Layered Double Hydroxide/Graphene Quantum Dots as a New Sorbent for the Dispersive Solid-Phase Microextraction of Selected Benzophenones, Phenols, and Parabens
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis Optimization
2.1.1. Carbonate-Containing and Carbonate-Free Mg-Al LDH
2.1.2. Different Types of GQDs
2.1.3. Amount of GQDs
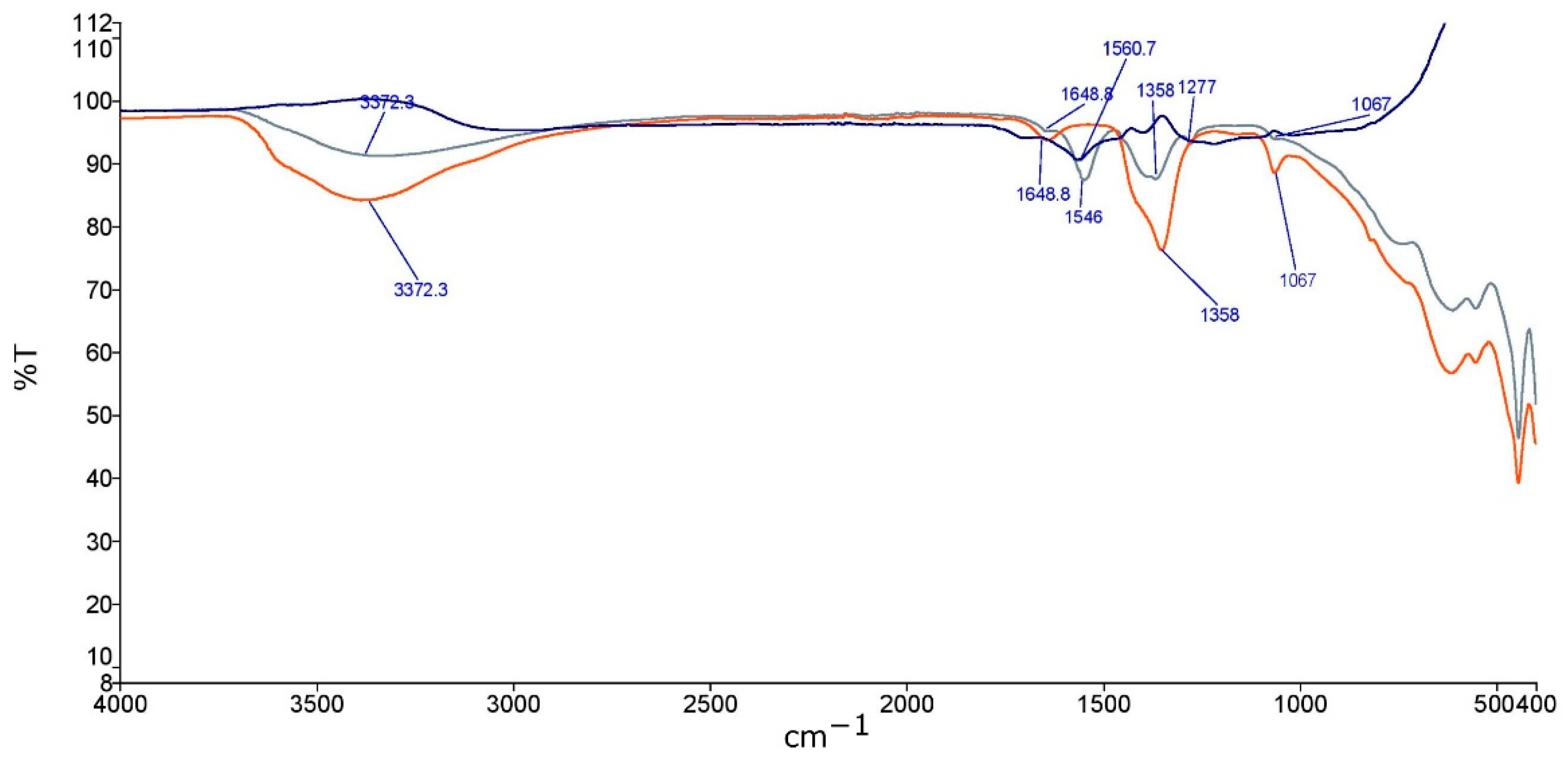
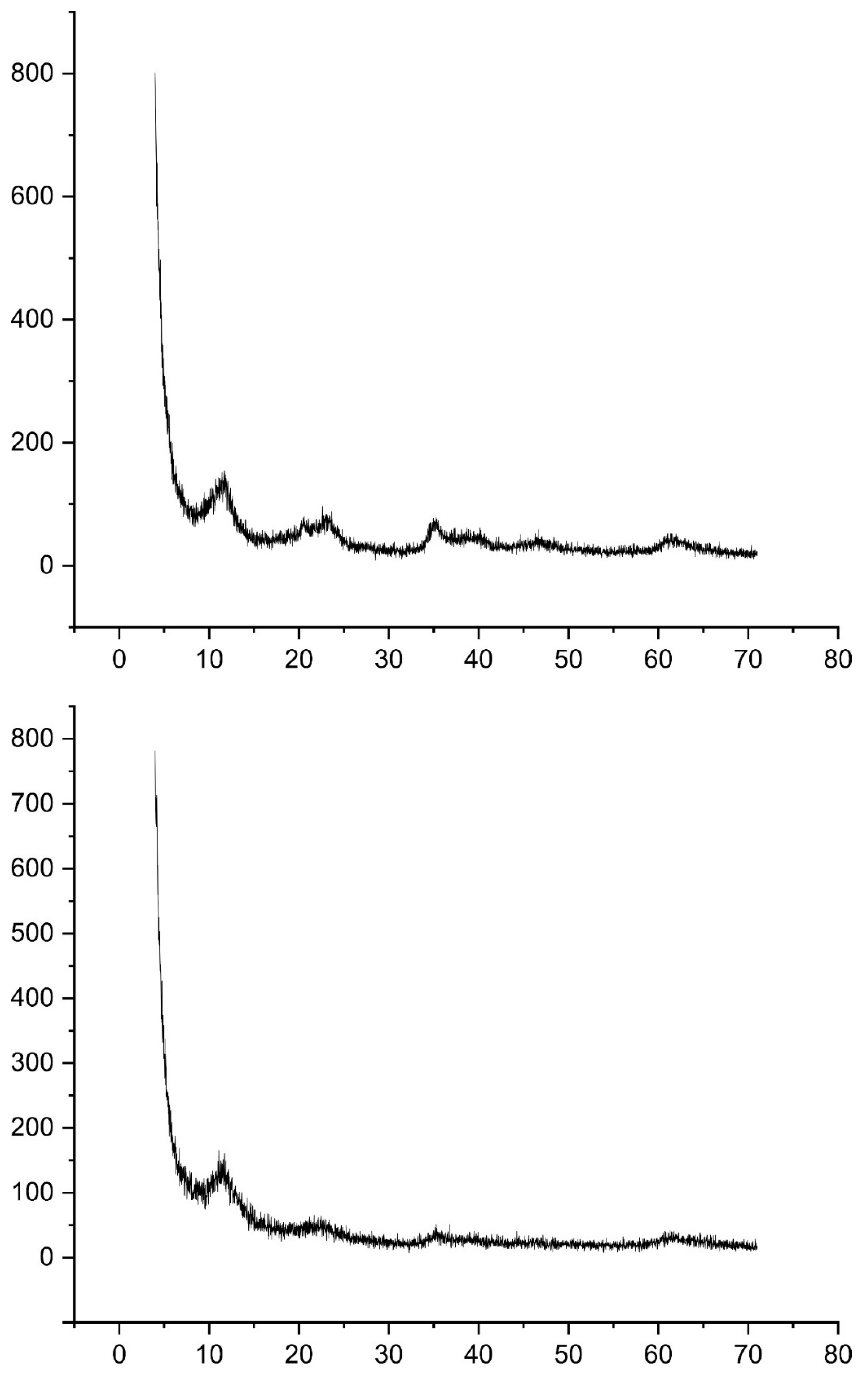
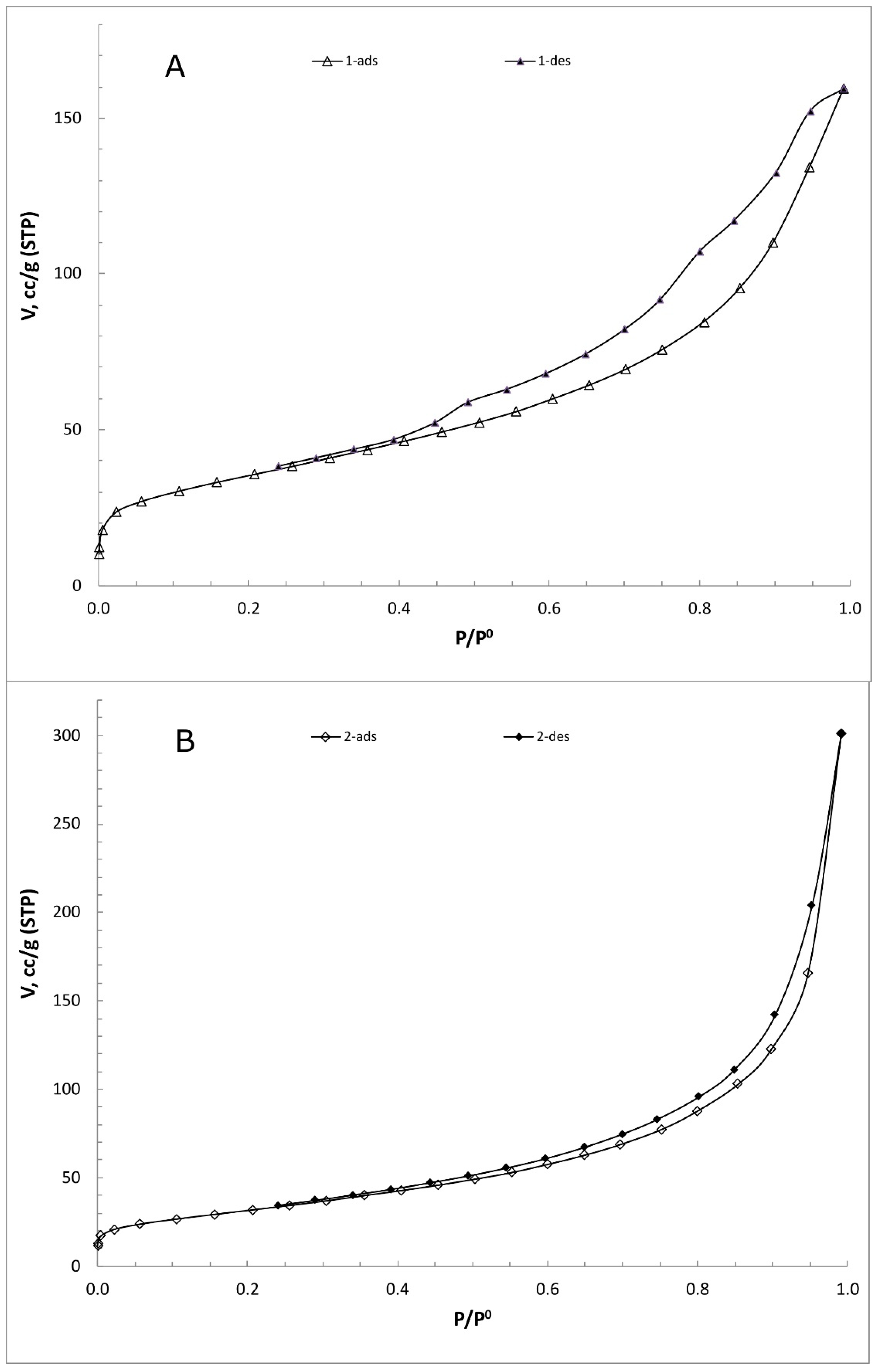
2.2. Material Characterization
2.3. Optimization of the Extraction Procedure
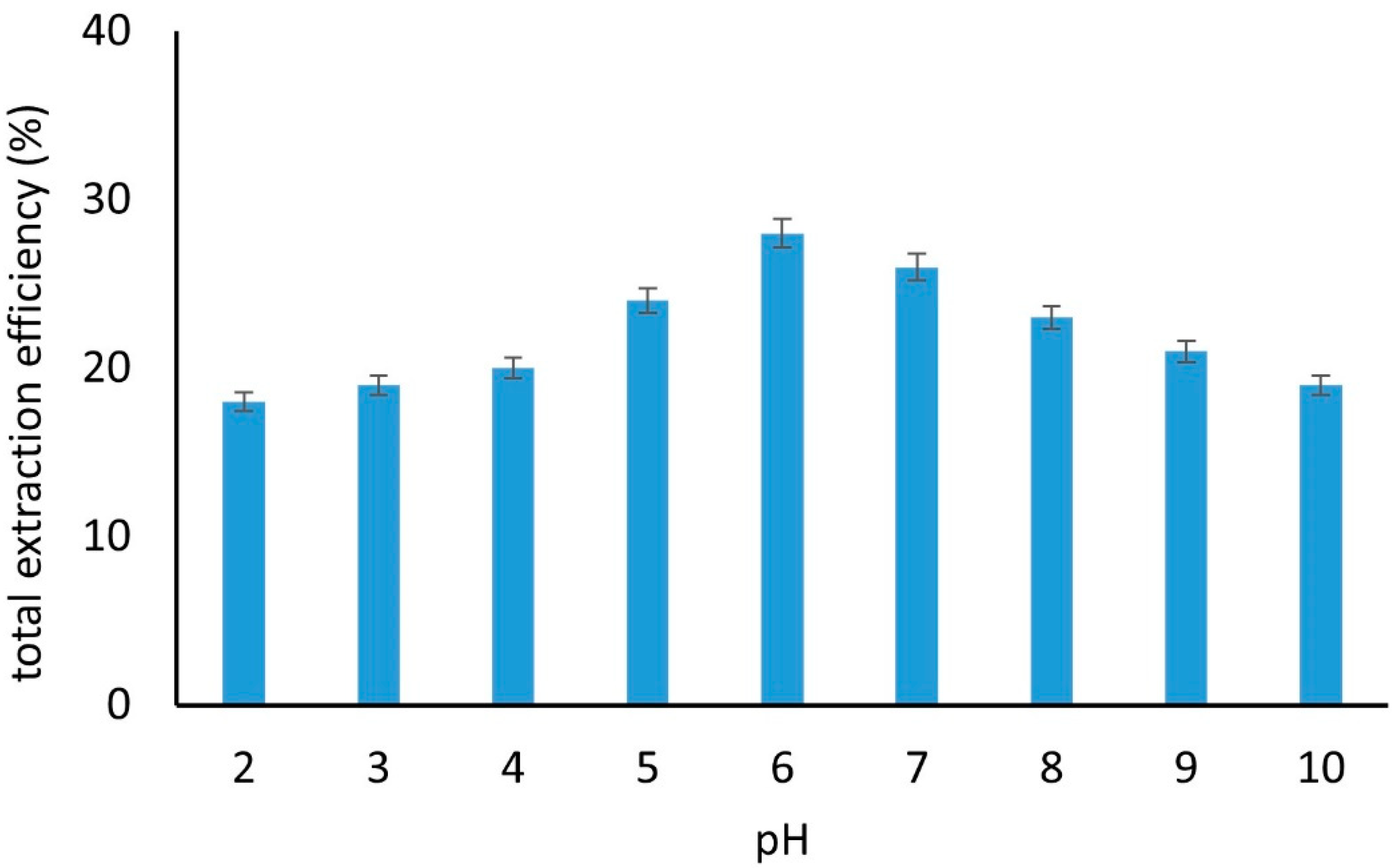
2.3.1. pH and Mechanism of Interaction
2.3.2. Ionic Strength
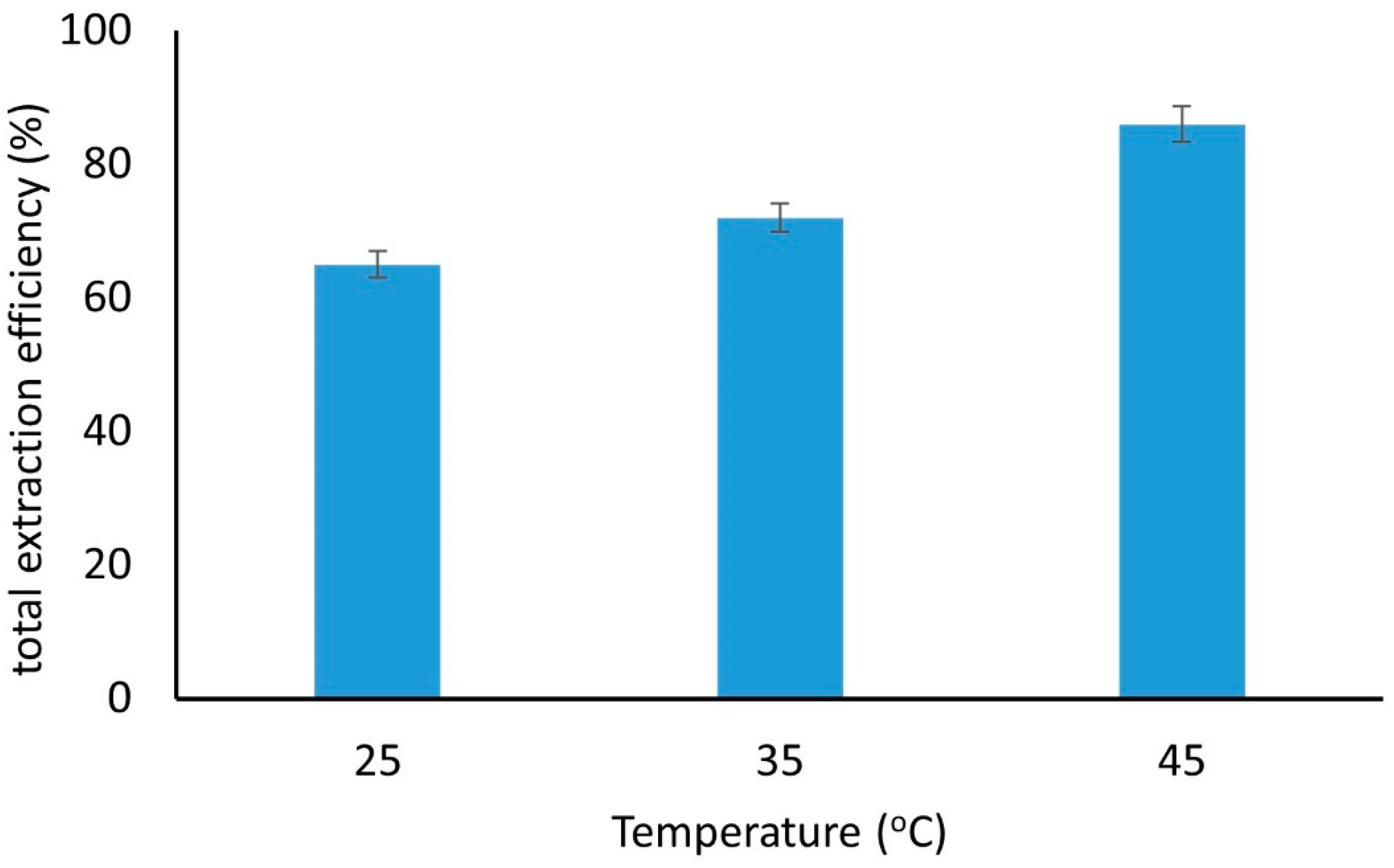
2.3.3. Temperature of Extraction
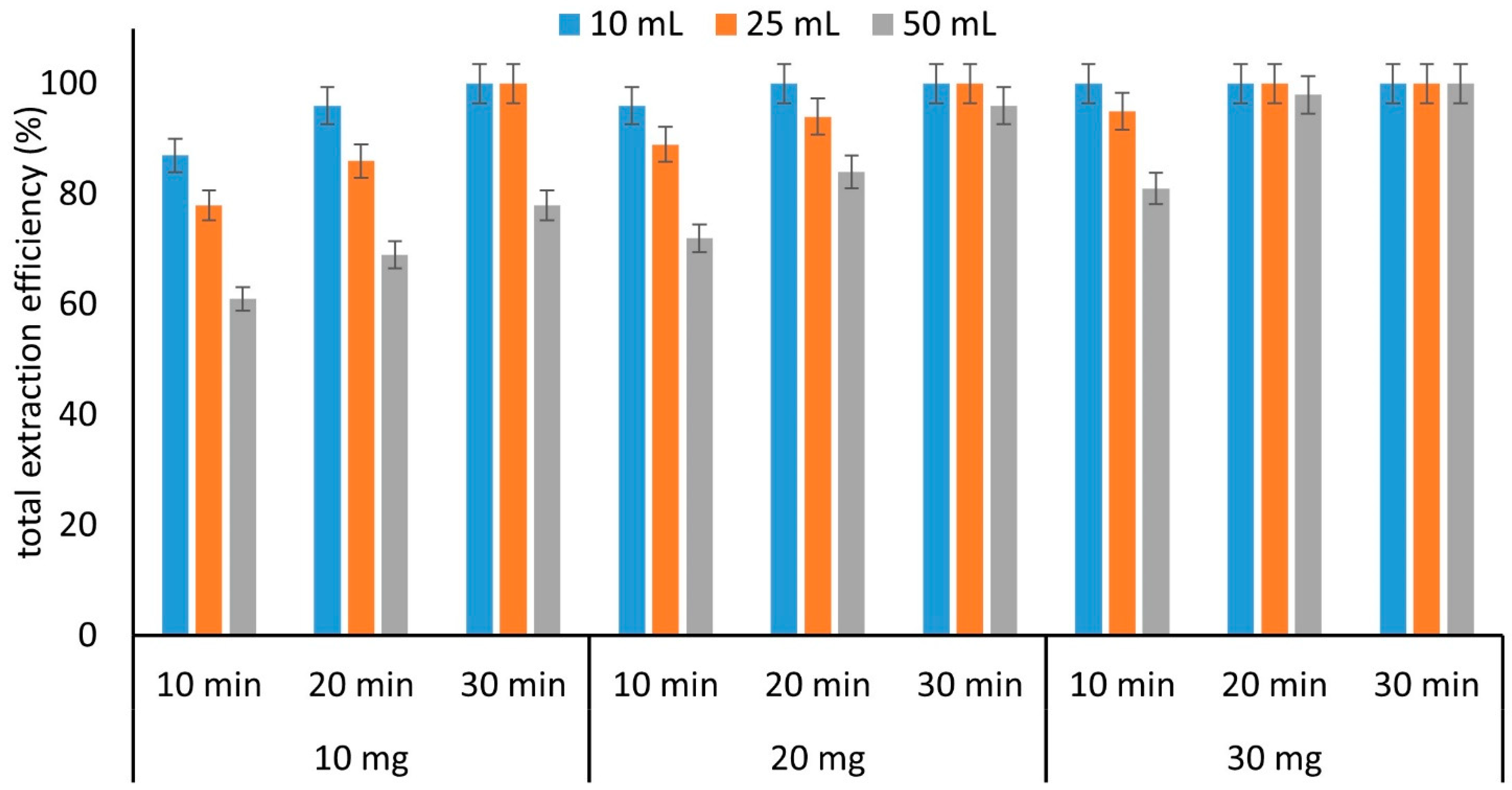
2.3.4. Other Extraction Parameters
2.4. Optimization of Elution Conditions and Reuse of the Material
2.5. Analytical Figures of Merit
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Graphene Quantum Dots (GQDs) Synthesis
3.4. Synthesis of Carbonate-Free Mg-Al LDH and Mg-Al LDH-Incorporating GQDs
3.5. Optimized Extraction Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Samanidou, V.F.; Karageorgou, E. Carbon Nanotubes in Sample Preparation. Curr. Org. Chem. 2012, 16, 1645–1669. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Wabaidur, S.M. Application of carbon nanotubes in extraction and chromatographic analysis: A review. Arab. J. Chem. 2019, 12, 633–651. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging Applications of Carbon Nanodots: A Review. C 2019, 5, 19. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Carbon nanodots from natural (re)sources: A new perspective on analytical chemistry. In Handbook of Nanomaterials in Analytical Chemistry: Modern Trends in Analysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–28. ISBN 9780128166994. [Google Scholar]
