Abstract
The palladium-catalyzed direct arylation of azoles with (hetero)aryl halides is nowadays one of the most versatile and efficient procedures for the selective synthesis of heterobiaryls. Although this procedure is, due to its characteristics, also of great interest in the industrial field, the wide use of a reaction medium such as DMF or DMA, two polar aprotic solvents coded as dangerous according to environmental, health, safety (EHS) parameters, strongly limits its actual use. In contrast, the use of aromatic solvents as the reaction medium for direct arylations, although some of them show good EHS values, is poorly reported, probably due to their low solvent power against reagents and their potential involvement in undesired side reactions. In this paper we report an unprecedented selective C-5 arylation procedure involving anisole as an EHS green reaction solvent. In addition, the beneficial role of benzoic acid as an additive was also highlighted, a role that had never been previously described.
1. Introduction
(Hetero)arylazoles are key structural cores frequently found in bioactive compounds [1,2,3,4,5,6,7,8,9] and organic functional materials such as liquid crystals [10] and fluorescent dyes [10,11,12,13,14,15]. Due to their widespread applications, the development of straightforward functional group-tolerant synthetic methods that enable selective heteroaromatic elaboration under mild conditions aroused considerable attention.
Among the methods able to functionalize azole scaffolds, the palladium-catalyzed activation of Csp2-H bonds represents an attractive strategy for the direct elaboration of their heteroaromatic core [2,4,16,17,18,19,20,21,22,23,24,25,26,27,28], since a pre-activation of both the coupling partners, which is instead required in traditional metal-catalyzed cross-coupling protocols [29] such as Suzuki–Miyaura [30,31,32,33,34,35,36], Migita–Stille [37,38,39,40,41], or Negishi [42,43,44] cross-couplings, is unnecessary. Starting from the pioneering studies by Ohta [45,46] and by Miura [47], synthetic procedures are now available that allow the direct arylation of several azoles, including imidazoles [48,49,50,51,52,53,54,55,56], oxazoles [50,55,57,58,59,60,61,62], thiazoles [10,50,55,63,64], and pyrazoles [50,65,66,67,68,69,70,71]. These reactions are characterized by a wide tolerance towards almost all the main functional groups, and thanks to the ubiquitous presence of C-H bonds they find advantageous application in late-stage functionalization (LSF) protocols useful for introducing molecular diversification in the last step of a synthetic sequence [72,73,74].
However, the presence of different reactive C-H bonds sometimes poses selectivity issues, leading to the formation of regioisomeric monoarylation products and, sometimes, to di- or triarylated azoles as side products.
Although the presence of one or more heteroatoms introduces a “native” differencing of the diverse C-H bonds [75,76], directing the arylation towards the desired position on the heteroaromatic ring is not simple considering the different operational mechanisms that have been suggested over time for these reactions. In fact, mechanistic hypotheses related to the stage of activation of the C-H bond of the catalytic cycle, on which the selectivity of the reaction depends, such as the classic aromatic electrophilic substitution via electrophilic palladation [47,56], the deprotonation–metalation concerted (CMD) [77,78,79,80], or non-concerted (n-CMD) [81,82], up to the most recent hypothesis of concerted electrophilic metalation–deprotonation (e-CMD) [83], highlight how there are many factors that influence the real reactivity of the C-H bonds of azoles.
To overcome this relevant issue, research has almost always been oriented towards an optimization of the pre-catalyst/ligand system along with the search for the best inorganic base, while the potential effect of the solvent on the outcome of the coupling has been rarely discussed [84,85,86,87]. In fact, even in cases where a solvent screening has been reported, no comment has been added to justify the different outcome of the arylation. This is much more important if we consider the fact that direct arylation procedures, precisely because of simple operating conditions and high chemoselectivity, can also be very useful in the industrial field. In this regard, an analysis of the solvent used in direct C-5 arylation reactions of 1,3-azoles, carried out by us in August 2022 using SciFindern, clearly shows that the most used solvents are DMA and DMF (over 66% of the total) and that apart from 1,4-dioxane only a little more than 6% of the reactions were conducted in aromatic solvents, therefore different from the polar aprotic ones (Figure 1).
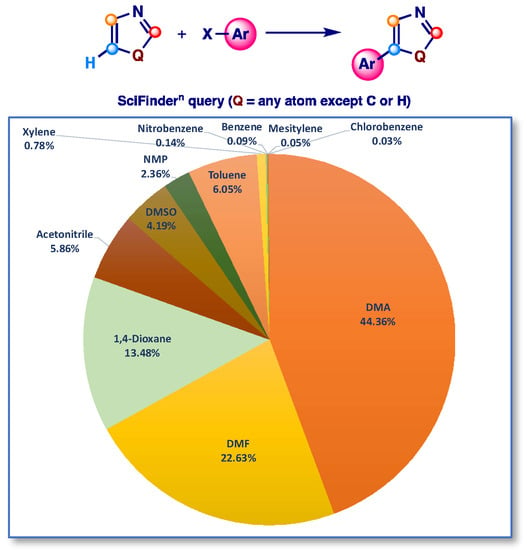
Figure 1.
Type of solvents used in Pd-catalyzed direct arylation reactions (from SciFindern, August 2022).
This analysis, despite its limitations, shows that the most widely used solvents, DMA and DMF, are solvents that have been coded as dangerous according to environmental, health, safety (EHS) parameters, while among the “green” EHS solvents only chlorobenzene was employed, while the “green” anisole is totally absent (Figure 2) [88,89]. The sporadic use of aromatic solvents as a reaction medium for direct arylations is probably related to their low solvent power against reagents, and also to their potential involvement in undesired side reactions.
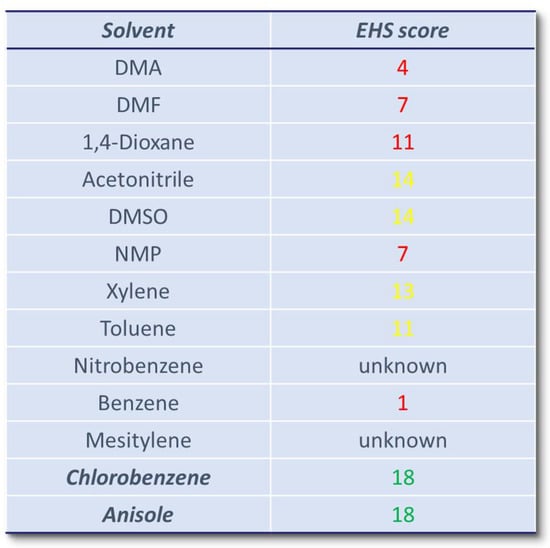
Figure 2.
Summarized environmental, health, and safety (EHS) score data label [88,89]. Solvent raws have been color-coded using a traffic light metric: green = recommended (few issues), yellow = problematic (some issues), red = hazardous (major issues). The higher the score, the more favorable the EHS profile of the solvent.
Over recent years we were interested in studies aimed to broaden the substrate scope of the direct functionalization of azoles and, in particular, to develop efficient synthetic protocols for the carbon–carbon bond forming reaction by selective palladium-catalyzed Csp2-H bond activation of imidazole derivatives [48,50,52,53,54,55,56,90,91].
During these studies, we discovered that the outcome of the Pd-catalyzed arylation of imidazoles with aryl bromides is deeply influenced by the nature of the reaction solvent. Specifically, while it is well known that the Pd-catalyzed direct arylation of imidazoles with aromatic halides selectively leads to C-5 monoarylation products when polar aprotic solvents such as DMF (or DMA) are used as a reaction medium [23,47,50,52,56,90], we have recently observed the preferential formation of C-2,5 double arylation products simply by using xylene as the reaction solvent (Scheme 1) [48].
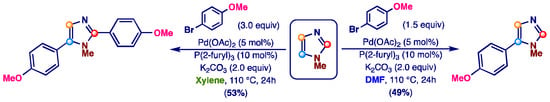
Scheme 1.
