Understanding the Regioselectivity and the Molecular Mechanism of [3 + 2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: A DFT Computational Study
Abstract
1. Introduction
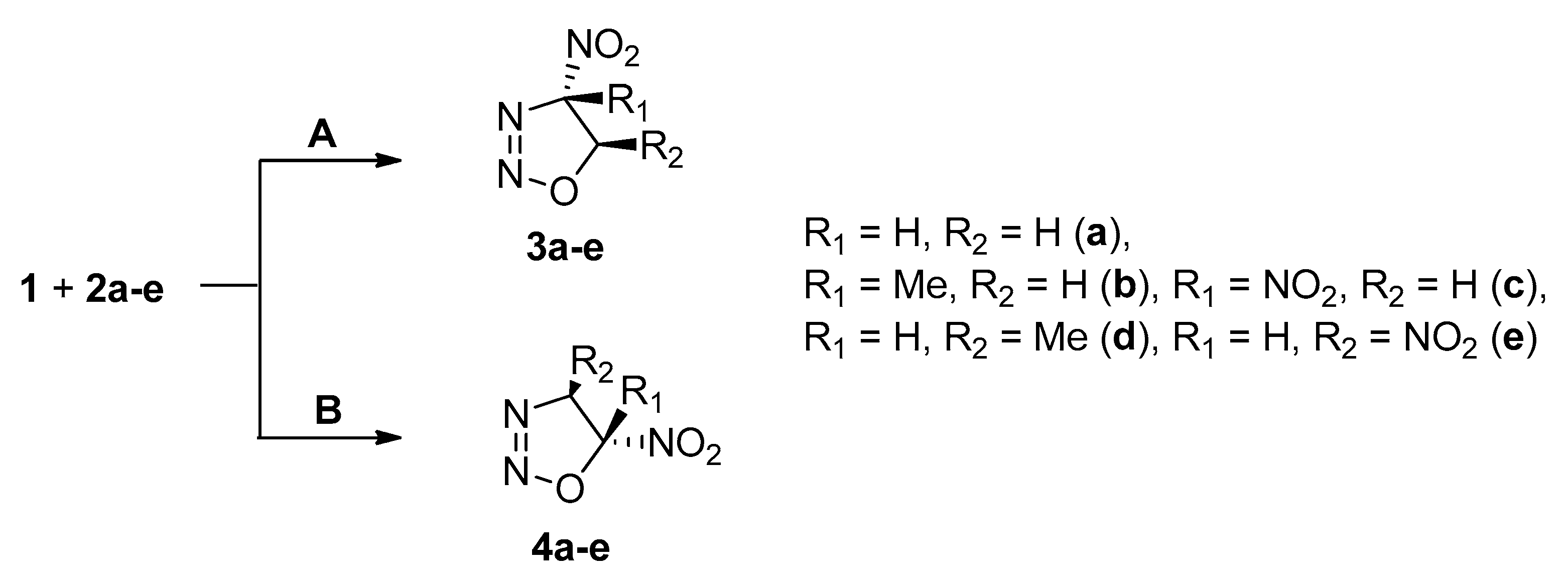
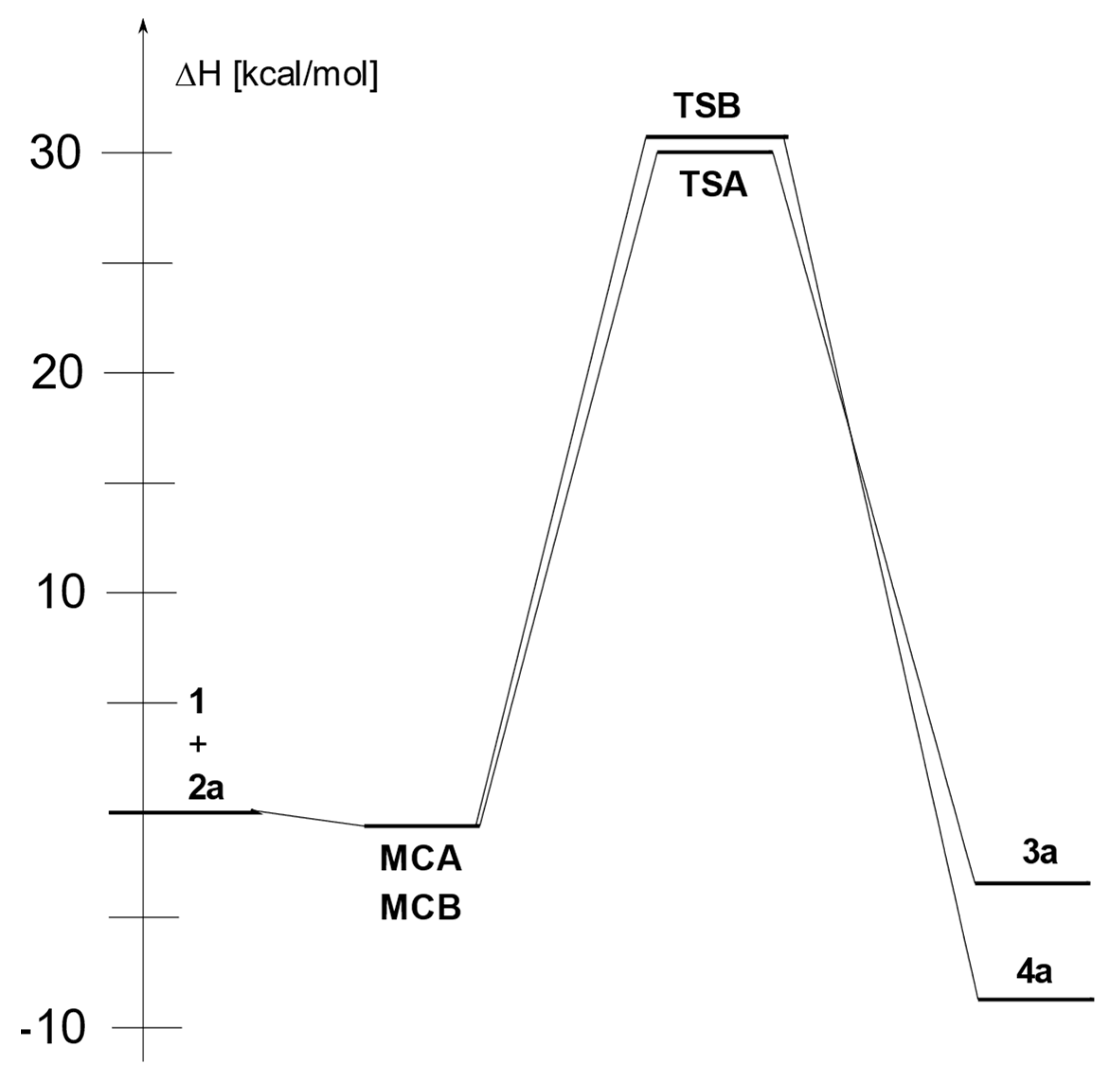
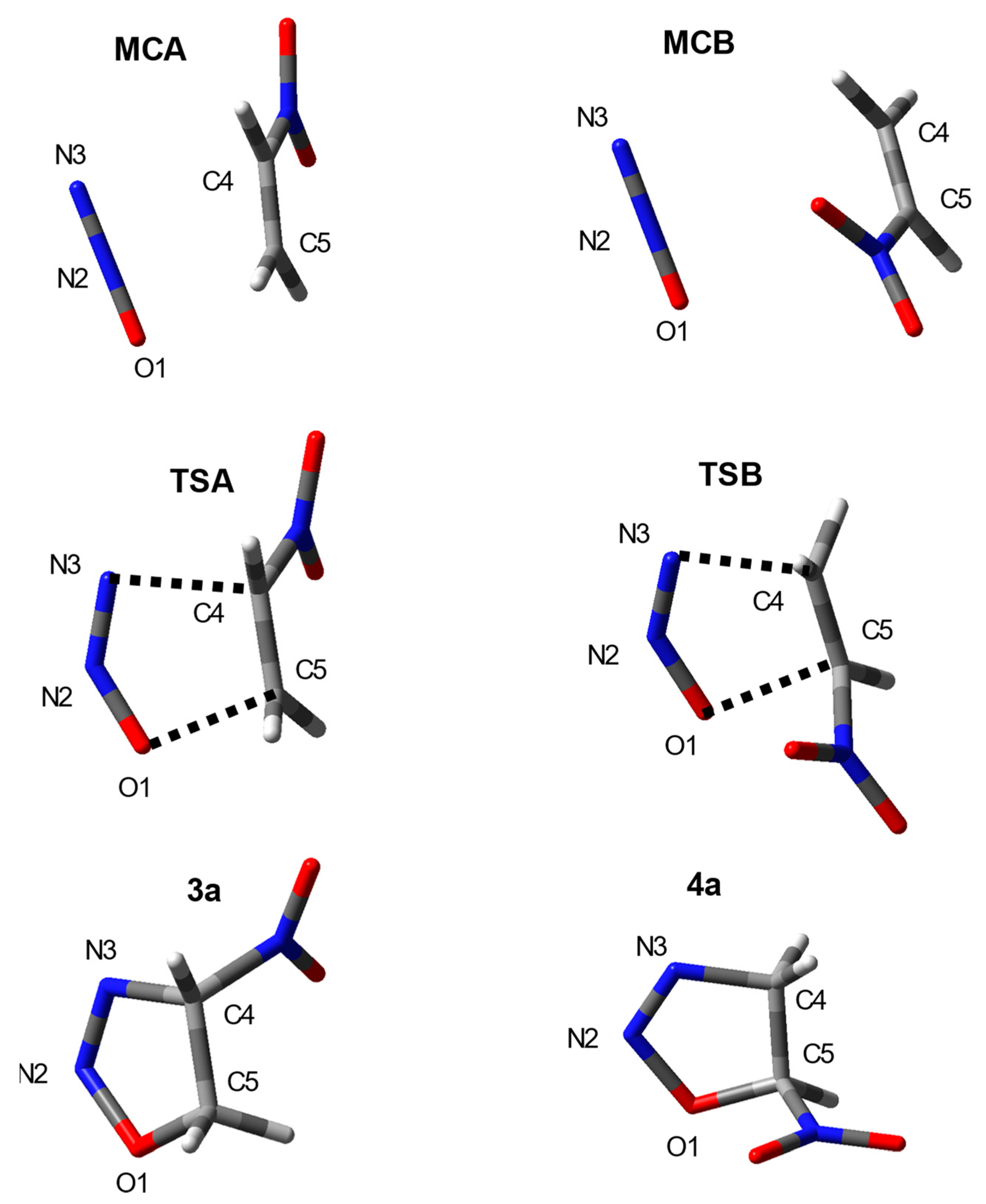
2. Results and Discussion
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Luo, L.; Wang, Q.; Xiang, Y.; Peng, X.; Hu, C. Synthesis and biological evaluation of novel thiazolo[4,5-d]pyrimidin-7(6H)-ones as topoisomerase I inhibitors. Chem. Heterocycl. Compd. 2021, 57, 1220–1229. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Ngo, T.H.; Tran, H.T.; Dinh, T.P.; Do, P.T.; Nguyen, H.B.; Tran, L.T.P.; Ta, H.M. Synthesis and Anticancer Activity of 11-azaartemisinin Derivatives Bearing 1,2,3-triazole Moiety. Chem. Heterocycl. Compd. 2021, 57, 1037–1044. [Google Scholar] [CrossRef]
- Bhat, S.I.; Kigga, M.; Heravi, M.M. Multicomponent reactions based on in situ generated isocyanides for the construction of heterocycles. Chem. Heterocycl. Compd. 2021, 57, 709–719. [Google Scholar] [CrossRef]
- Noriega, S.; Cardoso-Ortiz, J.; López-Luna, A.; Cuevas-Flores, M.D.R.; Flores De La Torre, J.A. The Diverse Biological Activity of Recently Synthesized Nitro Compounds. Pharmaceuticals 2022, 15, 717. [Google Scholar] [CrossRef]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Comppounds to Amines. Org. Process Res. Dev. 2018, 22, 430–445. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Understanding the molecular mechanism of γ-elimination of nitrous acid in the framework of the molecular electron density theory. J. Comput. Chem. 2021, 42, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, S.; Zhao, Y.; Wang, D.Z. New Approach to Oximes through Reduction of Nitro Compounds Enabled by Visible Light Photoredox Catalysis. Org. Lett. 2013, 15, 2660–2663. [Google Scholar] [CrossRef]
- Kornblum, N.; Brown, R.A. The Synthesis and Characterization of Nitronic Esters. J. Am. Chem. Soc. 1964, 86, 2681–2687. [Google Scholar] [CrossRef]
- Hao, F.; Nishiwaki, N. Recent Progress in Nitro-Promoted Direct Functionalization of Pyridones and Quinolones. Molecules 2020, 25, 673. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Łapczuk-Krygier, A.; Kula, K.; Demchuk, O.M.; Dresler, E.; Jasiński, R. Regio- and stereoselective synthesis of nitrofunctionalized 1,2-oxazolidine analogs of nicotine. Chem. Heterocycl. Compd. 2020, 56, 120–122. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Dresler, E.; Hordyjewicz-Baran, Z.; Kulesza, R.; Jasiński, R. A unique example of noncatalyzed [3+2] cycloaddition involving (2E)-3-aryl-2-nitroprop-2-enenitriles. Chem. Heterocycl. Compd. 2017, 53, 1161–1162. [Google Scholar] [CrossRef]
- Jasiński, R. In the searching for zwitterionic intermediates on reaction paths of [3 + 2] cycloaddition reactions between 2,2,4,4-tetramethyl-3-thiocyclobutanone S-methylide and polymerizable olefins. RSC Adv. 2015, 5, 101045–101048. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Kącka-Zych, A.; Demchuk, O.M.; Mirosław, B.; Woliński, P.; Jasiński, R. Green synthesis of nitrocyclopropane-type precursors of inhibitors for the maturation of fruits and vegetables via domino reactions of diazoalkanes with 2-nitroprop-1-ene. J. Clean. Prod. 2021, 292, 126079. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Ríos-Gutiérrez, M.; Kula, K.; Woliński, P.; Mirosław, B.; Krawczyk, T.; Jasiński, R. The Participation of 3,3,3-Trichloro-1-nitroprop-1-ene in the [3+2] Cycloaddition Reaction with Selected Nitrile N-Oxides in the Light of the Experimental and MEDT Quantum Chemical Study. Molecules 2021, 26, 6774. [Google Scholar] [CrossRef] [PubMed]
- Woliński, P.; Kącka-Zych, A.; Demchuk, O.M.; Łapczuk-Krygier, A.; Mirosław, B.; Jasiński, R. Clean and molecularly programmable protocol for preparation of bis-heterobiarylic systems via a domino pseudocyclic reaction as a valuable alternative for TM-catalyzed cross-couplings. J. Clean. Prod. 2020, 275, 122086. [Google Scholar] [CrossRef]
- Jasiński, R. Nitroacetylene as dipolarophile in [2+3] cycloaddition reactions with allenyl-type three-atom components: DFT computational study. Monatsh. Chem. 2015, 146, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 5. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Kula, K.; Zawadzińska, K. Local nucleophile-electrophile interactions in [3+2] cycloaddition reactions between benzonitrile N-oxide and selected conjugated nitroalkenes in the light of MEDT computational study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Analysis of the possibility and molecular mechanism of carbon dioxide consumption in the Diels-Alder processes. Pure Appl. Chem. 2021, 93, 427–446. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Pérez, P. Perfluorobicyclo[2.2.0]hex-1(4)-ene as unique partner for Diels–Alder reactions with benzene: A density functional theory study. Theor. Chem. Acc. 2021, 140, 17. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Understanding the uniqueness of the stepwise [4+1]cycloaddition reaction between conjugated nitroalkenesand electrophilic carbene systems with a molecular electrondensity theory perspective. Int. J. Quantum Chem. 2021, 121, 26440. [Google Scholar] [CrossRef]
- Kacka-Zych, A. Push-pull nitronates in the [3+2] cycloaddition with nitroethylene: Molecular Electron Density Theory study. J Mol. Graph. Model. 2020, 97, 1075492. [Google Scholar] [CrossRef]
- Kącka-Zych, A. The Molecular Mechanism of the Formation of Four-Membered Cyclic Nitronates and Their Retro (3+2) Cycloaddition: A DFT Mechanistic Study. Molecules 2021, 26, 4786. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R. Stepwise, zwitterionic course of hetero-Diels–Alder reaction between 1,2,4-triazine molecular systems and 2-cyclopropylidene-1,3-dimethylimidazoline. Chem. Heterocycl. Comp. 2022, 58, 260–262. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
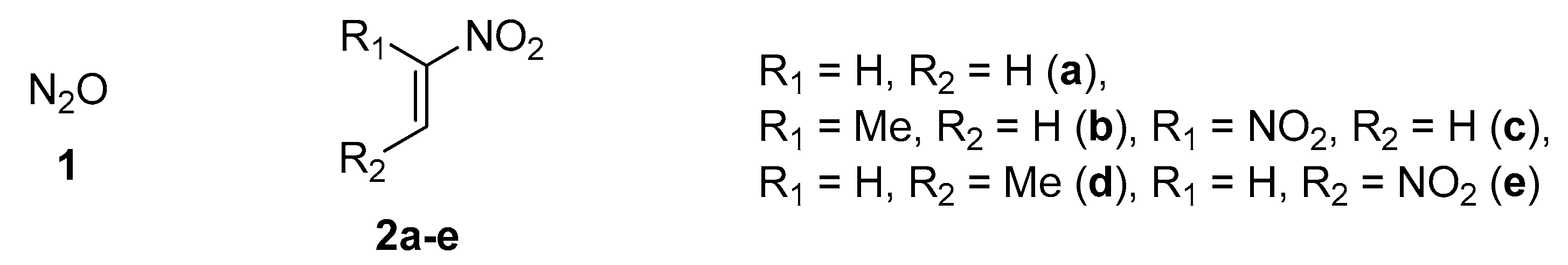
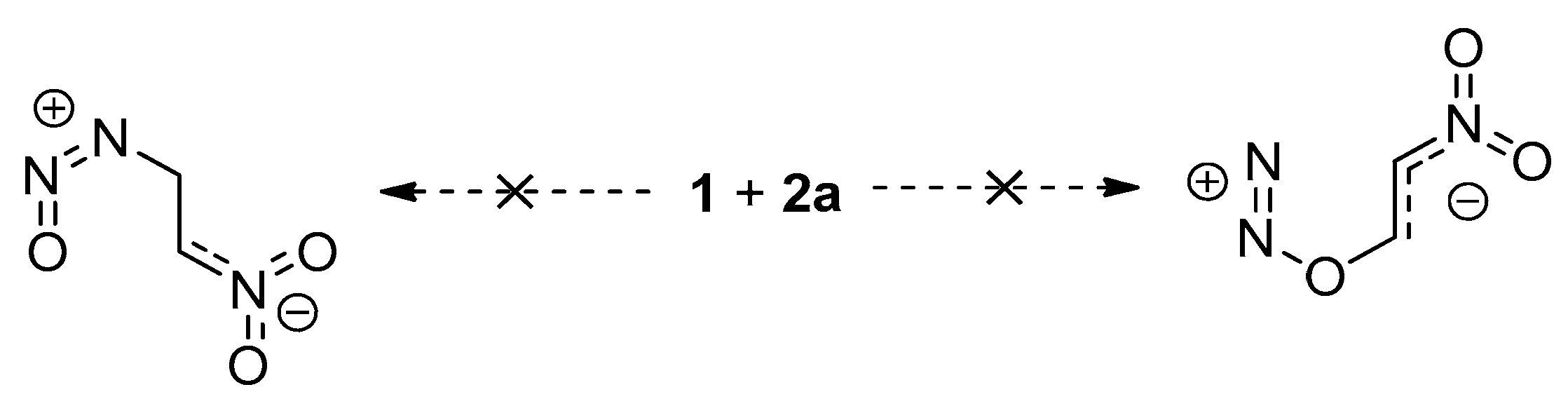
| μ (eV) | η (eV) | ω (eV) | Δω (eV) | |
|---|---|---|---|---|
| 1 | −4.92 | 8.79 | 1.37 | |
| 2a | −5.33 | 5.45 | 2.61 | 1.23 |
| 2b | −5.16 | 5.48 | 2.43 | 1.05 |
| 2c | −5.98 | 5.03 | 3.56 | 2.18 |
| 2d | −5.08 | 5.48 | 2.35 | 0.98 |
| 2e | −6.49 | 4.66 | 4.52 | 3.15 |
| Solvent | Transition | ΔH | ΔS | ΔG |
|---|---|---|---|---|
| Toluene | 1 + 2a→ MCA | −0.2 | −14.1 | 4.0 |