- Yao, W.; Wang, X.; Liang, Y.; Yu, S.; Gu, P.; Sun, Y.; Xu, C.; Chen, J.; Hayat, T.; Alsaedi, A.; et al. Synthesis of novel flower-like layered double oxides/carbon dots nanocomposites for U(VI) and 241Am(III) efficient removal: Batch and EXAFS studies. Chem. Eng. J. 2018, 332, 775–786. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Yue, X.; Yang, Q.; Liu, F.; Wang, Y.; Zhang, D.; Li, Z.; Wang, J. One-pot synthesis of multifunctional magnetic ferrite-MoS2-carbon dot nanohybrid adsorbent for efficient Pb(II) removal. J. Mater. Chem. A 2016, 4, 3893–3900. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.; Huang, H.; Liu, Y.; Kang, Z. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon N. Y. 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Wang, H.; Liu, C.; Fu, Y.; Yuan, S.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots decorated magnetic ZnFe2O4 nanoparticles with enhanced adsorption capacity for the removal of dye from aqueous solution. Appl. Surf. Sci. 2018, 433, 790–797. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Carbon dots in sample preparation. In Carbon Dots in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 59–66. [Google Scholar]
- Aladaghlo, Z.; Javanbakht, S.; Fakhari, A.R.; Shaabani, A. Gelatin microsphere coated Fe3O4@graphene quantum dots nanoparticles as a novel magnetic sorbent for ultrasound-assisted dispersive magnetic solid-phase extraction of tricyclic antidepressants in biological samples. Microchim. Acta 2021, 188, 73. [Google Scholar] [CrossRef]