Influence of reaction solvent on the Pd-catalyzed direct arylation of 1-methylimidazole with 4-bromoanisole [48,90].
Intrigued by the influence of aromatic solvents on the reactivity of Csp2-H heteroaromatic bonds and by the good EHS parameters of aromatic solvents, as mentioned above, we started a study devoted to evaluating the influence of the aromatic solvents on the efficiency and the selectivity of the direct arylation of imidazoles and other azoles. In particular, in this paper we will discuss the possibility of achieving C-5 selectivity in aromatic solvents, studying how the ratio between mono- and diarylated products changes in function of the nature of the aromatic solvent and of the electronic characteristic of aromatic bromide (Scheme 2).
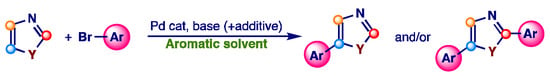
Scheme 2.
Pd-catalyzed direct arylation of 1,3-azoles with aryl bromides using aromatic solvents.
2. Results and Discussion
As discussed in the Introduction, the results obtained using xylene as an aromatic solvent for the direct arylation of imidazoles showed a substantially lower selectivity towards monoarylation, combined with a greater efficiency of coupling when electron-rich aromatic bromides, such as 4-bromoanisole or 4-bromoaniline, were used as coupling partners [48].
To verify the possible influence of the aromatic solvent on the efficiency and selectivity of azole arylation, we started the study by evaluating the outcome of the coupling of 1-methyl-1H-imidazole (1a), chosen as the model azole, with aromatic bromides in four different aromatic solvents: xylenes, anisole, chlorobenzene, and nitrobenzene. These solvents have been selected because they all have a boiling point equal to or greater than 140 °C, the temperature at which we have decided to conduct the initial screening. As coupling partners we chose three aromatic bromides, selected for their different electronic characteristics: 4-bromoanisole (2a) (Hammett’s σp = −0.27 [92]), 4-bromotoluene (2b) (Hammett’s σp = −0.17 [92]), and 1-bromo-4-nitrobenzene (2c) (Hammett’s σp = 0.78 [92]). The screening was carried out using 5 mol% of Pd(OAc)2 as the palladium pre-catalyst, 2.0 equiv of K2CO3 as the base, 1.0 mmol of 1a, 3.0 equiv of bromides 2a–c, in 5.0 mL of the aromatic solvent at 140 °C for 24 h (Scheme 3). To better highlight the possible effect of individual solvents, we decided to carry out the screening under ligandless conditions.
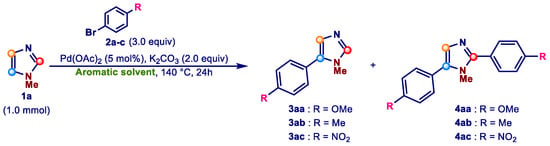
Scheme 3.
First screening of aromatic solvents for the Pd-catalyzed direct arylation of 1-methylimidazole 1a with aryl bromides 2a–c.
The results of the screening are summarized in Table 1, where the GLC yields of 3 and 4 along with the selectivity of the monoarylation vs. the diarylation are reported.

Table 1.
Screening of aromatic solvents 1.
Examination of the data given in Table 1 shows that the use of anisole as the reaction solvent allowed us to obtain selectively the monoarylated 5-arylimidazole 3, regardless of the electronic nature of the aromatic bromides (Entries 10–12, Table 1). On the contrary, the selectivity of 3 vs. 4 when the other three aromatic solvents were used seems to be clearly influenced by the substituent present on the aromatic ring of bromides 2. In fact, in nitrobenzene (Entries 1–3, Table 1), chlorobenzene (Entries 4–6, Table 1), and xylenes (Entries 7–9, Table 1), the highest selectivity was observed with 1-bromo-4-nitrobenzene (2c) (Entries 3, 6, and 9, Table 1), and the lowest when 4-bromoanisole (2a) (Entries 1, 4, and 7, Table 1) was used as a coupling partner. It is also worth mentioning that the selectivity of 3 vs. 4 is reversed using anisole as the reaction solvent (Entries 10–12, Table 1).
Intrigued by the fact that by using anisole as the reaction solvent we observed selective arylation toward the monoarylated 3aa–ac products regardless of the electronic nature of the bromides 2a–c (despite the 1:2 molar ratio being 1:3), and that the trend in selectivity as a function of the electronic nature of the aryl bromide 2 was inverse to that found with the other three aromatic solvents, we decided to start a new screening using anisole as the reaction solvent, and choosing 1-bromo-4-nitrobenzene (2c) because it had given the worst selectivity in the same solvent.
To our surprise, the first results of the screening in anisole showed that the selectivity of the reaction, conducted under the exact experimental conditions shown in Table 1, was dependent on the commercial origin of anisole (Table 2).

Table 2.
Direct arylation of 1-methylimidazole 1a with 1-bromo-4-nitrobenzene (2c) in anisole from different suppliers 1.
To understand these selectivity results a GLC analysis of the different reaction solvents was carried out. In detail, GLC-MS analysis of small aliquots of commercial anisole from the three suppliers (Table 2) showed that in AC anisole methyl benzoate was present as an impurity, in addition to 2-methylanisole (Figure 3).
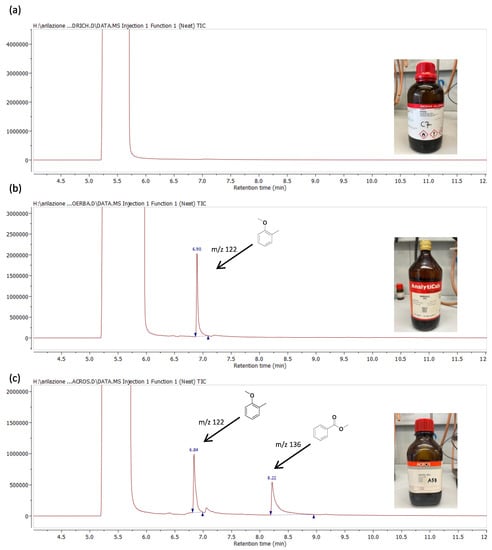
Figure 3.
GLC-EIMS of anisole from Acros (a), Carlo Erba (b), and Sigma-Aldrich (c).
The presence of this ester only in the AC anisole, together with the greater selectivity observed in this specific solvent, has led us to think that this compound or, much more likely, the benzoate analogue that can be formed in a basic environment, not strictly anhydrous (the solvents were simply deaerated with argon), may have an important role in the efficiency and selectivity of the reaction. It is in fact well known that aliphatic carboxylic acids [93], such as pivalic acid or its salts, can effectively promote the selective C-5 arylation of imidazoles and other azoles by means of a CMD mechanism [78,79,80,94]. In this circumstance, the alkyl carboxylate acts as a base-shuttle between the inorganic base and the catalytically active palladium complexes, entering the coordination sphere of the transition metal and favoring the extraction of the proton in the activation step of the heteroaromatic C-H bond.
Therefore, assuming that the same role could be effectively played by benzoic acid in an aromatic solvent such as anisole, we conducted a first test using SA anisole as solvent, the purest of the three batches examined (Scheme 4).
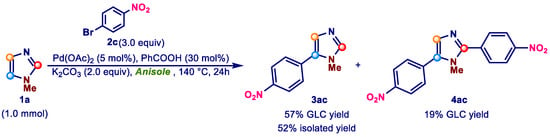
Scheme 4.
Direct arylation of 1-methylimidazole (1a) with 1-bromo-4-nitrobenzene (2c) in SA anisole using benzoic acid as an additive.
As shown in Scheme 4, the addition of 30 mol% benzoic acid was beneficial to reaction outcome. In fact, 3ac was recovered in 52% isolated yield, and the selectivity was substantially identical to that found when AC anisole was used (compare Scheme 4 with Entry 12, Table 1).
With the aim of further increasing the selectivity and yield of the monoarylation reaction, we performed an additional screening using only 1.5 equiv of 1-bromo-4-nitrobenzene (2c) (Table 3).

