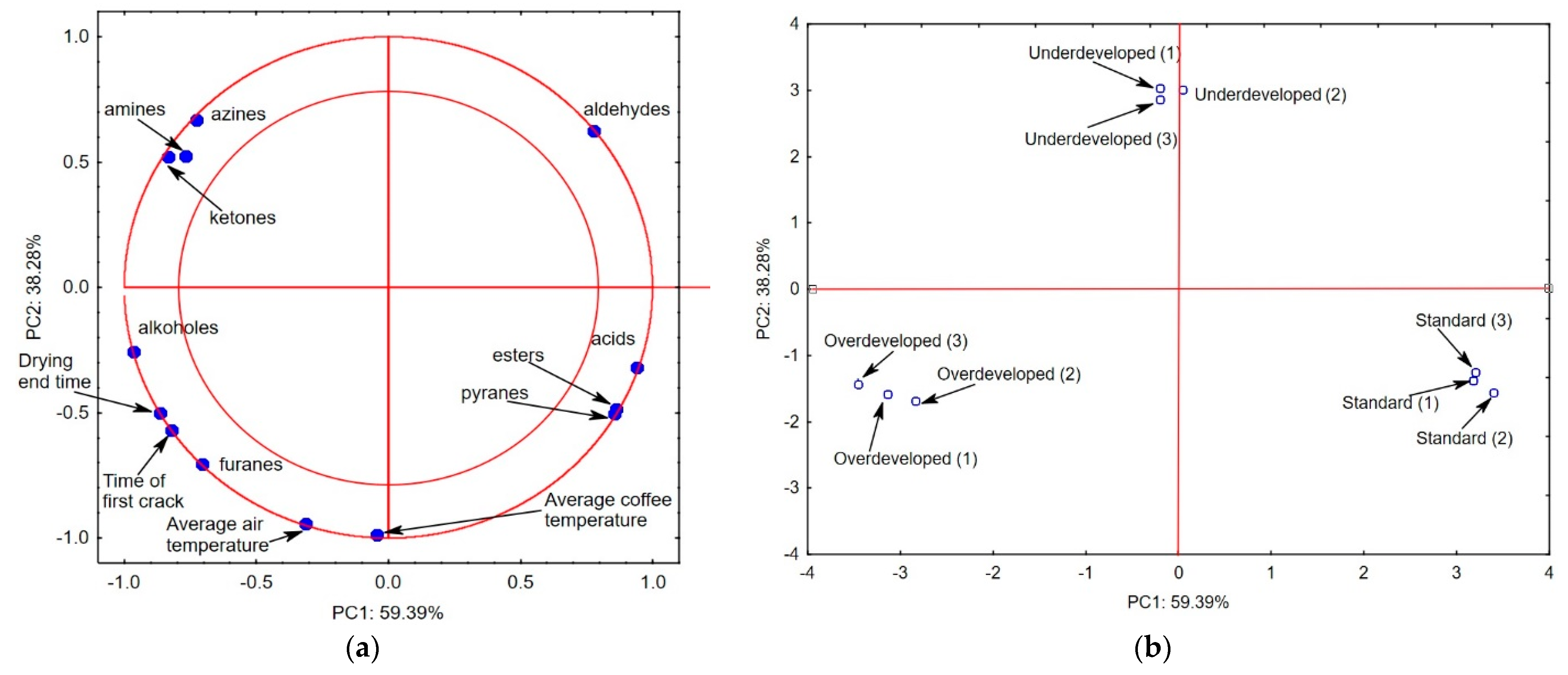
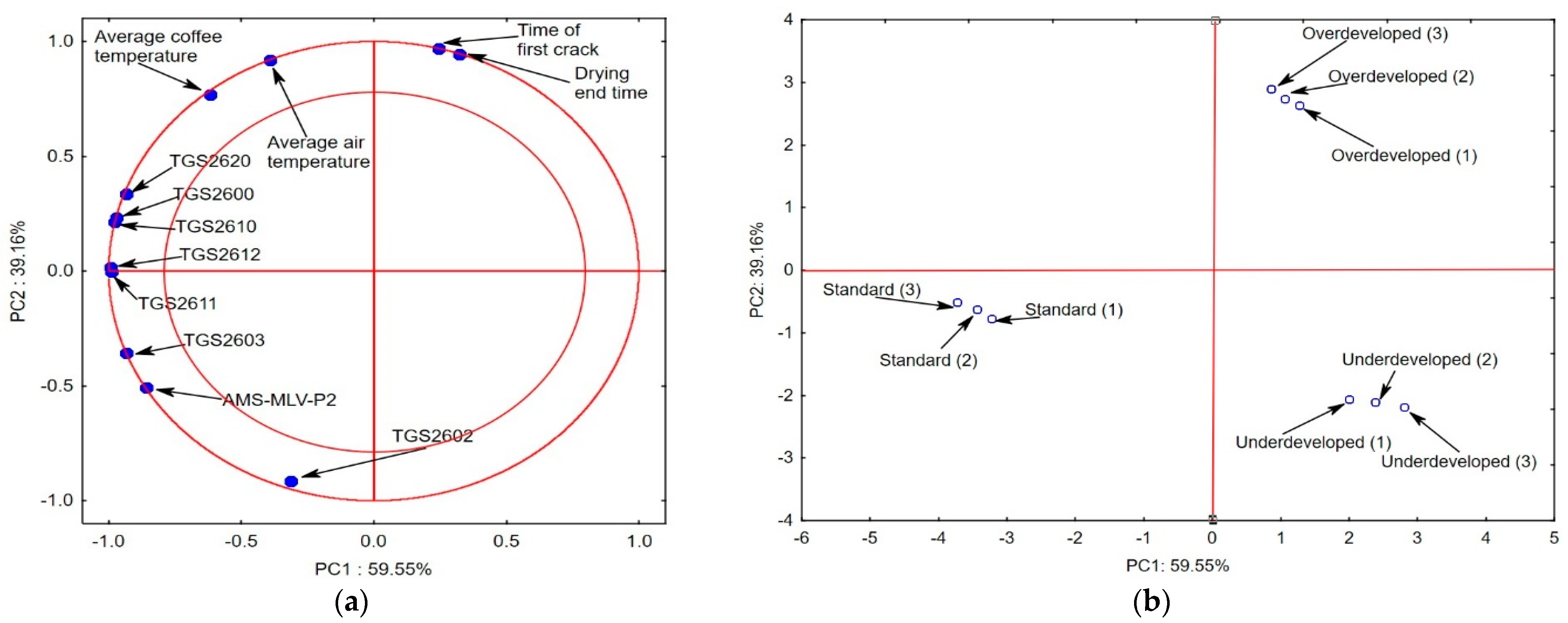
2.3. Statistical Analysis
3. Materials and Methods
3.1. Materials
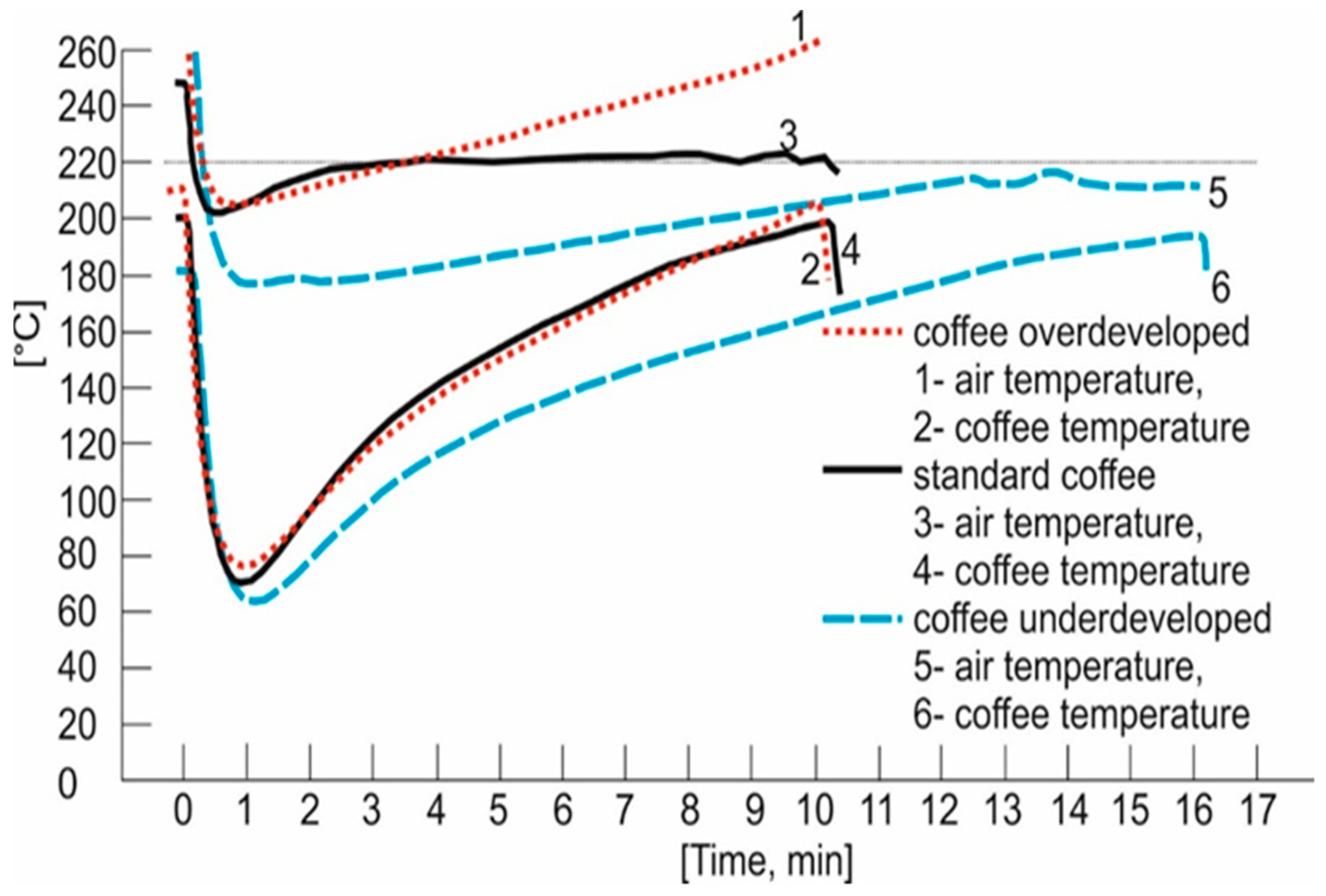
3.2. Roasting Procedure
3.3. Electronic Nose
3.4. GC–MSAnalysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization. Growing divergence between Arabica and Robusta exports. Vending Marketwatch, 9 May 2018. [Google Scholar]
- Jung, S.; Gu, S.; Lee, S.H.; Jeong, Y. Effect of roasting degree on the antioxidant properties of espresso and drip coffee extracted from coffea arabica cv. Java. Appl. Sci. 2021, 11, 7025. [Google Scholar] [CrossRef]
- Yu, J.M.; Chu, M.; Park, H.; Park, J.; Lee, K.G. Analysis of volatile compounds in coffee prepared by various brewing and roasting methods. Foods 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Asy’Ari Hasbullah, U.H.; Rini Umiyati, D. Antioxidant Activity and Total Phenolic Compounds of Arabica and Robusta Coffee at Different Roasting Levels. J. Phys. Conf. Ser. 2021, 1764. [Google Scholar] [CrossRef]
- Marek, G.; Dobrzański, B.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef] [PubMed]
- Seninde, D.R.; Chambers, E., IV. Coffee Flavor: A Review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Yener, S.; Romano, A.; Cappellin, L.; Granitto, P.M.; Aprea, E.; Navarini, L.; Märk, T.D.; Gasperi, F.; Biasioli, F. Tracing coffee origin by direct injection headspace analysis with PTR/SRI-MS. Food Res. Int. 2015, 69, 235–243. [Google Scholar] [CrossRef]
- Bolka, M.; Emire, S. Effects of coffee roasting technologies on cup quality and bioactive compounds of specialty coffee beans. Food Sci. Nutr. 2020, 8, 6120–6130. [Google Scholar] [CrossRef]
- Tsai, C.F.; Jioe, I.P.J. The analysis of chlorogenic acid and caffeine content and its correlation with coffee bean color under different roasting degree and sources of coffee (Coffea arabica typica). Processes 2021, 9, 2040. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Ricci, I.; Fiore, A.G.; Caporizzi, R. How Much Caffeine in Coffee Cup? Effects of Processing Operations, Extraction Methods and Variables. Quest. Caffeine 2017. [Google Scholar] [CrossRef]