- Cayuela, A.; Benítez-Martínez, S.; Soriano, M.L. Carbon nanotools as sorbents and sensors of nanosized objects: The third way of analytical nanoscience and nanotechnology. TrAC-Trends Anal. Chem. 2016, 84, 172–180. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, T.; Chen, M.L.; Wang, J.H. Supported carbon dots serve as high-performance adsorbent for the retention of trace cadmium. Talanta 2018, 180, 18–24. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Li, Y.; Wei, H.P.; Li, X.S.; Zhang, X.H.; Chen, S.M.; Chen, X.Q. Synthesis of polyethyleneimine capped carbon dots for preconcentration and slurry sampling analysis of trace chromium in environmental water samples. Talanta 2015, 134, 16–23. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Rezvani, Z.; Sadeghi, G.H.; Zorufi, E. Layered double hydroxides: A novel nano-sorbent for solid-phase extraction. Anal. Chim. Acta 2011, 685, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Basheer, C. Layered double hydroxides: Emerging sorbent materials for analytical extractions. TrAC-Trends Anal. Chem. 2016, 75, 174–182. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Gunawan, P.; Xu, R. Lanthanide-doped layered double hydroxides intercalated with sensitizing anions: Efficient energy transfer between host and guest layers. J. Phys. Chem. C 2009, 113, 17206–17214. [Google Scholar] [CrossRef]
- Perioli, L.; Posati, T.; Nocchetti, M.; Bellezza, F.; Costantino, U.; Cipiciani, A. Intercalation and release of antiinflammatory drug diclofenac into nanosized ZnAl hydrotalcite-like compound. Appl. Clay Sci. 2011, 53, 374–378. [Google Scholar] [CrossRef]
