Energetic [1,2,5]oxadiazolo [2,3-a]pyrimidin-8-ium Perchlorates: Synthesis and Characterization
Abstract
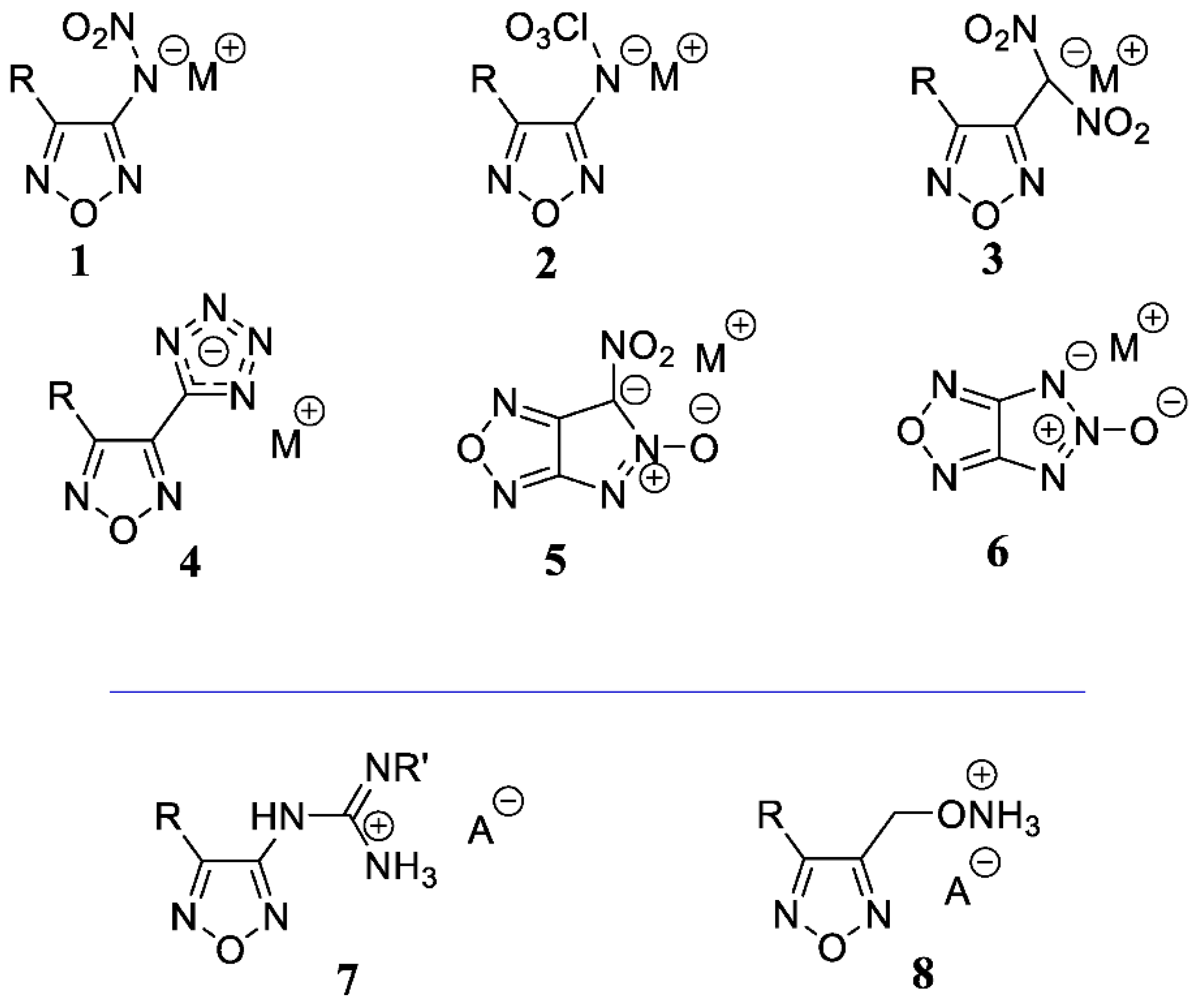
1. Introduction
2. Results
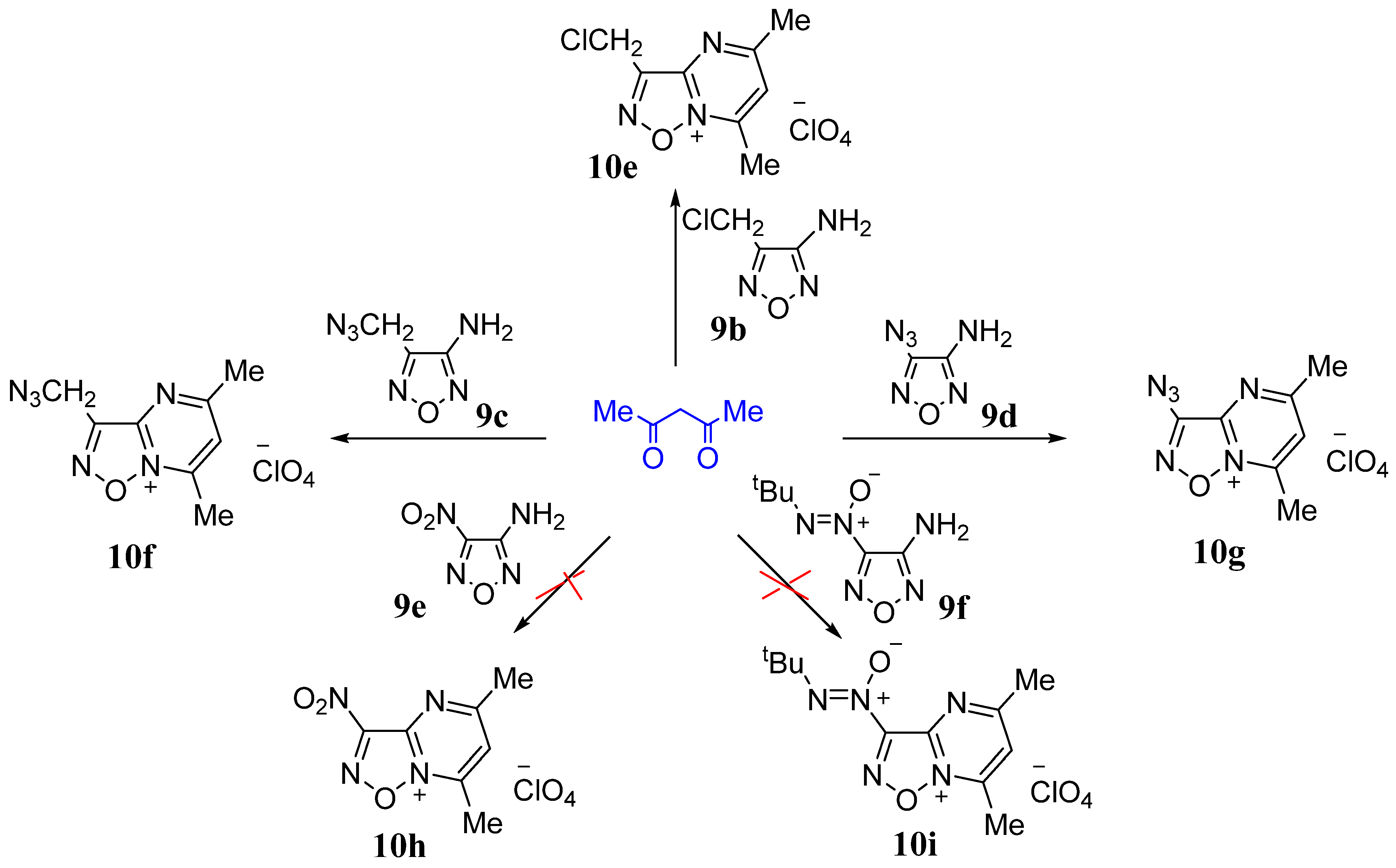
2.1. Synthesis
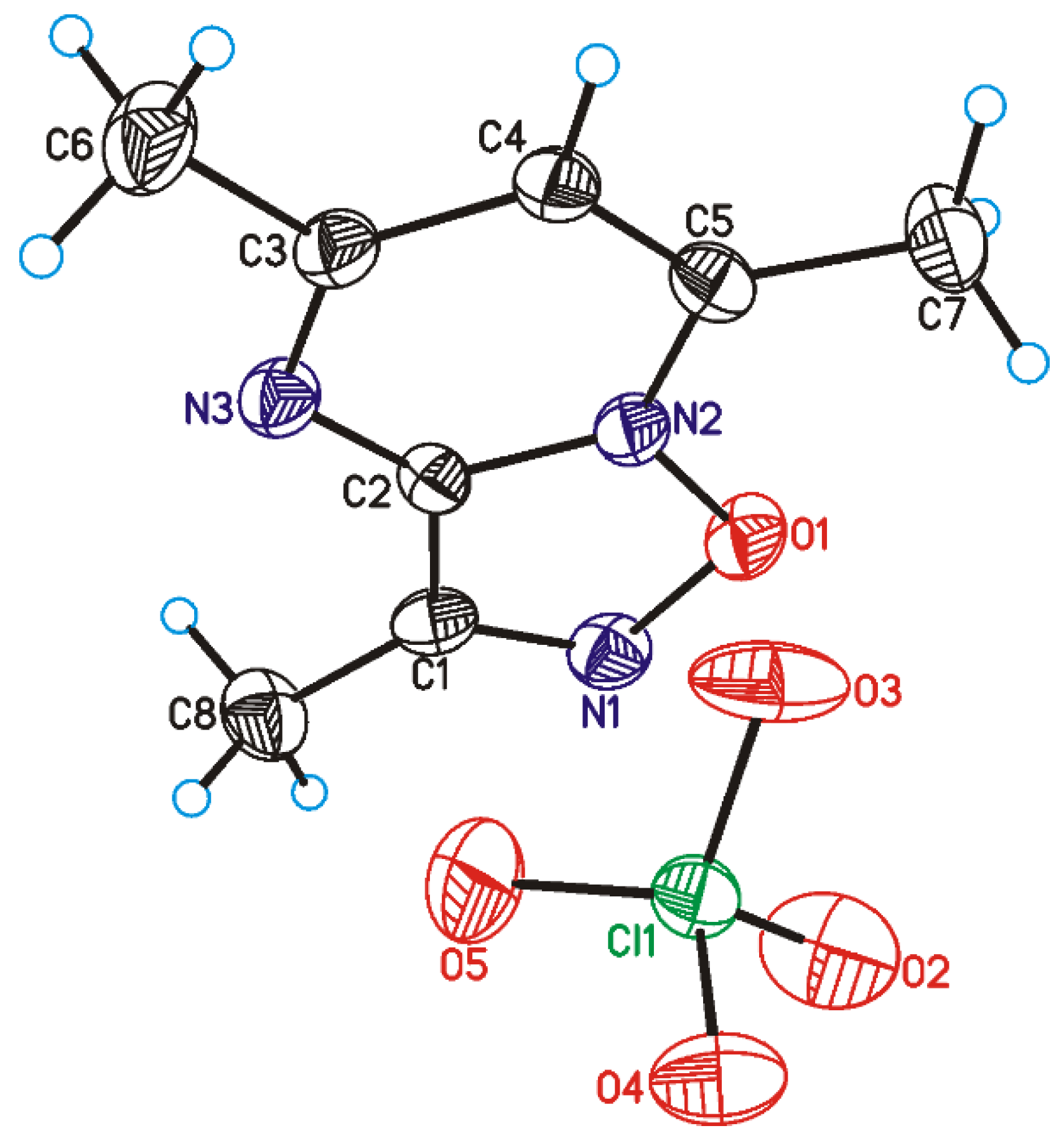
2.2. X-ray Analysis
2.3. Initial Safety Testing
2.4. Thermal Analysis
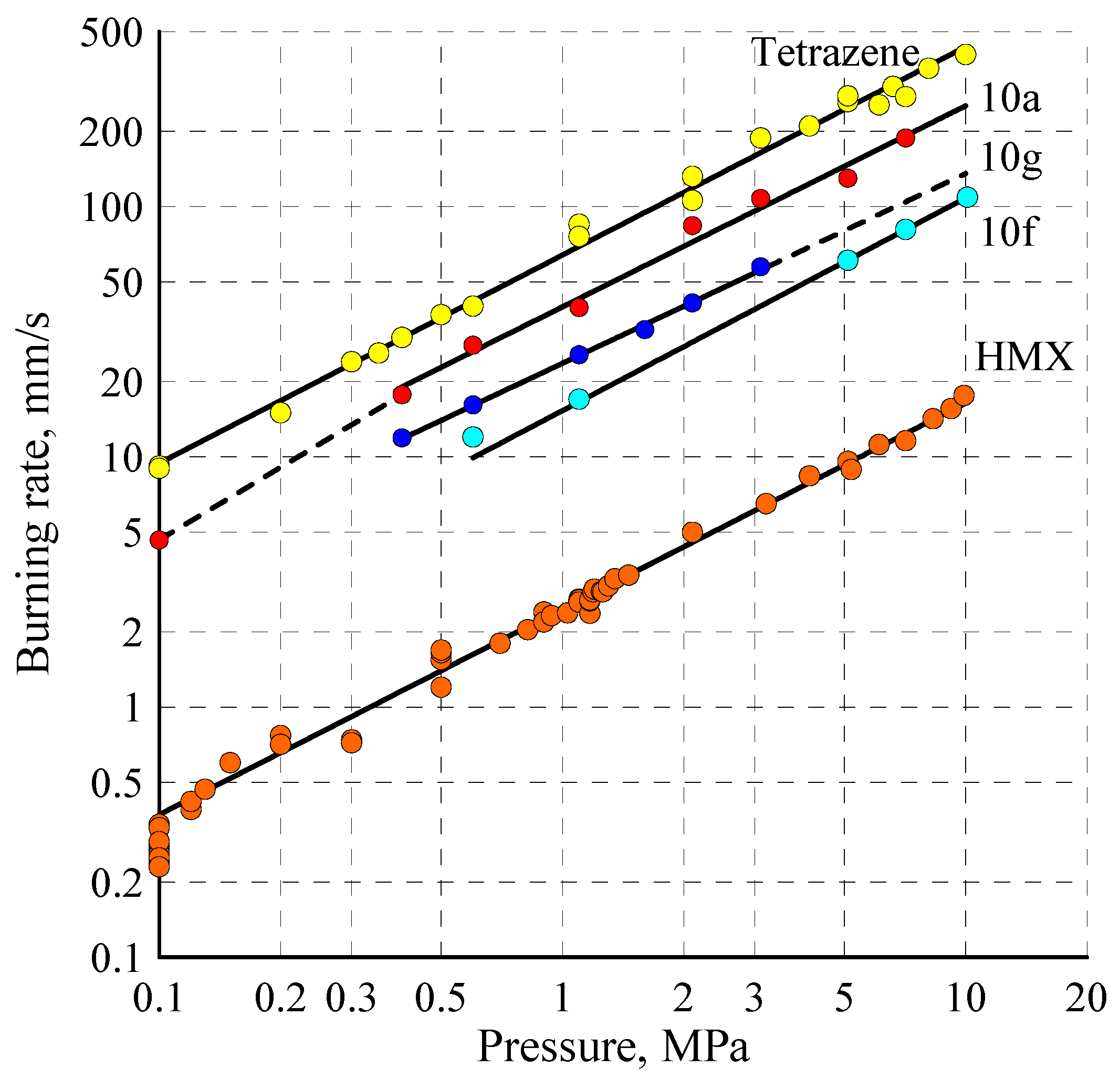
2.5. Combustion
2.6. Explosive Performance
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rogov, N.G.; Ischenko, M.A. Solid Composite Propellants: Components, Requirements, Properties; SPSTU: St. Petersburg, Russia, 2005. (In Russian) [Google Scholar]
- Madyakin, F.P. Components and Combustion Products of Pyrotechnic Compositions; KSTU: Kazan, Russia, 2006. (In Russian) [Google Scholar]
- Singh, H.; Shekhar, H. Solid Rocket Propellants: Science and Technology Challenges; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Zhang, L.; Lin, Q.-Q.; Cheng, B.; Wang, P.; Lu, M.; Lin, Q.-H. Effect of hexanitroethane (HNE) and hydrazinium nitroformate (HNF) on energy characteristics of composite solid propellants. FirePhysChem 2021, 1, 116–122. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. Azole-Based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shreeve, J.M. Energetic Ionic Liquids as Explosives and Propellant Fuels: A New Journey of Ionic Liquid Chemistry. Chem. Rev. 2014, 114, 10527–10574. [Google Scholar] [CrossRef]
- Sebastiao, E.; Cook, C.; Hub, A.; Murugesu, M. Recent developments in the field of energetic ionic liquids. J. Mater. Chem. A 2014, 2, 8153–8173. [Google Scholar] [CrossRef]
- Chingin, K.; Perry, R.H.; Chambreau, S.D.; Vaghjiani, G.L.; Zare, R.N. Generation of melamine polymer condensates upon hypergolic ignition of dicyanamide ionic liquids. Angew. Chem. Int. Ed. 2011, 50, 8634–8637. [Google Scholar] [CrossRef] [PubMed]
- Sheremetev, A.B. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russia. 2001; Unpublished work. [Google Scholar]
- Dorofeenko, G.N.; Krivun, S.V.; Dulenko, V.I.; Zhdanov, Y.A. Perchloric acid and its compounds in organic synthesis. Russ. Chem. Rev. 1965, 34, 88–104, [Translation of Usp. Khim. 1965, 34, 219–252]. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Kumar, A. Activation of Organic Reactions by Perchlorates. Adv. Org. Synth. 2005, 1, 215–232. [Google Scholar] [CrossRef]
- Dalpozzo, R.; Bartoli, G.; Sambri, L.; Melchiorre, P. Perchloric Acid and Its Salts: Very Powerful Catalysts in Organic Chemistry. Chem. Rev. 2010, 110, 3501–3551. [Google Scholar] [CrossRef] [PubMed]
