Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones
Abstract
1. Introduction
2. The Antibacterial Activity Study
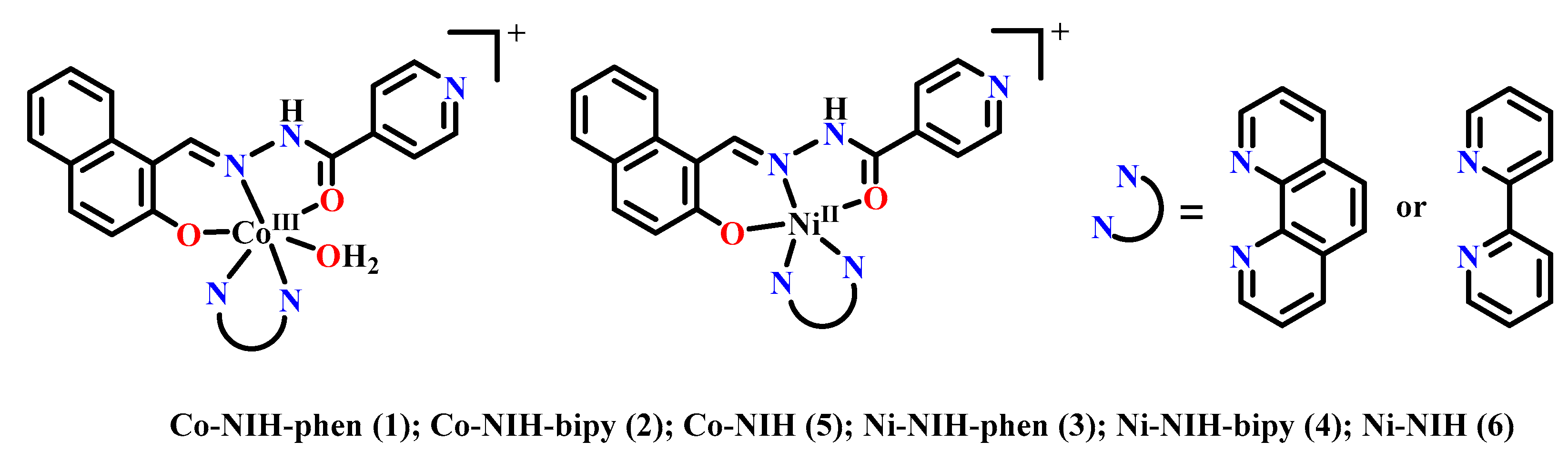
2.1. Antibacterial Metal Complexes of Naphthylhydrazone
2.1.1. The First Transition Metal Complexes of Naphthylhydrazone with Antibacterial Activities
2.1.2. The Second and Third Transition Metal Complexes of Naphthylhydrazone with Antibacterial Activities
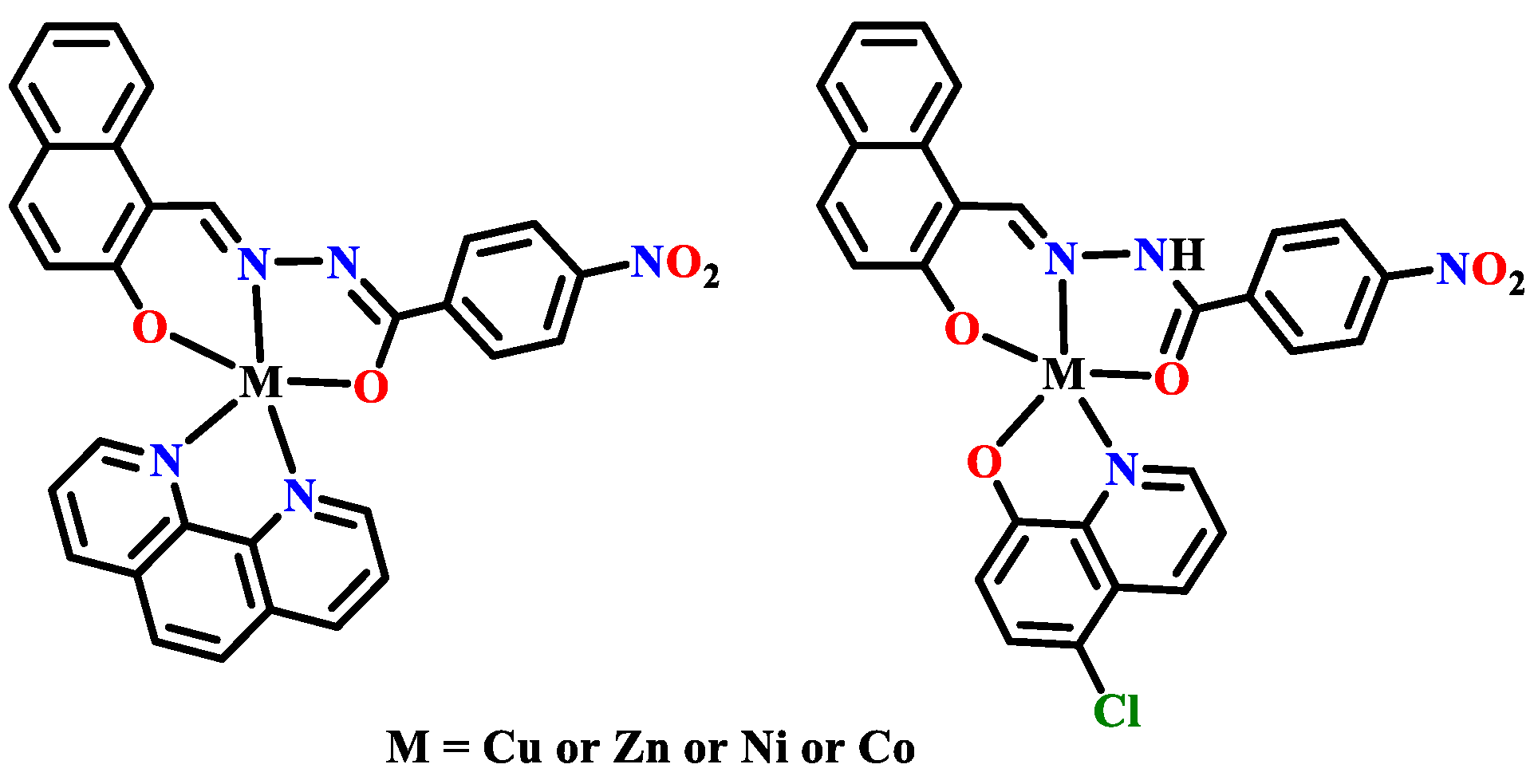
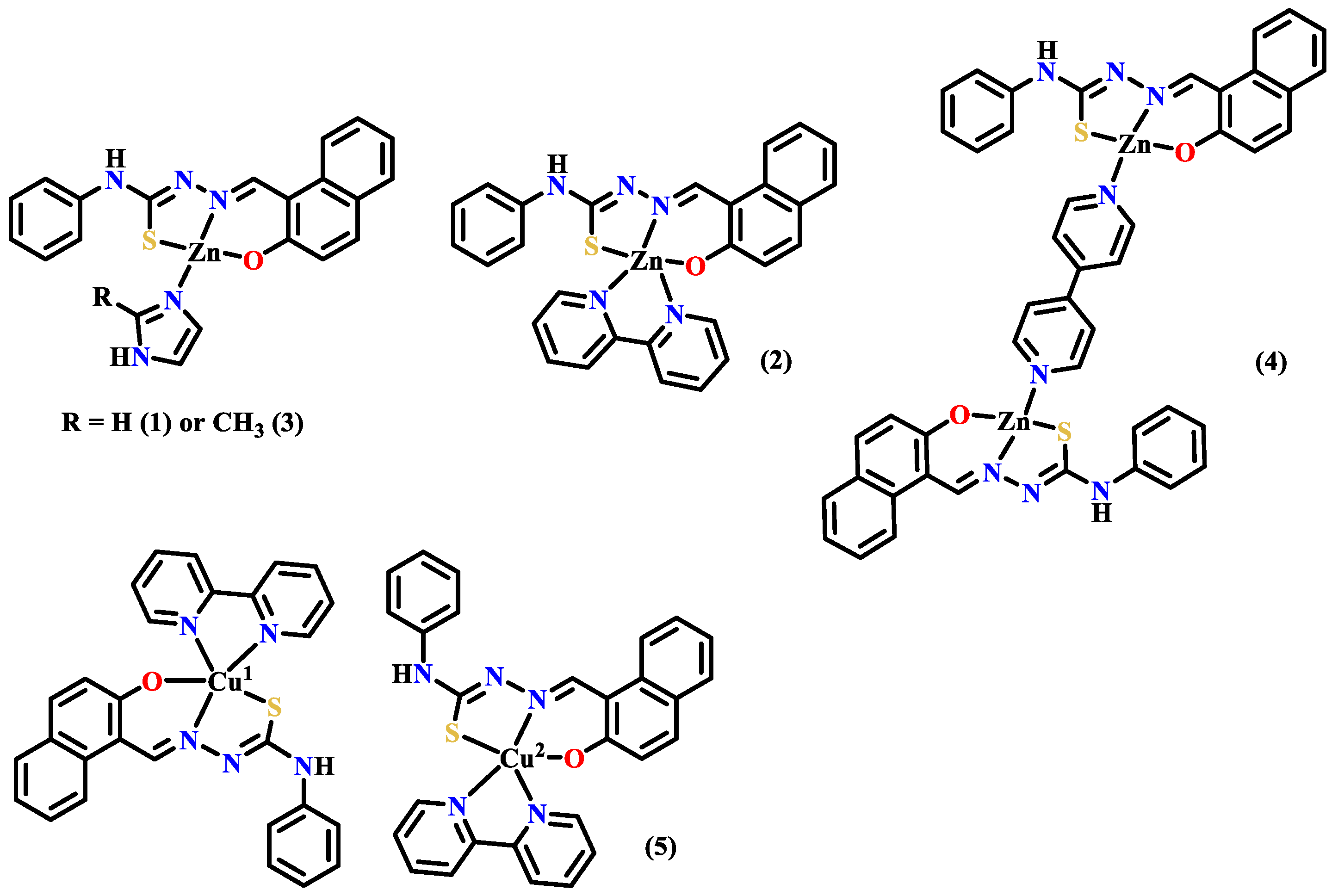
2.2. Antibacterial Metal Complexes of the Other Polycyclic Aromatic Hydrazones
3. The Anticancer Activity Study
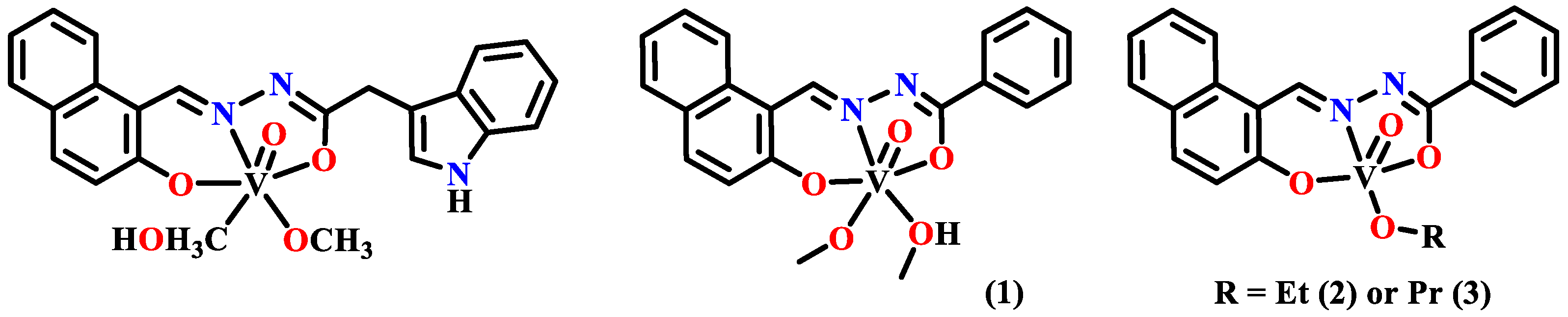
3.1. Anticancer Metal Complexes of Naphthylhydrazone
3.1.1. The First Transition Metal Complexes with Anticancer Activities
3.1.2. The Second and Third Transition Metal Complexes of Naphthylhydrazone with Anticancer Activities
3.2. Anticancer Metal Complexes of Anthrahydrazone
3.2.1. The First Transition Metal Complexes of Anthrahydrazone with Anticancer Activities
3.2.2. The Second and Third Transition Metal Complexes of Anthrahydrazone with Anticancer Activities
3.3. Anticancer Metal Complexes of the Other Polycyclic Aromatic Hydrazones
4. The Metal Complexes of Polycyclic Hydrazones for the Bioactive-Related Targets
5. Conclusions
- We found that there is a significant structure—activity relationship for the anticancer activity of the metal complexes of hydrazones, but this relationship is very complicated because the influencing factors include the central metal, number of hydrazone rings, side chain groups, substituents of the side chain groups, and co-ligands (as illustrated in Scheme 1). Therefore, in order to further understand and clarify the structure—activity relationship of these anticancer metal complexes, it is necessary to guide the rational design of metal drugs and to synthesize and test more aromatic hydrazone metal complexes with anticancer activity. However, through the combination of available reports presented here, we have found some rules and characteristics regarding SAR on the metal complexes of polycyclic aromatic hydrazones. From the perspective of side chains, thiourea and acylhydrazone showed better bioactivity, which seems to be related to the addition of auxiliary chelating sites. Furthermore, this also makes the complexes more stable, which contributes to exerting the synergistic effect between the central metal and the hydrazone. In addition, a more alkaline N-heterocyclic (such as imidazoline) seems to be beneficial to increase the activity if the side chain has a N-heterocyclic pharmacophore, as suggested by our reported and unreported results [123]. Considering that it is easier for a stronger alkaline N atom to be protonated into quaternary ammonium salt, it is speculated that this could be related to the influence of the charged property of the side chain when acting on its potential intracellular targets. In addition, when viewed from the metal center, the copper(II) complexes of such hydrazones exhibited significantly higher pharmacological activity, which was primarily ascribed to the bioactivity of copper(II) itself. However, the potential toxicity of copper(II) complexes should also be considered seriously. Other metal centers, however, did not show distinct and predictable activity patterns, which means that different metal complexes of polycyclic aromatic hydrazones are still worth exploring more widely.
- Furthermore, according to the complexes reported in this review, the antitumor and antibacterial activities of the complexes of naphthylhydrazone were almost found to be higher than those of the corresponding phenylhydrazone [58,76,78,85], although the metal complexes of phenylhydrazone were not specifically discussed here. In addition, when used as co-ligands, o-phenanthroline and bipyridine could effectively improve the antitumor activity, and o-phenanthroline was even better than bipyridine, as reported in the mentioned vanadium and ruthenium complexes as well as in some instructional reviews [49,50,76,78,85,129]. In fact, this pattern was also observed in our unreported study on the metal complexes of anthrahydrazone. We think that the expansion of the aromatic ring and the addition of the co-ligand obviously increase the lipophilicity of the complex, which could enhance its ability to penetrate the cell membrane and thus improve its absorption efficiency by cells or bacteria. However, the metal complexes of anthrahydrazone should not be taken lightly. Although it is generally believed that the toxicity and carcinogenicity risks of anthracyclines increase as the number of cycles increases, it is worthwhile to further enrich the metal complexes of anthrahydrazone by considering the successful application of anthracyclines such as doxorubicin. We also think that aromatic hydrazone compounds with more than four rings are not only rarely studied, but also do not exhibit the prospect of medicinal applications due to their obvious high toxicity and poor solubility.