- Chu, Y.-F. Coffee: Emerging Health Effects and Disease Prevention; John Wiley & Sons: New York, NY, USA, 2012; ISBN 9780470958780. [Google Scholar]
- Fowble, K.L.; Okuda, K.; Cody, R.B.; Musah, R.A. Spatial distributions of furan and 5-hydroxymethylfurfural in unroasted and roasted Coffea arabica beans. Food Res. Int. 2019, 119, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Getaneh, E.; Fanta, S.W.; Satheesh, N. Effect of Broken Coffee Beans Particle Size, Roasting Temperature, and Roasting Time on Quality of Coffee Beverage. J. Food Qual. 2020, 2020, 8871577. [Google Scholar] [CrossRef]
- Stasiak, M.; Rusinek, R.; Molenda, M.; Fornal, J.; Błaszczak, W. Effect of potato starch modification on mechanical parameters and granules morphology. J. Food Eng. 2011, 102, 154–162. [Google Scholar] [CrossRef]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef]
- da Silva, C.Q.; Fernandes, A.D.S.; Teixeira, G.F.; França, R.J.; Marques, M.R.D.C.; Felzenszwalb, I.; Falcão, D.Q.; Ferraz, E.R.A. Risk assessment of coffees of different qualities and degrees of roasting. Food Res. Int. 2021, 141. [Google Scholar] [CrossRef]
- Houessou, J.K.; Maloug, S.; Leveque, A.-S.; Delteil, C.; Heyd, B.; Camel, V. Effect of roasting conditions on the polycyclic aromatic hydrocarbon content in ground Arabica coffee and coffee brew. J. Agric. Food Chem. 2007, 55, 9719–9726. [Google Scholar] [CrossRef]
- Gancarz, M.; Dobrzański, B.; Malaga-Toboła, U.; Tabor, S.; Combrzyński, M.; Ćwikła, D.; Strobel, W.R.; Oniszczuk, A.; Karami, H.; Darvishi, Y.; et al. Impact of Coffee Bean Roasting on the Content of Pyridines Determined by Analysis of Volatile Organic Compounds. Molecules 2022, 27, 1559. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Hu, G.; Hong, D.; Guo, T.; Li, J.; Li, Z.; Qiu, M. Review on factors affecting coffee volatiles: From seed to cup. J. Sci. Food Agric. 2022, 102, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joët, T. Climatic factors directly impact the volatile organic compound fingerprint in green Arabica coffee bean as well as coffee beverage quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-G.; Shibamoto, T. Analysis of volatile components isolated from Hawaiian green coffee beans (Coffea arabica L.). Flavour Fragr. J. 2002, 17, 349–351. [Google Scholar] [CrossRef]