Table 3.
Synthesis of 5-arylimidazole 3c by direct arylation of 1-methylimidazole (1a) with 1-bromo-4-nitrobenzene (2c) in SA anisole using benzoic acid as additive.1
As can be seen from Entry 1, Table 3, by reducing the aryl bromide to 1.5 equiv the conversion of 1a remained high and the percentage of the diarylation product (4ac) decreased, so 3ac was obtained with a GLC yield of 75% (72% isolated). A reduction in catalytic loading to 2.5 mol% (Entry 2, Table 3) resulted in a slight decrease in conversion of 1a and GLC yield of 3ac. Lowering the reaction temperature from 140 to 120 °C (Entry 3, Table 3) led to a decrease in conversion of 1a and yield of 3ac. Carrying out the reaction in xylenes as the reaction solvent (Entry 4, Table 3) gave a high conversion of precursor 1a but also a higher amount of the diarylation product (4ac) than the reaction conducted in anisole, giving 3ac in 63% yield. When other additives were tried, i.e., pivalic acid and phenol (Entries 5 and 6, Table 3), worse results in terms of 3ac yield were obtained. Tests were also carried out with different kinds of phosphines as palladium ligands (tris(o-tolyl)phosphine, tri(2-furyl)phosphine, tricyclohexylphosphine, and dppf), but in all cases the 3ac yields were lower than that observed under ligandless conditions (Table S1, Supporting Information).
The satisfactory result obtained in the preparation of 3ac from 1a and 2c under the experimental conditions summarized in Entry 1, Table 3 prompted us to extend this methodology to the selective synthesis of several 5-arylazoles in anisole as the reaction solvent.
In detail, performing the reaction in the presence of 5 mol% Pd(OAc)2, 30 mol% benzoic acid, and 2.0 equiv K2CO3 in 5 mL of anisole under ligandless conditions, we were able to recover the required 5-aryl substituted derivatives 3ac–3ec and 3aa–3aj in 40–72% isolated yield after 24 h at 140 °C (Scheme 5).
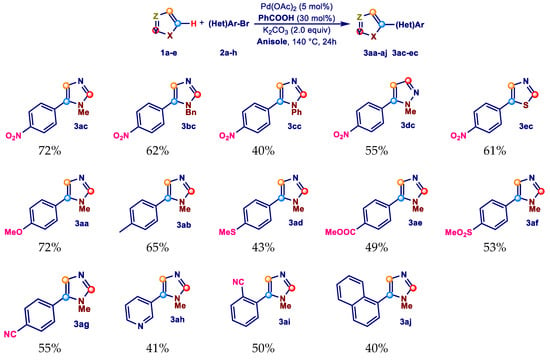
Scheme 5.
Selective Pd-catalyzed direct arylation of azoles 1a–e with aryl bromides 2a–h.
As can be seen from Scheme 5, the coupling also works efficiently when 1-benzyllimidazole (1b) and 1-phenylimidazole (1c) were used as partner of coupling with 1-bromo-4-nitrobenzene (2c), giving the desired products 3bc and 3cc in 62 and 40% isolated yield. Satisfactory results were also obtained with 1-methylpyrazole (2d) and thiazole (2e), giving the products 3dc and 3ec with isolated yields of 55 and 61%, respectively.
Subsequent tests were performed by varying the nature of the aryl bromide. In particular, the electron-rich bromides 4-bromoanisole (2a), 4-bromotoluene (2b), and 4-bromothioanisole (2d) gave the respective 5-aryl products 3aa, 3ab, and 3ad in 72, 65, and 43% isolated yields. Good results were also obtained with the electron-poor methyl 4-bromobenzoate (2e), 1-bromo-4-(methylsulfonyl)benzene (2f), 4-bromobenzonitrile (2g), and 3-bromopyridine (2h), which resulted in the respective products 3ae–3ah with isolated yields of 49, 53, 55, and 41%. In the end, the procedure was tested with the sterically hindered bromides 2-bromobenzonitrile (2i) and 1-bromonaphthalene (2j), and it proved to be effective; products 3ai and 3aj, in fact, were obtained with yields of 50 and 40%.
Further studies are required to elucidate the operative mechanism in aromatic solvents. However, while it was demonstrated that in DMA a solvate complex with palladium A seems to play an important role in the catalytic cycle [95], in an aromatic solvent having poorer coordinating ability azole-ligated organo-palladium intermediates B and C could be the active catalytic species (Figure 4) [96].
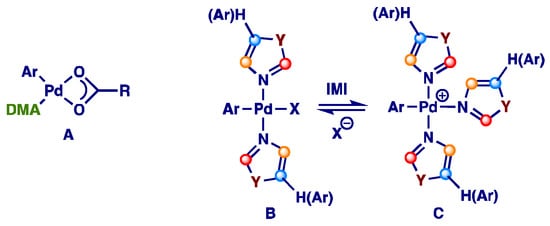
Figure 4.
Supposed Pd(II)-catalyzed direct arylation mechanism in aromatic solvents. A: DMA solvate Pd complex; B and C: structures of azole-ligated arylpallaium intermediates.
Moreover, the fact that the observed reactivity of azoles parallels that of classical electrophilic aromatic substitution (EAS) [97], and that higher efficiency was obtained when benzoic acid was added, an electrophilic concerted metalation–deprotonation (e-CMD) mechanistic pathway [83] seems to be the most plausible among the various mechanistic hypotheses formulated for the palladium-catalyzed direct arylation of azoles.
3. Materials and Methods
3.1. General Information
Melting points were recorded on a hot-stage microscope (Reichert, Wien, Austria, Thermovar). Precoated silica gel PET foils (Sigma-Aldrich, St. Louis, MI, USA) were used for TLC analyses. GLC-FID analyses were performed on a Dani (Milan, Italy) GC 1000 chromatograph equipped with a PTV injector, using an Agilent (Santa Clara, USA) J&W DB-1 column (15 m × 0.25 mm × 0.25 μm) and recorded with a Dani DDS 1000 data station. GLC-MS analyses were recorded with an Agilent 6890N gas chromatograph interfaced with an Agilent MS5973 mass detector, using an Agilent J&W DB-5ms (30 m × 0.25 mm × 0.25 μm) column. Purifications by flash chromatography were performed using Merck 60 silica gel. 1H-NMR and 13C-NMR spectra were recorded at 400 and 100 MHz, respectively, with a Jeol (Tokyo, Japan) 400 spectrometer referring chemical shifts to the residual solvent signal. The following notation was used to report NMR spectra: s = singlet, d = doublet, dd = double doublet, t = triplet, dt = double triplet, q = quadruplet. All the commercially available reagents and solvents were used as received.
3.2. Procedure for the Screening of the aromatic solvents for the Pd-Catalyzed 5-Arylation of 1-Methyl-1H-imidazole (1a) with Aryl Bromides 2a–c
Pd(OAc)2 (11.2 mg, 0.05 mmol), aryl bromide (2a–c) (3.0 mmol), if solid, and K2CO3 (276 mg, 2.0 mmol) were placed in a reaction vessel. The reaction vessel was fitted with a silicon septum, evacuated, and backfilled with argon. This sequence was repeated twice. The selected deaerated solvent (5 mL), aryl bromide 2 (3.0 mmol), if a liquid, and 1-methyl-1H-imidazole (1a) (82 mg, 80 µL, 1.0 mmol) were then added successively under a stream of argon by syringe. The resulting mixture was stirred under argon for 24 h at 140 °C. After cooling to room temperature, the crude reaction mixture was diluted with DCM and AcOEt, biphenyl was added as internal standard, and the resulting mixture was analyzed by GLC and GC–MS. The results of this screening are summarized in Table 1 and Table 2.