- Liang, R.; Wei, M.; Peng, L.; Mei, X. The fabrication of layered double hydroxides based bio-materials and their application in theranostic and drug delivery. Sci. Sin. Chim. 2017, 47, 431–441. [Google Scholar] [CrossRef][Green Version]
- Yan, S.; Xu, K.; Li, L.; Gu, W.; Rolfe, B.E.; Xu, Z.P. The pathways for layered double hydroxide nanoparticles to enhance antigen (cross)-presentation on immune cells as adjuvants for protein vaccines. Front. Pharmacol. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Violante, A.; Pucci, M.; Cozzolino, V.; Zhu, J.; Pigna, M. Sorption/desorption of arsenate on/from Mg-Al layered double hydroxides: Influence of phosphate. J. Colloid Interface Sci. 2009, 333, 63–70. [Google Scholar] [CrossRef]
- You, Y.; Zhao, H.; Vance, G.F. Adsorption of dicamba (3,6-dichloro-2-methoxy benzoic acid) in aqueous solution by calcined-layered double hydroxide. Appl. Clay Sci. 2002, 21, 217–226. [Google Scholar] [CrossRef]
- Pavan, P.C.; de Gomes, G.A.; Valim, J.B. Adsorption of sodium dodecyl sulfate on layered double hydroxides. Microporous Mesoporous Mater. 1998, 21, 659–665. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Hu, J.; Chen, C.; Wang, X. The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem. Eng. J. 2011, 171, 167–174. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, Q.; Chen, B. Metal composition of layered double hydroxides (LDHs) regulating ClO-4 adsorption to calcined LDHs via the memory effect and hydrogen bonding. J. Environ. Sci. (China) 2014, 26, 493–501. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Q.; Lu, C.; Li, Z.; Wang, W. Layered double hydroxide-carbon dot composite: High-performance adsorbent for removal of anionic organic dye. ACS Appl. Mater. Interfaces 2014, 6, 20225–20233. [Google Scholar] [CrossRef]