- Rodríguez Méndez, M.L. Electronic Noses and Tongues in Food Science; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128004029. [Google Scholar]
- Wolska, J.; Janda, K.; Jakubczyk, K.; Szymkowiak, M.; Chlubek, D.; Gutowska, I. Levels of Antioxidant Activity and Fluoride Content in Coffee Infusions of Arabica, Robusta and Green Coffee Beans in According to their Brewing Methods. Biol. Trace Elem. Res. 2017, 179, 327–333. [Google Scholar] [CrossRef]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Fluoride contents in tea and soil from tea plantations and the release of fluoride into tea liquor during infusion. Environ. Pollut. 1999, 104, 197–205. [Google Scholar] [CrossRef]
- Revel’skii, I.A.; Chivarzin, M.E.; Gerasimov, M.A.; Frolova, A.V.; Dolgonosov, A.M.; Skalnyi, A.V.; Revel’skii, A.I.; Buryak, A.K. A New Approach to the Assessment of the Safety of Tea, Coffee, Cocoa, and Vegetable Oils, Based on the Rapid Screening of Samples for the Total Concentration of Fluorine-, Chlorine-, and Bromine-Containing Organic Compounds. J. Anal. Chem. 2021, 76, 613–620. [Google Scholar] [CrossRef]
- Procida, G.; Lagazio, C.; Cateni, F.; Zacchigna, M.; Cichelli, A. Characterization of Arabica and Robusta volatile coffees composition by reverse carrier gas headspace gas chromatography–mass spectrometry based on a statistical approach. Food Sci. Biotechnol. 2020, 29, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Kao, T.-H.; Chen, B.-H. Development of a GC-MS/MS Method Coupled with HS-SPME-Arrow for Studying Formation of Furan and 10 Derivatives in Model Systems and Commercial Foods. Food Chem. 2022, 395, 133572. [Google Scholar] [CrossRef]
