Abstract
A rapid, cheap and feasible new approach was used to synthesize the Mg0.375Fe0.375Al0.25-LDH in the presence of tetramethylammonium hydroxide (TMAH), as a nontraditional hydrolysis agent, applying both mechano-chemical (MC) and co-precipitation methods (CP). For comparison, these catalysts were also synthesized using traditional inorganic alkalis. The mechano-chemical method brings several advantages since the number of steps and the energy involved are smaller than in the co-precipitation method, while the use of organic alkalis eliminates the possibility of contaminating the final solid with alkaline cations. The memory effect was also investigated. XRD studies showed Fe3O4 as stable phase in all solids. Regardless of the alkalis and synthesis methods used, the basicity of catalysts followed the trend: mixed oxides > parent LDH > hydrated LDH. The catalytic activity of the catalysts in the Claisen–Schmidt condensation between benzaldehyde and cyclohexanone showed a linear dependence to the basicity values. After 2 h, the calcined sample cLDH-CO32−/OH−-CP provided a conversion value of 93% with a total selectivity toward 2,6-dibenzylidenecyclohexanone. The presence of these catalysts in the reaction media inhibited the oxidation of benzaldehyde to benzoic acid. Meanwhile, for the self-condensation of cyclohexanone, the conversions to mono- and di-condensed compounds did not exceed 3.8%.
1. Introduction
Layered double hydroxides (LDHs) are a class of ionic solids characterized by a layered structure with a generic layer sequence showing a general formula [M2+1−xM3+x(OH)2]x+[An−x/n]·mH2O [1], with M2+ and M3+ as bi- and trivalent cations in an octahedral geometry [2], A as an anion with charge n, x as the M3+/(M2+ + M3+) ratio and m as the number of water molecules [3,4]. While these kind of solids were firstly discovered in Sweden in mineral ores around 1842, their first artificial synthesis was covered one century later by a patent in 1970 [5]. However, the real interest in this class of solids with base properties started with the work of Cavani et al. [1]. It has been strengthened by potential applications in different areas of strategic importance such as medicine [6], environmental protection, ion exchange, adsorption [7,8], polymer stabilization [9,10,11], corrosion inhibition [12], catalysis [13,14], batteries [15], filtration [16], etc.
Such materials are constituted from positively charged brucite-like layers with negatively charged interlayers compensating anions as well as water molecules, resulting in a charge-balanced architecture [17]. These structures can incorporate a large number of cations with dimensions close to those of Mg2+, such as Ni2+, Cu2+, Zn2+, Co2+, Fe2+, Mn2+, Ga3+, Fe3+, Al3+, In3+, Ti4+, Zn4+ [1,18], etc. Thus, depending on the number of the cations, LDHs with binary [19], ternary [20], or quaternary [21] structures can be produced. Further tuning of the particular electronegativity of the cations may lead to the optimization of the acid-base properties (ditopic character) of the synthesized materials. Furthermore, due to the flexibility of the interlayer space, larger anions such as phthalocyanines [22], polyoxometalate [23], citrate [24], etc. can be also inserted in the structure. Even more, recent publications reported the intercalation of large structures such as graphene [25], carbon nanotubes [26], polymers [27], etc., opening opportunities to more complex materials.
Traditionally, the LDH can be synthesized by several methods [1] such as (i) co-precipitation at constant pH, at low/high supersaturation, or increasing pH, by titration with NaOH and/or NaHCO3; (ii) hydrothermal treatments under pressure in an autoclave at a higher temperature or by aging at a lower temperature; and (iii) anionic exchange or memory effect reconstruction, which enables the reformation of the layered structure following the hydration of the mixed oxides generated by thermal treatments applied to the parent LDH at temperatures that do not exceed 600 °C. However, these methods have some disadvantages such as numerous steps, high energy, time consumption, large volumes of water for washing the solids, etc. Other routes such as urea hydrolysis, sol-gel, electro-synthesis or the mechano-chemical method may offer alternatives for the preparation of these materials. Even though, the ball milling in the absence of water or other solvents [28,29,30,31] has some limitations. Larger cations such as La (1.032 Å) or Y (0.9 Å) [18] can be accommodated into the octahedral positions of the layered network only in small amounts.
The use of organic alkalis seems to be an extremely good alternative to these drawbacks despite a higher price compared to that of the inorganic ones. Several LDH materials containing larger cations such as Cu/Mn-LDH [32], Zn/Al-LDH [33], Ni/Cr, Ni/Fe, or Ni/Co-LDHs [34] were prepared in this way. Our group also investigated LDH syntheses in the presence of organic alkali focusing on the size of the chain [35].
Among the investigated reactions in the presence of the LDH catalysts [36], aldol condensation, Claisen–Schmidt and Knoevenagel reactions, Michael addition, Baeyer–Villiger oxidation, Weitz–Scheffer epoxidation, nitriles hydrolysis, alkylation, and acylation or polymerization are most frequently considered as references. Specifically, Claisen–Schmidt condensation [37,38] is dedicated to the formation of new C–C bonds. The condensation of cyclohexanone and benzaldehyde to mono-, 2-benzylidene-cyclohexanone, or di-condensation, 2,6-dibenzylidene-cyclohexanone, is also an important subject [39,40,41,42,43,44,45,46]. However, the Claisen–Schmidt condensation has some limitations due to the possibility of the appearance of side reactions, such as self-condensation, as an effect of the additional presence of the acid sites.
Already published articles showed that for this reaction, the conversions may reach up to 60% in the presence of mixed oxide type catalysts with pronounced basicity obtained from layered materials modified with rare-earth cations [47]. The same activity was also displayed by a perfluorosulfonic acid resin catalyst, HRF5015 [48]. On the other hand, benzaldehyde may be oxidized with oxygen from air, both in the absence/presence of the catalyst. In that case the performances are related to the acidity strength of the catalyst [49].
Starting from this state of the art, the aims of this paper are: (i) to bring new perspectives in adapting the physicochemical properties of Mg0.325Fe0.325Al0.25-LDH through two non-traditional parameters, e.g., the mechano-chemical preparation method and the use of organic alkali (TMAH) as a hydrolysis agent; (ii) the possibility of protecting Fe2+ against oxidation by its inclusion in a solid LDH network; (iii) to optimize the Claisen–Schmidt condensation between benzaldehyde and cyclohexanone to the desired condensation product.
2. Results and Discussion
2.1. Characterization of Catalysts
Except for the LDH-TMAH-MC (Figure 1D, in black) the XRD patterns of the parent LDHs presented the typical reflections of layered materials with intense and symmetric reflections at small angles and broad and weak reflections at higher angles [1].