| 1 + 2a→ TSA | 30.3 | −30.3 | 39.3 | |
| 1 + 2a→ 3a | −2.8 | −31.0 | 6.4 | |
| 1 + 2a→ MCB | −0.4 | −16.0 | 4.4 | |
| 1 + 2a→ TSB | 31.2 | −30.4 | 40.2 | |
| 1 + 2a→ 4a | −8.1 | −30.8 | 1.1 | |
| Acetone | 1 + 2a→ MCA | 0.0 | −11.5 | 3.5 |
| 1 + 2a→ TSA | 30.5 | −31.2 | 39.8 | |
| 1 + 2a→ 3a | −3.4 | −32.4 | 6.2 | |
| 1 + 2a→ MCB | −0.7 | −18.7 | 4.9 | |
| 1 + 2a→ TSB | 31.5 | −31.4 | 40.9 | |
| 1 + 2a→ 4a | −9.0 | −32.1 | 0.6 | |
| Nitromethane | 1 + 2a→ MCA | 0.1 | −11.2 | 3.4 |
| 1 + 2a→ TSA | 30.5 | −31.2 | 39.8 | |
| 1 + 2a→ 3a | −3.5 | −32.4 | 6.2 | |
| 1 + 2a→ MCB | −0.7 | −18.6 | 4.9 | |
| 1 + 2a→ TSB | 31.6 | −31.4 | 40.9 | |
| 1 + 2a→ 4a | −9.0 | −32.2 | 0.5 | |
| Water | 1 + 2a→ MCA | 0.1 | −11.2 | 3.4 |
| 1 + 2a→ TSA | 30.5 | −31.2 | 39.8 | |
| 1 + 2a→ 3a | −3.5 | −32.4 | 6.1 | |
| 1 + 2a→ MCB | −0.6 | −18.7 | 4.9 | |
| 1 + 2a→ TSB | 31.6 | −31.4 | 40.9 | |
| 1 + 2a→ 4a | −9.1 | −32.2 | 0.5 | |
| Toluene | 1 + 2b→ MCA | −0.6 | −15.0 | 3.9 |
| 1 + 2b→ TSA | 31.1 | −31.8 | 40.6 | |
| 1 + 2b→ 3b | −3.8 | −33.1 | 6.1 | |
| 1 + 2b→ MCB | −0.9 | −16.5 | 4.0 | |
| 1 + 2b→ TSB | 30.5 | −31.8 | 40.0 | |
| 1 + 2b→ 4b | −8.7 | −33.4 | 1.3 | |
| Toluene | 1 + 2c→ MCA | −0.4 | −15.5 | 4.2 |
| 1 + 2c→ TSA | 31.0 | −33.4 | 40.9 | |
| 1 + 2c→ 3c | −2.3 | −34.2 | 7.9 | |
| 1 + 2c→ MCB | −0.5 | 1169.2 | 4.8 | |
| 1 + 2c→ TSB | 32.8 | −33.5 | 42.8 | |
| 1 + 2c→ 4c | −9.7 | −33.8 | 0.4 | |
| Toluene | 1 + 2d→ MCA | −0.6 | −13.8 | 3.5 |
| 1 + 2d→ TSA | 30.8 | −30.6 | 40.0 | |
| 1 + 2d→ 3d | −2.5 | −32.2 | 7.1 | |
| 1 + 2d→ MCB | −0.6 | −16.3 | 4.3 | |
| 1 + 2d→ TSB | 33.4 | −30.8 | 42.6 | |