- When testing the anticancer activity, more normal human cells should be selected as cell lines to reflect the cytotoxicity of the metal complexes of naphthylhydrazone more comprehensively. Furthermore, it is regrettable to note that many of the metal complexes of naphthylhydrazone that have been reported to show very high anticancer activity in vitro have not been thoroughly studied and evaluated for their anticancer activity in animals. It is suggested that further work should be conducted in this area to promote research on the drug properties of hydrazone metal complexes, especially those candidate complexes that have shown high activity in vitro.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, H.; Wang, Z.; Wei, C.; Wang, J.; Xu, Y.; Bai, G.; Yao, Q.; Zhang, L.; Chen, Y. Anticancer potential of indirubins in medicinal chemistry: Biological activity, structural modification, and structure-activity relationship. Eur. J. Med. Chem. 2021, 223, 113652. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kumar, K. Synthetic and medicinal perspective of quinolines as antiviral agents. Eur. J. Med. Chem. 2021, 215, 113220. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.J.; Kaye, S.B. Aspects of cytotoxic drug penetration, with particular reference to anthracyclines. Cancer Chemoth. Pharm. 1987, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, F. Mechanism of action of anthracycline drugs. Lancet Oncol. 2013, 14, e296. [Google Scholar] [CrossRef]
- Braña, M.F.; Cacho, M.; Gradillas, A.; de Pascual-Teresa, B.; Ramos, A. Intercalators as anticancer drugs. Curr. Pharm. Design 2001, 7, 1745–1780. [Google Scholar] [CrossRef]
- Jackson, B.A.; Barton, J.K. Probing nucleic acid structure with shape-selective rhodium and ruthenium complexes. In Current Protocols in Nucleic Acid Chemistry; Chapter 6, Unit 6.2; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Kisko, J.L.; Barton, J.K. Recognition of DNA base pair mismatches by a cyclometalated Rh(III) intercalator. Inorg. Chem. 2000, 39, 4942–4949. [Google Scholar] [CrossRef] [PubMed]
- Stinner, C.; Wightman, M.D.; Kelley, S.O.; Hill, M.G.; Barton, J.K. Synthesis and spectroelectrochemistry of Ir(bpy)(phen)(phi)3+, a tris(heteroleptic) metallointercalator. Inorg. Chem. 2001, 40, 5245–5250. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Zhang, S.; Zhao, F.; Gao, C.; Feng, L.S.; Lv, Z.S.; Xu, Z.; Wu, X. Isoniazid derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 133, 255–267. [Google Scholar] [CrossRef]
- Shao, B.; Aprahamian, I. Hydrazones as new molecular tools. Chem. 2020, 6, 2162–2173. [Google Scholar] [CrossRef]
- Popiołek, Ł. The bioactivity of benzenesulfonyl hydrazones: A short review. Biomed. Pharmacother. 2021, 141, 111851. [Google Scholar] [CrossRef]
- Sartorelli, A.C.; Booth, B.A. Inhibition of the growth of sarcoma 180 ascites cells by combinations of inhibitors of nucleic acid biosynthesis and the cupric chelate of kethoxal bis-(thiosemicarbazone). Cancer Res. 1967, 27, 1614–1619. [Google Scholar] [PubMed]
- Sartorelli, A.C.; Agrawal, K.C.; Moore, E.C. Mechanism of inhibition of ribonucleoside diphosphate reductase by ga-(n)-heterocyclic aldehyde thiosemicarbazones. Biochem. Pharmacol. 1971, 20, 3119–3123. [Google Scholar] [CrossRef]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the old to new: Iron chelators that are more than just ribonucleotide reductase inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [Google Scholar] [CrossRef] [PubMed]
- Carcelli, M.; Tegoni, M.; Bartoli, J.; Marzano, C.; Pelosi, G.; Salvalaio, M.; Rogolino, D.; Gandin, V. In vitro and in vivo anticancer activity of tridentate thiosemicarbazone copper complexes: Unravelling an unexplored pharmacological target. Eur. J. Med. Chem. 2020, 194, 112266. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, C.; Zhang, D.; Niu, J.; Ji, B. Mn(II), Co(II) and Zn(II) complexes with heterocyclic substituted thiosemicarbazones: Synthesis, characterization, X-ray crystal structures and antitumor comparison. Eur. J. Med. Chem. 2010, 45, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Murdock, K.C.; Child, R.G.; Yang, I.L.; Warren, J.D.; Angier, R.B. NEXT Antitumor agents. II. Bis(guanylhydrazones) of anthracene-9,10-dicarboxaldehydes. J. Med. Chem. 1982, 25, 505–518. [Google Scholar] [CrossRef]
- Leszek, J.; Trypka, E.; Tarasov, V.V.; Ashraf, G.M.; Aliev, G. Type 3 diabetes mellitus: A novel implication of alzheimers disease. Curr. Top. Med. Chem. 2017, 17, 1331–1335. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef]
- Swaminathan, S.; Haribabu, J.; Subarkhan, M.K.M.; Gayathri, D.; Balakrishnan, N.; Bhuvanesh, N.; Echeverria, C.; Karvembu, R. Impact of aliphatic acyl and aromatic thioamide substituents on the anticancer activity of Ru(II)-p-cymene complexes with acylthiourea ligands—In vitro and in vivo studies. Dalton Trans. 2021, 50, 16311–16325. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Z. Copper in medicine: Homeostasis, chelation therapy and antitumor drug design. Curr. Med. Chem. 2006, 13, 525–537. [Google Scholar] [CrossRef]
- Erxleben, A. Interactions of copper complexes with nucleic acids. Coordin. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, M.; Li, S.; Deng, J.; Li, J.; Fang, G.; Yang, F.; Huang, G. Dithiocarbazate-copper complexes for bioimaging and treatment of pancreatic cancer. J. Med. Chem. 2021, 64, 5485–5499. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, F.; Zhang, J.; Sun, J.; Zhao, X.; Zhu, Y.; Wei, W.; Zhao, J.; Guo, Z. A ferroptosis-inducing iridium(III) complex. Sci. China Chem. 2020, 63, 65–72. [Google Scholar] [CrossRef]
- Wang, W.J.; Ling, Y.Y.; Zhong, Y.M.; Li, Z.Y.; Tan, C.P.; Mao, Z.W. Ferroptosis-enhanced cancer immunity by a ferrocene-appended iridium(III) diphosphine complex. Angew. Chem. Int. Ed. 2022, 61, e202115247. [Google Scholar]