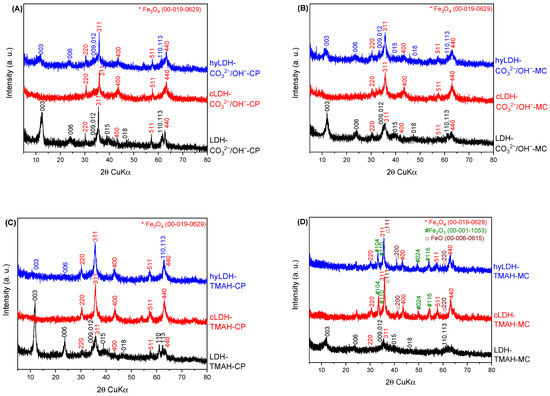
Figure 1.
XRD patterns of the synthesized materials (A)—co-precipitation method/inorganic alkali; (B)—mechano-chemical method/inorganic alkali; (C)—co-precipitation method/organic alkali; (D)—mechano-chemical method/organic alkali; * Fe3O4; # Fe2O3; □ FeO).
The presence of iron was identified from the distinguishable lines of Fe3O4 (ICDD 00-019-0629, intense 311 and weaker 220, 400, 511, and 440) which highlight the oxidation of Fe2+ to Fe3+ with the formation of the stable FeO·Fe2O3 structure. While the network parameter a (expressing the cation-cation distance in the layered structure) did not change significantly, the c parameter changed, proving a slight increase for the co-precipitated samples compared to the mechano-chemical ones (Table 1) associated with the presence of small amounts of TMAH and tri-methyl amine (an impurity present in TMAH) [39].

Table 1.
The structural parameters of the prepared samples.
At the same time, the value of c parameter is slightly higher in the case of mechano-chemically obtained samples compared to those obtained by co-precipitation due to the higher amount of embedded Fe3O4 phase. It should be highlighted that the pattern with the most intense diffraction lines is that of the sample obtained in the presence of TMAH, i.e., LDH-TMAH-CP, which also has the lowest value of crystallite size (111 Å). The LDH-TMAH-MC sample contains a high amount of amorphous phase with the largest dimension of crystallite size of 600 Å. The impurity phases, obtained during the synthesis, are probably located in the interlayer space as well as onto the surface of the LDH lamellae, as is suggested by the decreasing of the I003/I110 ratio value, e.g., 4.30 (LDH-CO32−/OH−-CP); 3.50 (LDH-CO32−/OH−-MC); 3.73 (LDH-TMAH-CP); 1.13 (LDH-TMAH-MC) as well as by the shift to lower 2θ values of the 003 diffraction lines (12.6607; 11.9400; 11.7720; 11.2666) [50]. The calcination of LDH samples at 460 °C for 18 h lead to corresponding mixed oxides where Fe3O4 is the prevailing crystalline phase, Figure 1 (red lines). Using TMAH in the mechano-chemical preparation generated more crystalline phases of FexOy, e.g., FeO (ICDD 00-006-0615), Fe2O3 (ICDD 00-001-1053) and Fe3O4 (ICDD 00-019-0629). The presence of these separate oxides in the calcined samples may be a consequence of the co-existence of Fe2+ and Fe3+ amorphous hydroxyl/hydroxycarbonate phases in the parent LDH material. Therefore, it can be concluded that part of Fe2+ is oxidized to Fe3+ and the presence of the organic alkali does not succeed in avoiding the oxidation.
The reconstruction of the layered structure by memory effect occurs slightly for the materials prepared with inorganic alkalis, while in the case of the materials synthesized in the presence of TMAH the diffraction lines corresponding to the LDH structure have a very low intensity. The I003/I006 ratio shows a sudden decrease from the parent LDHs to the rehydrated samples, a fact that sustains the above statement.
The presence of stable oxides in the rehydrated samples is also highlighted by the increased values of the IFS parameter for the LDH phase for the samples hyLDH-CO32−/OH−-CP, hyLDH-CO32−/OH−-MC, and hyLDH-TMAH-CP, compared to the corresponding parent LDHs. Both cLDH-TMAH-MC and hyLDH-TMAH-MC present similar diffraction lines, suggesting a higher stability of the oxide phases and, as a consequence, a poor memory effect. Meanwhile, for the sample hyLDH-TMAH-MC that has the largest crystallite size D003 (1296 Å), the IFS parameter decreases to 2.63 compared to the 2.84 of LDH-TMAH-MC. This fact may be a consequence of the formation of a compact mixture of oxide phases.
Diffuse reflectance infrared Fourier transform (DRIFT) spectra of the parent LDH (Figure 2, black line) present a large band in the 3700–3400 cm−1 domain corresponding to the vibration of hydroxyl groups, υO-H, while that at 3000 cm−1 is assigned to hydrogen bonds present between carbonate anion and water molecules from the interlayer space [29].
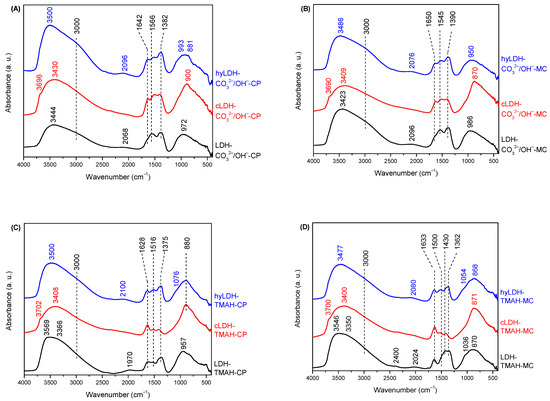
Figure 2.
DRIFT spectra of the materials ((A)—co-precipitation method/inorganic alkali; (B)—mechano-chemical method/inorganic alkali; (C)—co-precipitation method/organic alkali; (D)—mechano-chemical method/organic alkali).
The band at 1628–1650 cm−1 is characteristic to the H2O bending vibration in the LDH interlayer, while the one at 1500–1566 cm−1 is assigned to C=O bond from the surface. The characteristic vibrations for bidentate carbonate ν(CO32−) are noticed at 1362–1390 cm−1, and 1200–600 cm−1. The bands below 600 cm−1 correspond to Mg–O and Al–O bonds while the one at 450–460 cm−1 is characteristic to Fe-O vibrations. The calcined samples show a new band at 3690–3702 cm−1 characteristic to the stretching vibrations of isolated structural hydroxyl groups connected to hexacoordinated Mg species [52] highly dispersed in the solid matrix. The presence of an inflexion point around 3000 cm−1 in the spectra of the calcined samples prepared in the presence of inorganic alkalis is due to the residual CO32− and OH− after the calcination at a temperature of 460 °C. Noteworthy, this temperature is the key factor responsible for the memory effect. Higher temperatures lead to the stabilization of the cations in the tetrahedral positions [53].
As an interesting fact, only the LDH-TMAH-CP sample showed a very weak band characteristic to Fe0-CO at 1970 cm−1 stretching [54]. The samples prepared in the presence of inorganic alkalis presented bands at 2068 and 2096 cm−1, respectively, attributed to Fe2+-CO stretching vibration. Hence it may be inferred that iron carbonic phases are present in the parent LDH under an amorphous state and are not visible in the XRD patterns. It should be also highlighted that regardless of the type of LDH, Fe2+ is oxidized to Fe3+ during the hydration process, as it is evidenced by the presence of the weak bands at 2011–2076 cm−1 corresponding to the Fe3+-CO stretching [54].