- Sheremetev, A.B.; Makhova, N.N.; Friedrichsen, W. Monocyclic Furazans and Furoxans. Adv. Heterocycl. Chem. 2001, 78, 65–188. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. 1,2,5-Oxadiazole-Based High-Energy-Density Materials: Synthesis and Performance. ChemPlusChem 2020, 85, 13–42. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Bi, F.; Wang, B. Energetic materials based on poly furazan and furoxan structures. Chin. Chem. Lett. 2020, 31, 2375–2394. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, J.M.; Zhao, W.; Qu, Y.; Xing, X.W.; Meng, J.W.; Liu, Y.C. Application and Development of 3,4-Bis(3-nitrofurazan-4-yl)furoxan (DNTF). Russ. J. Gen. Chem. 2021, 91, 445–455. [Google Scholar] [CrossRef]
- Tang, J.; Yang, H.; Cui, Y.; Cheng, G. Nitrogen-rich tricyclic-based energetic materials. Mater. Chem. Front. 2021, 5, 7108–7118. [Google Scholar] [CrossRef]
- Willer, R.L.; Day, R.S.; Park, D.J. Composition of Salts of 3-Nitramino-4-Nitrofurazan for Propellants. U.S. Patent 5,460,669, 28 June 1995. [Google Scholar]
- Williams, G.K.; Brill, T.B. Thermal decomposition of energetic materials 72: Unusual behavior of substituted furazan compounds upon flash pyrolysis. Combust. Flame 1998, 114, 569–576. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Yudin, I.L.; Aleksandrova, N.S.; Aronova, S.M.; Kryazhevskikh, I.A.; Lempert, D.B. Hydroxylammonium salts of furazan family. Int. Annu. Conf. ICT 2003, 34, 101/1–101/10. [Google Scholar]
- Blomquist, H.R. (Nitramino)Nitrofurazan-Based Monopropellant Smokeless Gas Generating Compositions, Especially for Airbags. U.S. Patent 6,513,834, 29 August 2003. [Google Scholar]
- Wang, B.; Xiong, H.; Cheng, G.; Yang, H. Incorporating Energetic Moieties into Four Oxadiazole Ring Systems for the Generation of High-Performance Energetic Materials. ChemPlusChem 2018, 83, 439–447. [Google Scholar] [CrossRef]
- Ma, J.; Chinnam, A.K.; Cheng, G.; Yang, H.; Zhang, J.; Shreeve, J.M. 1,3,4-Oxadiazole Bridges: A Strategy to Improve Energetics at the Molecular Level. Angew. Chem. Int. Ed. 2021, 60, 5497–5504. [Google Scholar] [CrossRef] [PubMed]
- Sheremetev, A.B. (Perchlorylamino)furazans and its salts: New high-energy-density materials with high sensitivity. Mendeleev Commun. 2020, 30, 490–493. [Google Scholar] [CrossRef]
- Li, H.; Zhao, F.; Wang, B.; Zhai, L.; Lai, W.; Liu, N. A new family of energetic salts based on oxybridged bis(dinitromethyl)furazan: Syntheses, characterization and properties. RSC Adv. 2015, 5, 21422–21429. [Google Scholar] [CrossRef]
- Zhai, L.; Fan, X.; Wang, B.; Bi, F.; Liab, Y.; Zhua, Y. A green high-initiation-power primary explosive: Synthesis, 3D structure and energetic properties of dipotassium 3,4-bis(3-dinitromethylfurazan-4-oxy)furazan. RSC Adv. 2015, 5, 57833–57841. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, H.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Energetic dinitromethyl group functionalized azofurazan and its azofurazanates. RSC Adv. 2016, 6, 91477–91482. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Yang, J.; Pan, R.; Lin, X. Materials with good energetic properties resulting from the smart combination of nitramino and dinitromethyl group with furazan. New J. Chem. 2017, 41, 7697–7704. [Google Scholar] [CrossRef]