- Yuan, H.; Han, Z.; Chen, Y.; Qi, F.; Fang, H.; Guo, Z.; Zhang, S.; He, W. Ferroptosis photoinduced by new cyclometalated iridium(III) complexes and its synergism with apoptosis in tumor cell inhibition. Angew. Chem. Int. Ed. 2021, 60, 8174–8181. [Google Scholar] [CrossRef]
- Małecka, M.; Skoczyńska, A.; Goodman, D.M.; Hartinger, C.G.; Budzisz, E. Biological properties of ruthenium(II)/(III) complexes with flavonoids as ligands. Coord. Chem. Rev. 2021, 436, 213849. [Google Scholar] [CrossRef]
- Sandbhor, U.; Padhye, S.; Billington, D.; Rathbone, D.; Franzblau, S.; Anson, C.E.; Powell, A.K. Metal complexes of carboxamidrazone analogs as antitubercular agents. J. Inorg. Biochem. 2002, 90, 127–136. [Google Scholar] [CrossRef]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; El Mokhtar, E.; Aubert, G.; Laurent, R.; Caminade, A.-M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.-P. Original multivalent copper(II)-conjugated phosphorus dendrimers and corresponding mononuclear copper(II) complexes with antitumoral activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.U.; Bhatti, M.Z.; Ali, A.; Duong, H.Q.; Zhang, Y.; Yang, B.; Koppireddi, S.; Lin, Y.; Wang, H.; Li, Z.T.; et al. Homo- and heteroleptic Pt(II) complexes of ONN donor hydrazone and 4-picoline: A synthetic, structural and detailed mechanistic anticancer investigation. Eur. J. Med. Chem. 2018, 143, 1039–1052. [Google Scholar] [CrossRef]
- Liang, H.; Liu, Y.C.; Chen, Z.F.; Liu, R.X.; Wu, Y.S.; Du, X.Q. Copper (II) Complex with 9-Aldehyde-10-pyrimidinehydrazone as Ligand and Synthetic Method and Application Thereof. CN 110950896A, 3 April 2020. [Google Scholar]
- Liang, H.; Liu, Y.C.; Chen, Z.F.; Wu, Y.S.; Bao, Z.C.; Liu, R.X.; Tang, S.Y. Binuclear Copper Complex with 9-Aldehyde-10-benzothiazole Anthracene Hydrazone as Ligand and Synthetic Method and Application Thereof. CN 110950895A, 3 April 2020. [Google Scholar]
- Liu, Y.C.; Liang, H.; Chen, Z.F.; Liu, R.X.; Wu, Y.S.; Han, H.H. Lonic Metal Complex with 9-Aldehyde-10-pyrimidinehydrazone as Ligand and Synthetic Method and Application Thereof. CN 110950913A, 3 April 2020. [Google Scholar]
- Rodrigues, D.A.; Ferreira-Silva, G.A.; Ferreira, A.C.S.; Fernandes, R.A.; Kwee, J.K.; Sant’Anna, C.M.R.; Ionta, M.; Fraga, C.A.M. Design, synthesis, and pharmacological evaluation of novel N-acylhydrazone derivatives as potent histone deacetylase 6/8 dual inhibitors. J. Med. Chem. 2016, 59, 655–670. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Ma, C.; Lu, D. Synthesis and anticancer activities of novel 8-azapurine carbocyclic nucleoside hydrazones. Bioorg. Med. Chem. Lett. 2015, 25, 4461–4463. [Google Scholar] [CrossRef]
- Lindgren, E.B.; de Brito, M.A.; Vasconcelos, T.R.A.; de Moraes, M.O.; Montenegro, R.C.; Yoneda, J.D.; Leal, K.Z. Synthesis and anticancer activity of (E)-2-benzothiazole hydrazones. Eur. J. Med. Chem. 2014, 86, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Zhang, S.; Shao, Y.; Fan, L.; Xu, H.; Yu, X.; Zhi, X.; Yao, X.; Zhang, R. Synthesis and quantitative structure–activity relationship (QSAR) study of novel N-arylsulfonyl-3-acylindole arylcarbonyl hydrazone derivatives as nematicidal agents. J. Agric. Food Chem. 2013, 61, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, X.; Ding, L.; Zhang, Y.; Cui, L.; Sun, L.; Li, W.; Wang, D.; Zhao, Y. Synthesis and antibacterial activity evaluation of novel biaryloxazolidinone analogues containing a hydrazone moiety as promising antibacterial agents. Eur. J. Med. Chem. 2018, 158, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Salgin-Goksen, U.; Gokhan-Kelekci, N.; Goktas, O.; Koysal, Y.; Kilic, E.; Isik, S.; Aktay, G.; Ozalp, M. 1-acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, M.; Utku, S.; Küpeli, E. Synthesis and analgesic and anti-inflammatory activities 6-substituted-3(2H)-pyridazinone-2-acetyl-2-(p-substituted/nonsubstituted benzal)hydrazone derivatives. Eur. J. Med. Chem. 2009, 44, 3760–3764. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Luo, D.; Guo, S.; He, F.; Dai, A.; Song, B.; Wu, J. Synthesis of anthranilic diamide derivatives containing moieties of trifluoromethylpyridine and hydrazone as potential anti-viral agents for plants. J. Agric. Food. Chem. 2019, 67, 13344–13352. [Google Scholar] [CrossRef]
- El-Sabbagh, O.I.; Rady, H.M. Synthesis of new acridines and hydrazones derived from cyclic β-diketone for cytotoxic and antiviral evaluation. Eur. J. Med. Chem. 2009, 44, 3680–3686. [Google Scholar] [CrossRef]
- Gudasi, K.B.; Patil, M.S.; Vadavi, R.S.; Shenoy, R.V.; Patil, S.A.; Nethaji, M. X-ray crystal structure of the N-(2-hydroxy-1-naphthalidene)phenylglycine schiff base. synthesis and characterization of its transition metal complexes. Transit. Metal Chem. 2006, 31, 580–585. [Google Scholar] [CrossRef]
- Murukan, B.; Mohanan, K.J. Synthesis, characterization and antibacterial properties of some trivalent metal complexes with [(2-hydroxy-1-naphthaldehyde)-3-isatin]-bishydrazone. Enzyme Inhib. Med. Chem. 2007, 22, 65–70. [Google Scholar] [CrossRef]
- Gulya, A.P.; Prisakar, V.I.; Tsapkov, V.I.; Buracheva, S.A.; Spynu, S.N.; Bezhenar, N.P. Synthesis and antimicrobial activity of sulfanilamide-containing copper(II) naphthalidenethiosemicarbazidates. Pharm. Chem. J. 2008, 42, 326–328. [Google Scholar] [CrossRef]
- Patil, S.A.; Unki, S.N.; Kulkarni, A.D.; Naik, V.H.; Badami, P.S. Synthesis, characterization, in vitro antimicrobial and DNA cleavage studies of Co(II), Ni(II) and Cu(II) complexes with ONOO donor coumarin schiff bases. J. Mol. Struct. 2011, 985, 330–338. [Google Scholar] [CrossRef]
- Aslan, H.G.; Karacan, N.; Aslan, E. Synthesis, characterization and antimicrobial activity of a new aromatic sulfonyl hydrazone derivative and its transition metal complexes. J. Chin. Chem. Soc. 2013, 60, 212–217. [Google Scholar] [CrossRef]