- Giordano, L.; Calabrese, R.; Davoli, E.; Rotilio, D. Quantitative analysis of 2-furfural and 5-methylfurfural in different Italian vinegars by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry using isotope dilution. J. Chromatogr. A 2003, 1017, 141–149. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Idrees, A.; Majeed, M.Z.; Majeed, M.I.; Shehzad, M.Z.; Ullah, M.I.; Afzal, A.; Li, J. Synergized Toxicity of Promising Plant Extracts and Synthetic Chemicals against Fall Armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Pakistan. Agronomy 2022, 12, 1289. [Google Scholar] [CrossRef]
- Gopu, C.; Chirumamilla, P.; Daravath, S.B.; Vankudoth, S.; Taduri, S. GC-MS analysis of bioactive compounds in the plant parts of methanolic extracts of Momordica cymbalaria Fenzl. J. Med. Plants Stud. 2021, 9, 209–218. [Google Scholar] [CrossRef]
- Farooq, A. Evaluation of Antioxidant, Antimicrobial activity and GC-MS analysis of Phlomis stewartii. Pure Appl. Biol. 2019, 8, 2420–2433. [Google Scholar] [CrossRef]
- Petisca, C.; Pérez-Palacios, T.; Farah, A.; Pinho, O.; Ferreira, I.M.P.L.V.O. Furans and other volatile compounds in ground roasted and espresso coffee using headspace solid-phase microextraction: Effect of roasting speed. Food Bioprod. Process. 2013, 91, 233–241. [Google Scholar] [CrossRef]
- Bressanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee aroma: Chemometric comparison of the chemical information provided by three different samplings combined with GC–MS to describe the sensory properties in cup. Food Chem. 2017, 214, 218–226. [Google Scholar] [CrossRef] [PubMed]
- World Coffee Research. Arabica Coffee Varieties. 2018. Available online: https://varieties.worldcoffeeresearch.org/info/coffee/about-varieties/main-types (accessed on 14 December 2021).
- Anh-Dao, L.-T.; Nhon-Duc, L.; Cong-Hau, N.; Thanh-Nho, N. Variability of total polyphenol contents in ground coffee products and their antioxidant capacities through different reaction mechanisms. Biointerface Res. Appl. Chem. 2022, 12, 4857–4870. [Google Scholar] [CrossRef]
- Rusinek, R.; Jelen, H.; Malaga-tobola, U.; Molenda, M. Influence of Changes in the Level of Volatile Compounds Emitted During Rapeseed Quality Degradation on the Reaction of MOS Type. Sensors 2020, 20, 3135. [Google Scholar] [CrossRef]