- Ma, Q.; Gu, H.; Huang, J.; Liu, D.; Li, J.; Fan, G. Synthesis and Characterization of New Melt-cast Energetic Salts: Dipotassium and Diaminoguanidinium N,N′-Dinitro-N,N′-Bis(3-dinitromethyl-furazanate-4-yl)methylenediamine. Propellants Explos. Pyrotech. 2018, 43, 90–95. [Google Scholar] [CrossRef]
- Guo, T.; Wang, Z.; Tang, W.; Wang, W.; Bi, F.; Wang, B.; Zhou, Z.; Meng, Z.; Ge, Z. A good balance between the energy density and sensitivity from assembly of bis(dinitromethyl) and bis(fluorodinitromethyl) with a single furazan ring. J. Anal. Appl. Pyrolysis 2018, 134, 218–230. [Google Scholar] [CrossRef]
- Yu, Q.; Chinnam, A.K.; Yin, P.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Finding furoxan rings. J. Mater. Chem. A 2020, 8, 5859–5864. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.; Shreeve, J.M. Dinitromethyl groups enliven energetic salts. Energ. Mater. Front. 2020, 1, 2–15. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Huo, H.; Fan, Y.; Fan, X. Synthesis, characterization and thermal properties of energetic compounds derived from 3-amino-4-(tetrazol-5-yl)furazan. Chin. J. Chem. 2011, 29, 919–924. [Google Scholar] [CrossRef]
- Ilyushin, M.A.; Shugaley, I.V.; Tselinskii, I.V.; Garabadzhiu, A.V. Environmental Problems and Their Solutions of Using Energy-Rich Substances for Initiating Devices. Russ. J. Gen. Chem. 2013, 83, 2624–2632. [Google Scholar] [CrossRef]
- Ilyushin, M.A.; Tselinsky, I.V.; Shugalei, I.V. Environmentally Friendly Energetic Materials for Initiation Devices. Cent. Eur. J. Energ. Mater. 2012, 9, 293–327. [Google Scholar]
- Stepanov, A.I.; Sannikov, V.S.; Dashko, D.V.; Roslyakov, A.G.; Astrat’yev, A.A.; Stepanova, E.V.; Aliev, Z.G.; Goncharov, T.K.; Aldoshin, S.M. Synthesis and properties of 3-azido-4-(2H-tetrazol-5-yl)furazan. Chem. Heterocycl. Compd. 2017, 53, 779–785. [Google Scholar] [CrossRef]
- Dong, Z.; An, D.; Yang, R.; Ye, Z. Insensitive and Thermostable Energetic Materials Based on 3-Ureido-4-tetrazole-furazan: Synthesis, Characterization, and Properties. Z. Anorg. Allg. Chem. 2019, 645, 1285–1290. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, H.; Ju, X.; Lu, C.; Lin, Q.; Zhang, J.; Cheng, G. 1D Energetic Metal-Organic Framework: Sodium 6-Nitro-5-oxidopyrazolo [3,4-c][1,2,5]oxadiazol-4-ide with Good Thermal Stability. ChemistrySelect 2017, 2, 4673–4677. [Google Scholar] [CrossRef]
- Tang, Y.; He, C.; Shreeve, J.M. A furazan-fused pyrazole N-oxide via unusual cyclization. J. Mater. Chem. A 2017, 5, 4314–4319. [Google Scholar] [CrossRef]