- Gowda, K.R.S.; Naik, H.S.B.; Kumar, B.V.; Sudhamani, C.N.; Sudeep, H.V.; Naik, T.R.R.; Krishnamurthy, G. Synthesis, antimicrobial, DNA-binding and photonuclease studies of cobalt(III) and nickel(II) schiff base complexes. Spectrochim. Acta A 2013, 105, 229–237. [Google Scholar] [CrossRef]
- McCann, M.; Kellett, A.; Kavanagh, K.; Devereux, M.; Santos, A.L.S. Deciphering the antimicrobial activity of phenanthroline chelators. Curr. Med. Chem. 2012, 19, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The antibacterial activity of metal complexes containing 1, 10-phenanthroline: Potential as alternative therapeutics in the era of antibiotic resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Adly, O.M.I.; Taha, A. Coordination diversity of new mononuclear ONS hydrazone with transition metals: Synthesis, characterization, molecular modeling and antimicrobial studies. J. Mol. Struct. 2013, 1038, 250–259. [Google Scholar] [CrossRef]
- Rajam, K.G.; Kumar, M.P.; Kiran, K.J.; Shivaraj. Mixed ligand complexes derived from semicarbazone schiff base and heterocyclic ligands: Structure and antimicrobial activity. Russ. J. Gen. Chem. 2018, 88, 1000–1008. [Google Scholar] [CrossRef]
- Azarkish, M.; Akbari, A.; Sedaghat, T.; Simpson, J. Ternary complexes of Zn(II) and Cu(II) with 1-((2-hydroxynaphthalen-1-yl)methylene)-4-phenylthiosemicarbazide in the presence of heterocyclic bases as auxiliary ligands: Synthesis, spectroscopic and structural characterization and antibacterial activity. J. Mol. Struct. 2018, 1156, 34–42. [Google Scholar] [CrossRef]
- El-Sawaf, A.K.; Abdel-Monem, Y.K.; Azzam, M.A. Synthesis, spectroscopic, electrochemical characterization, density functional theory (DFT), time dependent density functional theory (TD-DFT), and antibacterial studies of some Co(II), Ni(II), and Cu(II) chelates of (E)-4-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1-(3-hydroxynaphthalen-2-yl)methylene) thiosemicarbazide schiff base ligand. Appl. Organomet. Chem. 2020, 34, e5729 (1–18). [Google Scholar]
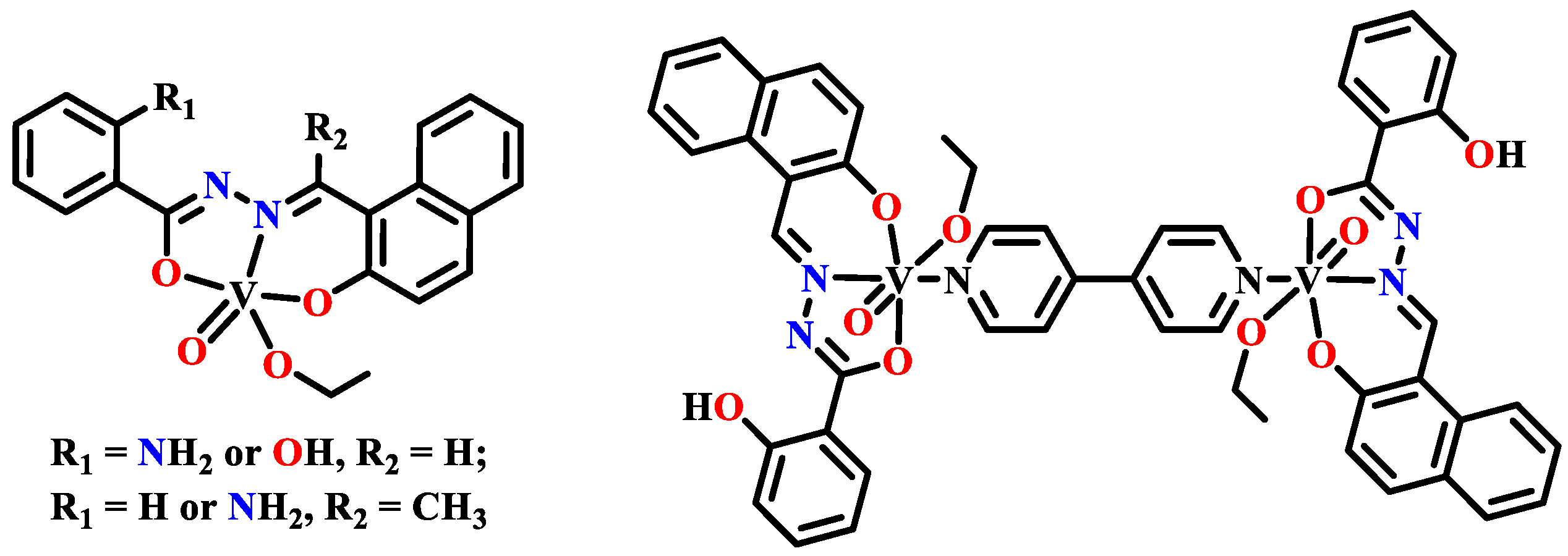
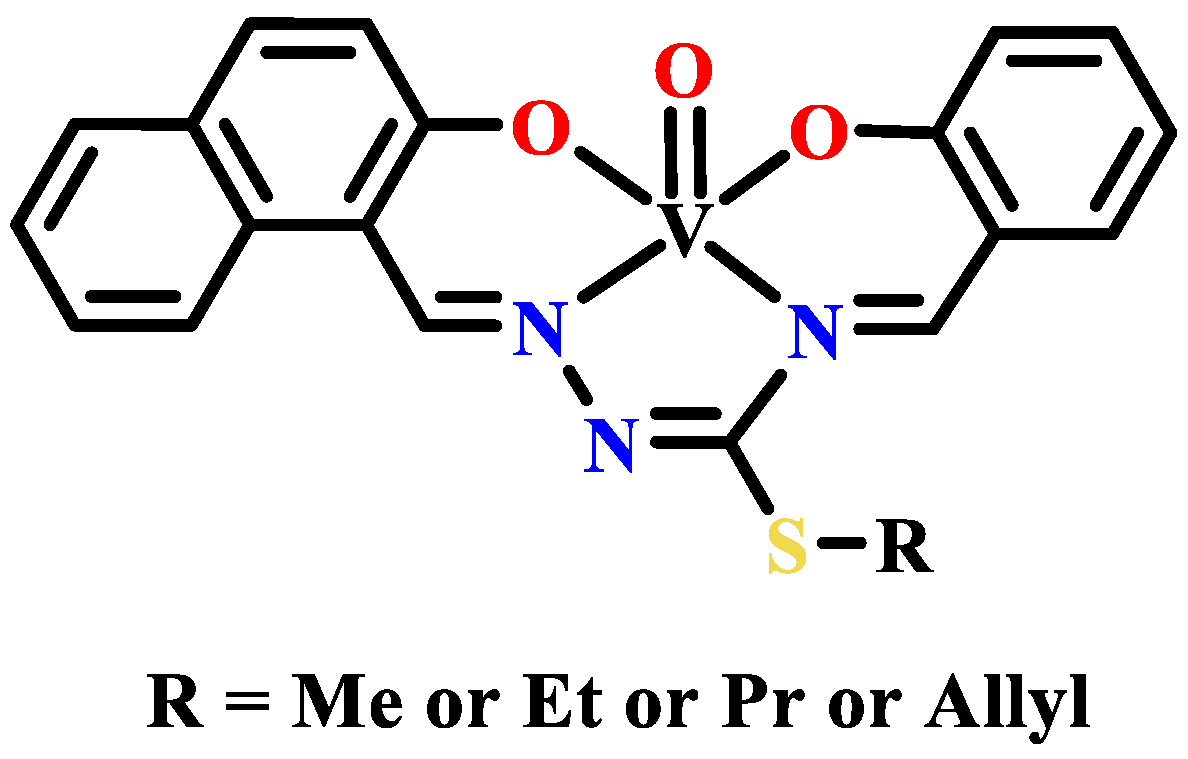
- Li, W.; Han, X.; Ding, Y. Synthesis, crystal structure, and preliminary antibacterial activity of oxovanadium(V) complex with hydrazone ligand. Russ. J. Coord. Chem. 2015, 41, 442–446. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Sheikhshoaie, I.; Simpson, J.; Ebrahimnejad, H.; Dusek, M.; Kharazmi, N.; Eigner, V. Antimicrobial activity of aroylhydrazone-based oxido vanadium(V) complexes: In vitro and in silico studies. New J. Chem. 2016, 40, 2401–2412. [Google Scholar] [CrossRef]
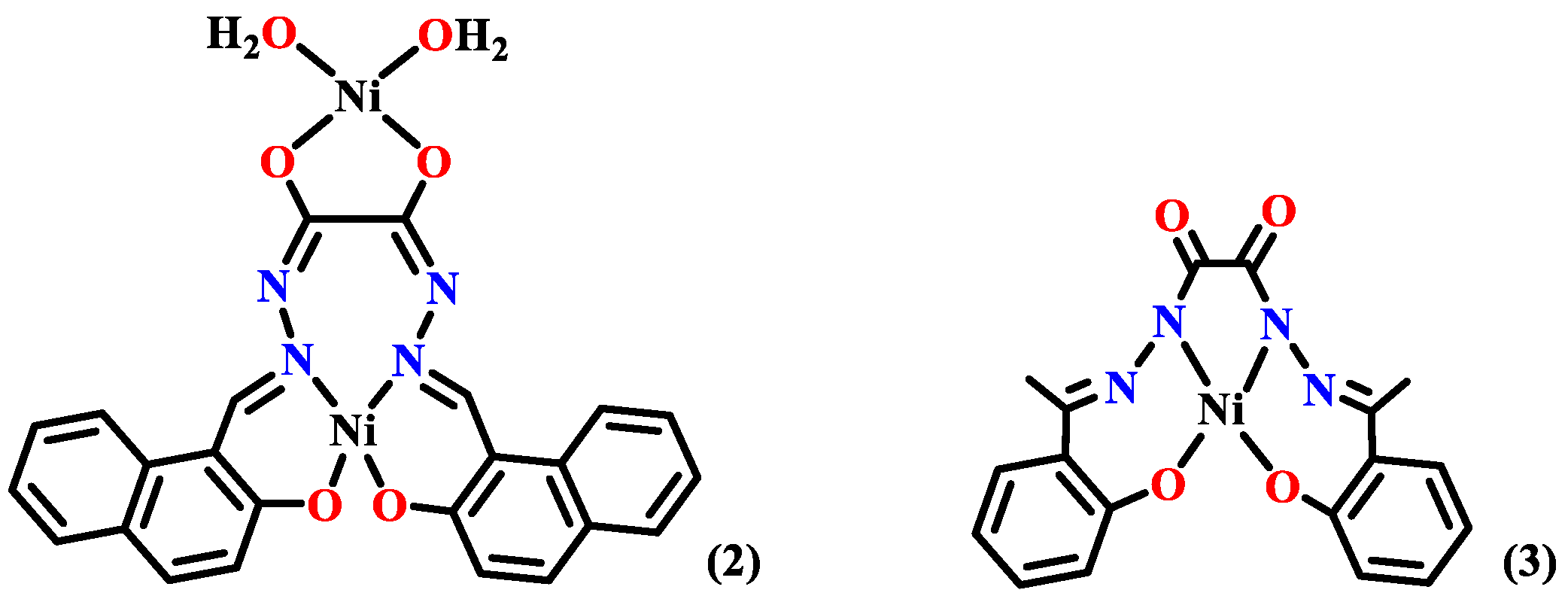
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M. Nickel(II)-oxaloyldihydrazone complexes: Characterization, indirect band gap energy and antimicrobial evaluation. Cogent Chem. 2016, 2, 1142820. [Google Scholar] [CrossRef]
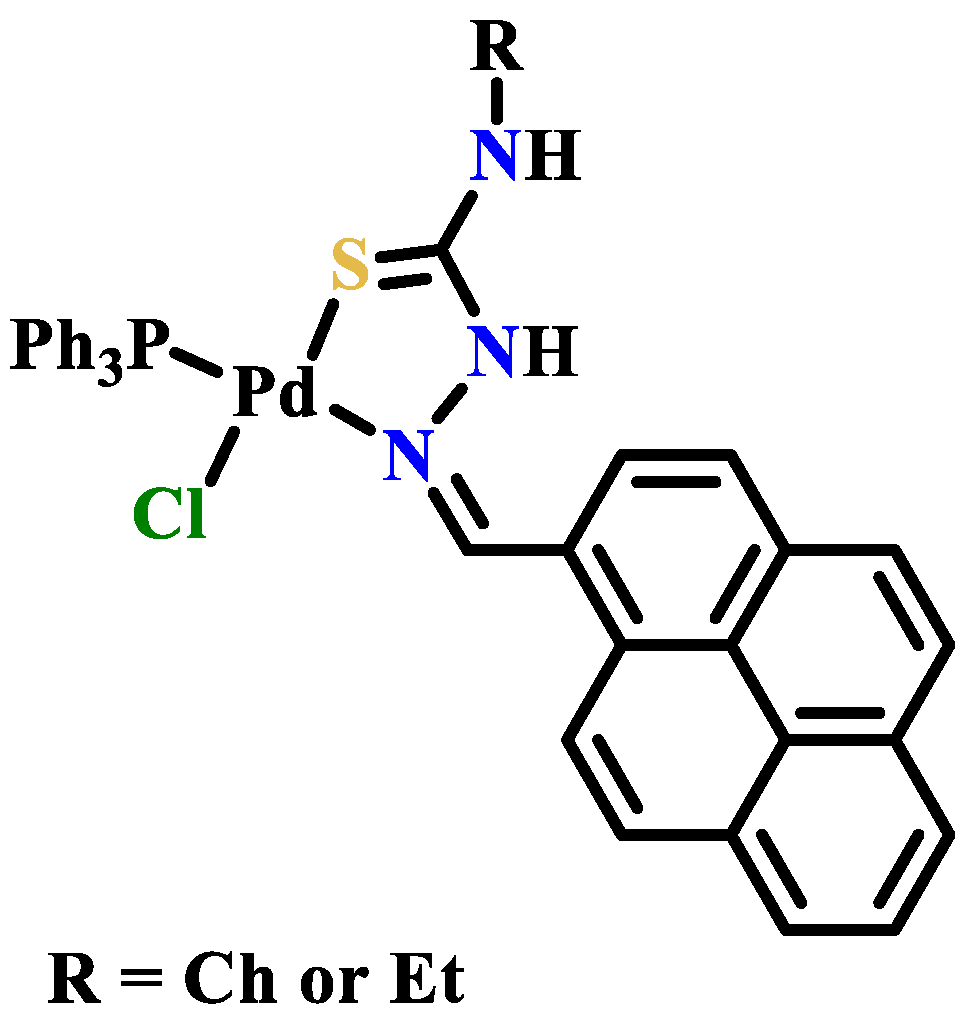
- Prabhakaran, R.; Renukadevi, S.V.; Karvembu, R.; Huang, R.; Mautz, J.; Huttner, G.; Subashkumar, R.; Natarajan, K. Structural and biological studies of mononuclear palladium(II) complexes containing N-substituted thiosemicarbazones. Eur. J. Med. Chem. 2008, 43, 268–273. [Google Scholar] [CrossRef]
- Bandyopadhyay, N.; Das, M.; Samanta, A.; Zhu, M.; Lu, L.; Naskar, J.P. Promising antimicrobial activity of an oxime based palladium(II) complex. ChemistrySelect 2017, 2, 230–240. [Google Scholar] [CrossRef]
- Raja, G.; Sathya, N.; Jayabalakrishnan, C. Spectroscopic, catalytic, and biological studies on mononuclear ruthenium(II) ONSN chelating thiosemicarbazone complexes. J. Coord. Chem. 2011, 64, 817–831. [Google Scholar] [CrossRef]
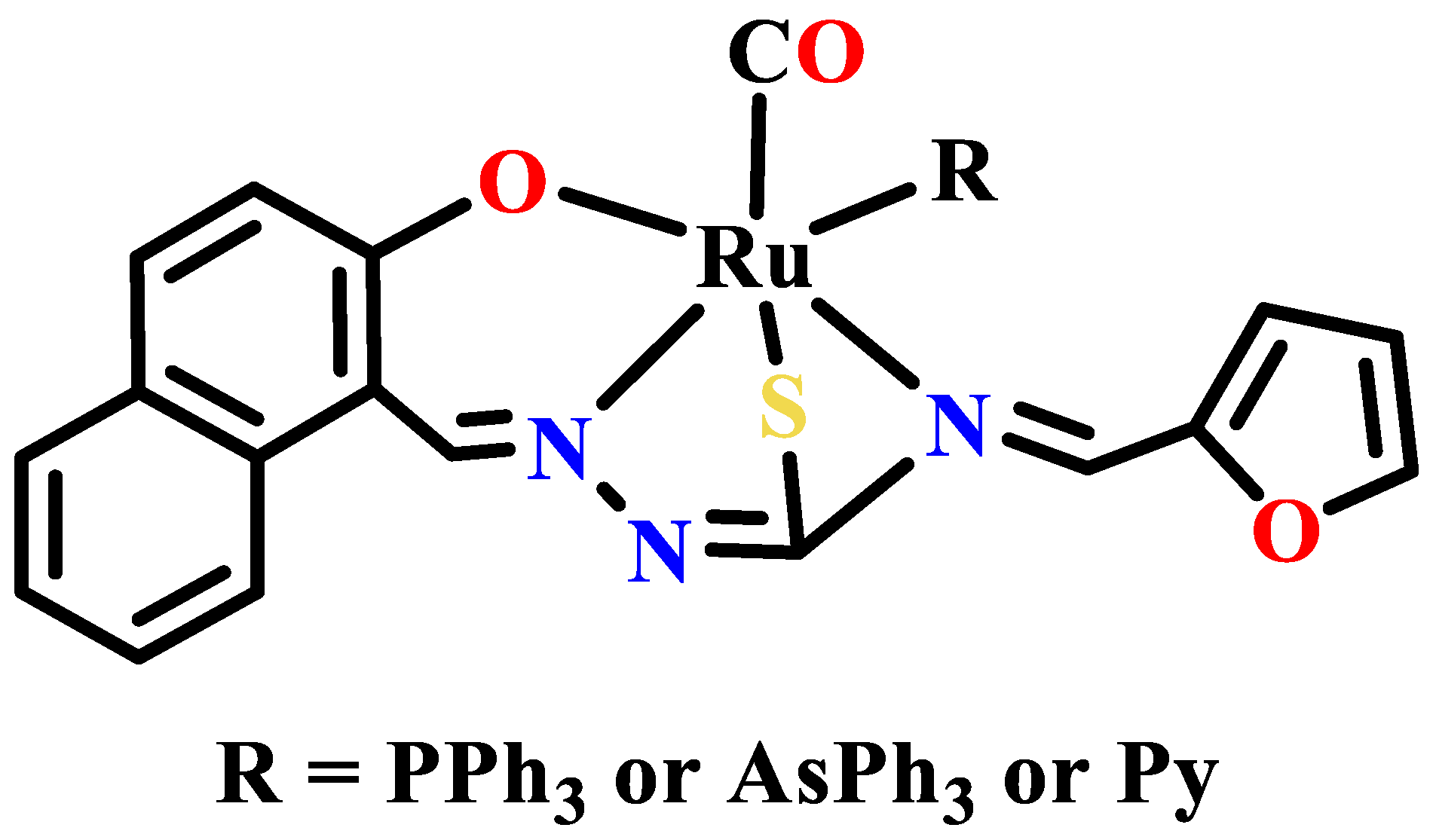
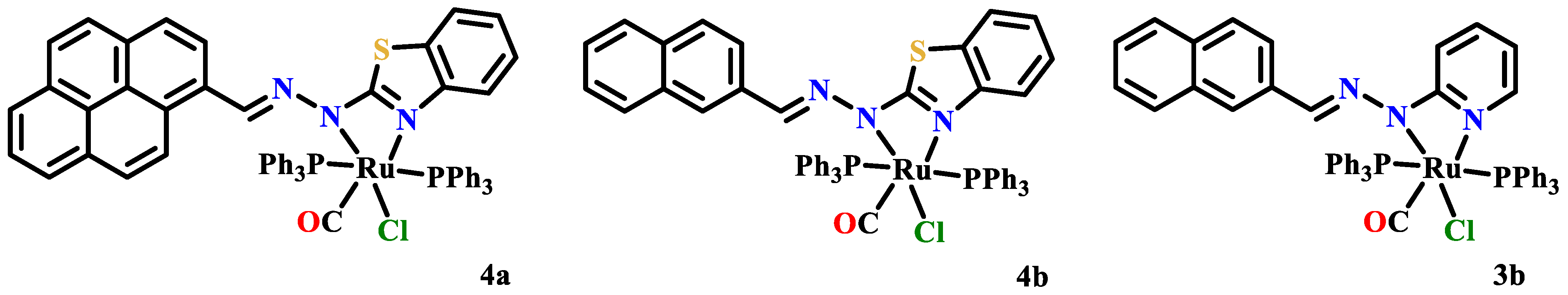
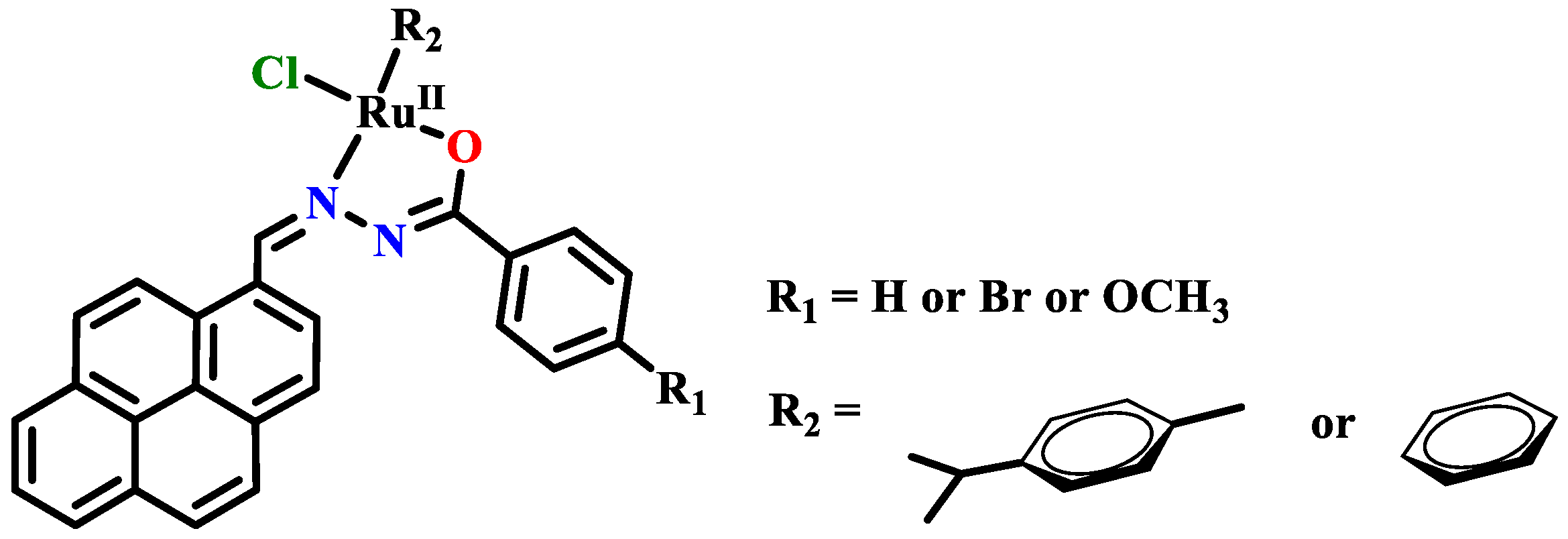
- Dinda, S.; Sultana, T.; Sultana, S.; Patra, S.C.; Mitra, A.K.; Roy, S.; Pramanik, K.; Ganguly, S. Ruthenocycles of benzothiazolyl and pyridyl hydrazones with ancillary PAHs: Synthesis, structure, electrochemistry and antimicrobial activity. New J. Chem. 2020, 44, 11022–11034. [Google Scholar] [CrossRef]
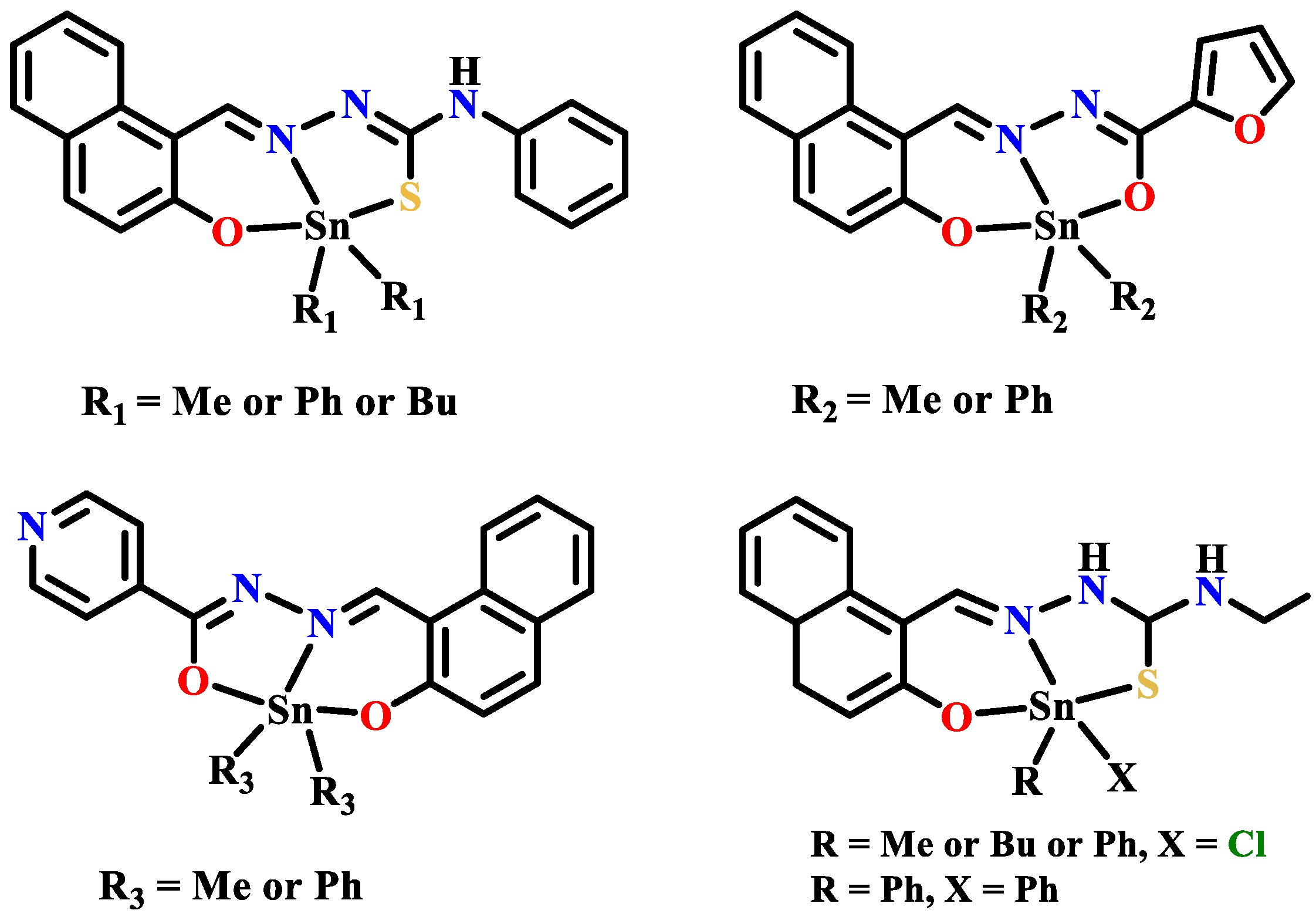
- Sedaghat, T.; Aminian, M.; Bruno, G.; Rudbari, H.A. Binuclear organotin(IV) complexes with adipic dihydrazones: Synthesis, spectral characterization, crystal structures and antibacterial activity. J. Organomet. Chem. 2013, 737, 26–31. [Google Scholar] [CrossRef]
- Sedaghat, T.; Aminian, M.; Azarkish, M. New bis-diphenyltin(IV) complexes with oxalyldihydrazone derivatives: Synthesis, characterization and antibacterial activity. Phosphorus Sulfur. 2015, 190, 352–359. [Google Scholar] [CrossRef]
- Yousefi, M.; Sedaghat, T.; Simpson, J.; Motamedi, H.; Dayer, M.R. Bis-substituted diphenylamine arylidene hydrazones for the synthesis of new binuclear organotin(IV) complexes: Crystal structure, DNA cleavage and molecular docking. Polyhedron 2018, 155, 153–162. [Google Scholar] [CrossRef]
- Sedaghat, T.; Golalzadeh, A.; Motamedi, H. Diorganotin complexes with N(4)-phenylthiosemicarbazones: Synthesis, spectrocopic characterization and antibacterial activity. Phosphorus Sulfur. 2013, 188, 1694–1702. [Google Scholar] [CrossRef]
- Sedaghat, T.; Tahmasbi, L.; Motamedi, H.; Reyes-Martinez, R.; Morales-Morales, D. Diorganotin(IV) complexes with furan-2-carbohydrazone derivatives: Synthesis, characterization, crystal structure and antibacterial activity. J. Coord. Chem. 2013, 66, 712–724. [Google Scholar] [CrossRef]
- Sedaghat, T.; Yousefi, M.; Bruno, G.; Rudbari, H.A.; Motamedi, H.; Nobakht, V. Synthesis, spectral characterization, crystal structure and antibacterial studies of diorganotin(IV) complexes with isonicotinoyl hydrazone derivatives. Polyhedron 2014, 79, 88–96. [Google Scholar] [CrossRef]
- Salam, M.A.; Arafath, M.A.; Hussein, M.A.; Basri, R.; Pervin, R. Organotin(IV) complexes with 2-hydroxynaphthaldehyde-N(4)-ethylthiosemicarbazone: Synthesis, characterization, and in vitro antibacterial activity. Phosphorus Sulfur. 2016, 191, 1101–1107. [Google Scholar] [CrossRef]