3.3. Procedure for the Screening of the Reaction Conditions for the Pd-Catalyzed 5-Arylation of 1-Methyl-1H-imidazole (1a) with 1-Bromo-4-Nitrobenzene (2c) in Anisole (SA)
Pd(OAc)2 (11.2 mg, 0.05 mmol), additive (30 mol%), 1-bromo-4-nitrobenzene (2c) (303 mg, 1.5 mmol), and K2CO3 (276 mg, 2.0 mmol) were placed in a reaction vessel. The reaction vessel was fitted with a silicon septum, evacuated, and backfilled with argon. This sequence was repeated twice. The selected deaerated solvent (5 mL) and 1-methyl-1H-imidazole (1a) (82 mg, 80 µL, 1.0 mmol), were then added successively under a stream of argon by syringe. The resulting mixture was stirred under argon for 24 h at the selected temperature. After cooling to room temperature, the crude reaction mixture was diluted with DCM and AcOEt, biphenyl was added as internal standard, and the resulting mixture was analyzed by GLC and GC–MS. Table 3 summarizes the results of this screening.
3.4. Procedure for the Screening of the Reaction Scope for the Pd-Catalyzed 5-Arylation of Azoles (1a–e) with Aryl Bromides (2a–j) in Anisole (SA)
Pd(OAc)2 (11.2 mg, 0.05 mmol), benzoic acid (36 mg, 0.30 mmol), azole 1 (1 mmol), if solid, aryl bromide 2 (1.5 mmol), if solid, and K2CO3 (276 mg, 2.0 mmol) were placed in a reaction vessel. The reaction vessel was fitted with a silicon septum, evacuated, and backfilled with argon. This sequence was repeated twice. Anisole (5 mL), azole 1 (1 mmol), if liquid, and aryl bromide 2, if liquid, were then added successively under a stream of argon by syringe. The resulting mixture was stirred under argon for 24 h at 140 °C. After cooling to room temperature, the crude reaction mixture was diluted with DCM and AcOEt. The resulting mixture was analyzed by GLC and GC–MS and concentrated under reduced pressure and the residue purified by flash chromatography on silica gel. This procedure was used to prepare compounds 3ac–3aj and 3bc–3ec. For the product 3aa and 3ab the reaction was carried out with 1.0 mmol of the aryl bromide 2 and 1.5 mmol of 1-Methyl-1H-imidazole (1a). The results are summarized in Scheme 5.
3.4.1. 1-methyl-5-(4-nitrophenyl)-1H-imidazole (3ac)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 1-bromo-4-nitrobenzene (2c) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (93:7) as eluent to give 3ac as a yellow-orange solid, (145 mg, 72%), m.p. 165–167 °C (lit. m.p. 169–171 °C) [50]. ESI-MS m/z 204 [M+H]+. EI-MS, m/z (%): 203 (100), 173 (19), 130 (17), 103 (16), 89 (32). 1H NMR (400 MHz, CDCl3) δ 8.32–8.28 (m, 2H), 7.61–7.55 (m, 3H), 7.27 (s, 1H), 3.76 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [50].
3.4.2. 1-benzyl-5-(4-nitrophenyl)-1H-imidazole (3bc)
The crude reaction product obtained by the coupling reaction of 1-benzyl-1H-imidazole (1b) with 1-bromo-4-nitrobenzene (2c) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (95:5) as eluent to give 3bc as an orange solid, (173 mg, 62%), m.p. 108–109 °C (lit. m.p. 106–108 °C) [98]. ESI-MS m/z 280 [M+H]+. EI-MS, m/z (%): 91 (100), 279 (46), 65 (10), 280 (9), 92 (8). 1H NMR (400 MHz, CDCl3) δ 8.24–8.16 (m, 2H), 7.67 (s, 1H), 7.49–7.41 (m, 2H), 7.40–7.24 (m, 4H), 7.04–6.97 (m, 2H), 5.23 (s, 2H). The spectral properties of this compound are in agreement with those previously reported [98].
3.4.3. 5-(4-nitrophenyl)-1-phenyl-1H-imidazole (3cc)
The crude reaction product obtained by the coupling reaction of 1-phenyl-1H-imidazole (1c) with 1-bromo-4-nitrobenzene (2c) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of toluene and ethyl acetate (80:20) as eluent to give 3cc as a yellow solid, (106 mg, 40%), m.p. 155–156 °C (lit. m.p. 162–164 °C) [99]. ESI-MS m/z 266 [M+H]+. EI-MS, m/z (%): 265 (100), 191 (19), 266 (17), 165 (16), 192 (15). 1H NMR (400 MHz, CDCl3) δ 8.15–8.07 (m, 2H), 7.77 (d, J = 1.0 Hz, 1H), 7.48–7.43 (m, 4H), 7.30–7.26 (m, 2H), 7.25–7.17 (m, 2H). The spectral properties of this compound are in agreement with those previously reported [100].
3.4.4. 1-methyl-5-(4-nitrophenyl)-1H-pyrazole (3dc)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-pyrazole (1d) with 1-bromo-4-nitrobenzene (2c) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of dichloromethane and acetone (98:2) as eluent to give 3dc as a yellow solid, (112 mg, 55%), m.p. 85–86 °C (lit. m.p. 75–77 °C) [50]. ESI-MS m/z 204 [M+H]+. EI-MS, m/z (%): 203 (100), 173 (20), 204 (12), 103 (11), 89 (10). 1H NMR (400 MHz, CDCl3) δ 8.37–8.29 (m, 2H), 7.64–7.59 (m, 2H), 7.57 (d, J = 2.0 Hz, 1H), 6.43 (d, J = 2.0 Hz, 1H), 3.95 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [50].
3.4.5. 5-(4-nitrophenyl)thiazole (3ec)
The crude reaction product obtained by the coupling reaction of thiazole (1e) with 1-bromo-4-nitrobenzene (2c) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of toluene and ethyl acetate (80:20) as eluent to give 3ec as a yellow solid, (126 mg, 61%), m.p. 142–143 °C (lit. m.p. 139–141 °C) [50]. ESI-MS m/z 207 [M+H]+. EI-MS, m/z (%): 206 (100), 176 (26), 148 (20), 133 (27), 89 (53). 1H NMR (400 MHz, CDCl3) δ 8.88 (s, 1H), 8.33–8.22 (m, 2H), 8.23 (s, 1H), 7.77–7.72 (m, 2H). The spectral properties of this compound are in agreement with those previously reported [50].
3.4.6. 5-(4-methoxyphenyl)-1-methyl-1H-imidazole (3aa)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 1-bromo-4-methoxybenzene (2a) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (96:4) as eluent to give 3aa as a white solid, (135 mg, 72%), m.p. 77–78 °C (lit. m.p. 73–75 °C) [50]. ESI-MS m/z 189 [M+H]+. EI-MS, m/z (%): 188 (100), 173 (82), 145 (17), 189 (12), 174 (10). 1H NMR (400 MHz, CDCl3) δ 7.49 (s, 1H), 7.34–7.27 (m, 2H), 7.03 (s, 1H), 7.00–6.93 (m, 2H), 3.85 (s, 2H), 3.63 (s, 2H). The spectral properties of this compound are in agreement with those previously reported [50].
3.4.7. 1-methyl-5-(p-tolyl)-1H-imidazole (3ab)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 1-bromo-4-methylbenzene (2b) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (95:5) as eluent to give 3ab as a yellow oil, (112 mg, 65%). ESI-MS m/z 173 [M+H]+. EI-MS, m/z (%): 172 (100), 171 (17), 130 (16), 144 (14), 173 (13). 1H NMR (400 MHz, CDCl3) δ 7.51 (s, 1H), 7.30–7.22 (m, 5H), 7.07 (d, J = 1.1 Hz, 1H), 3.65 (s, 3H), 2.40 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [50].