The DR-UV-Vis spectra of the samples (Figure 3) display a quasi-similar shape, proving the simultaneous presence of Fe2+ and Fe3+ species. The band at 638 nm corresponding to the Fe2+ species is more intense for the fresh sample prepared using the co-precipitation method with inorganic alkali. It has similar intensities for the fresh samples prepared with TMAH, and the lowest intensity for the fresh LDH-CO32−/OH−-MC, most probably caused by the oxidation during the mechano-chemical process. It should be noted that the fresh sample prepared with TMAH by the mechano-chemical method was not oxidized to the same extent as the one prepared with inorganic alkali. The intensity of the absorbance in this region decreased significantly for the calcined and rehydrated samples, confirming the oxidation of Fe2+ species during these treatments.
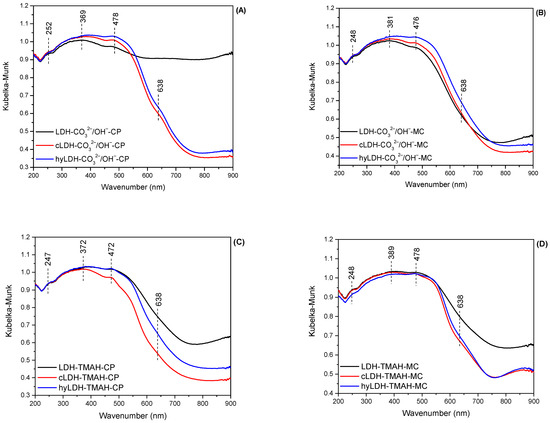
Figure 3.
DR-UV-Vis spectra of the materials ((A)—co-precipitation method/inorganic alkali; (B)—mechano-chemical method/inorganic alkali; (C)—co-precipitation method/organic alkali; (D)—mechano-chemical method/organic alkali).
All the spectra showed strong absorption below 500 nm due to the presence of Fe3+ species [55]. However, several specific species were also distinguished from the bands at 247–252 nm assigned to isolated Fe3+, at 369–389 nm characteristic to FexOy oligomers, and at 472–478 nm assigned to Fe2O3 particles [56].
The characterization by Raman spectroscopy (Figure 4) confirmed the presence of the iron oxides even in the parent LDH samples, a fact also certified by the above presented characterization techniques. Thus, the characteristic bands of hematite Fe3O4, undergoing a slight shifting depending on the spatial disposition were observed (E1g 200–247 cm−1; E1g 291–293 cm−1; E1g 315–336 cm−1; E1g 403–408 cm−1; A1g 483–488 cm−1; E1g 605–612 cm−1) [57]. The calcined/hydrated mechano-chemically synthesized samples presented weak bands corresponding to maghemite (γ-Fe2O3) at 340 cm−1 (T1), 483–485 cm−1 (E), and 700–730 cm−1 (A1). This fact is somewhat unexpected because in accordance to the literature data at 400 °C, i.e., below the calcination temperature (460 °C), the Fe3O4 phase usually turns into α-Fe2O3, while the γ-Fe2O3 phase is stable in the temperature range of 200–400 °C [57]. However, the variable intensity ratio of E1g 200–247 cm−1 and E1g 291–293 cm−1 levels indicated the presence of a certain heterogeneity at the micro-scale level of the iron mixed oxide samples [58]. A weak band at 656–663 cm−1 corresponding to FeO (wustite) was also observed for the calcined/hydrated samples [59].
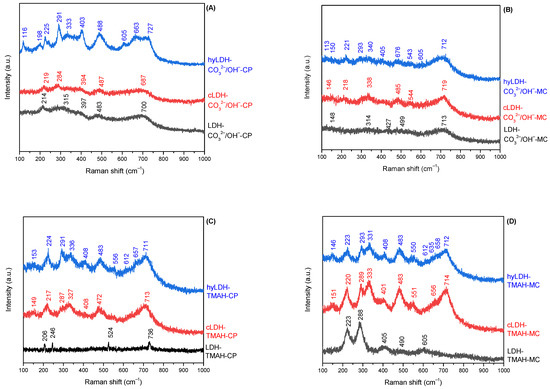
Figure 4.
Raman spectra of the materials ((A)—co-precipitation method/inorganic alkali; (B)—mechano-chemical method/inorganic alkali; (C)—co-precipitation method/organic alkali; (D)—mechano-chemical method/organic alkali).
Regardless of the preparation route or the base, the basicity of the iron-containing samples (Table 2) presented an atypical variation, i.e., mixed oxides > parent LDH > reconstructed LDH, also showing the lowest value for the hydrated samples. It was also different to Mg(MII)/Al(MIII)-LDH [3,29,39] where the trend was mixed oxides > reconstructed LDH > parent LDH. This behavior can be assigned to the presence of the iron oxides in the layered materials (parent or hydrated). Nevertheless, a certain influence of the CO32−/OH− ratio cannot be totally eliminated, since these are present in the hydrated materials prepared by the co-precipitation with inorganic alkalis. We can therefore speculate that the lower basicity of the hydrated LDH compared to the parent LDH is due to the structural alterations of the oxides in the presence of water. These alterations were already evidenced by the characterization techniques.

Table 2.
The surface area and basicity of catalysts.
All the samples showed a Type IV adsorption–desorption isotherm with a shape characteristic to mesoporous materials (Figure 5). Only LDH-TMAH-MC showed a deviation from the traditional shape due to the presence of a mixture of amorphous phases.
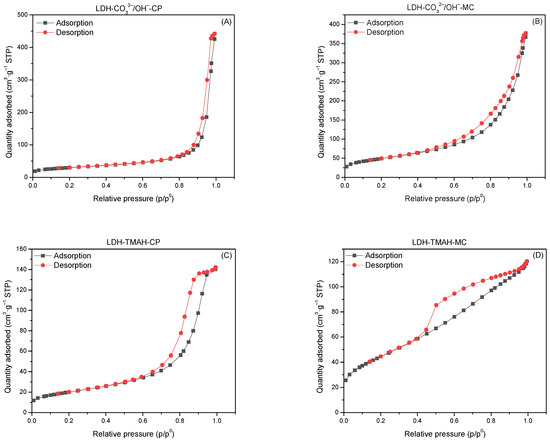
Figure 5.
The adsorption–desorption isotherms of LDH sample.
In addition to the hydrolysis agent activity, TMAH also behaves in the synthesis of LDH as a template molecule that generates a porosity that does not exceed the value of 200 Å, while inorganic alkalis generate porosity on a wide range up to 1200 Å (Figure 6). If in the case of mechanochemically obtained materials there is only one domain of pores of 108 Å for LDH-TMAH-CP and, respectively, 37 Å for LDH-TMAH-MC, the co-precipitation method leads to obtaining several domains, e.g., 31 Å, 214 Å, and 288 Å for LDH-CO32−/OH−-CP and 38 Å, 66 Å, and 244 Å for LDH-CO32−/OH−-MC. Unlike these, LDH-TMAH-MC showed an extremely narrow pore distribution that was even narrower compared to LDH-TMAH-CP.