- Hermann, T.S.; Klapotke, T.M.; Krumm, B. Formation and Characterization of Heavy Alkali and Silver Salts of the 4-Nitro-pyrazolo-(3,4-c)-furazan-5-N-oxide Anion. Propellants Explos. Pyrotech. 2018, 43, 54–61. [Google Scholar] [CrossRef]
- Voronin, A.A.; Fedyanin, I.V.; Churakov, A.M.; Pivkina, A.N.; Muravyev, N.V.; Strelenko, Y.A.; Klenov, M.S.; Lempert, D.B.; Tartakovsky, V.A. 4H-[1,2,3]Triazolo [4,5-c][1,2,5]oxadiazole 5-oxide and Its Salts: Promising Multipurpose Energetic Materials. ACS Appl. Energy Mater. 2020, 3, 9401–9407. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Yudin, I.L.; Aleksandrova, N.S. High nitrogen furazan derivatives for gas generetors. In Proceedings of the Twenty-Third International Pyrotechnics Seminar, Tsukuba, Japan, 30 September 1997; pp. 377–388. [Google Scholar]
- Davis, M.; Deady, L.W.; Homfeld, E. Rates of N-methylation of 2-Methybenzotriazole, 2,1,3-Benzoxadiazole, 2,1,3-Benzothia(or Selena)diazole, 1,2,5-Oxadiazole and 1,2,5-Thiadiazole. Aust. J. Chem. 1974, 27, 1917–1921. [Google Scholar] [CrossRef]
- Bachkovsky, I.P.; Mikhailovsky, A.P.; Chuiguk, V.A. 1,2,5-Oxadiazolo [2,3-a]-pyrimidinium salts. Ukr. Khim. Zh. 1980, 46, 637–639. [Google Scholar]
- Struchkov, Y.T.; Batsanov, A.S.; Chuiguk, V.A.; Batog, L.V.; Kulikov, A.S.; Pivina, T.S.; Strelenko, Y.A. 5,7-Dimethyl-3-phenylfurazano- and -furoxano [5,4-a]pyrimidinium perchlorates: A new type of condensed system. Chem. Heterocycl. Comp. 1992, 24, 193–197, [Translation of Khim. Geterotsikl. Soedin. 1992, 2, 233–238]. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, B.; Qiu, L.; Sheremetev, A.B. Recent Synthetic Efforts towards High Energy Density Materials: How to Design High-Performance Energetic Structures? FirePhysChem 2022, 2, 83–139. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Shamshina, Y.L.; Dmitriev, D.E. Synthesis of 3-alkyl-4-aminofurazans. Russ. Chem. Bull. 2005, 54, 1032–1037, [Translation of Izv. Akad. Nauk. Ser. Khim. 2005, 4, 1007–1012]. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Mel’nikova, S.F.; Kokareva, E.S.; Nekrutenko, R.E.; Strizhenko, K.V.; Suponitsky, K.Y.; Pham, T.D.; Pivkina, A.N.; Sinditskii, V.P. Nitroxy- and azidomethyl azofurazans as advanced energetic materials. Def. Technol. 2022, 18, 1369–1381. [Google Scholar] [CrossRef]
- Tselinskii, I.V.; Mel’nikova, S.F.; Vergizov, S.N. Azidofurazans in the synthesis of condensed systems. Zhurnal Org. Khimii 1981, 17, 1123–1124. [Google Scholar]
- Rakitin, O.A.; Zalesova, O.A.; Kulikov, A.S.; Makhova, N.N.; Godovikova, T.I.; Khmel’nitskii, L.I. Synthesis and reactivity of furazanyl- and furoxanyldiazonium salts. Russ. Chem. Bull. 1993, 42, 1865–1870, [Translation of Izv. Akad. Nauk. Ser. Khim. 1993, 11, 1949–1955]. [Google Scholar] [CrossRef]