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiącek, D.; Nawrocka, A.; Tadla, M.; Rusinek, R. Application of electronic nose with MOS sensors to prediction of rapeseed quality. Meas. J. Int. Meas. Confed. 2017, 103, 227–234. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H.; Fuentes, S.; Kaveh, M.; Rusinek, R.; Gancarz, M. Preliminary study non-destructive sorting techniques for pepper (Capsicum annuum L.) using odor parameter. LWT 2022, 164, 113667. [Google Scholar] [CrossRef]
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiącek, D.; Nawrocka, A.; Rusinek, R. Electronic nose with polymer-composite sensors for monitoring fungal deterioration of stored rapeseed. Int. Agrophys. 2017, 31, 317–325. [Google Scholar] [CrossRef]
- Rusinek, R.; Gawrysiak-Witulska, M.; Siger, A.; Oniszczuk, A.; Ptaszyńska, A.A.; Knaga, J.; Malaga-Toboła, U.; Gancarz, M. Effect of supplementation of flour with fruit fiber on the volatile compound profile in bread. Sensors 2021, 21, 2812. [Google Scholar] [CrossRef]
| No. | Rtime | Name of Compound | Chemical Formula | Chemical Class | Percentage |
|---|---|---|---|---|---|
| 1 | 1.87 | 4,5-difluoroctane isomer | C8H10F2 | hydrocarbons | 11.79 ± 0.39 |
| 2 | 3.09 | 8,11,14-eicosatrienoic acid, methyl ester | C21H36O2 | esters | 5.71 ± 0.30 |
| 3 | 4.05 | 2-furanmethanol | C5H6O2 | alcohols | 6.07 ± 0.14 |
| 4 | 5.82 | 2-methyl-3-(2-methylpropyl) pyrazine | C9H14N2 | azines | 4.29 ± 0.27 |
| 5 | 6.47 | 1,3,7-octatriene, 3,7-dimethyl-, E- | C10H16 | terpenes | 5.00 ± 0.13 |
| 6 | 7.44 | 5-amino-1-benzoyl-1H-pyrazole-3,4-dicarbonitrile | C12H7N5O | amines | 4.64 ± 0.19 |
| 7 | 8.35 | Propane, 2-methyl-1-nitro- | C4H9NO2 | esters | 33.57 ± 0.83 |
| 8 | 8.47 | 2-furanmethanol, acetate | C7H8O3 | alcohols | 6.43 ± 0.30 |
| 9 | 9.61 | Oxiranecarboxamide, 2-ethyl-3-propyl- | C8H15NO2 | others | 1.79 ± 0.09 |
| 10 | 9.70 | 6,6-dimethyl-2-methylene-bicyclo [3.1.1] heptane | C10H16 | terpenes | 5.36 ± 0.15 |