3.4.8. 1-methyl-5-(4-(methylthio)phenyl)-1H-imidazole (3ad)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 1-(4-bromophenyl)(methyl)sulfane (2d) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (94:6) as eluent to give 3ad as a white solid, (88 mg, 43%), m.p. 71–73 °C. ESI-MS m/z 205 [M+H]+. EI-MS, m/z (%): 204 (100), 189 (63), 205 (13), 190 (8), 162 (7). 1H NMR (400 MHz, CDCl3) δ 7.48 (s, 1H). 7.30–7.25 (m, 4H), 7.05 (s, 1H), 3.62 (s, 3H), 2.49 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 139.12, 138.60, 132.99, 128.81, 128.04, 126.53, 126.41, 32.55, 15.63. Elemental analysis calcd. for C11H12N2S: C, 64.67; H, 5.92; N, 13.71; found C, 64.71; H, 5.91; N, 13.69.
3.4.9. methyl 4-(1-methyl-1H-imidazol-5-yl)benzoate (3ae)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with methyl 4-bromobenzoate (2e) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (96:4) as eluent to give 3ae as a white solid, (106 mg, 49%), m.p. 128–130 °C. ESI-MS m/z 217 [M+H]+. EI-MS, m/z (%): 216 (100), 185 (78), 217 (14), 89 (13), 130 (12). 1H NMR (400 MHz, CDCl3) δ 8.12–8.07 (m, 2H), 7.55 (s, 1H), 7.50–7.46 (m, 2H), 7.20 (s, 1H), 3.94 (s, 3H), 3.72 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [101].
3.4.10. 1-methyl-5-(4-(methylsulfonyl)phenyl)-1H-imidazole (3af)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 1-bromo-4-(methylsulfonyl)benzene (2f) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (95:5) as eluent to give 3af as a white solid, (130 mg, 53%), m.p. 189–190 °C. ESI-MS m/z 237 [M+H]+. EI-MS, m/z (%): 236 (100), 173 (33), 157 (26), 89 (18), 130 (17). 1H NMR (400 MHz, CDCl3) δ 8.01–7.97 (m, 2H), 7.61–7.58 (m, 2H), 7.57 (s, 1H), 7.21 (s, 1H), 3.73 (s, 3H), 3.09 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 140.65, 139.50, 135.47, 131.68, 130.03, 128.67, 128.07, 44.62, 33.02. Elemental analysis calcd. for C11H12N2O2S: C, 55.92; H, 5.12; N, 11.86; found C, 55.97; H, 5.13; N, 11.88.
3.4.11. 4-(1-methyl-1H-imidazol-5-yl)benzonitrile (3ag)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 4-bromobenzonitrile (2g) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (95:5) as eluent to give 3ag as a yellow solid, (101 mg, 55%), m.p. 146–147 °C (lit. m.p. 148–151 °C) [50]. ESI-MS m/z 184 [M+H]+. EI-MS, m/z (%): 183 (100), 155 (14), 184 (13), 128 (11), 114 (10). 1H NMR (400 MHz, CDCl3) δ 7.74–7.70 (m, 2H), 7.57 (s, 1H), 7.53–7.50 (m, 2H), 7.22 (s, 1H), 3.73 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [50].
3.4.12. 3-(1-methyl-1H-imidazol-5-yl)pyridine (3ah)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 3-bromopyridine (2h) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (95:5) as eluent to give 3ah as a pale green oil, (65 mg, 41%), ESI-MS m/z 160 [M+H]+. EI-MS, m/z (%): 160 (11), 159 (100), 158 (9), 131 (32), 104 (10). 1H NMR (400 MHz, CDCl3) δ 8.61–8.58 (m, 1H), 8.55–8.51 (m, 1H), 7.67–7.63 (m, 1H), 7.50 (s, 1H), 7.34–7.29 (m, 1H), 7.09 (s, 1H), 3.62 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [90].
3.4.13. 2-(1-methyl-1H-imidazol-5-yl)benzonitrile (3ai)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 2-bromobenzonitrile (2i) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (95:5) as eluent to give 3ai as a yellow solid, (91 mg, 50%), m.p. 153–155 °C (lit. m.p. 156–158 °C) [102]. ESI-MS m/z 184 [M+H]+. EI-MS, m/z (%): 183 (100), 155 (31), 129 (27), 156 (20), 182 (19). 1H NMR (400 MHz, CDCl3) δ 7.79 (ddd, J = 7.7, 1.4, 0.6 Hz, 1H), 7.67 (td, J = 7.7, 1.4 Hz, 1H), 7.60 (s, 1H), 7.51 (td, J = 7.7, 1.2 Hz, 1H), 7.44 (ddd, J = 7.7, 1.2, 0.6 Hz, 1H), 7.24 (s, 1H), 3.64 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [102].
3.4.14. 1-methyl-5-(naphthalen-1-yl)-1H-imidazole (3aj)
The crude reaction product obtained by the coupling reaction of 1-methyl-1H-imidazole (1a) with 1-bromonaphthalene (2j) (Scheme 5) was purified by flash chromatography on silica gel with a mixture of DCM and MeOH (95:5) as eluent to give 3aj as an orange solid, (94 mg, 45%), m.p. 140–144 °C. ESI-MS m/z 209 [M+H]+. EI-MS, m/z (%): 208 (100), 207 (36), 166 (20), 153 (19), 180 (15). 1H NMR (400 MHz, CDCl3) δ 7.95–7.89 (m, 2H), 7.67–7.63 (m, 2H), 7.56–7.43 (m, 4H), 7.16 (s, 1H), 3.42 (s, 3H). The spectral properties of this compound are in agreement with those previously reported [103].
4. Conclusions
In conclusion, in this work, we developed a simple and efficient ligandless Pd-catalyzed selective C-5 direct arylation of imidazoles and other azoles with aryl bromides, using anisole as the reaction solvent. In fact, with the aim of verifying the possible influence of aromatic solvents on the efficiency and selectivity arylation of azoles, having good environmental, health, safety (EHS) parameters, we started a study with 1-methyl-1H-imidazole (1a), chosen as the model azole, and aromatic bromides in four different solvents: xylenes, anisole, chlorobenzene, and nitrobenzene. After this preliminary screening, we discovered a high C-5 selectivity in anisole; specifically, when the anisole used was from a specific supplier, and we observed that in this solvent ethyl benzoate was present as an impurity. This has led us to think that this compound or, much more likely, the benzoate analogue that can be formed in a basic environment, not strictly anhydrous, may have an important role on the efficiency and selectivity of the reaction. Therefore, assuming that the same role could be effectively played by benzoic acid, we conducted a test with the addition of 30 mol% benzoic acid and this was beneficial to reaction outcome, increasing the monoarylation selectivity. So, after a final fine-tuning of the conditions, we found that by performing the reaction in the presence of Pd(OAc)2 as a pre-catalyst, benzoic acid as an additive, and K2CO3 as a base in anisole, we recovered several 5-aryl substituted azoles in 72–40% isolated yield after 24 h at 140 °C. Further studies on the interesting role of aromatic solvents in direct arylation reactions and on their role in the reaction mechanism are undergoing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238454/s1, Table S1: Screening of the ligands, Figures S1–S16: NMR spectra of compounds 3ac–3ec and 3aa–3aj.