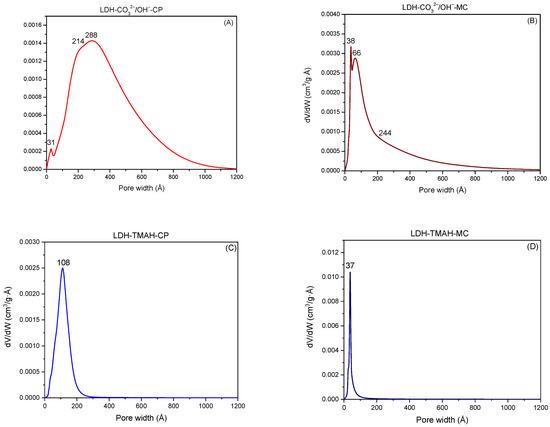
Figure 6.
The representation of LDH sample pore widths.
2.2. Catalytic Activity
2.2.1. Claisen–Schmidt Condensation
The blank tests at room temperature, after 2h, led to a conversion of cyclohexanone below 1%, that further increased at 10% at 120 °C for a total selectivity to 2,6-dibenzylidene-cyclohexanone (the di-condensed product). The samples synthesized by the co-precipitation method showed a higher activity compared to those prepared mechano-chemically (Figure 7). The samples prepared in the presence of TMAH showed slightly lower values compared to those prepared with inorganic alkalis. However, the variation of the catalytic activity kept a linear trend versus the variation of the total basicity, e.g., mixed oxides > parent LDH > hydrated LDH (Figure 8). A total selectivity to the di-condensed product has been preserved in all the cases being in accordance to the twisting ability of the mono-condensed product. This behavior can also be associated with the possibility of a further adsorption on the surface active sites leading to the di-condensed product (Scheme 1) [39]. This assumption is also supported by the cyclohexanone self-condensation where the main product is a mono-condensed compound as an effect of a steric hindrance.
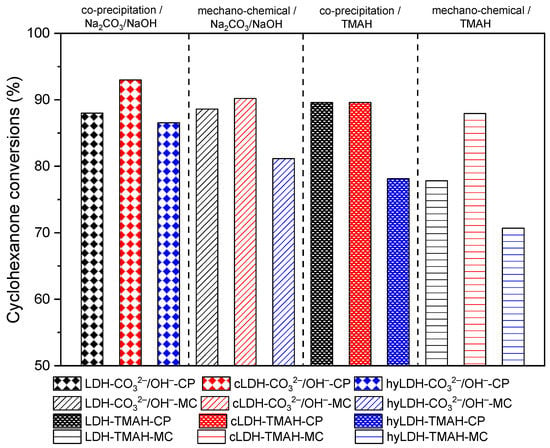
Figure 7.
Cyclohexanone conversion of samples (2 h, 120 °C, 20 mg of catalyst, solvent-free).
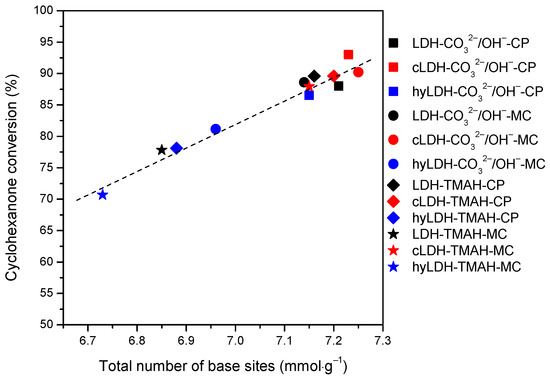
Figure 8.
The cyclohexanone conversion vs. total number of base sites for investigated catalysts (2 h, 120 °C, 20 mg of catalyst, solvent-free).
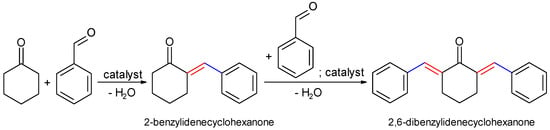
Scheme 1.
Claisen–Schmidt condensation (benzaldehyde (0.002 mol), cyclohexanone (0.001 mol) and 20 mg of catalyst, 600 rpm, 2 h, 120 °C, solvent-free conditions).
Under similar reaction conditions, the investigated mixed oxide catalysts showed superior activities compared to those reported with activated NaOH/fly ash catalyst [41], and comparable to the cesium salts of 12-tungstophosphoric acid catalysts [60] or ultrasound activation [61].
2.2.2. Benzaldehyde Oxidation as Possible Side Reaction
Considering that benzaldehyde can be easily oxidized to benzoic acid, we further investigated the behavior of these catalysts in this oxidation (Scheme 2). The blank test at room temperature after 2 h displays a benzaldehyde conversion of 9%, while after 2 h at 120 °C it increased up to 31.5%. Meanwhile, the presence of these catalysts inhibited the oxidation. As an effect, even the highest conversion was below 6% (cLDH-TMAH-MC), (Table 3). Furthermore, there was no correlation between the catalysts’ basicity and the conversion levels.
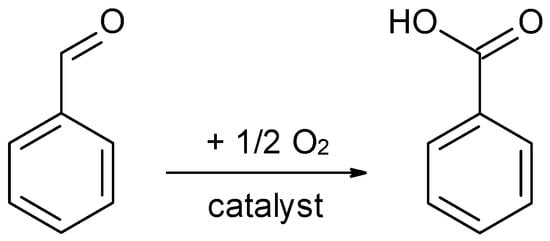
Scheme 2.
The oxidation of benzaldehyde (0.01 mol benzaldehyde, 53 mg catalyst under solvent-free conditions, 600 rpm, 2 h, 120 °C).

Table 3.
The benzaldehyde conversion in oxidation reaction with molecular oxygen from air under ambient pressure (2 h, 120 °C, 53 mg of catalyst, solvent-free).
2.2.3. Self-Cyclohexanone Condensation as Possible Side Reaction
The conversion of cyclohexanone in the blank test after 2 h at room temperature did not exceed 1%, while at 120 °C the conversion increased up to 6% with a selectivity of 91.5% to 2-(1-cyclohexenyl)-cyclohexanone (mono-condensed product/Mono-A). This reaction commonly proceeds via an enolate ion intermediate where the double bonds are able to cyclically migrate between the two cycles generating (Di-A) and (Di-B) products (Scheme 3) [47].