| 11 | 9.86 | 2-cyclopropyl-2-methylspiro [2.2]pentane-1-carboxylic acid | C10H14O2 | acids | 3.93 ± 0.10 |
| 12 | 15.22 | 4-hydroxy-4-methyl-hex-5-enoic acid tert-butyl ester | C11H20O3 | esters | 2.5 ± 0.10 |
| 13 | 15.42 | 9,12,15-octadecatrienoic acid, methyl ester | C19H32O2 | esters | 1.07 ± 0.06 |
| 14 | 18.15 | 16-methylene-1,20-dioxopregn-4-en-17-yl acetate | C24H32O4 | esters | 1.07 ± 0.07 |
| 15 | 21.06 | 1,4-methanoazulene, decahydro-4,8,8-trimethyl-9-methylene-, [1S-(1α,3Aβ,4α,8Aβ)]- | C15H24 | terpenes | 1.07 ± 0.02 |
| 16 | 23.37 | Ethanone, 1-(5,6,7,8-tetrahydro-2,8,8-trimethyl-4H-cyclohepta[B]furan-5-yl)- | C14H20O2 | ketones | 1.43 ± 0.03 |
| 17 | 31.28 | Alanine, 3-(benzyloxy)-, L- | C10H13NO3 | acids | 1.07 ± 0.01 |
| 18 | 32.19 | Hexadecadienoic acid, methyl ester | C17H30O2 | esters | 3.21 ± 0.02 |
| No. | Rtime | Name of Compound | Chemical Formula | Chemical Class | Underdeveloped | Standard | Overdeveloped |
|---|---|---|---|---|---|---|---|
| 1 | 1.29 | 2-acetonyl-3-cyano-2,3-dimethylcyclobutane-1-carboxylic acid | C11H15NO3 | acids | n.d. | 9.91 ± 0.24 | n.d. |
| 2 | 1.61 | Furan, 2-methyl- | C5H6O | furanes | 5.67 ± 0.27 | 4.19 ± 0.22 | 6.7 ± 0.40 |
| 3 | 1.85 | Butanal, 2-methyl- | C5H10O | aldehydes | 2.77 ± 0.05 | 2.66 ± 0.11 | 2.37 ± 0.22 |
| 4 | 2.01 | (2E)-2-(hydroxyimino)etyl acetate | C4H7NO3 | esters | 1.45 ± 0.10 | n.d. | n.d. |
| 5 | 2.42 | Pyridine | C5H5N | azines | 9.89 ± 0.35 | 6.33 ± 0.14 | 11.03 ± 0.69 |
| 6 | 3.14 | Pregnane-3,11,20,21-tetrol, cyclic 20,21-(butyl boronate), (3α,5β,11β,20R)- | C25H43BO4 | others | 0.79 ± 0.03 | n.d. | n.d. |
| 7 | 3.17 | 2-butanone | C4H8O | ketones | 3.69 ± 0.30 | 2.76 ± 0.08 | 2.51 ± 0.13 |
| 8 | 3.20 | 2-thiopheneethanol, 5-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)- | C11H15NO2S | alcohols | n.d. | n.d. | 2.37 ± 0.13 |
| 9 | 3.48 | Pyrimidine, 2-methyl- | C5H6N2 | azines | 8.58 ± 0.30 | 8.17 ± 0.28 | 8.94 ± 0.34 |
| 10 | 3.60 | 2-furancarboxaldehyde | C5H4O2 | aldehydes | 5.8 ± 0.40 | 6.23 ± 0.25 | n.d. |
| 11 | 4.01 | 2-furanmethanol | C5H6O2 | alcohols | 9.76 ± 0.05 | 8.17 ± 0.22 | 13.69 ± 0.51 |