| 1 + 2d→ 4d | −6.8 | −32.1 | 2.8 | |
| Toluene | 1 + 2e→ MC | −0.3 | −20.5 | 5.8 |
| 1 + 2e→ TS | 31.1 | −35.3 | 41.7 | |
| 1 + 2e→ 3e | −4.2 | −35.4 | 6.3 |
| Solvent | Reaction | Structure | Interatomic Distances r (Å) | lC3C4 | lC5O1 | Δl | GEDT (e) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O1-N2 | N2-N3 | N3-C4 | C4-C5 | C5-O1 | |||||||
| Toluene | 1 + 2a | 1 | 1.179 | 1.119 | |||||||
| 2a | 1.320 | ||||||||||
| MCA | 1.179 | 1.118 | 3.570 | 1.321 | 3.326 | ||||||
| TSA | 1.225 | 1.159 | 2.058 | 1.383 | 1.971 | 0.601 | 0.630 | 0.03 | 0.19 | ||
| 3a | 1.364 | 1.226 | 1.471 | 1.517 | 1.438 | ||||||
| MCB | 1.179 | 1.118 | 3.558 | 1.321 | 3.315 | ||||||
| TSB | 1.217 | 1.165 | 1.919 | 1.382 | 2.079 | 0.690 | 0.488 | 0.20 | 0.19 | ||
| 4a | 1.456 | 1.206 | 1.464 | 1.529 | 1.375 | ||||||
| Acetone | 1 + 2a | 1 | 1.179 | 1.118 | |||||||
| 2a | 1.321 | ||||||||||
| MCA | 1.178 | 1.118 | 3.407 | 1.321 | 3.294 | ||||||
| TSA | 1.224 | 1.158 | 2.068 | 1.383 | 1.970 | 0.594 | 0.633 | 0.04 | 0.18 | ||
| 3a | 1.361 | 1.227 | 1.471 | 1.516 | 1.441 | ||||||
| MCB | 1.179 | 1.118 | 3.612 | 1.321 | 3.308 | ||||||
| TSB | 1.217 | 1.165 | 1.913 | 1.382 | 2.091 | 0.693 | 0.478 | 0.21 | 0.17 | ||
| 4a | 1.458 | 1.206 | 1.464 | 1.528 | 1.375 | ||||||
| Nitromethane | 1 + 2a | 1 | 1.179 | 1.118 | |||||||
| 2a | 1.321 | ||||||||||
| MCA | 1.178 | 1.118 | 3.406 | 1.322 | 3.294 | ||||||
| TSA | 1.224 | 1.158 | 2.069 | 1.383 | 1.970 | 0.594 | 0.634 | 0.04 | 0.18 | ||
| 3a | 1.361 | 1.227 | 1.471 | 1.516 | 1.441 | ||||||
| MCB | 1.179 | 1.118 | 3.612 | 1.321 | 3.308 | ||||||