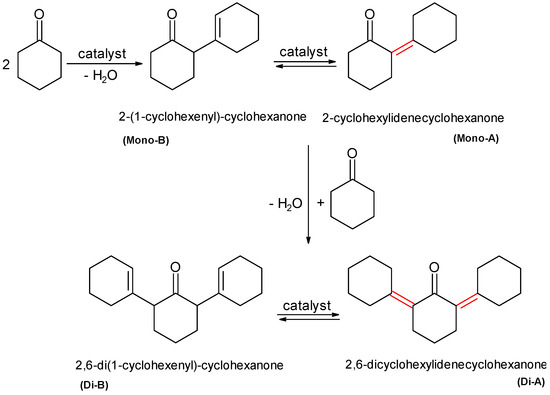
Scheme 3.
The aldol self-condensation (0.01 mol cyclohexanone, 50 mg catalyst under solvent-free conditions, 600 rpm, 5 h, 120 °C).
Compared to the layered materials, the mixed oxides presented better conversion, but the resulted products were no longer similar to the ones in the Claisen–Schmidt condensation (Figure 9). The variation trend of the conversions on the parent LDHs was also dissimilar to that on the hydrated LDHs. Concerning the selectivity to the mono-condensation product, these were superior to those for the di-condensed product (Table 4). This certifies the fact that the double bond between the fragments of the mono-condensed product (red bond, Scheme 3) stiffens the molecule, preventing its adsorption for a subsequent condensation step. The presence of small amounts of di-condensed products is only attributed to the active sites placed on the external geometric surface of the catalysts.
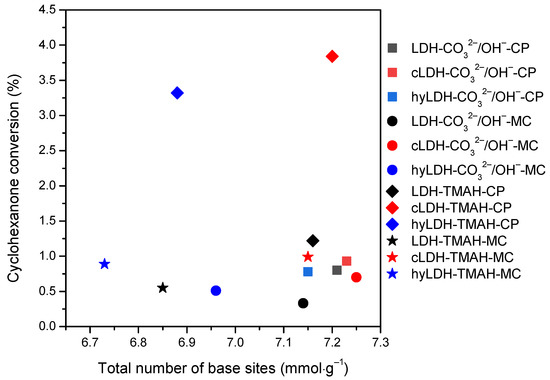
Figure 9.
Cyclohexanone conversion vs. total number of base sites of investigated catalysts in self cyclohexanone condensation (2 h, 120 °C, 50 mg catalyst, solvent-free).

Table 4.
Experimental data for self-cyclohexanone conversion (2 h, 120 °C, 50 mg catalyst, solvent-free).
2.3. Catalyst Reusability
The stability of mixed oxides as the best catalysts was checked in several consecutive Claisen–Schmidt condensation runs. After the third cycle, the conversion decreased by less than 5.1% (Figure 10), reaching a plateau level in the fourth and fifth cycle. The stability of the catalysts was confirmed by the fact that the XRD patterns of the solids recovered after the third cycle did not show modifications of the diffraction lines compared to the patterns of the fresh samples (Figure 11).
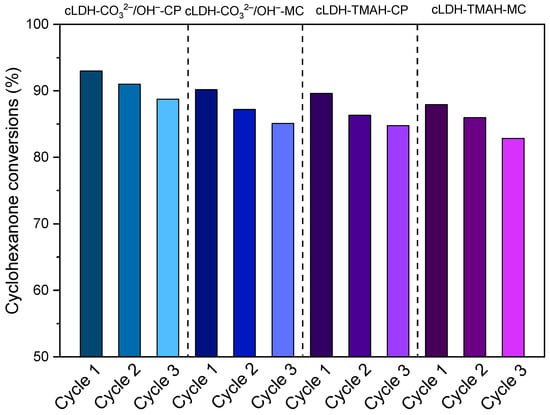
Figure 10.
The catalyst reusability after 3 cycles in Claisen–Schmidt condensation for mixed oxides.
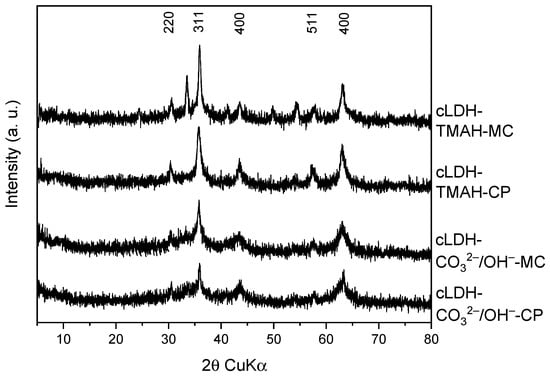
Figure 11.
XRD patterns of the reused mixed oxide catalysts after 3 reaction cycles in the Claisen–Schmidt condensation.
3. Materials and Methods
3.1. Catalyst Preparation
The Mg0.325Fe0.325Al0.25-LDH was synthesized using (i) a co-precipitation and (ii) a mechano-chemical method using either inorganic or organic alkalis for the pH adjustment. The co-precipitation was achieved in the presence of inorganic alkalis following a methodology already reported [29,39] by mixing two salt solutions. The first solution contained Mg(NO3)2·6H2O, FeCl2·4H2O, and Al(NO3)3·9H2O in a 0.325/0.325/0.25 molar ratio at a 1.5 M concentration. The second solution consisted of NaOH and Na2CO3 at a NaOH/Na2CO3 = 2.5 molar ratio and 1 M Na2CO3 concentration. These solutions were mixed at 600 rpm and pH of 10 at RT by using a TIM854, NB pH/EP/Stat pH-STAT Titrator at a feed rate of 60 mL·h−1. After the complete precipitation, the suspension was aged for 18 h at 80 °C in an air atmosphere, cooled at room temperature, filtered, washed with bi-distilled water until a pH of 7, and dried for 24 h in air at 120 °C (LDH-CO32−/OH−-CP). The calcination of the resulting solid was carried out at 460 °C for 18 h in air atmosphere leading to the corresponding mixed oxides (cLDH-CO32−/OH−-CP). The reconstruction of the layered structure was carried out through the memory effect by hydration of cLDH-CO32−/OH−-CP with bi-distilled water for 24 h at RT, followed by drying for 24 h at 120 °C in air (hyLDH-CO32−/OH−-CP). The mechano-chemical method was performed by direct milling of the above mentioned precursors in a Mortar Grinder RM 200 for 1 h at 100 rpm (LDH-CO32−/OH−-MC) at a pH close to 10 without the addition of bi-distilled water or submission to an aging process. Further protocols for the synthesis of the corresponding mixed oxides and reconstructed layered samples (cLDH-CO32−/OH−-MC and hyLDH-CO32−/OH−-MC) were identical to the above-described ones. The same co-precipitation and mechano-chemical protocols were also utilized for the synthesis of LDH in the presence of tetramethylammonium hydroxide (wt. 25% in water). Thus, in the case of co-precipitation, the inorganic alkaline solution (NaOH/Na2CO3) was replaced by an organic one of TMAH while all the synthesis steps and tools were maintained identical. The synthesized materials were denoted as LDH-TMAH-CP, cLDH-TMAH-CP, and hyLDH-TMAH-CP, respectively. For the mechano-chemical route, a volume of TMAH equal to the one employed in the co-precipitation was used, while maintaining all the other procedures identical to those employed for LDH-CO32−/OH−-MC. The initial sample and its derivatives were denoted as LDH-TMAH-MC, cLDH-TMAH-MC, and hyLDH-TMAH-MC, respectively. In the presence of TMAH the processes occurred with a significant decrease (10 times less) of bi-distilled water consumption in the washing step compared to the syntheses in the presence of inorganic alkalis.