- Beckford, F.; Holt, A.T. Organometallic Ruthenium Complexes of Novel Thiosemicarbazones. J. Arkansas Acad. Sci. 2006, 60, 27–31. [Google Scholar]
- Beckford, F.; Thessing, J.; Woods, J.; Didion, J.; Gerasimchuk, N.; Gonzalez-Sarrias, A.; Seeram, N.P. Synthesis and structure of [(η6-p-cymene)Ru(2-anthracen-9-ylmethylene-N-ethylhydrazinecarbothioamide)Cl]Cl; biological evaluation, topoisomerase II inhibition and reaction with DNA and human serum albumin. Metallomics 2011, 3, 491–502. [Google Scholar] [CrossRef]
- Holt, A.; Beckford, F. Coordination chemistry of polycyclic thiosemicarbazones. I. Reactions of the thiosemicarbazones from 9-anthraldehyde. J. Undergrad. Chem. Res. 2007, 6, 173–177. [Google Scholar]
- Kumar, S.; Hansda, A.; Chandra, A.; Kumar, A.; Kumar, M.; Sithambaresan, M.; Faizi, M.S.H.; Kumar, V.; John, R.P. Co(II), Ni(II), Cu(II) and Zn(II) complexes of acenaphthoquinone 3-(4-benzylpiperidyl)thiosemicarbazone: Synthesis, structural, electrochemical and antibacterial studies. Polyhedron 2017, 134, 11–21. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Yang, X. Bioactivity, molecular mechanism and drug discovery of vanadium complexes. Prog. Pharm. Sci. 2020, 44, 256–268. [Google Scholar]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef]
- Carpéné, C.; Garcia-Vicente, S.; Serrano, M.; Marti, L.; Belles, C.; Royo, M.; Galitzky, J.; Zorzano, A.; Testar, X. Insulin-mimetic compound hexaquis (benzylammonium) decavanadate is antilipolytic in human fat cells. World J. Diabetes. 2017, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, H.; Zeng, X.; Zhang, Y.; Zhao, P.; Jiang, J.; Zang, L. Synthesis and characterization of unsymmetrical oxidovanadium complexes: DNA-binding, cleavage studies and antitumor activities. J. Inorg. Biochem. 2012, 112, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Tian, X.; Zeng, P.; Liu, W.; Ying, P.; Chen, H.; Lu, J.; Yang, N.; Chen, H. The anti-tumor activity of novel oxovanadium complexes derived from thiosemicarbazones and fluoro-phenanthroline derivatives. Polyhedron 2016, 117, 803–816. [Google Scholar] [CrossRef]
- Liao, X.; Lu, J.; Ying, P.; Zhao, P.; Bai, Y.; Li, W.; Liu, M. DNA binding, antitumor activities, and hydroxyl radical scavenging properties of novel oxovanadium(IV) complexes with substituted isoniazid. J. Biol. Inorg. Chem. 2013, 18, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Ying, P.; Zeng, P.; Lu, J.; Chen, H.; Liao, X.; Yang, N. New oxidovanadium complexes incorporating thiosemicarbazones and 1, 10-phenanthroline derivatives as DNA cleavage, potential anticancer agents, and hydroxyl radical scavenger. Chem. Biol. Drug Des. 2015, 86, 926–937. [Google Scholar] [CrossRef]
- Zeng, P.; He, L.; Ye, Z.; Yang, N.; Song, Y.; Lu, J. DNA interactions, antitumor activities and radical scavenging properties of oxovanadium complexes with pyrazino[2,3-f] [1,10]phenanthroline ligands. Transit. Metal Chem. 2015, 40, 779–787. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Zhang, Y.-W.; Xiao, J.-Y.; Guo, H.-W.; Liao, X.-W.; Li, W.-J.; Zhang, Y.-C. Oxovanadium phenanthroimidazole derivatives: Synthesis, DNA binding and antitumor activities. Transit. Metal Chem. 2018, 43, 171–183. [Google Scholar] [CrossRef]
- Dash, S.P.; Panda, A.K.; Pasayat, S.; Dinda, R.; Biswas, A.; Tiekink, E.R.T.; Mukhopadhyay, S.; Bhutia, S.K.; Kaminsky, W.; Sinn, E. Oxidovanadium(V) complexes of aroylhydrazones incorporating heterocycles: Synthesis, characterization and study of DNA binding, photoinduced DNA cleavage and cytotoxic activities. RSC Adv. 2015, 5, 51852–51867. [Google Scholar] [CrossRef]
- Dash, S.P.; Pasayat, S.; Bhakat, S.; Roy, S.; Dinda, R.; Tiekink, E.R.T.; Mukhopadhyay, S.; Bhutia, S.K.; Hardikar, M.R.; Joshi, B.N.; et al. Highly stable hexacoordinated nonoxidovanadium(IV) complexes of sterically constrained ligands: Syntheses, structure, and study of antiproliferative and insulin mimetic activity. Inorg. Chem. 2013, 52, 14096–14107. [Google Scholar] [CrossRef]
- Banerjee, A.; Dash, S.P.; Mohanty, M.; Sahu, G.; Sciortino, G.; Garribba, E.; Carvalho, M.F.N.N.; Marques, F.; Pessoa, J.C.; Kaminsky, W.; et al. New VIV, VIVO, VVO, and VVO2 systems: Exploring their interconversion in solution, protein interactions, and cytotoxicity. Inorg. Chem. 2020, 59, 14042–14057. [Google Scholar] [CrossRef]
- Banerjee, A.; Mohanty, M.; Lima, S.; Samanta, R.; Garribba, E.; Sasamori, T.; Dinda, R. Synthesis, structure and characterization of new dithiocarbazate-based mixed ligand oxidovanadium(IV) complexes: DNA/HAS interaction, cytotoxic activity and DFT studies. New J. Chem. 2020, 44, 10946–10963. [Google Scholar] [CrossRef]
- Qi, J.; Gou, Y.; Zhang, Y.; Yang, K.; Chen, S.; Liu, L.; Wu, X.; Wang, T.; Zhang, W.; Yang, F. Developing anticancer ferric prodrugs based on the N-donor residues of human serum albumin carrier IIA subdomain. J. Med. Chem. 2016, 59, 7497–7511. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, S.; Ghorai, A.; Ghosh, U.; Stoeckli-Evans, H. Synthesis, structure, interaction with DNA and cytotoxicity of a luminescent copper(II) complex with a hydrazone ligand. Polyhedron 2013, 51, 228–234. [Google Scholar] [CrossRef]
- Pradeepa, S.M.; Naik, H.S.B.; Kumar, B.V.; Priyadarsini, K.I.; Barik, A.; Naik, T.R.R.; Prabhakara, M.C. Metal based photosensitizers of tetradentate schiff base: Promising role in anti-tumor activity through singlet oxygen generation mechanism. Spectrochim. Acta A 2013, 115, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sheikhshoaie, I.; Ebrahimipour, S.Y.; Sheikhshoaie, M.; Mohamadi, M.; Abbasnejad, M.; Rudbari, H.A.; Bruno, G. Synthesis, characterization, X-ray crystal structure, electrochemical evaluation and anti-cancer studies of a mixed ligand Cu(II) complex of (E)-N’-((2-hydroxynaphthalen-1-yl)methylene)acetohydrazide. J. Chem. Sci. 2015, 127, 2193–2200. [Google Scholar] [CrossRef]