| TSB | 1.217 | 1.165 | 1.913 | 1.382 | 2.092 | 0.694 | 0.478 | 0.22 | 0.17 | ||
| 4a | 1.458 | 1.206 | 1.464 | 1.528 | 1.375 | ||||||
| Water | 1 + 2a | 1 | 1.179 | 1.118 | |||||||
| 2a | 1.321 | ||||||||||
| MCA | 1.178 | 1.118 | 3.398 | 1.322 | 3.296 | ||||||
| TSA | 1.224 | 1.158 | 2.069 | 1.383 | 1.970 | 0.594 | 0.634 | 0.04 | 0.18 | ||
| 3a | 1.360 | 1.227 | 1.471 | 1.516 | 1.442 | ||||||
| MCB | 1.179 | 1.118 | 3.612 | 1.321 | 3.309 | ||||||
| TSB | 1.217 | 1.165 | 1.913 | 1.382 | 2.093 | 0.694 | 0.477 | 0.22 | 0.17 | ||
| 4a | 1.459 | 1.206 | 1.464 | 1.528 | 1.375 | ||||||
| Toluene | 1 + 2b | 2b | 1.325 | ||||||||
| MCA | 1.178 | 1.119 | 3.305 | 1.325 | 3.329 | ||||||
| TSA | 1.225 | 1.158 | 2.071 | 1.388 | 1.985 | 0.598 | 0.617 | 0.02 | 0.21 | ||
| 3b | 1.369 | 1.223 | 1.477 | 1.526 | 1.435 | ||||||
| MCB | 1.179 | 1.118 | 3.512 | 1.325 | 3.153 | ||||||
| TSB | 1.216 | 1.166 | 1.926 | 1.386 | 2.122 | 0.683 | 0.466 | 0.22 | 0.19 | ||
| 4b | 1.442 | 1.211 | 1.463 | 1.527 | 1.383 | ||||||
| Toluene | 1 + 2c | 2c | 1.317 | ||||||||
| MCA | 1.181 | 1.118 | 3.981 | 1.317 | 3.150 | ||||||
| TSA | 1.227 | 1.152 | 2.098 | 1.389 | 1.886 | 0.556 | 0.686 | 0.13 | 0.34 | ||
| 3c | 1.353 | 1.232 | 1.452 | 1.512 | 1.436 | ||||||
| MCB | 1.179 | 1.118 | 3.675 | 1.317 | 3.113 | ||||||
| TSB | 1.212 | 1.166 | 1.866 | 1.386 | 2.057 | 0.714 | 0.463 | 0.25 | 0.34 | ||
| 4c | 1.528 | 1.193 | 1.451 | 1.536 | 1.338 | ||||||
| Toluene | 1 + 2d | 2d | 1.326 | ||||||||
| MCA | 1.180 | 1.118 | 3.468 | 1.327 | 3.174 | ||||||
| TSA | 1.224 | 1.161 | 2.041 | 1.389 | 2.015 | 0.607 | 0.608 | 0.00 | 0.20 | ||