3.2. Catalyst Characterization
Solid powder XRD diffraction patterns were recorded on a Shimadzu XRD 7000 diffractometer with Cu Kα radiation (λ = 1.5418 Å, 40 kV, 40 mA) at a scanning speed of 0.10°·min−1 using the 2θ range between 5–80°. DRIFTS spectra were recorded with JASCO FT/IR-4700 spectrometer by acquisition of 128 scans in 4000–400 cm−1 domain. DR UV-VIS spectra were recorded in the range 900–200 nm on Jasco V-650 UV-VIS spectrophotometer equipped with an integration sphere and using Spectralon as white reference. Raman spectra were collected with a Horiba Jobin Yvon–Labram HR UV–Visible–NIR Raman Microscope Spectrometer, using a 632 nm laser. The spectra were collected at the average of 10 scans at a resolution of 2 cm−1 between 100–4000 cm−1 Raman Shift. N2 adsorption-desorption isotherms were determined using a Micromeritics ASAP 2010 instrument, where prior to N2 adsorption-desorption, a vacuum for 24 h at 120 °C was involved for samples degassing. The total number of base sites in the catalysts was determined by the irreversible adsorption of acrylic acid (pKa = 4.2) [62,63,64].
3.3. Catalytic Tests
3.3.1. Claisen–Schmidt Condensation
Claisen–Schmidt condensation was performed in a thermo-stated glass reactor equipped with a water cooled condenser, where a mixture of cyclohexanone (0.001 mol, >99%, Sigma–Aldrich (St. Louis, MO, USA)), benzaldehyde (0.002 mol, >99%, Sigma–Aldrich) and 20 mg of catalyst was stirred (600 rpm) under solvent-free conditions for 2 h at 120 °C [41]. After that, the catalyst was removed by filtration, washed with 1 mL of toluene, and the liquid mixture was analyzed by Thermo-Quest GC provided with a FID detector and a capillary column of 30 m length with DB5 stationary phase. The compounds were also identified using a GC/MS/MS Varian Saturn 2100 T equipped with a CP-SIL 8 CB Low Bleed/MS column of 30 m length and 0.25 mm diameter.
3.3.2. The Aldol Cyclohexanone Self-Condensation
The cyclohexanone self-condensation was carried out in a reactor similar to the above-described one by mixing 0.01 mol of cyclohexanone with 50 mg catalyst under solvent-free conditions [47]. After 2 h at 120 °C, the catalyst was removed from the mixture by filtration and the liquid reaction mixture was analyzed by GC-FID. Furthermore, GC/MS was used for compound identification.
3.3.3. Benzaldehyde Oxidation
The benzaldehyde oxidation by air was carried out under ambient pressure in the same reactor taking 0.01 moles of benzaldehyde and 53 mg catalyst under solvent-free conditions. After 2 h at 120 °C, the catalyst was removed from the mixture by filtration and the reaction mixture was analyzed by GC-FID. Furthermore, GC/MS was used for the compound identification.
3.4. Catalyst Recycling
The catalytic activity of mixed oxides was evaluated in 3 successive tests. The catalysts were separated from the reaction mixture by filtration, washed with 1 mL of toluene, and dried for 5 h at 120 °C in air before subsequent use.
4. Conclusions
The synthesized Fe-LDH materials contained Fe3O4 regardless of the preparation method or alkalis used in the syntheses. The highest crystallinity was displayed by LDH-TMAH-CP, whereas LDH-TMAH-MC exhibited the lowest one. The hydroxyl/hydroxycarbonate phases of Fe2+ and Fe3+ were encapsulated in an amorphous form in the parent LDH. FeO, Fe2O3, and Fe3O4 were also present in the sample prepared by the mechano-chemical method with TMAH. The catalysts basicity paralleled the conversion of cyclohexanone in the Claisen–Schmidt condensation, i.e., mixed oxides > parent LDH > hydrated LDH. The calcination of LDH provided an enhanced catalytic activity (93% conversion) for a total selectivity to 2,6-dibenzylidenecyclohexanone. The self-condensation of cyclohexanone is directed towards the mono-condensation product, 2-(1-cyclohexenyl)-cyclohexanone at a maximal conversion of 3.8% (cLDH-TMAH-CP). The presence of a single or double bond between the fragments of the mono-condensed product, corroborated with the size of the pores in the catalyst, are the essential factors in orientating the transformation.
Author Contributions
Conceptualization, O.D.P. and V.I.P.; methodology, O.D.P. and B.C.; investigation, M.T., B.C. and O.D.P.; resources, R.Z., B.C. and O.D.P.; writing—original draft preparation, O.D.P. and R.Z.; writing—review and editing, O.D.P. and V.I.P.; visualization, O.D.P., M.T. and B.C.; supervision, V.I.P.; funding acquisition, R.Z., B.C. and O.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CCCDI-UEFISCDI, project number PN-III-P2-2.1-PED-2021-1870, within PNCDI III. This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, project number PN-III-P4-ID-PCE-2020-2207, within PNCDI III. Octavian Dumitru Pavel extend his thanks to the University of Bucharest C1.2.PFE_CDI.2021-587/Contract no.41PFE/30.12.2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds LDH-CO32−/OH−-CP; LDH-CO32−/OH−-MC; LDH-TMAH-CP and LDH-TMAH-MC are available from the authors.
References
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Quim. Nova 2004, 27, 601–614. [Google Scholar] [CrossRef]
- Angelescu, E.; Pavel, O.; Bîrjega, R.; Zăvoianu, R.; Costentin, G.; Che, M. Solid base catalysts obtained from hydrotalcite precursors, for Knoevenagel synthesis of cinamic acid and coumarin derivatives. Appl. Catal. A Gen. 2006, 308, 13–18. [Google Scholar] [CrossRef]
- Teodorescu, F.; Slabu, A.; Pavel, O.; Zăvoianu, R. A comparative study on the catalytic activity of ZnAl, NiAl, and CoAl mixed oxides derived from LDH obtained by mechanochemical method in the synthesis of 2-methylpyrazine. Catal. Commun. 2020, 133, 105829. [Google Scholar] [CrossRef]
- Bröcker, F.J.; Kainer, L. German Patent 2.024.282 (1970), to BASF AG, and UK Patent 1.342.020 (1971), to BASF AG.