Author Contributions
Methodology, E.R.; Formal analysis, M.L.; Investigation, F.B. (Federico Banchini), S.B. and L.P.; Supervision, F.B. (Fabio Bellina). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Università di Pisa (Project PRA_2020_219).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R.; Lessi, M.; Manzini, C.; Marianetti, G. Direct (Hetero)arylation Reactions of (Hetero)arenes as Tools for the Step- and Atom-Economical Synthesis of Biologically Active Unnatural Compounds Including Pharmaceutical Targets. Synthesis 2016, 48, 3821–3862. [Google Scholar] [CrossRef]
- Bellina, F.; Guazzelli, N.; Lessi, M.; Manzini, C. Imidazole analogues of resveratrol: Synthesis and cancer cell growth evaluation. Tetrahedron 2015, 71, 2298–2305. [Google Scholar] [CrossRef]
- Bellina, F.; Rossi, R.; Lessi, M.; Manzini, C.; Perego, L. Synthesis of Multiply Arylated Heteroarenes, Including Bioactive Derivatives, via Palladium-Catalyzed Direct C–H Arylation of Heteroarenes with (Pseudo)Aryl Halides or Aryliodonium Salts. Synthesis 2014, 46, 2833–2883. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.M.; Damu, G.L.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Shalini, K.; Sharma, P.K.; Kumar, N. Imidazole and its biological activities: A review. Der Chem. Sin. 2010, 1, 36–47. [Google Scholar]
- Bonezzi, K.; Taraboletti, G.; Borsotti, P.; Bellina, F.; Rossi, R.; Giavazzi, R. Vascular disrupting activity of tubulin-binding 1,5-diaryl-1H-imidazoles. J. Med. Chem. 2009, 52, 7906–7910. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron 2007, 63, 4571–4624. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Monti, S.; Rossi, R. Novel imidazole-based combretastatin A-4 analogues: Evaluation of their in vitro antitumor activity and molecular modeling study of their binding to the colchicine site of tubulin. Bioorganic Med. Chem. Lett. 2006, 16, 5757–5762. [Google Scholar] [CrossRef]
- Mori, A.; Sekiguchi, A.; Masui, K.; Shimada, T.; Horie, M.; Osakada, K.; Kawamoto, M.; Ikeda, T. Facile synthesis of 2,5-diarylthiazoles via palladium-catalyzed tandem C-H substitutions. Design of tunable light emission and liquid crystalline characteristics. J. Am. Chem. Soc. 2003, 125, 1700–1701. [Google Scholar] [CrossRef]
- Marianetti, G.; Lessi, M.; Barone, V.; Bellina, F.; Pucci, A.; Minei, P. Solar collectors based on luminescent 2,5-diarylimidazoles. Dye. Pigment. 2018, 157, 334–341. [Google Scholar] [CrossRef]
- Lessi, M.; Manzini, C.; Minei, P.; Perego, L.A.; Bloino, J.; Egidi, F.; Barone, V.; Pucci, A.; Bellina, F. Synthesis and Optical Properties of Imidazole-Based Fluorophores having High Quantum Yields. ChemPlusChem 2014, 79, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Chen, Y. Synthesis and photophysical properties of thiolactone derivatives. Tetrahedron 2013, 69, 1872–1876. [Google Scholar] [CrossRef]
- Kulhanek, J.; Bures, F. Imidazole as a parent pi-conjugated backbone in charge-transfer chromophores. Beilstein J. Org. Chem. 2012, 8, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Fridman, N.; Speiser, S.; Kaftory, M. Structures and Chromogenic Properties of Bisimidazole Derivatives. Cryst. Growth Des. 2006, 6, 1653–1662. [Google Scholar] [CrossRef]
- Basak, S.; Dutta, S.; Maiti, D. Accessing C2-Functionalized 1,3-(Benz)azoles through Transition Metal-Catalyzed C-H Activation. Chem. Eur. J. 2021, 27, 10533–10557. [Google Scholar] [CrossRef]
- Dhawa, U.; Kaplaneris, N.; Ackermann, L. Green strategies for transition metal-catalyzed C–H activation in molecular syntheses. Org. Chem. Front. 2021, 8, 4886–4913. [Google Scholar] [CrossRef]
- Bohra, H.; Wang, M. Direct C–H arylation: A “Greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 2017, 5, 11550–11571. [Google Scholar] [CrossRef]
- Bheeter, C.B.; Chen, L.; Soulé, J.-F.; Doucet, H. Regioselectivity in palladium-catalysed direct arylation of 5-membered ring heteroaromatics. Catal. Sci. Technol. 2016, 6, 2005–2049. [Google Scholar] [CrossRef]
- Bellina, F. Recent Developments in Pd-Catalyzed Direct Arylations of Heteroarenes with Aryl Halides. In C-H Bond Activation and Catalytic Functionalization I; Springer: Berlin, Germany, 2015; pp. 77–102. [Google Scholar] [CrossRef]
- Roger, J.; Gottumukkala, A.L.; Doucet, H. Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds with Aryl Halides by CH Bond Activation. ChemCatChem 2010, 2, 20–40. [Google Scholar] [CrossRef]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition-metal-catalyzed direct arylation of (hetero)arenes by C-H bond cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9826. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R. Recent advances in the synthesis of (hetero)aryl-substituted heteroarenes via transition metal-catalysed direct (hetero)arylation of heteroarene C–H bonds with aryl halides or pseudohalides, diaryliodonium salts, and potassium aryltrifluoroborates. Tetrahedron 2009, 65, 10269–10310. [Google Scholar] [CrossRef]
- Liegault, B.; Lapointe, D.; Caron, L.; Vlassova, A.; Fagnou, K. Establishment of broadly applicable reaction conditions for the palladium-catalyzed direct arylation of heteroatom-containing aromatic compounds. J. Org. Chem. 2009, 74, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- McGlacken, G.P.; Fairlamb, I.J.S. Palladium-Catalysed Cross-Coupling and Related Processes: Some Interesting Observations That Have Been Exploited in Synthetic Chemistry. Eur. J. Org. Chem. 2009, 2009, 4011–4029. [Google Scholar] [CrossRef]
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar] [CrossRef]
- Satoh, T.; Miura, M. Catalytic Direct Arylation of Heteroaromatic Compounds. Chem. Lett. 2007, 36, 200–205. [Google Scholar] [CrossRef]
- Miura, M.; Nomura, M. Direct Arylation via Cleavage of Activated and Unactivated C-H Bonds. In Cross-Coupling Reactions; Springer: Berlin, Germany, 2002; pp. 211–241. [Google Scholar] [CrossRef]
- Negishi, E.-I. Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; Casals-Cruanas, E.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Chem. Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef]
- Pagett, A.B.; Lloyd-Jones, G.C. Suzuki–Miyaura Cross-Coupling. In Organic Reactions; Wiley: Hoboken, NJ, USA, 2019; pp. 547–620. [Google Scholar] [CrossRef]
- Rossi, R.; Bellina, F.; Carpita, A. Palladium Catalysts for the Suzuki Cross-Coupling Reaction: An Overview of Recent Advances. Synthesis 2004, 2004, 2419–2440. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Suzuki, A. Overview of the Suzuki Protocol with B. In Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002; pp. 249–262. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Cordovilla, C.; Bartolomé, C.; Martínez-Ilarduya, J.M.; Espinet, P. The Stille Reaction, 38 Years Later. ACS Catal. 2015, 5, 3040–3053. [Google Scholar] [CrossRef]
- Kosugi, M.; Fugami, K. Overview of the Stille Protocol with Sn. In Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002; pp. 263–283. [Google Scholar] [CrossRef]