| 3d | 1.361 | 1.228 | 1.465 | 1.525 | 1.447 | ||||||
| MCB | 1.178 | 1.119 | 3.463 | 1.326 | 3.362 | ||||||
| TSB | 1.220 | 1.166 | 1.928 | 1.388 | 2.070 | 0.690 | 0.491 | 0.20 | 0.19 | ||
| 4d | 1.460 | 1.206 | 1.472 | 1.536 | 1.371 | ||||||
| Toluene | 1 + 2e | 2e | 1.318 | ||||||||
| MC | 1.180 | 1.118 | 3.493 | 1.319 | 3.038 | ||||||
| TS | 1.221 | 1.160 | 1.982 | 1.386 | 1.968 | 0.647 | 0.574 | 0.07 | 0.37 | ||
| 3e | 1.412 | 1.217 | 1.465 | 1.523 | 1.380 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Regioselectivity and the Molecular Mechanism of [3 + 2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: A DFT Computational Study. Molecules 2022, 27, 8441. https://doi.org/10.3390/molecules27238441
Dresler E, Wróblewska A, Jasiński R. Understanding the Regioselectivity and the Molecular Mechanism of [3 + 2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: A DFT Computational Study. Molecules. 2022; 27(23):8441. https://doi.org/10.3390/molecules27238441
Chicago/Turabian StyleDresler, Ewa, Aneta Wróblewska, and Radomir Jasiński. 2022. "Understanding the Regioselectivity and the Molecular Mechanism of [3 + 2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: A DFT Computational Study" Molecules 27, no. 23: 8441. https://doi.org/10.3390/molecules27238441
APA StyleDresler, E., Wróblewska, A., & Jasiński, R. (2022). Understanding the Regioselectivity and the Molecular Mechanism of [3 + 2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: A DFT Computational Study. Molecules, 27(23), 8441. https://doi.org/10.3390/molecules27238441