- Jin, W.; Lee, D.; Jeon, Y.; Park, D.-H. Biocompatible Hydrotalcite Nanohybrids for Medical Functions. Minerals 2020, 10, 172. [Google Scholar] [CrossRef]
- Ulibarri, M.; Pavlovic, I.; Hermosín, M.; Cornejo, J. Hydrotalcite-like compounds as potential sorbents of phenols from water. Appl. Clay Sci. 1995, 10, 131–145. [Google Scholar] [CrossRef]
- Yamaoka, T.; Abe, M.; Tsuji, M. Synthesis of Cu–Al hydrotalcite like compound and its ion exchange property. Mater. Res. Bull. 1989, 24, 1183–1199. [Google Scholar] [CrossRef]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar] [CrossRef]
- Vu, V.N.; Pham, T.H.T.; Chanthavong, M.; Do, T.H.; Nguyen, T.H.L.; Nguyen, Q.D.; Tran, T.K.N. Enhanced Photocatalytic Degradation of Rhodamine-B under Led Light Using CuZnAl Hydrotalcite Synthesized by Co-Precipitation Technique. Inorganics 2022, 10, 89. [Google Scholar] [CrossRef]
- van der Ven, L.; van Gemert, M.; Batenburg, L.; Keern, J.; Gielgens, L.; Koster, T.; Fischer, H. On the action of hydrotalcite-like clay materials as stabilizers in polyvinylchloride. Appl. Clay Sci. 2000, 17, 25–34. [Google Scholar] [CrossRef]
- Jing, C.; Dong, B.; Raza, A.; Zhang, T.; Zhang, Y. Corrosion inhibition of layered double hydroxides for metal-based systems. Nano Mater. Sci. 2020, 3, 47–67. [Google Scholar] [CrossRef]
- Tichit, D.; Coq, B. Catalysis by Hydrotalcites and Related Materials. CATTECH 2003, 7, 206–217. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, J.; Fan, Y.; Zhan, Y.; Chen, C.; Li, D.; Jiang, L. Mg–Al hydrotalcite-supported Pd catalyst for low-temperature CO oxidation: Effect of Pdn+ species and surface hydroxyl groups. Dalton Trans. 2018, 47, 14938–14944. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Huang, H.; Niederberger, M. Layered cobalt hydrotalcite as an advanced lithium-ion anode material with high capacity and rate capability. J. Mater. Chem. A 2019, 7, 21264–21269. [Google Scholar] [CrossRef]
- Trujillano, R.; González-García, I.; Morato, A.; Rives, V. Controlling the Synthesis Conditions for Tuning the Properties of Hydrotalcite-Like Materials at the Nano Scale. ChemEngineering 2018, 2, 31. [Google Scholar] [CrossRef]
- Kwon, S.; Fan, M.; DaCosta, H.F.M.; Russell, A.G.; Berchtold, K.A.; Dubey, M.K. Chapter 10–CO2 Sorption. In Coal Gasification and Its Applications; Bell, D.A., Towler, B.F., Fan, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 293–339. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Ardhayanti, L.I.; Santosa, S.J. Synthesis of Magnetite-Mg/Al Hydrotalcite and Its Application as Adsorbent for Navy Blue and Yellow F3G Dyes. Procedia Eng. 2016, 148, 1380–1387. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Behaviour of SOx-traps derived from ternary Cu/Mg/Al hydrotalcite materials. Catal. Today 2007, 127, 219–229. [Google Scholar] [CrossRef]
- Das, N.N.; Das, R. Synthesis, characterization and activation of quaternary layered double hydroxides for the one-pot synthesis of methyl isobutyl ketone. React. Kinet. Mech. Catal. 2010, 99, 397–408. [Google Scholar] [CrossRef]
- Barbosa, C.A.; Dias, P.M.; Ferreira, A.M.D.C.; Constantino, V.R. Mg–Al hydrotalcite-like compounds containing iron-phthalocyanine complex: Effect of aluminum substitution on the complex adsorption features and catalytic activity. Appl. Clay Sci. 2005, 28, 147–158. [Google Scholar] [CrossRef]
- Jana, S.K.; Kubota, Y.; Tatsumi, T. Cobalt-substituted polyoxometalate pillared hydrotalcite: Synthesis and catalysis in liquid-phase oxidation of cyclohexanol with molecular oxygen. J. Catal. 2008, 255, 40–47. [Google Scholar] [CrossRef]
- Li, Q.; Kirkpatrick, R.J. Organic anions in layered double hydroxides: An experimental investigation of citrate hydrotalcite. Am. Min. 2007, 92, 397–402. [Google Scholar] [CrossRef]
- Jana, A.; Roy, O.; Ravuru, S.S.; De, S. Tuning of graphene oxide intercalation in magnesium aluminium layered double hydroxide and their immobilization in polyacrylonitrile beads by single step mussel inspired phase inversion: A super adsorbent for lead. Chem. Eng. J. 2020, 391, 123587. [Google Scholar] [CrossRef]
- Duarte, M.F.; Rocha, I.M.; Figueiredo, J.L.; Freire, C.; Pereira, M.F.R. CoMn-LDH@carbon nanotube composites: Bifunctional electrocatalysts for oxygen reactions. Catal. Today 2018, 301, 17–24. [Google Scholar] [CrossRef]
- Ju, M.; Cai, R.; Ren, J.; Chen, J.; Qi, L.; Long, X.; Yang, S. Conductive Polymer Intercalation Tunes Charge Transfer and Sorption–Desorption Properties of LDH Enabling Efficient Alkaline Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 37063–37070. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2015, 119, 185–192. [Google Scholar] [CrossRef]
- Pavel, O.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V. Mechanochemical versus co-precipitated synthesized lanthanum-doped layered materials for olefin oxidation. Appl. Catal. A Gen. 2017, 542, 10–20. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Bucur, I.C.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechano-chemical versus co-precipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A Gen. 2020, 605, 117797. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Bacalum, E.; Cojocaru, B.; Zăvoianu, R.; Pârvulescu, V.I. Catalytic behavior of Li-Al-LDH prepared via mechanochemical and co-precipitation routes for cyanoethylation reaction. Catal. Today 2021, 366, 227–234. [Google Scholar] [CrossRef]
- Wang, C.; Li, F.; Sun, Z.; Song, Q. A highly active K/Cu-Mn-O catalyst for the removal of nitric oxide in indoor air. Indoor Built. Environ. 2019, 28, 7–16. [Google Scholar] [CrossRef]
- Ciotta, E.; Pizzoferrato, R.; Di Vona, M.L.; Ferrari, I.V.; Richetta, M.; Varone, A. Increasing the Electrical Conductivity of Layered Double Hydroxides by Intercalation of Ionic Liquids. Mater. Sci. Forum 2018, 941, 2209–2213. [Google Scholar] [CrossRef]
- Rong, F.; Zhao, J.; Yang, Q.; Li, C. Nanostructured hybrid NiFeOOH/CNT electrocatalysts for oxygen evolution reaction with low overpotential. RSC Adv. 2016, 6, 74536–74544. [Google Scholar] [CrossRef]