- Farina, V.; Krishnamurthy, V.; Scott, W.J. The Stille Reaction; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Mitchell, T.N. Palladium-Catalysed Reactions of Organotin Compounds. Synthesis 1992, 1992, 803–815. [Google Scholar] [CrossRef]
- Stille, J.K. The Palladium-Catalyzed Cross-Coupling Reactions of Organotin Reagents with Organic Electrophiles [New Synthetic Methods(58)]. Angew. Chem. Int. Ed. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Diner, C.; Organ, M.G. The Negishi Cross-Coupling Reaction. In Organic Reactions; Wiley: Hoboken, NJ, USA, 2019; pp. 1–62. [Google Scholar] [CrossRef]
- Haas, D.; Hammann, J.M.; Greiner, R.; Knochel, P. Recent Developments in Negishi Cross-Coupling Reactions. ACS Catal. 2016, 6, 1540–1552. [Google Scholar] [CrossRef]
- Negishi, E.-I. Overview of the Negishi Protocol with Zn, Al, Zr, and Related Metals. In Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002; pp. 229–247. [Google Scholar] [CrossRef]
- Ohta, A.; Aoyagi, Y.; Inoue, A.; Koizumi, I.; Hashimoto, R.; Tokunaga, K.; Gohma, K.; Komatsu, J.; Sekine, K.; Miyafuji, A.; et al. Palladium-catalyzed Cross-coupling Ractions of Chloropyrazines with Aromatic Heterocycles. Heterocycles 1992, 33, 257–272. [Google Scholar] [CrossRef]
- Akita, Y.; Itagaki, Y.; Takizawa, S.; Ohta, A. Cross-coupling reactions of chloropyrazines with 1-substituted indoles. Chem. Pharm. Bull. 1989, 37, 1477–1480. [Google Scholar] [CrossRef]
- Pivsa-Art, S.; Satoh, T.; Kawamura, Y.; Miura, M.; Nomura, M. Palladium-Catalyzed Arylation of Azole Compounds with Aryl Halides in the Presence of Alkali Metal Carbonates and the Use of Copper Iodide in the Reaction. Bull. Chem. Soc. Jpn. 1998, 71, 467–473. [Google Scholar] [CrossRef]
- Bellina, F.; Lessi, M.; Panzetta, G.; Marianetti, G. Improved Synthesis of Symmetrical 2,5-Diarylimidazoles by One-Pot Palladium-Catalyzed Direct Arylation Tailored on the Electronic Features of the Aryl Halide. Synthesis 2017, 49, 4676–4686. [Google Scholar] [CrossRef]
- Takfaoui, A.; Zhao, L.; Touzani, R.; Soulé, J.-F.; Dixneuf, P.H.; Doucet, H. One pot Pd(OAc)2-catalysed 2,5-diarylation of imidazoles derivatives. Tetrahedron 2014, 70, 8316–8323. [Google Scholar] [CrossRef]
- Bellina, F.; Lessi, M.; Manzini, C. Mild Palladium-Catalyzed Regioselective Direct Arylation of Azoles Promoted by Tetrabutylammonium Acetate. Eur. J. Org. Chem. 2013, 2013, 5621–5630. [Google Scholar] [CrossRef]
- Roger, J.; Doucet, H. Phosphine-free palladium-catalysed direct 5-arylation of imidazole derivatives at low catalyst loading. Tetrahedron 2009, 65, 9772–9781. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Di Fiore, A.; Marchetti, C.; Rossi, R. Highly selective synthesis of 4(5)-aryl-, 2,4(5)-diaryl-, and 4,5-diaryl-1H-imidazoles via Pd-catalyzed direct C-5 arylation of 1-benzyl-1H-imidazole. Tetrahedron 2008, 64, 6060–6072. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Efficient and practical synthesis of 4(5)-aryl-1H-imidazoles and 2,4(5)-diaryl-1H-imidazoles via highly selective palladium-catalyzed arylation reactions. J. Org. Chem. 2007, 72, 8543–8546. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Mannina, L.; Rossi, R.; Viel, S. Regiocontrolled Synthesis of 1,2-Diaryl-1H-imidazoles by Palladium- and Copper-Mediated Direct Coupling of 1-Aryl-1H-imidazoles with Aryl Halides under Ligandless Conditions. Eur. J. Org. Chem. 2006, 2006, 693–703. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Palladium- and Copper-Mediated Direct C-2 Arylation of Azoles—Including Free (NH)-Imidazole, -Benzimidazole and -Indole—Under Base-Free and Ligandless Conditions. Eur. J. Org. Chem. 2006, 2006, 1379–1382. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Mannina, L.; Rossi, R.; Viel, S. Regioselective synthesis of 1,5-diaryl-1H-imidazoles by palladium-catalyzed direct arylation of 1-aryl-1H-imidazoles. J. Org. Chem. 2005, 70, 3997–4005. [Google Scholar] [CrossRef]
- Shi, X.; Soulé, J.F.; Doucet, H. Reaction Conditions for the Regiodivergent Direct Arylations at C2- or C5-Positions of Oxazoles using Phosphine-Free Palladium Catalysts. Adv. Synth. Catal. 2019, 361, 4748–4760. [Google Scholar] [CrossRef]
- Liu, X.W.; Shi, J.L.; Yan, J.X.; Wei, J.B.; Peng, K.; Dai, L.; Li, C.G.; Wang, B.Q.; Shi, Z.J. Reigoselective arylation of thiazole derivatives at 5-position via Pd catalysis under ligand-free conditions. Org. Lett. 2013, 15, 5774–5777. [Google Scholar] [CrossRef]
- Strotman, N.A.; Chobanian, H.R.; Guo, Y.; He, J.; Wilson, J.E. Highly regioselective palladium-catalyzed direct arylation of oxazole at C-2 or C-5 with aryl bromides, chlorides, and triflates. Org. Lett. 2010, 12, 3578–3581. [Google Scholar] [CrossRef]
- Derridj, F.; Djebbar, S.; Benali-Baitich, O.; Doucet, H. Direct arylation of oxazole and benzoxazole with aryl or heteroaryl halides using a palladium–diphosphine catalyst. J. Organomet. Chem. 2008, 693, 135–144. [Google Scholar] [CrossRef]
- Ohnmacht, S.A.; Mamone, P.; Culshaw, A.J.; Greaney, M.F. Direct arylations on water: Synthesis of 2,5-disubstituted oxazoles balsoxin and texaline. Chem. Commun. 2008, 10, 1241–1243. [Google Scholar] [CrossRef]
- Verrier, C.; Martin, T.; Hoarau, C.; Marsais, F. Palladium-catalyzed direct (hetero)arylation of ethyl oxazole-4-carboxylate: An efficient access to (hetero)aryloxazoles. J. Org. Chem. 2008, 73, 7383–7386. [Google Scholar] [CrossRef]
- Tani, S.; Uehara, T.N.; Yamaguchi, J.; Itami, K. Programmed synthesis of arylthiazoles through sequential C–H couplings. Chem. Sci. 2014, 5, 123–135. [Google Scholar] [CrossRef]
- Gottumukkala, A.L.; Doucet, H. Activated Aryl Chlorides: Useful Partners for the Coupling with 2-Substituted Thiazoles in the Palladium-Catalysed C-H Activation/Functionalisation Reaction. Eur. J. Inorg. Chem. 2007, 2007, 3629–3632. [Google Scholar] [CrossRef]
- Kumpulainen, E.T.T.; Pohjakallio, A. Selective Palladium-Catalyzed Direct C—H Arylation of Unsubstituted. N-Protected Pyrazoles. Adv. Synth. Catal. 2014, 356, 1555–1561. [Google Scholar] [CrossRef]
- Derridj, F.; Roger, J.; Djebbar, S.; Doucet, H. Catalytic System for Inhibition of Amination-Type Reaction and Palladium-Catalysed Direct Arylation using Non-Protected Pyrazole Derivatives. Adv. Synth. Catal. 2012, 354, 747–750. [Google Scholar] [CrossRef]
- Yan, T.; Chen, L.; Bruneau, C.; Dixneuf, P.H.; Doucet, H. Palladium-catalyzed direct arylation of 5-chloropyrazoles: A selective access to 4-aryl pyrazoles. J. Org. Chem. 2012, 77, 7659–7664. [Google Scholar] [CrossRef]
- Doucet, H.; Ammar, H.; Beladhria, A.; Beydoun, K.; Salem, R. Pd-Catalysed Direct 5-Arylation of 1-Methylpyrazole with Aryl Bromides. Synthesis 2011, 2011, 2553–2560. [Google Scholar] [CrossRef]
- Mateos, C.; Mendiola, J.; Carpintero, M.; Minguez, J.M. Regioselective palladium-catalyzed arylation of 4-chloropyrazoles. Org. Lett. 2010, 12, 4924–4927. [Google Scholar] [CrossRef]