- Hou, L.; Jia, X.; Wu, Y.; Li, J.; Yao, D.; Gou, Y.; Huang, G. Aroylhydrazone Cu(II) complexes: Syntheses, crystal structures, and anticancer properties. J. Mol. Struct. 2021, 130469. [Google Scholar] [CrossRef]
- Gou, Y.; Li, J.; Fan, B.; Xu, B.; Zhou, M.; Yang, F. Structure and biological properties of mixed-ligand Cu(II) schiff base complexes as potential anticancer agents. Eur. J. Med. Chem. 2017, 134, 207–217. [Google Scholar] [CrossRef]
- Gou, Y.; Qi, J.; Ajayi, J.P.; Zhang, Y.; Zhou, Z.; Wu, X.; Yang, F.; Liang, H. Developing anticancer copper(II) pro-drugs based on the nature of cancer cells and the human serum albumin carrier IIA subdomain. Mol. Pharm. 2015, 12, 3597–3609. [Google Scholar] [CrossRef]
- Ribeiro, N.; Roy, S.; Butenko, N.; Cavaco, I.; Pinheiro, T.; Alho, I.; Marques, F.; Avecilla, F.; Pessoa, J.C.; Correia, I. New Cu(II) complexes with pyrazolyl derived schiff base ligands: Synthesis and biological evaluation. J. Inorg. Biochem. 2017, 174, 63–75. [Google Scholar] [CrossRef]
- Qi, J.; Yao, Q.; Tian, L.; Wang, Y. Piperidylthiosemicarbazones Cu(II) complexes with a high anticancer activity by catalyzing hydrogen peroxide to degrade DNA and promote apoptosis. Eur. J. Med. Chem. 2018, 158, 853–862. [Google Scholar] [CrossRef]
- Saswati; Chakraborty, A.; Dash, S.P.; Panda, A.K.; Acharyya, R.; Biswas, A.; Mukhopadhyay, S.; Bhutia, S.K.; Crochet, A.; Patil, Y.P.; et al. Synthesis, X-ray structure and in vitro cytotoxicity studies of Cu(I/II) complexes of thiosemicarbazone: Special emphasis on their interactions with DNA. Dalton Trans. 2015, 44, 6140–6157. [Google Scholar] [CrossRef] [PubMed]
- Saswati; Mohanty, M.; Banerjee, A.; Biswal, S.; Horn, A., Jr.; Schenk, G.; Brzezinski, K.; Sinn, E.; Reuter, H.; Dinda, R. Polynuclear zinc(II) complexes of thiosemicarbazone: Synthesis, X-ray structure and biological evaluation. J. Inorg. Biochem. 2020, 203, 110908. [Google Scholar] [CrossRef] [PubMed]
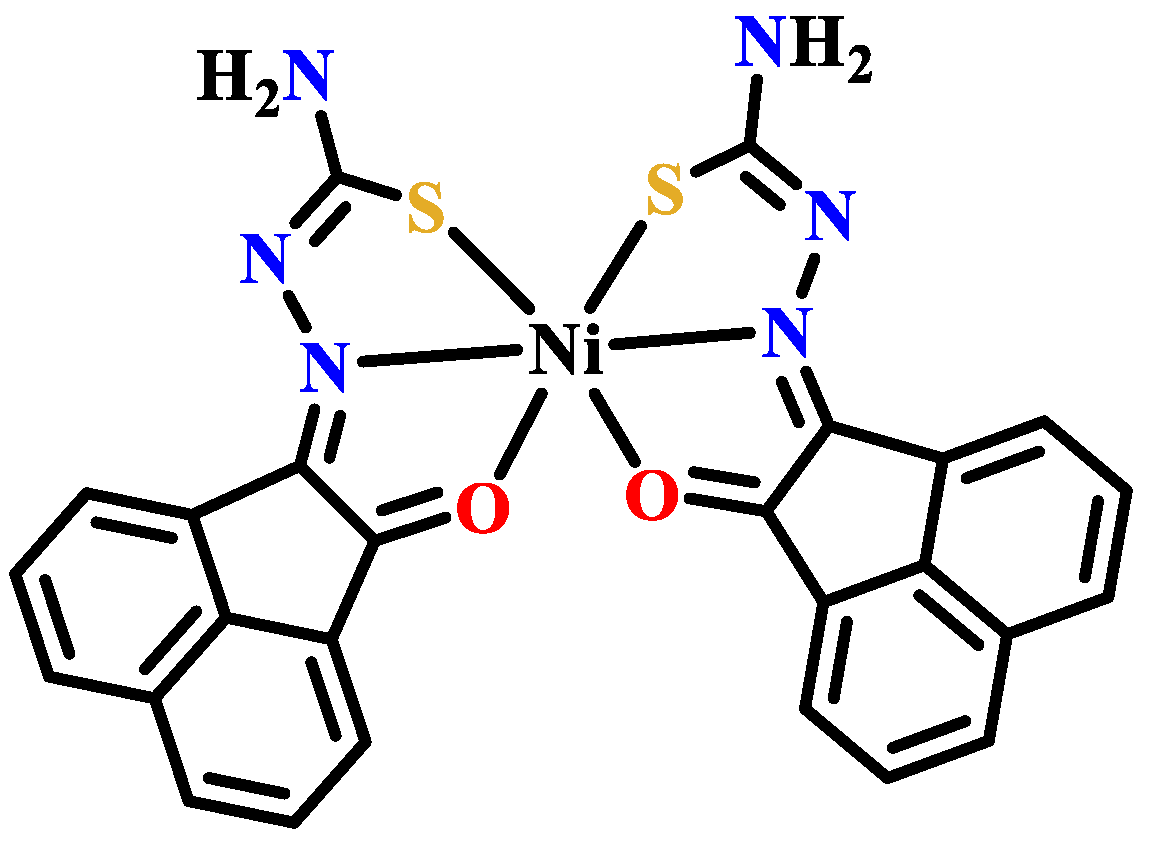
- Krishnamoorthy, P.; Sathyadevi, P.; Butorac, R.R.; Cowley, A.H.; Bhuvanesh, N.S.P.; Dharmaraj, N. Variation in the biomolecular interactions of nickel(II) hydrazone complexes upon tuning the hydrazide fragment. Dalton Trans. 2012, 41, 6842–6854. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Seth, D.K.; Gangopadhyay, S.; Karmakar, P.; Bhattacharya, S. Nickel complexes of some thiosemicarbazones: Synthesis, structure, catalytic properties and cytotoxicity studies. Inorg. Chim. Acta 2012, 392, 118–130. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Kalaivani, P.; Huang, R.; Poornima, P.; Padma, V.V.; Dallemer, F.; Natarajan, K. DNA binding, antioxidant, cytotoxicity (MTT, lactate dehydrogenase, NO), and cellular uptake studies of structurally different nickel(II) thiosemicarbazone complexes: Synthesis, spectroscopy, electrochemistry, and X-ray crystallography. J. Biol. Inorg. Chem. 2013, 18, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Nanjundan, N.; Selvakumar, P.; Narayanasamy, R.; Haque, R.A.; Velmurugan, K.; Nandhakumar, R.; Silambarasan, T.; Dhandapani, R.J. Synthesis, structure, DNA/BSA interaction and in vitro cytotoxic activity of nickel(II) complexes derived from S-allyldithiocarbazate. Photochem. Photobiol. B 2014, 141, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Di Paolo, R.E.; Galvão, A.M.; Marques, F.; Pessoa, J.C.; Correia, I. Photophysical properties and biological evaluation of a Zinc(II)-5-methyl-1H-pyrazole Schiff base complex. Spectrochim. Acta A 2018, 204, 317–327. [Google Scholar] [CrossRef]
- Halder, S.; Peng, S.-M.; Lee, G.-H.; Chatterjee, T.; Mukherjee, A.; Dutta, S.; Sanyal, U.; Bhattacharya, S. Synthesis, structure, spectroscopic properties and cytotoxic effect of some thiosemicarbazone complexes of palladium. New J. Chem. 2008, 32, 105–114. [Google Scholar] [CrossRef]
- Hernández, W.; Paz, J.; Carrasco, F.; Vaisberg, A.; Spodine, E.; Manzur, J.; Hennig, L.; Sieler, J.; Blaurock, S.; Beyer, L. Synthesis and characterization of new palladium(II) thiosemicarbazone complexes and their cytotoxic activity against various human tumor cell lines. Bioinorg. Chem. Appl. 2013, 2013, 524701. [Google Scholar] [CrossRef]
- Hernández, W.; Vaisberg, A.J.; Tobar, M.; Álvarez, M.; Manzur, J.; Echevarría, Y.; Spodine, E. In vitro antiproliferative activity of palladium(II) thiosemicarbazone complexes and the corresponding functionalized chitosan coated magnetite nanoparticles. New J. Chem. 2016, 40, 1853–1860. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Kalaivani, P.; Poornima, P.; Dallemer, F.; Huang, R.; Padma, V.V.; Natarajan, K. Synthesis, DNA/protein binding and in vitro cytotoxic studies of new palladium metallothiosemicarbazones. Bioorg. Med. Chem. 2013, 21, 6742–6752. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, J.; Khan, M.; Yu, P.; Yang, F.; Liang, H. Structure and biological properties of five Pt(II) complexes as potentialanticancer agents. J. Inorg. Biochem. 2018, 185, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, V.; Đilović, I.; Rubčić, M.; Pavelić, S.K.; Kralj, M.; Matković-Čalogović, D.; Piantanida, I.; Novak, P.; Rožman, A.; Cindrić, M. Synthesis and characterisation of thiosemicarbazonato molybdenum(VI) complexes and their in vitro antitumor activity. Eur. J. Med. Chem. 2010, 45, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Dinda, R.; Panda, A.; Banerjee, A.; Mohanty, M.; Pasayat, S.; Tiekink, E.R.T. Investigation of DNA interaction and antiproliferative activity of mixed ligand dioxidomolybdenum(VI) complexes incorporating ONO donor aroylhydrazone ligands. Polyhedron 2020, 183, 114533. [Google Scholar] [CrossRef]