- Saraji, M.; Ghani, M. Dissolvable layered double hydroxide coated magnetic nanoparticles for extraction followed by high performance liquid chromatography for the determination of phenolic acids in fruit juices. J. Chromatogr. A 2014, 1366, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined graphene/MgAl-layered double hydroxides for enhanced Cr(VI) removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Z.; Sun, D.; Wu, T.; Li, Y. Enhanced adsorption capacity of dyes by surfactant-modified layered double hydroxides from aqueous solution. J. Ind. Eng. Chem. 2017, 49, 208–218. [Google Scholar] [CrossRef]
- Rahmanian, O.; Dinari, M.; Abdolmaleki, M.K. Carbon quantum dots/layered double hydroxide hybrid for fast and efficient decontamination of Cd(II): The adsorption kinetics and isotherms. Appl. Surf. Sci. 2018, 428, 272–279. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. Synthesis and in situ mechanism of nuclei growth of layered double hydroxides. Bull. Mater. Sci. 2011, 34, 1599–1604. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols-Sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Seidel, F. Reproductive toxicity of benzophenone-3. Arch. Toxicol. 2020, 94, 3593–3594. [Google Scholar] [CrossRef]
- Petric, Z.; Ruzić, J.; Zuntar, I. The controversies of parabens—An overview nowadays. Acta Pharm. 2021, 71, 17–32. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, P.; Xu, B.; Zhao, R.; Qiao, S.; Chen, X.; Tang, R.; Wu, D.; Song, L.; Wang, S.; et al. Determination of nine environmental phenols in urine by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2012, 36, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Neri, I.; Russo, G.; Laneri, S.; Grumetto, L. Tracking Down of a Selected Panel of Parabens: A Validated Method to Evaluate Their Occurrence in Skin Layers. Cosmetics 2022, 9, 102. [Google Scholar] [CrossRef]