- Novikova, T.S.; Melnikova, T.M.; Kharitonova, O.V.; Kulagina, V.O.; Aleksandrova, N.S.; Sheremetev, A.B.; Pivina, T.S.; Khmelnitskii, L.I.; Novikov, S.S. An Effective Method for the Oxidation of Aminofurazans to Nitrofurazans. Mendeleev Commun. 1994, 4, 138–140. [Google Scholar] [CrossRef]
- Apasov, E.T.; Sheremetev, A.B.; Dzhetigenov, B.A.; Kalinin, A.V.; Tartakovsky, V.A. Reaction of Silylated Aminonitrofurazan with N-Magnesium Amine Derivatives. Bull. Russ. Acad. Sci. Div. Chem. Sci. 1992, 41, 1500–1501. [Google Scholar] [CrossRef]
- Skibsted, J.; Jakobsen, H.J. 35Cl and 37Cl Magic-Angle Spinning NMR Spectroscopy in the Characterization of Inorganic Perchlorates. Inorg. Chem. 1999, 38, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Suponitsky, K.Y.; Lyssenko, K.A.; Antipin, M.Y.; Aleksandrova, N.S.; Sheremetev, A.B.; Novikova, T.S. 4,4-Bis(nitramin)azofurazane and its Salts. Study of Molecular and Crystal Structure Based on X-ray and Quantum Chemical Data. Russ. Chem. Bull. 2009, 58, 2129–2136. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Lyssenko, K.A.; Ananyev, I.V.; Kozeev, A.M.; Sheremetev, A.B. Role of Weak Intermolecular Interactions in the Crystal Structure of Tetrakis-furazano [3,4-c:3′,4′-g:3″,4″-k:3‴,4‴-o][1,2,5,6,9,10,13,14]octaazacyclohexadecine and Its Solvates. Cryst. Growth Des. 2014, 14, 4439–4449. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Smol’yakov, A.F.; Ananyev, I.V.; Khakhalev, A.V.; Gidaspov, A.A.; Sheremetev, A.B. 3,4-Dinitrofurazan: Structural Nonequivalence of Ortho-Nitro Groups as a Key Feature of the Crystal Structure and Density. ChemistrySelect 2020, 5, 14543–14548. [Google Scholar] [CrossRef]
- Manelis, G.B.; Nazin, G.M.; Rubtsov, Y.I.; Strunin, V.A. Thermal Decomposition and Combustion of Explosives and Propellants; CRC Press: London, UK, 2003. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Burzhava, A.V.; Sheremetev, A.B.; Aleksandrova, N.S. Thermal and combustion properties of 3,4-Bis(3-nitrofurazan-4-yl)furoxan (DNTF). Propellants Explos. Pyrotech. 2012, 37, 575–580. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Burzhava, A.V.; Egorshev, V.Y.; Sheremetev, A.B.; Zelenov, V.P. Combustion of Furazanotetrazine Dioxide. Combust. Explos. Shock. Waves 2013, 49, 117–120, [Translation of Fiz. Goren. Vzriv. 2013, 49, 134–137]. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Burzhava, A.V.; Chernyi, A.N.; Shmelev, D.S.; Apalkova, V.N.; Palysaeva, N.V.; Sheremetev, A.B. A comparative study of two difurazanyl ethers. J. Therm. Anal. Calorim. 2016, 123, 1431–1438. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Smirnova, A.D.; Serushkin, V.V.; Aleksandrova, N.S.; Sheremetev, A.B. Furazan-fused azacyclic nitramines: Influence of structural features on the combustion and the thermolysis. ChemistrySelect 2020, 5, 13868–13877. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Burzhava, A.V.; Sheremetev, A.B. Macrocyclic tetra(azo-) and tetra(azoxyfurazan)s: Comparative study of decomposition and combustion with linear analogs. Energetic Mater. Front. 2021, 2, 87–95. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Smirnova, A.D.; Serushkin, V.V.; Yudin, N.V.; Vatsadze, I.A.; Dalinger, I.L.; Kiselev, V.G.; Sheremetev, A.B. Nitroderivatives of N-pyrazolyltetrazoles: Thermal decomposition and combustion. Thermochim. Acta 2021, 698, 178876. [Google Scholar] [CrossRef]
- Vereschagin, A.N. Inductive Effect. Constants of Substituents for Correlation Analysis; Nauka: Moscow, Russia, 1988. [Google Scholar]
- Whelan, D.J.; Spear, R.J.; Read, R.W. The thermal decomposition of some primary explosives as studied by differential scanning calorimetry. Thermochim. Acta 1984, 80, 149–163. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Egorshev, V.Y.; Berezin, M.V.; Serushkin, V.V. Mechanism of HMX combustion in a wide range of pressures. Combust. Explos. Shock. Waves 2009, 45, 461–477. [Google Scholar] [CrossRef]
- Fogelzang, A.E.; Egorshev, V.Y.; Pimenov, A.Y.; Sinditskii, V.P.; Saklanty, A.R.; Svetlov, B.S. Investigation of the Steady-State Burning of Primary Explosives at High Pressures. Dokl. Akad. Nauk SSSR 1985, 285, 1449–1452. [Google Scholar]
- Sinditskii, V.P.; Egorshev, V.Y.; Serushkin, V.V.; Levshenkov, A.I.; Berezin, M.V.; Filatov, S.A. Combustion of energetic materials governed by reactions in the condensed phase. Inter. J. Ener. Mat. Chem. Prop. 2010, 9, 147–192. [Google Scholar] [CrossRef]
- Kolesov, V.I.; Kapranov, K.O.; Tkacheva, A.V.; Kulagin, I.A. Explosive Characteristics of Tetrazene and MTX-1. Combust. Explos. Shock Waves. 2021, 57, 350–355. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Wozniak, D.R.; Piercey, D.G. Progress and performance of energetic materials: Open dataset, tool, and implications for synthesis. J. Mater. Chem. A 2022, 10, 11054–11073. [Google Scholar] [CrossRef]
- STANAG 4489; Explosives, Impact Sensitivity Tests. NATO: Brussels, Belgium, 1999.
- STANAG 4487; Explosives, Friction Sensitivity Tests. NATO: Brussels, Belgium, 2002.
- Bruker AXS Inc. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Roobottom, H.K.; Passmore, J.; Glasser, L. Relationships among Ionic Lattice Energies, Molecular (Formula Unit) Volumes, and Thermochemical Radii. Inorg. Chem. 1999, 38, 3609–3620. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Tudela, D.; Glasser, L. Lattice Potential Energy Estimation for Complex Ionic Salts from Density Measurements. Inorg. Chem. 2002, 41, 2364–2367. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Pople, J.A. Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J. Chem. Phys. 1997, 106, 1063. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schutz, M.; Celani, P.; Korona, T.; Lindh, R.; Mitrushenkov, A.; Rauhut, G.; et al. MOLPRO, Version 2010.1. Users Man. Version. 2010. Available online: https://www.molpro.net/pipermail/molpro-user/2010-September/003868.html (accessed on 1 November 2022).