- Kalaivani, P.; Prabhakaran, R.; Poornima, P.; Huang, R.; Hornebecq, V.; Dallemer, F.; Padma, V.V.; Natarajan, K. Synthesis and structural characterization of new ruthenium(II) complexes and investigation of their antiproliferative and metastatic effect against human lung cancer (A549) cells. RSC Adv. 2013, 3, 20363–20378. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Kalaivani, P.; Senthilkumar, K.; Natarajan, K. Synthesis, structural characterization, DNA/protein binding and in vitro cytotoxicity of three structurally different organoruthenium metallates from single pot. J. Organomet. Chem. 2016, 825–826, 83–99. [Google Scholar] [CrossRef]
- Kalaivani, P.; Prabhakaran, R.; Dallemer, F.; Natarajan, K. Photophysical properties and in vitro cytotoxicity studies of new Ru(II) carbonyl complexes and mixed geometrical Ru(II)–Ni(II) complex in HS-DNA/BSA protein and human lung (A549) and liver (HepG2) cells. RSC Adv. 2014, 4, 51850–51864. [Google Scholar] [CrossRef]
- Selvamurugan, S.; Ramachandran, R.; Viswanathamurthi, P. Ruthenium(II) carbonyl complexes containing S-methylisothiosemicarbazone based tetradentate ligand: Synthesis, characterization and biological applications. Biometals 2013, 26, 741–753. [Google Scholar] [CrossRef]
- Prakash, G.; Manikandan, R.; Viswanathamurthi, P.; Velmurugan, K.; Nandhakumar, R. Ruthenium(III) S-methylisothiosemicarbazone schiff base complexes bearing PPh3/AsPh3 coligand: Synthesis, structure and biological investigations, including antioxidant, DNA and protein interaction, and in vitro anticancer activities. J. Photochem. Photobiol. B 2014, 138, 63–74. [Google Scholar] [CrossRef]
- Jayanthi, E.; Kalaiselvi, S.; Padma, V.V.; Bhuvanesh, N.S.; Dharmaraj, N. Solvent assisted formation of ruthenium(III) and ruthenium(II) hydrazone complexes in one-pot with potential in vitro cytotoxicity and enhanced LDH, NO and ROS release. Dalton Trans. 2016, 45, 1693–1707. [Google Scholar] [CrossRef]
- Piska, K.; Jamrozik, M.; Koczurkiewicz-Adamczyk, P.; Bucki, A.; Żmudzki, P.; Kołaczkowski, M.; Pękala, E. Carbonyl reduction pathway in hepatic in vitro metabolism of anthracyclines: Impact of structure on biotransformation rate. Toxicol. Lett. 2021, 342, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Poornima, S.; Anbu, S.; Ravishankaran, R.; Sundaramoorthy, S.; Vennila, K.N.; Karande, A.A.; Velmurugan, D.; Kandaswamy, M. DNA and protein targeting 1,2,4-triazole based water soluble dinickel(II) complexes enhances antiproliferation and lactate dehydrogenase inhibition. Polyhedron 2013, 62, 26–36. [Google Scholar] [CrossRef]
- Kate, A.N.; Kumbhar, A.A.; Khan, A.A.; Joshi, P.V.; Puranik, V.G. Monitoring cellular uptake and cytotoxicity of copper(II) complex using a fluorescent anthracene thiosemicarbazone ligand. Bioconjug. Chem. 2014, 25, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.A.; Liu, F.; Seymour, L.; Magnusen, A.; Erves, T.R.; Arca, J.F.; Beckford, F.A.; Venkatraman, R.; González-Sarrías, A.; Fronczek, F.R.; et al. Synthesis, characterisation, and preliminary in vitro studies of vanadium(IV) complexes with a schiff base and thiosemicarbazones as mixed ligands. Eur. J. Inorg. Chem. 2012, 2012, 664–677. [Google Scholar] [CrossRef]
- Beebe, S.J.; Celestine, M.J.; Bullock, J.L.; Sandhaus, S.; Arca, J.F.; Cropek, D.M.; Ludvig, T.A.; Foster, S.R.; Clark, J.S.; Beckford, F.A.; et al. Synthesis, characterization, DNA binding, topoisomerase inhibition, and apoptosis induction studies of a novel cobalt(III) complex with a thiosemicarbazone ligand. J. Inorg. Biochem. 2020, 203, 110907. [Google Scholar] [CrossRef]
- Neethu, K.S.; Sivaselvam, S.; Theetharappan, M.; Ranjitha, J.; Bhuvanesh, N.S.P.; Ponpandian, N.; Neelakantan, M.A.; Kaveri, M.V. In vitro evaluations of biomolecular interactions, antioxidant and anticancer activities of nickel(II) and copper(II) complexes with 1:2 coordination of anthracenyl hydrazone ligands. Inorg. Chim. Acta 2021, 524, 120419. [Google Scholar] [CrossRef]
- Beckford, F.A.; Brock, A.; Gonzalez-Sarrias, A.; Seeram, N.P. Cytotoxic gallium complexes containing thiosemicarbazones derived from 9-anthraldehyde: Molecular docking with biomolecules. J. Mol. Struct. 2016, 1121, 156–166. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, Z.F.; Liang, H.; Chen, F.J. 9-anthracenecarboxaldehyde-4,5-dihydro-1H-imidazol-2-yl-hydrazone Cisplatin Complex and Synthesis Method and Use Thereof. CN 102268046A, 7 December 2011. [Google Scholar]
- Qin, Q.P.; Liu, Y.C.; Wang, H.L.; Qin, J.L.; Cheng, F.J.; Tang, S.F.; Liang, H. Synthesis and antitumor mechanisms of a copper(II) complex of anthracene-9-imidazoline hydrazone (9-AIH). Metallomics 2015, 7, 1124–1136. [Google Scholar] [CrossRef]
- Liu, R.X.; Wang, C.Y.; Wu, Y.S.; Luo, R.Y.; Jiang, X.H.; Tang, M.T.; Liu, Y.C.; Chen, Z.F.; Liang, H. The copper(II) complexes of new anthrahydrazone ligands: In vitro and in vivo antitumor activity and structure-activity relationship. J. Inorg. Biochem. 2020, 212, 111208. [Google Scholar] [CrossRef]
- Liu, R.X.; Luo, R.Y.; Tang, M.T.; Liu, Y.C.; Chen, Z.F.; Liang, H. The first copper(I) complex of anthrahydrazone with potential ROS scavenging activity showed significant in vitro anticancer activity by inducing apoptosis and autophagy. J. Inorg. Biochem. 2021, 218, 111390. [Google Scholar] [CrossRef]
- Liang, H.; Liu, Y.C.; Chen, Z.F.; Liu, R.X.; Wu, Y.S.; Yang, L.D. Dinuclear Metal Complex with 9-Aldehyde-10-mianthracene Hydrazone as Ligand and Synthetic Method and Application Thereof. CN 110903307A, 24 March 2020. [Google Scholar]
- Beckford, F.A.; Leblanc, G.; Thessing, J.; Shaloski, M., Jr.; Frost, B.J.; Li, L.; Seeram, N.P. Organometallic ruthenium complexes with thiosemicarbazone ligands: Synthesis, structure and cytotoxicity of [(η6-p-cymene)Ru(NS)Cl]+ (NS = 9-anthraldehyde thiosemicarbazones). Inorg. Chem. Commun. 2009, 12, 1094–1098. [Google Scholar] [CrossRef]