- Iyi, N.; Sasaki, T. Deintercalation of carbonate ions and anion exchange of an Al-rich MgAl-LDH (layered double hydroxide). Appl. Clay Sci. 2008, 42, 246–251. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon N. Y. 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Azzouz, A.; Aruş, V.A.; Platon, N.; Ghomari, K.; Nistor, I.D.; Shiao, T.C.; Roy, R. Polyol-modified layered double hydroxides with attenuated basicity for a truly reversible capture of CO2. Adsorption 2013, 19, 909–918. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Effect of synthesis conditions on the controlled growth of MgAl-LDH corrosion resistance film: Structure and corrosion resistance properties. Coatings 2019, 9, 30. [Google Scholar] [CrossRef]
- Chakraborty, J.; Sengupta, S.; Dasgupta, S.; Chakraborty, M.; Ghosh, S.; Mallik, S.; Das, K.L.; Basu, D. Determination of trace level carbonate ion in Mg-Al layered double hydroxide: Its significance on the anion exchange behaviour. J. Ind. Eng. Chem. 2012, 18, 2211–2216. [Google Scholar] [CrossRef]
- Chen, L.; Tu, Q.; Yang, X.; Hu, X.; Sun, X.; Li, H. MgAl Layered Double Hydroxides Intercalated with EDTA: Cu(II) Recovery and Mechanism. ChemistrySelect 2020, 5, 11299–11304. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef]
- Wang, L.; Zang, X.; Wang, C.; Wang, Z. Graphene oxide as a micro-solid-phase extraction sorbent for the enrichment of parabens from water and vinegar samples. J. Sep. Sci. 2014, 37, 1656–1662. [Google Scholar] [CrossRef]