- Karton, A.; Martin, J.M.L. Explicitly correlated Wn theory: W1-F12 and W2-F12. J. Chem. Phys. 2012, 136, 124114. [Google Scholar] [CrossRef]
- Kesharwani, M.K.; Brauer, B.; Martin, J.M.L. Frequency and Zero-Point Vibrational Energy Scale Factors for Double-Hybrid Density Functionals (and Other Selected Methods): Can Anharmonic Force Fields Be Avoided? J. Phys. Chem. A 2015, 119, 1701–1714. [Google Scholar] [CrossRef]
- Karton, A.; Schreiner, P.R.; Martin, J.M.L. Heats of formation of platonic hydrocarbon cages by means of high-level thermochemical procedures. J. Comput. Chem. 2016, 37, 49–58. [Google Scholar] [CrossRef]
- Kiselev, V.G.; Goldsmith, C.F. Accurate Prediction of Bond Dissociation Energies and Barrier Heights for High-Energy Caged Nitro and Nitroamino Compounds Using a Coupled Cluster Theory. J. Phys. Chem. A 2019, 123, 4883–4890. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, V.G.; Goldsmith, C.F. Accurate Thermochemistry of Novel Energetic Fused Tricyclic 1,2,3,4-Tetrazine Nitro Derivatives from Local Coupled Cluster Methods. J. Phys. Chem. A 2019, 123, 9818–9827. [Google Scholar] [CrossRef] [PubMed]
- Gorn, M.V.; Gritsan, N.P.; Goldsmith, C.F.; Kiselev, V.G. Thermal Stability of Bis-Tetrazole and Bis-Triazole Derivatives with Long Catenated Nitrogen Chains: Quantitative Insights from High-Level Quantum Chemical Calculations. J. Phys. Chem. A 2020, 124, 7665–7677. [Google Scholar] [CrossRef] [PubMed]
- Muravyev, N.V.; Monogarov, K.A.; Melnikov, I.N.; Pivkina, A.N.; Kiselev, V.G. Learning to Fly: Thermochemistry of Energetic Materials by Modified Thermogravimetric Analysis and Highly Accurate Quantum Chemical Calculations. Phys. Chem. Chem. Phys. 2021, 23, 15522–15542. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Taylor, P.R. A diagnostic for determining the quality of single-reference electron correlation methods. Int. J. Quantum Chem. 1989, 36, 199–207. [Google Scholar] [CrossRef]
- Chase, M.W., Jr. NIST-JANAF Thermochemical Tables; American Chemical Society: Washington, DC, USA, 1998; Volume 9. [Google Scholar]







| Compound | Formula | IS, a J | FS, b N | DSC, c °C |
|---|---|---|---|---|
| 10a | C8H10Cl1N3O5 (263.63) | 2.0 ± 0.4 | 32 ± 6 | 212 |
| 10b | C8H9Cl2N3O5 (298.08) | 2.1 ± 0.8 | 36 ± 8 | 177 |
| 10e | C8H9Cl2N3O5 (298.08) | 2 ± 1 | 60 ± 30 | 207 |
| 10f | C8H9Cl1N6O5 (304.65) | 2.6 ± 1.1 | 16 ± 8 | 142 |
| 10g | C7H7Cl1N6O5 (290.62) | 1.0 ± 0.3 | 5.9 ± 0.8 | 136 |