| 12 | 4.31 | 1,5-dimethyl-2,3-dihydro-1H-pyrrole | C6H11N | furanes | n.d. | 3.06 ± 0.10 | 5.17 ± 0.29 |
| 13 | 5.50 | Ethyl 2,3-pentadienoate | C7H10O2 | esters | 1.58 ± 0.10 | n.d. | 1.82 ± 0.13 |
| 14 | 5.63 | 2-amino-4-methyl-2-pentennitrile | C6H10N2 | amines | 3.03 ± 0.1 | 2.35 ± 0.12 | 2.93 ± 0.10 |
| 15 | 5.75 | Pyrimidine, 4,6-dimethyl- | C6H8N2 | azines | 12.93 ± 0.63 | 10.11 ± 0.56 | 11.31 ± 0,90 |
| 16 | 5.86 | Pyridine-2-D, 6-ethyl- | C7H8DN | azines | 4.09 ± 0.10 | 3.17 ± 0.20 | 3.49 ± 0.22 |
| 17 | 7.35 | 2-furancarboxaldehyde, 5-methyl- | C6H6O2 | aldehydes | 7.12 ± 0.29 | 5.92 ± 0.25 | 6.28 ± 0.25 |
| 18 | 8.45 | 2-furanmethanol, acetate | C7H8O3 | alcohols | 10.42 ± 0.56 | 6.54 ± 0.31 | 10.75 ± 0.53 |
| 19 | 8.60 | 2-pyridinecarbonitrile, 1,2,5,6-tetrahydro-1-methyl- | C7H10N2 | azines | 4.75 ± 0.23 | 3.37 ± 0.17 | 3.91 ± 0.10 |
| 20 | 8.79 | Pyrazine, 2-ethyl-5-methyl- | C7H10N2 | azines | 3.3 ± 0.16 | 2.25 ± 0.15 | 2.65 ± 0.20 |
| 21 | 11.28 | Pyrazine, 3-ethyl-2,5-dimethyl- | C8H12N2 | azines | 1.98 ± 0.06 | 1.33 ± 0.12 | 1.54 ± 0.03 |
| 22 | 11.42 | Furan, 2,2′-methylenebis- | C9H8O2 | furanes | 0.92 ± 0.07 | 0.61 ± 0.08 | n.d. |
| 23 | 11.69 | 2-cyclopenten-1-one, 3-ethyl-2-hydroxy- | C7H10O2 | ketones | n.d. | n.d. | 0.84 ± 0.03 |
| 24 | 14.51 | 1,1-dimethyl-1,3-dihydroisobenzofuran-3-one | C10H10O2 | ketones | 0.53 ± 0.05 | n.d. | 0.7 ± 0.05 |
| 25 | 14.79 | 2-hydroxymethylene-6-isopropyl-3-methyl-cyclohexanone | C11H18O2 | ketones | n.d. | 0.41 ± 0.05 | n.d. |
| 26 | 18.34 | 3,5-heptadienal, 2-ethylidene-6-methyl- | C10H14O | aldehydes | 0.26 ± 0.05 | 0.31 ± 0.02 | 0.42 ± 0.02 |
| 27 | 19.50 | Ethyl (2E,4E,6E)-9-formyl-10-oxo-2,4,6,8-decatetraenoate | C13H14O4 | esters | n.d. | n.d. | 0.14 ± 0.01 |
| 28 | 22.48 | 1H-2-benzopyran, 3-(3,4-dimethoxyphenyl)-6,7-dimethoxy-1-methyl- | C20H22O5 | pyranes | 0.13 ± 0.02 | 0.31 ± 0.01 | 0.14 ± 0.01 |
| 29 | 26.08 | 2,7-diphenyl-1,6-dioxopyridazino[4,5-2′,3′]pyrrolo[4′,5′-D]pyridazine | C20H13N5O2 | azines | 0.13 ± 0.03 | 0.31 ± 0.02 | n.d. |