- Jobbágy, M.; Regazzoni, A.E. Dissolution of nano-size Mg-Al-Cl hydrotalcite in aqueous media. Appl. Clay Sci. 2011, 51, 366–369. [Google Scholar] [CrossRef]
- García-Valverde, M.; Chatzimitakos, T.; Lucena, R.; Cárdenas, S.; Stalikas, C. Melamine Sponge Functionalized with Urea-Formaldehyde Co-Oligomers as a Sorbent for the Solid-Phase Extraction of Hydrophobic Analytes. Molecules 2018, 23, 2595. [Google Scholar] [CrossRef]
- Pavlovic, M.; Huber, R.; Adok-Sipiczki, M.; Nardin, C.; Szilagyi, I. Ion specific effects on the stability of layered double hydroxide colloids. Soft Matter 2016, 12, 4024–4033. [Google Scholar] [CrossRef]
- Samanta, T.; Bagchi, B. Temperature effects on the hydrophobic force between two graphene-like surfaces in liquid water. J. Chem. Sci. 2018, 130, 29. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Binellas, C.; Maidatsi, K.; Stalikas, C. Magnetic ionic liquid in stirring-assisted drop-breakup microextraction: Proof-of-concept extraction of phenolic endocrine disrupters and acidic pharmaceuticals. Anal. Chim. Acta 2016, 910, 53–59. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Veneti, G.; Chatzimitakos, T.; Stalikas, C. Melamine sponge functionalized with carbon nanodots for the extraction of polyaromatic hydrocarbons and musks from environmental samples prior to their determination by gas chromatography-mass spectrometry. J. Chromatogr. A 2022, 1679, 463375. [Google Scholar] [CrossRef] [PubMed]


| Compound | Linear Equation | Coefficient of Determination, R2 | LOQ (μg L−1) | Enrichment Factor |
|---|---|---|---|---|
| 4OH-BP | Y = 20,986 + 18,745 | 0.9987 | 0.13 | 178 |
| PPB | Y = 28,206x + 31,685 | 0.9986 | 0.10 | 175 |
| BPB | Y = 22,380x + 15,503 | 0.9980 | 0.12 | 186 |