| tetrazene | C2H8N10O1 (188.15) | 3 ± 2 | <5 | 127 |
| Sampl | Substituent | σIa | State | T, °C | logA b | Ea, c kJ mol−1 | k150C, d s−1 × 104 |
|---|---|---|---|---|---|---|---|
| 10a | CH3 | −0.07 | Solid | 160–180 | 14.5 | 164 | 0.015 |
| 10f | N3CH2 | 0.11 | Solid | 110–120 | 14.3 | 157 | 0.084 |
| 10g | N3 | 0.33 | Solid | 80–105 | 15.0 | 145 | 12 |
| Tetrazenee | Solid | 115–125 | 18.7 | 163 | 372 | ||
| 10a | CH3 | −0.07 | Liquid | 170–180 | 18.7 f | 183 f | 1.1 |
| 10f | N3CH2 | 0.11 | Liquid | 110–130 | 17.6 | 157 | 163 |
| 10g | N3 | 0.33 | Liquid | 90–110 | 18.8 | 152 | 530 |
| Compound | Δp, a MPa | Burning Rate rb = Apn | rb10, d mm s−1 | |
|---|---|---|---|---|
| A b | n c | |||
| 10a | 0.1–0.4 0.4–10.0 | 42.7 39.7 | 0.96 0.80 | 250 |
| 10g | 0.1–0.4 0.4–10.0 | 14.4 23.6 | 0.21 0.76 | 139 |
| 10f | 5.1–10.0 | 15.3 | 0.85 | 109 |
| Tetrazene | 0.1–10.0 | 64.0 | 0.83 | 435 |
| HMX | 0.1–10.0 | 2.47 | 0.82 | 16 |
| Compound | Formula Mw | d20, a g cm−3 | α b | ΔfH0, c kJ mol−1 (kJ g−1) | D, d m s−1 | PC-J, e GPa |
|---|---|---|---|---|---|---|
| 10a | C8H10Cl1N3O5 (263.63) | 1.502 | 0.262 | +147.1 (+0.56) | 6400 | 19 |
| 10f | C8H9Cl1N6O5 (304.65) | 1.599 | 0.268 | +552.8 (+1.81) | 7000 | 25 |
| 10g | C7H7Cl1N6O5 (290.62) | 1.562 | 0.314 | +557.2 (+1.92) | 7000 | 24 |
| Tetrazene | C2H8N10O1 (188.15) | 1.635 | 0.125 | +156 [69] (+0.83) | 7600 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strizhenko, K.V.; Smirnova, A.D.; Filatov, S.A.; Sinditskii, V.P.; Stash, A.I.; Suponitsky, K.Y.; Monogarov, K.A.; Kiselev, V.G.; Sheremetev, A.B. Energetic [1,2,5]oxadiazolo [2,3-a]pyrimidin-8-ium Perchlorates: Synthesis and Characterization. Molecules 2022, 27, 8443. https://doi.org/10.3390/molecules27238443
Strizhenko KV, Smirnova AD, Filatov SA, Sinditskii VP, Stash AI, Suponitsky KY, Monogarov KA, Kiselev VG, Sheremetev AB. Energetic [1,2,5]oxadiazolo [2,3-a]pyrimidin-8-ium Perchlorates: Synthesis and Characterization. Molecules. 2022; 27(23):8443. https://doi.org/10.3390/molecules27238443
Chicago/Turabian StyleStrizhenko, Kirill V., Anastasia D. Smirnova, Sergei A. Filatov, Valery P. Sinditskii, Adam I. Stash, Kyrill Yu. Suponitsky, Konstantin A. Monogarov, Vitaly G. Kiselev, and Aleksei B. Sheremetev. 2022. "Energetic [1,2,5]oxadiazolo [2,3-a]pyrimidin-8-ium Perchlorates: Synthesis and Characterization" Molecules 27, no. 23: 8443. https://doi.org/10.3390/molecules27238443
APA StyleStrizhenko, K. V., Smirnova, A. D., Filatov, S. A., Sinditskii, V. P., Stash, A. I., Suponitsky, K. Y., Monogarov, K. A., Kiselev, V. G., & Sheremetev, A. B. (2022). Energetic [1,2,5]oxadiazolo [2,3-a]pyrimidin-8-ium Perchlorates: Synthesis and Characterization. Molecules, 27(23), 8443. https://doi.org/10.3390/molecules27238443