| 30 | 26.18 | 4H-1-benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-3,5-dihydroxy-7-methoxy- | C18H16O7 | ketones | n.d. | 0.2 ± 0.01 | n.d. |
| 31 | 28.39 | Cholan-24-oic acid, 3,7,12-trihydroxy-, (3α,5β,7α,12α)- | C24H40O5 | acids | n.d. | 0.51 ± 0.01 | n.d. |
| 32 | 28.44 | Tridecanoic acid, 12-methyl-, methyl ester | C15H30O2 | esters | n.d. | 0.31 ± 0.01 | n.d. |
| 33 | 31.28 | 1-isothiocyanato-3-methyladamantane | C12H17NS | amines | 0.13 ± 0.02 | 0.1 ± 0.01 | 0.14 ± 0.01 |
| 34 | 32.19 | Methyl (7E)-7-hexadecenoate | C17H32O2 | esters | 0.26 ± 0.03 | 7.05 ± 0.28 | 0.14 ± 0.01 |
| 35 | 32.64 | Hexadecanoic acid, methyl ester | C17H34O2 | esters | n.d. | 2.96 ± 0.14 | n.d. |
| 36 | 34.60 | Heptadecanoic acid, methyl ester | C18H36O2 | esters | n.d. | 0.41 ± 0.03 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinek, R.; Dobrzański, B., Jr.; Oniszczuk, A.; Gawrysiak-Witulska, M.; Siger, A.; Karami, H.; Ptaszyńska, A.A.; Żytek, A.; Kapela, K.; Gancarz, M. How to Identify Roast Defects in Coffee Beans Based on the Volatile Compound Profile. Molecules 2022, 27, 8530. https://doi.org/10.3390/molecules27238530
Rusinek R, Dobrzański B Jr., Oniszczuk A, Gawrysiak-Witulska M, Siger A, Karami H, Ptaszyńska AA, Żytek A, Kapela K, Gancarz M. How to Identify Roast Defects in Coffee Beans Based on the Volatile Compound Profile. Molecules. 2022; 27(23):8530. https://doi.org/10.3390/molecules27238530
Chicago/Turabian StyleRusinek, Robert, Bohdan Dobrzański, Jr., Anna Oniszczuk, Marzena Gawrysiak-Witulska, Aleksander Siger, Hamed Karami, Aneta A. Ptaszyńska, Aleksandra Żytek, Krzysztof Kapela, and Marek Gancarz. 2022. "How to Identify Roast Defects in Coffee Beans Based on the Volatile Compound Profile" Molecules 27, no. 23: 8530. https://doi.org/10.3390/molecules27238530
APA StyleRusinek, R., Dobrzański, B., Jr., Oniszczuk, A., Gawrysiak-Witulska, M., Siger, A., Karami, H., Ptaszyńska, A. A., Żytek, A., Kapela, K., & Gancarz, M. (2022). How to Identify Roast Defects in Coffee Beans Based on the Volatile Compound Profile. Molecules, 27(23), 8530. https://doi.org/10.3390/molecules27238530