- Cojocaru, B.; Jurca, B.C.; Zăvoianu, R.; Bîrjega, R.; Pârvulescu, V.I.; Pavel, O.D. Tailored texture synthesized LDH catalysts in the presence of quaternary ammonium salts. Catal. Commun. 2022, 170, 106485. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Schmidt, J.G. Ueber die Einwirkung von Aldehyd auf Furfurol. Ber. Dtsch. Chem. Ges. 1880, 13, 2342–2345. [Google Scholar] [CrossRef]
- Claisen, L. Condensationen der Aldehyde mit Acetessig- und Malonsäureäther. Ber. Dtsch. Chem. Ges. 1881, 14, 345–349. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Mihăilă, S.-D.; Cojocaru, B.; Tudorache, M.; Pârvulescu, V.I.; Pavel, O.D.; Oikonomopoulos, S.; Jacobsen, E.E. An Advanced Approach for MgZnAl-LDH Catalysts Synthesis Used in Claisen-Schmidt Condensation. Catalysts 2022, 12, 759. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Gu, X. Modified calcium oxide as stable solid base catalyst for Aldol condensation reaction. J. Chem. Sci. 2013, 125, 313–320. [Google Scholar] [CrossRef]
- Jain, D.; Khatri, C.; Rani, A. Synthesis and characterization of novel solid base catalyst from fly ash. Fuel 2011, 90, 2083–2088. [Google Scholar] [CrossRef]
- Sluban, M.; Cojocaru, B.; Parvulescu, V.I.; Iskra, J.; Korošec, R.C.; Umek, P. Protonated titanate nanotubes as solid acid catalyst for aldol condensation. J. Catal. 2017, 346, 161–169. [Google Scholar] [CrossRef]
- Mortezaei, Z.; Zendehdel, M.; Bodaghifard, M.A. Synthesis and characterization of functionalized NaP Zeolite@CoFe2O4 hybrid materials: A micro–meso-structure catalyst for aldol condensation. Res. Chem. Intermed. 2020, 46, 2169–2193. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.G.; Liu, J.; Zeng, P.L.; Dong, Z.B. Synthesis of α,α′-bis(Substituted Benzylidene)Ketones Catalysed by a SOCl2/EtOH reagent. J. Chem. Res. 2004, 2004, 55–56. [Google Scholar] [CrossRef]
- Vashishtha, M.; Mishra, M.; Undre, S.; Singh, M.; Shah, D.O. Molecular mechanism of micellar catalysis of cross aldol reaction: Effect of surfactant chain length and surfactant concentration. J. Mol. Catal. A Chem. 2015, 396, 143–154. [Google Scholar] [CrossRef]
- Angelescu, E.; Birjega, R.; Pavel, O.D.; Che, M.; Costentin, G.; Popoiu, S. Hydrotalcites (HTs) and mesoporous mixed oxides obtained from HTs, basic solid catalysts for cyclohexanone condensation. Stud. Surf. Sci. Catal. 2005, 156, 257–264. [Google Scholar] [CrossRef]
- Peng, X.; Zeb, S.; Zhao, J.; Zhang, M.; Cui, Y.; Sun, G. Highly selective self-condensation of cyclohexanone: The distinct catalytic behaviour of HRF5015. R. Soc. Open Sci. 2020, 7, 200123. [Google Scholar] [CrossRef]
- Wu, X.; Xianmei, X.; Wu, Z.; Xie, X. Oxidation of Benzaldehyde to Benzoic Acid using Heterogenous Nial-Hydrotalcite-Like-Compounds as the Catalyst in Acetic Acid. Prog. React. Kinet. Mech. 2011, 36, 53–62. [Google Scholar] [CrossRef]
- Bîrjega, R.; Pavel, O.; Costentin, G.; Che, M.; Angelescu, E. Rare-earth elements modified hydrotalcites and corresponding mesoporous mixed oxides as basic solid catalysts. Appl. Catal. A: Gen. 2005, 288, 185–193. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties—I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Min. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Frost, R.L.; Cash, G.A.; Kloprogge, J. ‘Rocky Mountain leather’, sepiolite and attapulgite—an infrared emission spectroscopic study. Vib. Spectrosc. 1998, 16, 173–184. [Google Scholar] [CrossRef]
- Marchi, A.; Apesteguía, C. Impregnation-induced memory effect of thermally activated layered double hydroxides. Appl. Clay Sci. 1998, 13, 35–48. [Google Scholar] [CrossRef]
- Benziger, J.B.; Larson, L. An infrared spectroscopy study of the adsorption of CO on Fe/MgO. J. Catal. 1982, 77, 550–553. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Shi, R.; Waterhouse, G.I.N.; Wen, X.; Zhang, T. Fe-Based Catalysts for the Direct Photohydrogenation of CO 2 to Value-Added Hydrocarbons. Adv. Energy Mater. 2021, 11, 2002783. [Google Scholar] [CrossRef]
- Su, Y.; Wen, N.; Cheng, J.; Deng, W.; Zhou, H.; Zhao, B. Experimental Study on SCR-C3H6 Over Cu–Fe/Al-PILC Catalysts: Catalytic Performance, Characterization, and Mechanism. Ind. Eng. Chem. Res. 2020, 59, 14776–14788. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef] [PubMed]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.-F.; Cohen-Jonathan, S.; Soucé, M.; Marchais, H.; Dubois, P. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 2005, 130, 1395–1403. [Google Scholar] [CrossRef]
- Gunawardana, B.; Singhal, N.; Swedlund, P. Degradation of Chlorinated Phenols by Zero Valent Iron and Bimetals of Iron: A Review. Environ. Eng. Res. 2011, 16, 187–203. [Google Scholar] [CrossRef]
- Rafiee, E.; Rahimi, F. A green approach to the synthesis of chalcones via Claisen-Schmidt condensation reaction using cesium salts of 12-tungstophosphoric acid as a reusable nanocatalyst. Mon. Chem. 2013, 144, 361–367. [Google Scholar] [CrossRef]
- Li, J.-T.; Yang, W.-Z.; Chen, G.-F.; Li, T.-S. A Facile Synthesis of α,α′-bis(Substituted Benzylidene) Cycloalkanones Catalyzed by KF/Al2O3 Under Ultrasound Irradiation. Synth. Commun. 2003, 33, 2619–2625. [Google Scholar] [CrossRef]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, Tuning, and Exploiting the Basicity of Hydrotalcites for Applications in Heterogeneous Catalysis. Chem. Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef]
- Parida, K.; Das, J. Mg/Al hydrotalcites: Preparation, characterisation and ketonisation of acetic acid. J. Mol. Catal. A Chem. 2000, 151, 185–192. [Google Scholar] [CrossRef]
- Pavel, O.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E. The effect of ageing step elimination on the memory effect presented by Mg0.75Al0.25 hydrotalcites (HT) and their catalytic activity for cyanoethylation reaction. Catal. Commun. 2011, 12, 845–850. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).