| TCP | Y = 2582.3x + 1150.9 | 0.9983 | 1.33 | 169 |
| BP-3 | Y = 16,578x + 15,009 | 0.9984 | 0.17 | 179 |
| OCP | Y = 1696.6x + 1509.9 | 0.9985 | 1.28 | 170 |
| Analyte | RSD (%) | Relative Recovery (%) | ||
|---|---|---|---|---|
| Repeatability (n = 5) | Inter-Day Repeatability (n = 3 × 3) | 2 × LOQ | 10 × LOQ | |
| 4OH-BP | 5.0 | 6.7 | 93 | 94 |
| PPB | 5.5 | 7.9 | 99 | 100 |
| BPB | 5.9 | 8.0 | 92 | 93 |
| TCP | 5.6 | 7.8 | 92 | 96 |
| BP-3 | 6.4 | 7.3 | 94 | 95 |
| OCP | 6.9 | 8.2 | 94 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimitakos, T.; Vasilas, A.; Stalikas, C. Layered Double Hydroxide/Graphene Quantum Dots as a New Sorbent for the Dispersive Solid-Phase Microextraction of Selected Benzophenones, Phenols, and Parabens. Molecules 2022, 27, 8388. https://doi.org/10.3390/molecules27238388
Chatzimitakos T, Vasilas A, Stalikas C. Layered Double Hydroxide/Graphene Quantum Dots as a New Sorbent for the Dispersive Solid-Phase Microextraction of Selected Benzophenones, Phenols, and Parabens. Molecules. 2022; 27(23):8388. https://doi.org/10.3390/molecules27238388
Chicago/Turabian StyleChatzimitakos, Theodoros, Alkiviadis Vasilas, and Constantine Stalikas. 2022. "Layered Double Hydroxide/Graphene Quantum Dots as a New Sorbent for the Dispersive Solid-Phase Microextraction of Selected Benzophenones, Phenols, and Parabens" Molecules 27, no. 23: 8388. https://doi.org/10.3390/molecules27238388
APA StyleChatzimitakos, T., Vasilas, A., & Stalikas, C. (2022). Layered Double Hydroxide/Graphene Quantum Dots as a New Sorbent for the Dispersive Solid-Phase Microextraction of Selected Benzophenones, Phenols, and Parabens. Molecules, 27(23), 8388. https://doi.org/10.3390/molecules27238388