- Goikhman, R.; Jacques, T.L.; Sames, D. C-H bonds as ubiquitous functionality: A general approach to complex arylated pyrazoles via sequential regioselective C-arylation and N-alkylation enabled by SEM-group transposition. J. Am. Chem. Soc. 2009, 131, 3042–3048. [Google Scholar] [CrossRef]
- Santelli, M.; Doucet, H.; Fall, Y. Palladium-Catalyzed Direct Arylation of Pyrazole Derivatives: A Green Access to 4-Arylpyrazoles. Synthesis 2009, 2010, 127–135. [Google Scholar] [CrossRef]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Glorius, F. C-H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 2013, 5, 369–375. [Google Scholar] [CrossRef]
- Breslow, R.; Baldwin, S.; Flechtner, T.; Kalicky, P.; Liu, S.; Washburn, W. Remote oxidation of steroids by photolysis of attached benzophenone groups. J. Am. Chem. Soc. 1973, 95, 3251–3262. [Google Scholar] [CrossRef]
- Gorelsky, S.I. Origins of regioselectivity of the palladium-catalyzed (aromatic)CH bond metalation–deprotonation. Coord. Chem. Rev. 2013, 257, 153–164. [Google Scholar] [CrossRef]
- Lapointe, D.; Markiewicz, T.; Whipp, C.J.; Toderian, A.; Fagnou, K. Predictable and site-selective functionalization of poly(hetero)arene compounds by palladium catalysis. J. Org. Chem. 2011, 76, 749–759. [Google Scholar] [CrossRef]
- Korenaga, T.; Suzuki, N.; Sueda, M.; Shimada, K. Ligand effect on direct arylation by CMD process. J. Organomet. Chem. 2015, 780, 63–69. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the palladium-catalyzed (aromatic)C-H bond metalation-deprotonation mechanism spanning the entire spectrum of arenes. J. Org. Chem. 2012, 77, 658–668. [Google Scholar] [CrossRef]
- Lapointe, D.; Fagnou, K. Overview of the Mechanistic Work on the Concerted Metallation–Deprotonation Pathway. Chem. Lett. 2010, 39, 1118–1126. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the concerted metalation-deprotonation mechanism in palladium-catalyzed direct arylation across a broad range of aromatic substrates. J. Am. Chem. Soc. 2008, 130, 10848–10849. [Google Scholar] [CrossRef]
- Gandon, V.; Hoarau, C. Concerted vs. Nonconcerted Metalation-Deprotonation in Orthogonal Direct C-H Arylation of Heterocycles with Halides: A Computational Study. J. Org. Chem. 2021, 86, 1769–1778. [Google Scholar] [CrossRef]
- Théveau, L.; Querolle, O.; Dupas, G.; Hoarau, C. Ligand controlled orthogonal base-assisted direct C–H bond arylation in oxa(thia)zole-4-carboxylate series. New insights in nCMD mechanism. Tetrahedron 2013, 69, 4375–4380. [Google Scholar] [CrossRef]
- Wang, L.; Carrow, B.P. Oligothiophene Synthesis by a General C-H Activation Mechanism: Electrophilic Concerted Metalation-Deprotonation (eCMD). ACS Catal. 2019, 9, 6821–6836. [Google Scholar] [CrossRef]
- El Kazzouli, S.; Boujdi, K.; El Brahmi, N.; Collet, S.; Dubreuil, D.; Mathé-Allainmat, M.; Akssira, M.; Guillaumet, G.; Lebreton, J. Solvent/Ligand-Controlled Switchable C3 or C7 C–H Arylations of 1-Methyl-4-nitro-1H-indazole. Synthesis 2022, 54, 4834–4842. [Google Scholar] [CrossRef]
- Kanai, Y.; Müller-Borges, D.; Plenio, H. The Regioselective Arylation of 1,3-Benzodioxoles. Adv. Synth. Catal. 2021, 364, 679–688. [Google Scholar] [CrossRef]
- Matsidik, R.; Komber, H.; Sommer, M. Rational Use of Aromatic Solvents for Direct Arylation Polycondensation: C-H Reactivity versus Solvent Quality. ACS Macro Lett. 2015, 4, 1346–1350. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M. Synthesis of donor–acceptor conjugated polymers based on benzo[1,2-b:4,5-b′]dithiophene and 2,1,3-benzothiadiazole via direct arylation polycondensation: Towards efficient C–H activation in nonpolar solvents. Polym. Chem. 2014, 5, 5784–5792. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Di Fiore, A.; Rossi, R. Regioselective Synthesis of 4,5-Diaryl-1-methyl-1H-imidazoles Including Highly Cytotoxic Derivatives by Pd-Catalyzed Direct C-5 Arylation of 1-Methyl-1H-imidazole with Aryl Bromides. Eur. J. Org. Chem. 2008, 2008, 5436–5445. [Google Scholar] [CrossRef]
- Bellina, F.; Calandri, C.; Cauteruccio, S.; Rossi, R. Efficient and highly regioselective direct C-2 arylation of azoles, including free (NH)-imidazole, -benzimidazole and -indole, with aryl halides. Tetrahedron 2007, 63, 1970–1980. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 2002, 91, 165–195. [Google Scholar] [CrossRef]
- Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C-H bond functionalizations: Mechanism and scope. Chem. Rev. 2011, 111, 1315–1345. [Google Scholar] [CrossRef]
- Fagnou, K. Mechanistic considerations in the development and use of azine, diazine and azole N-oxides in palladium-catalyzed direct arylation. Top. Curr. Chem. 2010, 292, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hartwig, J.F. Assessment of the intermediacy of arylpalladium carboxylate complexes in the direct arylation of benzene: Evidence for C-H bond cleavage by “ligandless” species. J. Am. Chem. Soc. 2011, 133, 3308–3311. [Google Scholar] [CrossRef]
- Perego, L.A.; Grimaud, L.; Bellina, F. Mechanistic Studies on the Palladium-Catalyzed Direct C-5 Arylation of Imidazoles: The Fundamental Role of the Azole as a Ligand for Palladium. Adv. Synth. Catal. 2016, 358, 597–609. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Lagowski, J.M. Reactivity of Five-membered Rings with Two or More Heteroatoms. In Comprehensive Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 1984; pp. 39–110. [Google Scholar] [CrossRef]
- Lessi, M.; Lucci, A.; Cuzzola, A.; Bellina, F. Imidazo-Fused Isoindoles by Pd(II)/Ag(I)-Promoted Intramolecular Dehydrogenative Coupling. Eur. J. Org. Chem. 2020, 2020, 796–802. [Google Scholar] [CrossRef]
- Van Leusen, A.M.; Wildeman, J.; Oldenziel, O.H. Chemistry of sulfonylmethyl isocyanides. 12. Base-induced cycloaddition of sulfonylmethyl isocyanides to carbon,nitrogen double bonds. Synthesis of 1,5-disubstituted and 1,4,5-trisubstituted imidazoles from aldimines and imidoyl chlorides. J. Org. Chem. 1977, 42, 1153–1159. [Google Scholar] [CrossRef]
- Hu, L.-Q.; Deng, R.-L.; Li, Y.-F.; Zeng, C.-J.; Shen, D.-S.; Liu, F.-S. Developing Bis(imino)acenaphthene-Supported N-Heterocyclic Carbene Palladium Precatalysts for Direct Arylation of Azoles. Organometallics 2018, 37, 214–226. [Google Scholar] [CrossRef]
- He, X.-X.; Li, Y.; Ma, B.-B.; Ke, Z.; Liu, F.-S. Sterically Encumbered Tetraarylimidazolium Carbene Pd-PEPPSI Complexes: Highly Efficient Direct Arylation of Imidazoles with Aryl Bromides under Aerobic Conditions. Organometallics 2016, 35, 2655–2663. [Google Scholar] [CrossRef]
- Mao, S.X.; Shi, X.Z.; Soule, J.F.; Doucet, H. Exploring Green Solvents Associated to Pd/C as Heterogeneous Catalyst for Direct Arylation of Heteroaromatics with Aryl Bromides. Adv. Synth. Catal. 2018, 360, 3306–3317. [Google Scholar] [CrossRef]
- Ouyang, J.-S.; Li, Y.-F.; Shen, D.-S.; Ke, Z.; Liu, F.-S. Bulky α-diimine palladium complexes: Highly efficient for direct C–H bond arylation of heteroarenes under aerobic conditions. Dalton Trans. 2016, 45, 14919–14927. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).