- Beckford, F.A.; Stott, A.; Gonzalez-Sarrías, A.; Seeram, N.P. Novel microwave synthesis of half-sandwich [(η6-C6H6)Ru] complexes and an evaluation of the biological activity and biochemical reactivity. Appl. Organomet. Chem. 2013, 27, 425–434. [Google Scholar] [CrossRef]
- Beckford, F.A.; Shaloski, M., Jr.; Leblanc, G.; Thessing, J.; Lewis-Alleyne, L.C.; Holder, A.A.; Li, L.; Seeram, N.P. Microwave synthesis of mixed ligand diimine–thiosemicarbazone complexes of ruthenium(II): Biophysical reactivity and cytotoxicity. Dalton Trans. 2009, 10757–10764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Huang, K.B.; Chen, Z.F.; Qin, Q.P.; Wang, H.L.; Huang, Q.X.; Tang, S.F. 9-Benzothianthrene hydrazine-ruthenium (II) Complex as Well as Synthetic Method and Application Thereof. CN 105440085A, 30 March 2016. [Google Scholar]
- Liu, R.-X.; Wu, Y.-S.; Liu, Y.-C.; Luo, R.-Y.; Yang, L.-D.; Tang, M.-T.; Chen, Z.-F.; Liang, H. New anthrahydrazone derivatives and their cisplatin-like complexes: Synthesis, antitumor activity and structure–activity relationship. New J. Chem. 2019, 43, 18685–18694. [Google Scholar] [CrossRef]
- Chen, Z.F.; Liu, Y.C.; Peng, Y.; Hong, X.; Wang, H.H.; Zhang, M.M.; Liang, H. Synthesis, characterization, and in vitro antitumor properties of gold(III) compounds with the traditional Chinese medicine (TCM) active ingredient liriodenine. J. Biol. Inorg. Chem. 2012, 17, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Ingle, S.A.; Kate, A.N.; Kumbhar, A.A.; Khan, A.A.; Rao, S.S.; Gejji, S.P. Synthesis and biological evaluation of copper(II) pyrenethiosemicarbazone. RSC Adv. 2015, 5, 47476–47487. [Google Scholar] [CrossRef]
- Raja, N.; Devika, N.; Gupta, G.; Nayak, V.L.; Kamal, A.; Nagesh, N.; Therrien, B. Biological activities of pyrenyl-derived thiosemicarbazone half-sandwich complexes. J. Organomet. Chem. 2015, 794, 104–114. [Google Scholar] [CrossRef]
- Kumar, R.R.; Ramesh, R.; Małecki, J.G. Synthesis and structure of arene ruthenium(II) benzhydrazone complexes: Antiproliferative activity, apoptosis induction and cell cycle analysis. J. Organomet. Chem. 2018, 862, 95–104. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Romero-Canelón, I.; Silva, M.M.; Coverdale, J.P.C.; Maia, P.I.S.; Batista, A.A.; Castelli, S.; Desideri, A.; Sadler, P.J.; Deflon, V.M. Palladium(II) complexes with thiosemicarbazones derived from pyrene as topoisomerase IB inhibitors. Dalton Trans. 2019, 48, 16509–16517. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Romero-Canelón, I.; Coverdale, J.P.C.; Maia, P.I.S.; Clarkson, G.J.; Deflon, V.M.; Sadler, P.J. Novel tetranuclear PdII and PtII anticancer complexes derived from pyrene thiosemicarbazones. Dalton Trans. 2020, 49, 9595–9604. [Google Scholar] [CrossRef]
- Rodriguez-Argüelles, M.C.; Ferrari, M.B.; Fava, G.G.; Pelizzi, C.; Pelosi, G.; Albertini, R.; Bonati, A.; Dall’Aglio, P.P.; Lunghi, P.; Pinelli, S. Acenaphthenequinone thiosemicarbazone and its transition metal complexes: Synthesis, structure, and biological activity J. Inorg. Biochem. 1997, 66, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Wu, F. Study of the interaction between a new schiff-base complex and bovine serum albumin by fluorescence spectroscopy. Spectrochim. Acta A 2010, 77, 430–436. [Google Scholar] [CrossRef] [PubMed]
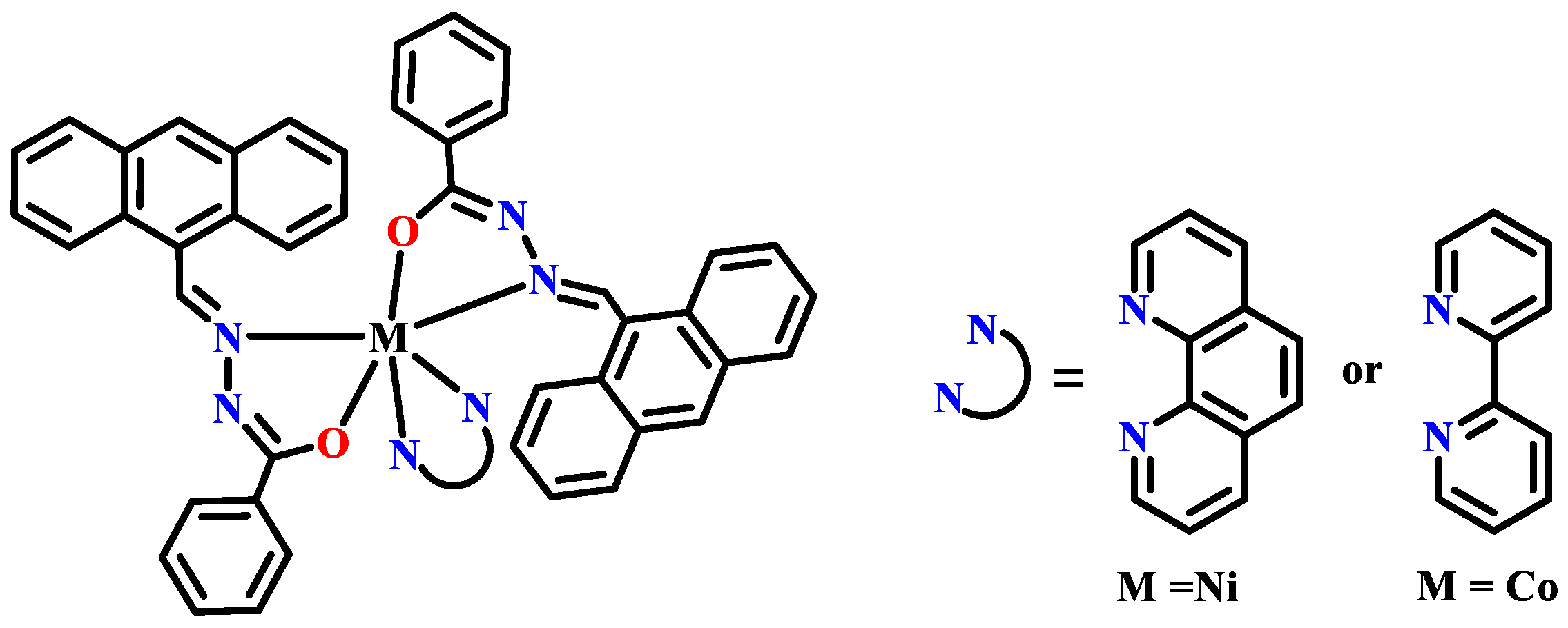
- Mondal, S.; Pakhira, B.; Blake, A.J.; Drew, M.G.B.; Chattopadhyay, S.K. Co(III) and Ni(II) complexes of an anthracene appended aroyl hydrazone: Synthesis, crystal structures, DNA binding and catecholase activity. Polyhedron 2016, 117, 327–337. [Google Scholar] [CrossRef]
- Kurbah, S.D.; Kumar, A.; Syiemlieh, I.; Lal, R.A. Crystal structure and biomimetic activity of homobinuclear dioxidovanadium(V) complexes containing succinoyldihydrazones ligands. Polyhedron 2018, 139, 80–88. [Google Scholar] [CrossRef]
- Yu, M.-K.; Liu, X.-R.; Ren, J.-W.; Liu, J.-J.; Yang, Z.-W.; Zhao, S.-S. Thermal properties and CT-DNA/BSA binding behavior of a binuclear Cu(II) complex with acylhydrazone containing naphthalene ring. J. Coord. Chem. 2018, 71, 1020–1034. [Google Scholar] [CrossRef]
- Dash, S.P.; Panda, A.K.; Dhaka, S.; Pasayat, S.; Biswas, A.; Maurya, M.R.; Majhi, P.K.; Crochet, A.; Dinda, R. A study of DNA/BSA interaction and catalytic potential of oxidovanadium(V) complexes with ONO donor ligands. Dalton Trans. 2016, 45, 18292–18307. [Google Scholar] [CrossRef]
- Ilhan-Ceylan, B.; Tuzun, E.; Kurt, Y.; Acikgoz, M.; Kahraman, S.; Atun, G.; Ulkuseven, B. Oxovanadium (IV) complexes based on S-alkyl-thiosemicarbazidato ligands. Synthesis, characterization, electrochemical, and antioxidant studies. J. Sulfur. Chem. 2015, 36, 434–449. [Google Scholar] [CrossRef]
- Palanimuthu, D.; Poon, R.; Sahni, S.; Anjum, R.; Hibbs, D.; Lin, H.Y.; Bernhardt, P.V.; Kalinowski, D.S.; Richardson, D.R. A novel class of thiosemicarbazones show multi-functional activity for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 612–632. [Google Scholar] [CrossRef] [PubMed]
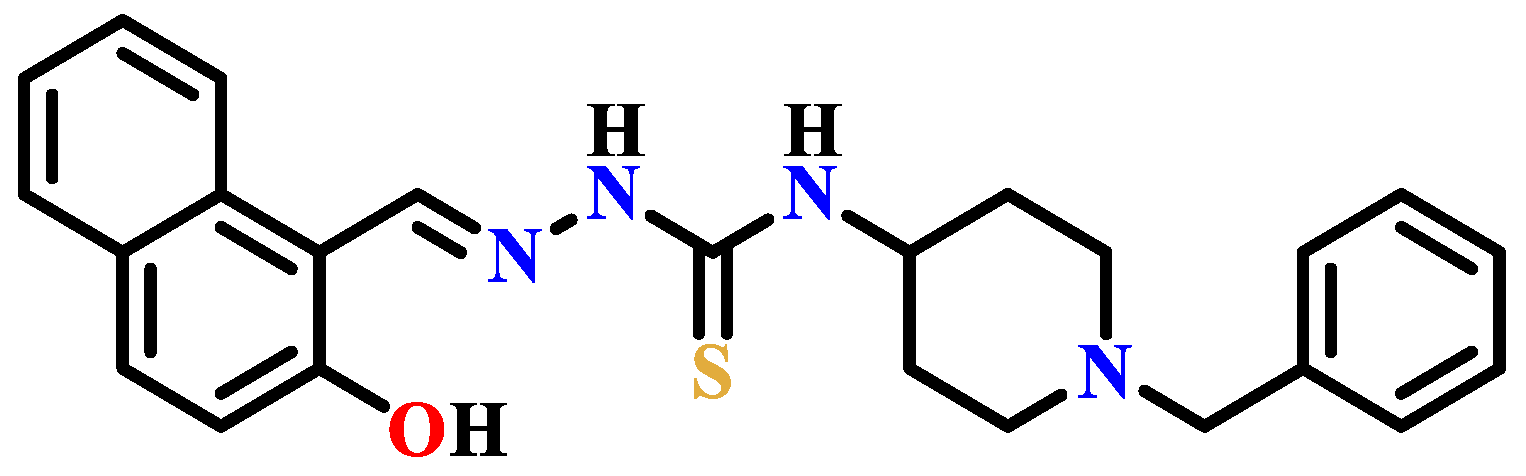
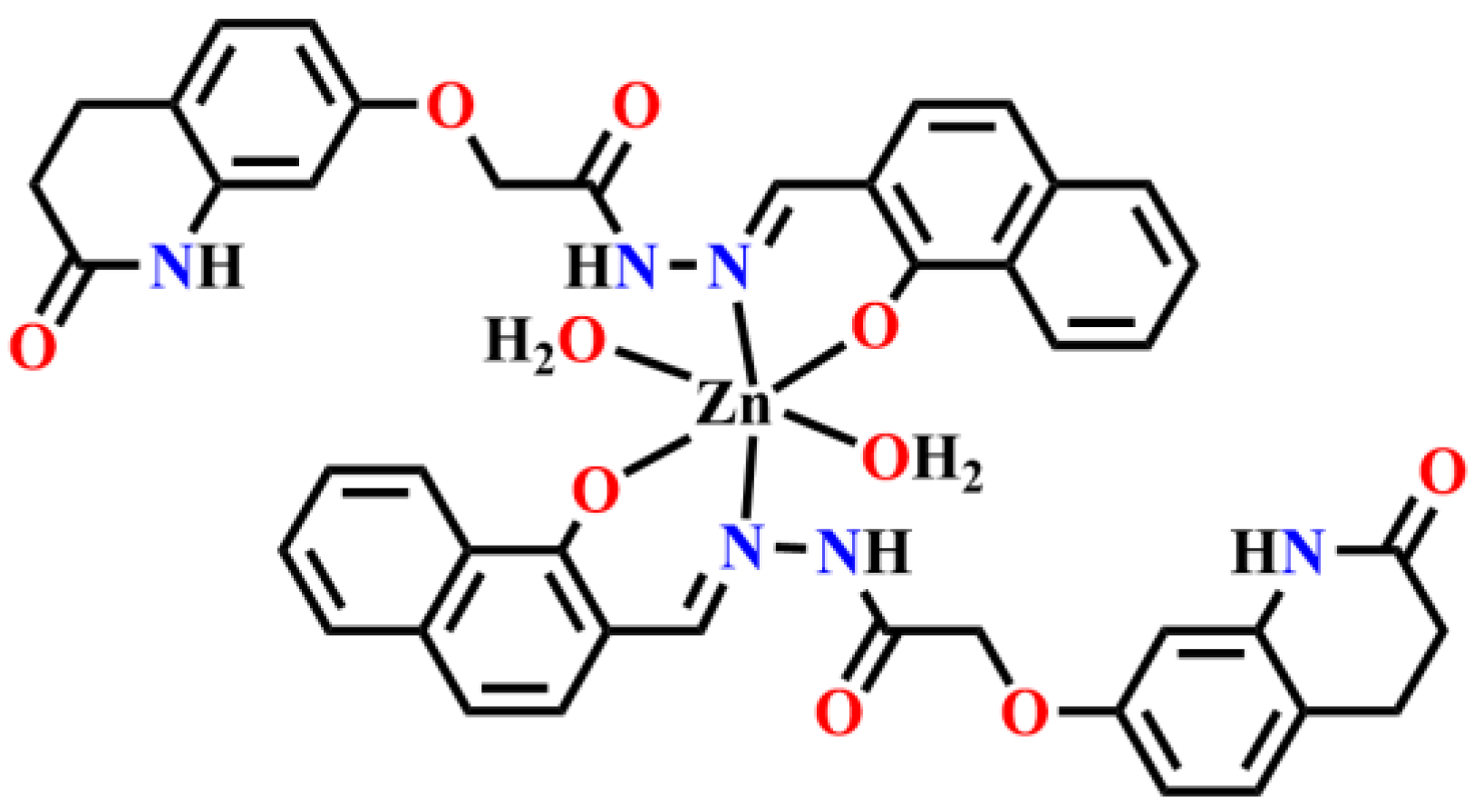
- Mandewale, M.C.; Kokate, S.; Thorat, B.; Sawant, S.; Yamgar, R. Zinc complexes of hydrazone derivatives bearing 3,4-dihydroquinolin-2(1H)-one nucleus as newanti-tubercular agents. Arab. J. Chem. 2016, 12, 4479–4489. [Google Scholar] [CrossRef]
- Scalese, G.; Machado, I.; Fontana, C.; Risi, G.; Salinas, G.; Pérez-Díaz, L.; Gambino, D. New heteroleptic oxidovanadium(V) complexes: Synthesis, characterization and biological evaluation as potential agents against Trypanosoma cruzi. J. Biol. Inorg. Chem. 2018, 23, 1265–1281. [Google Scholar] [CrossRef]






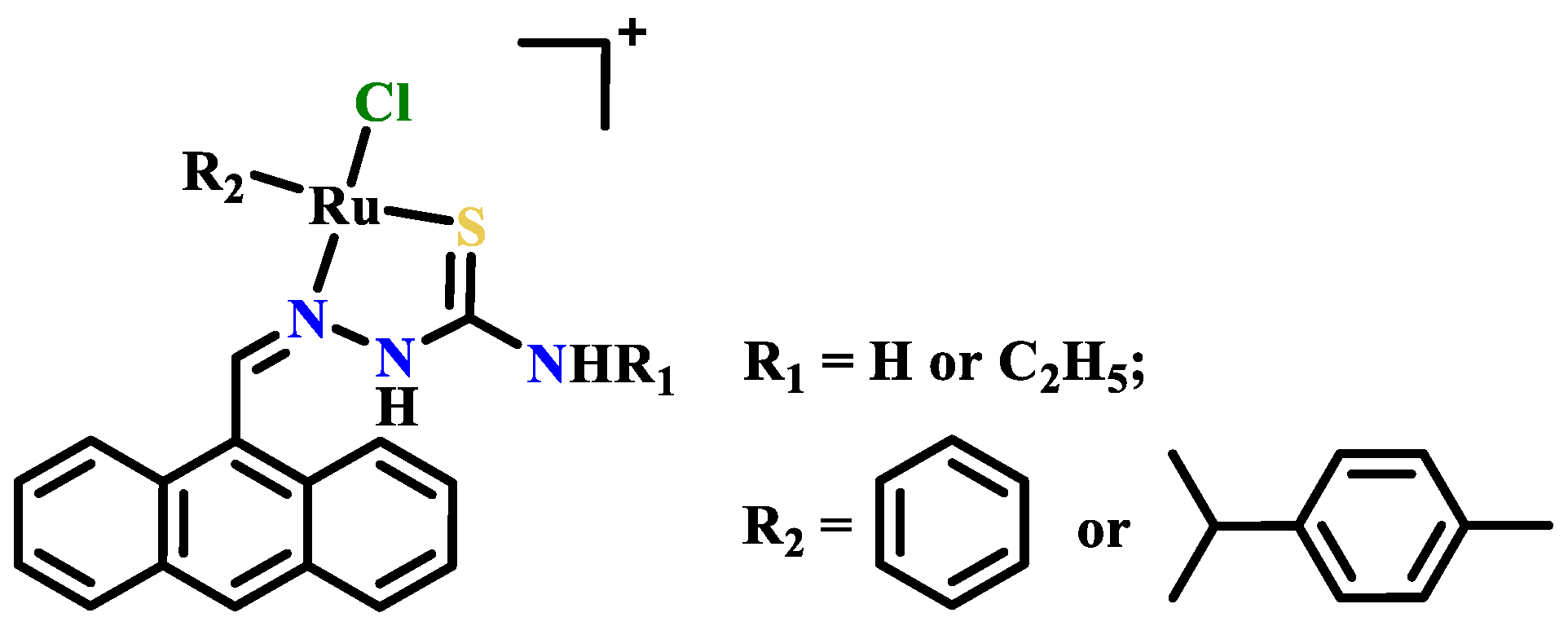


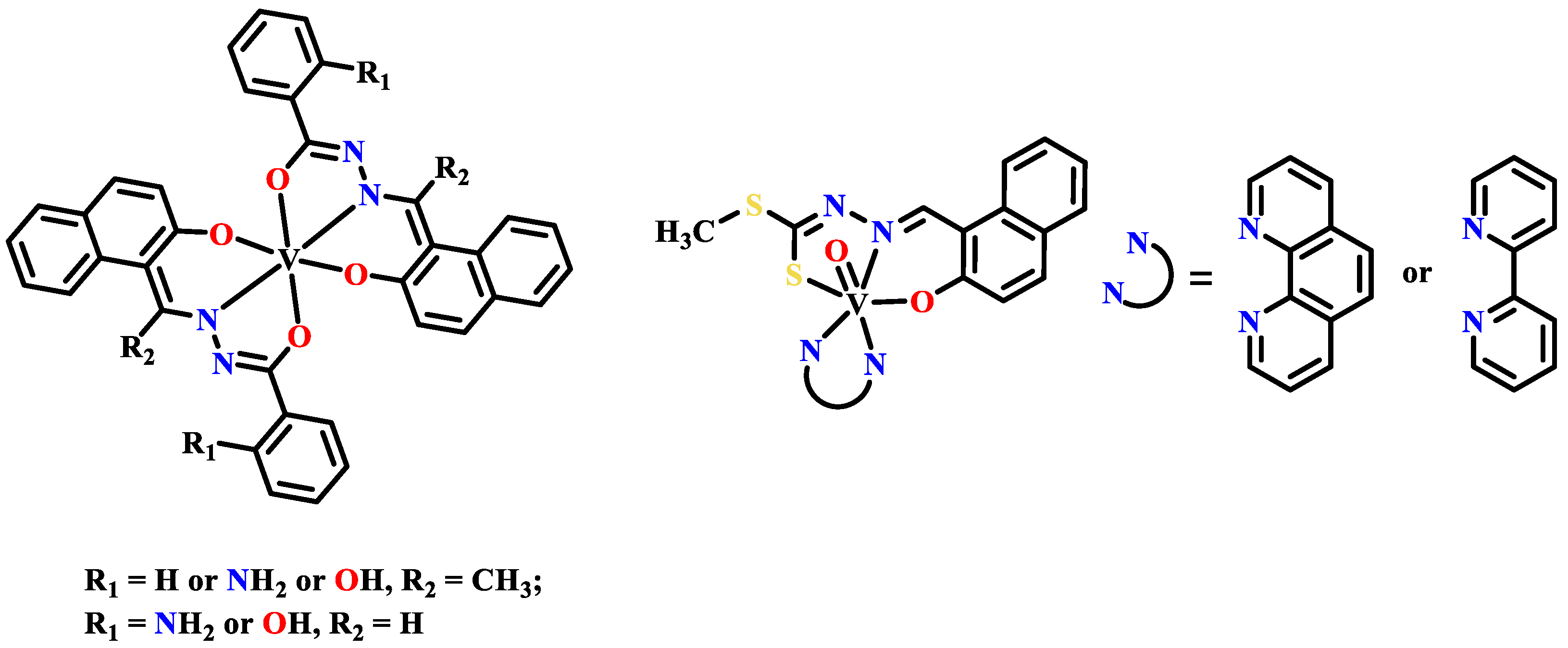

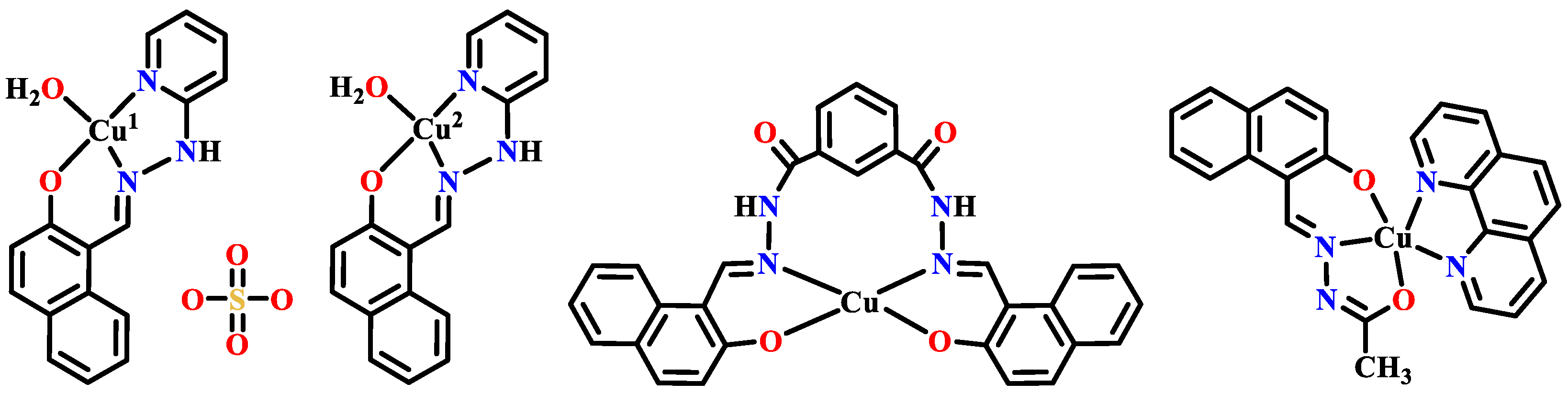

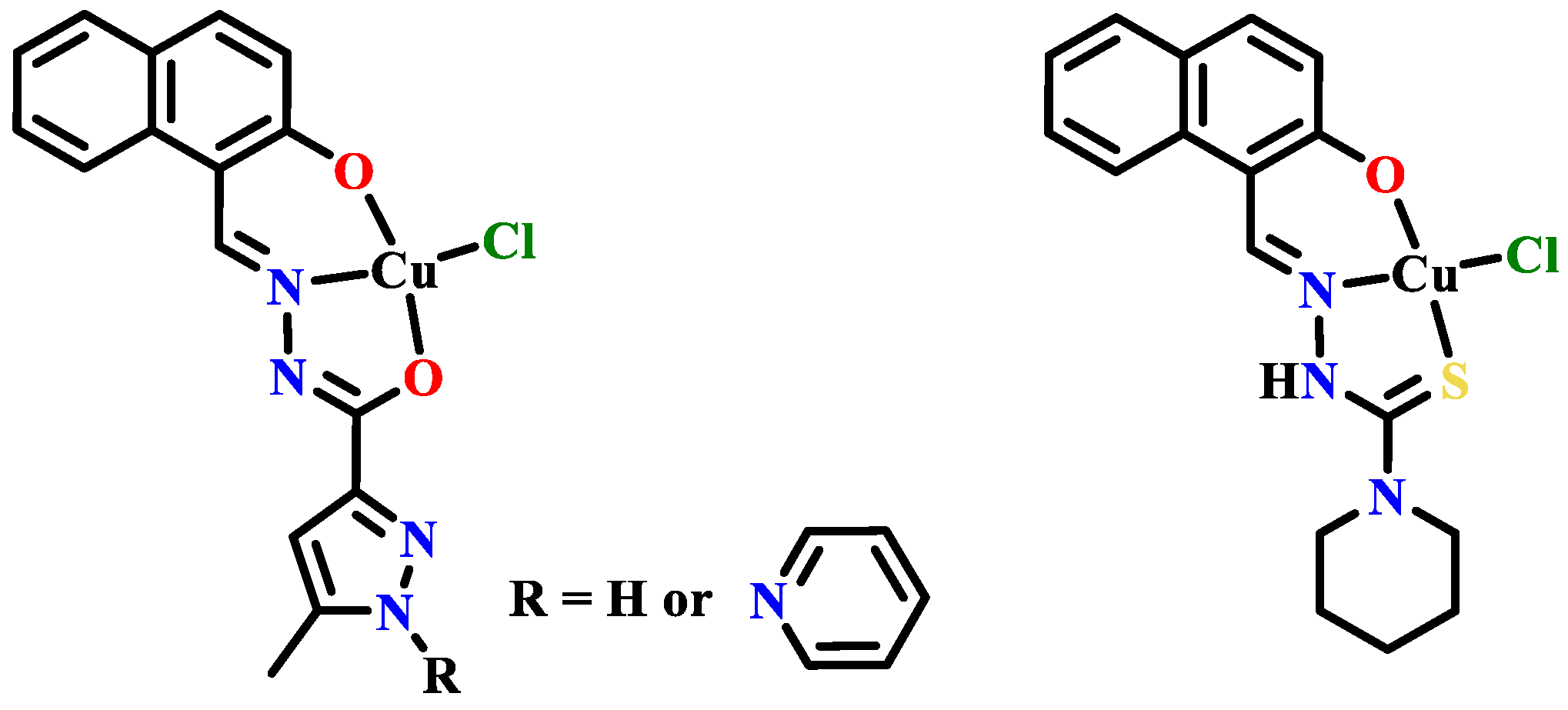

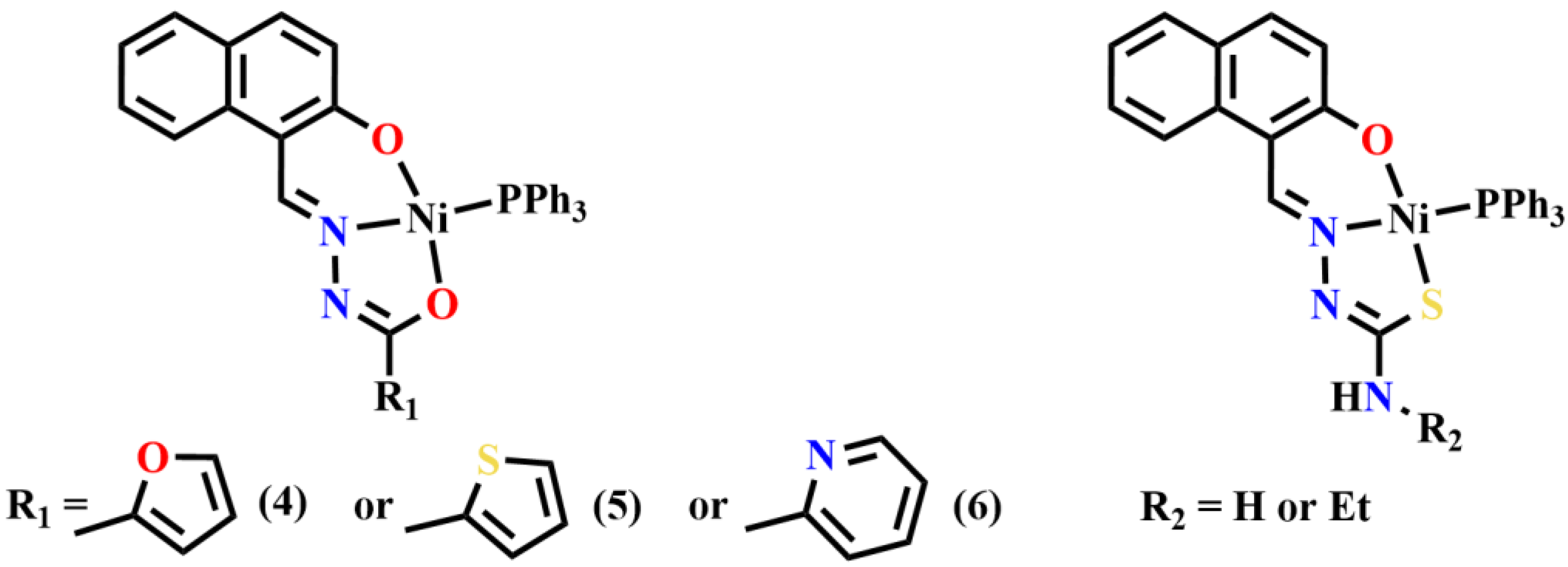
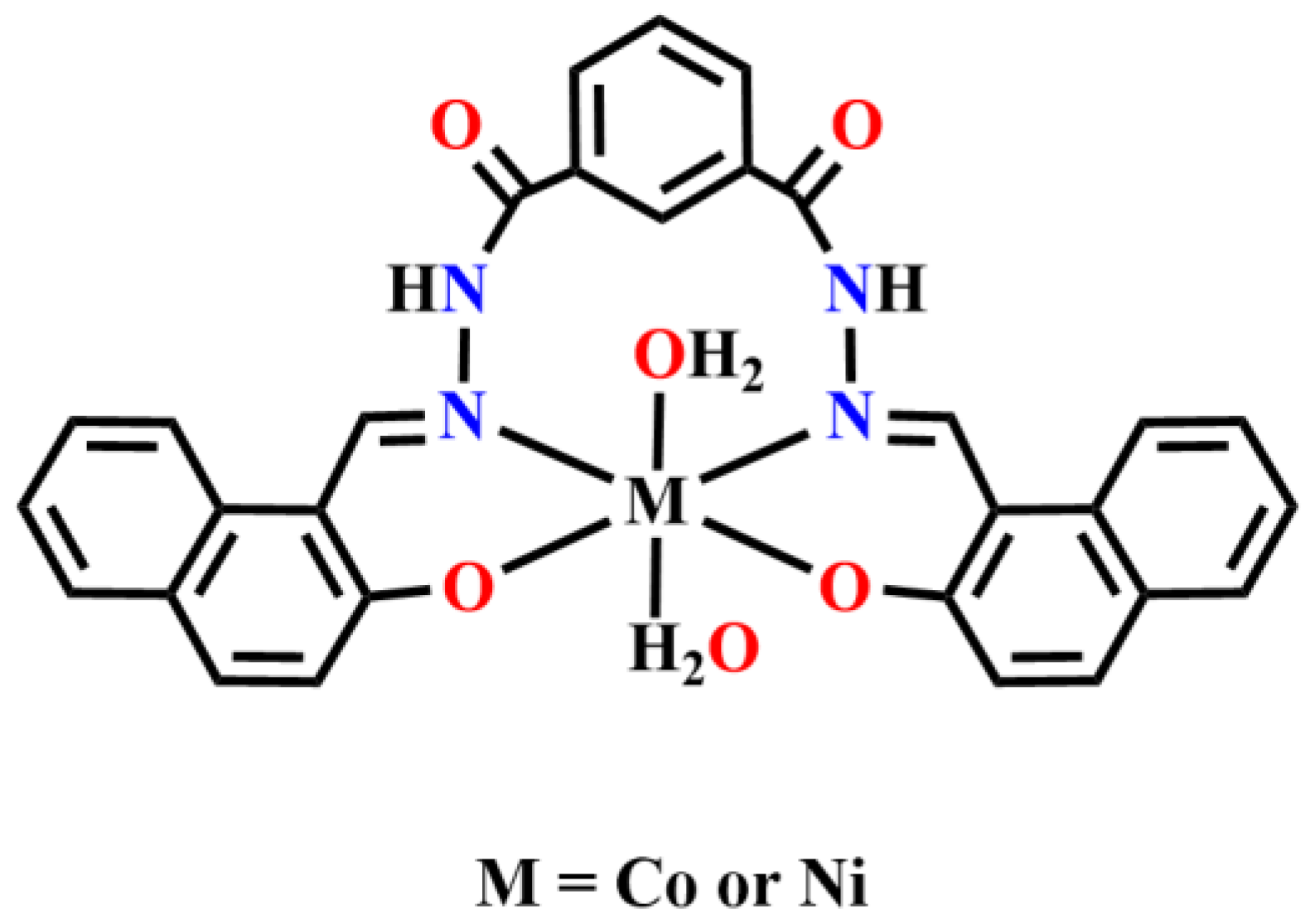

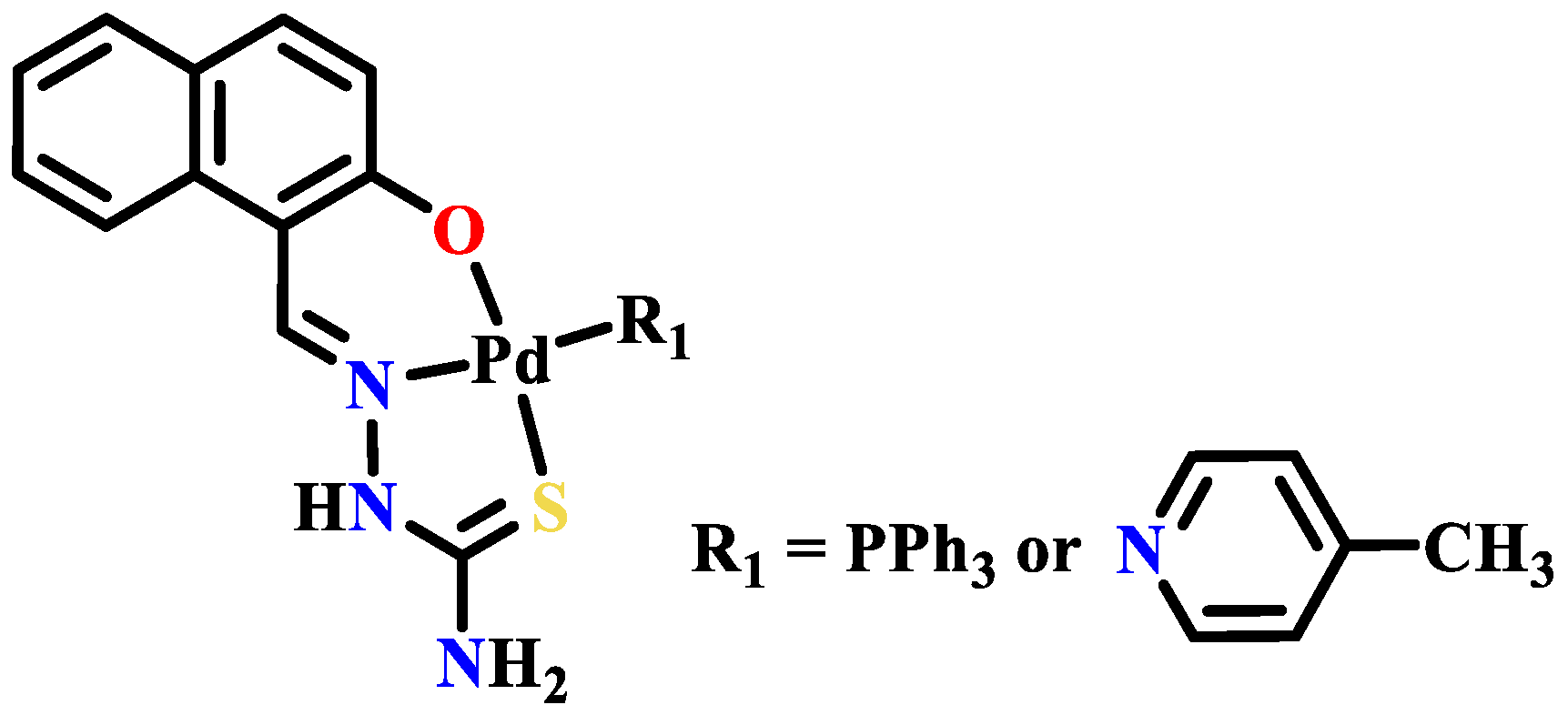


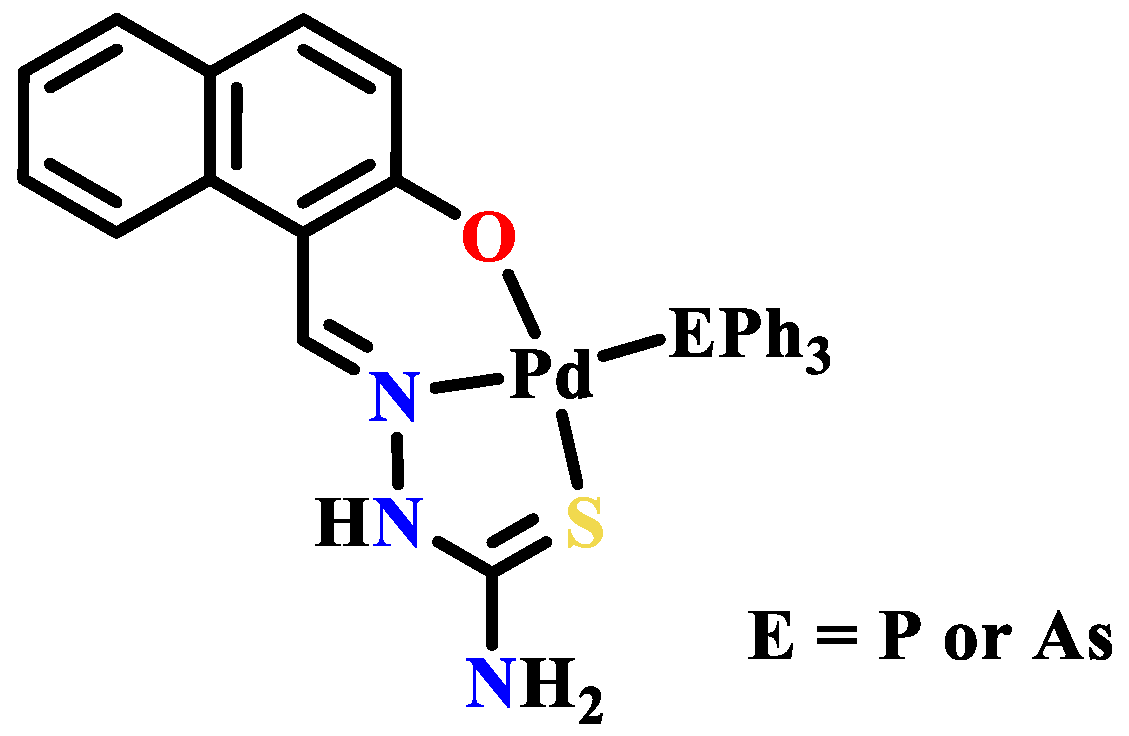



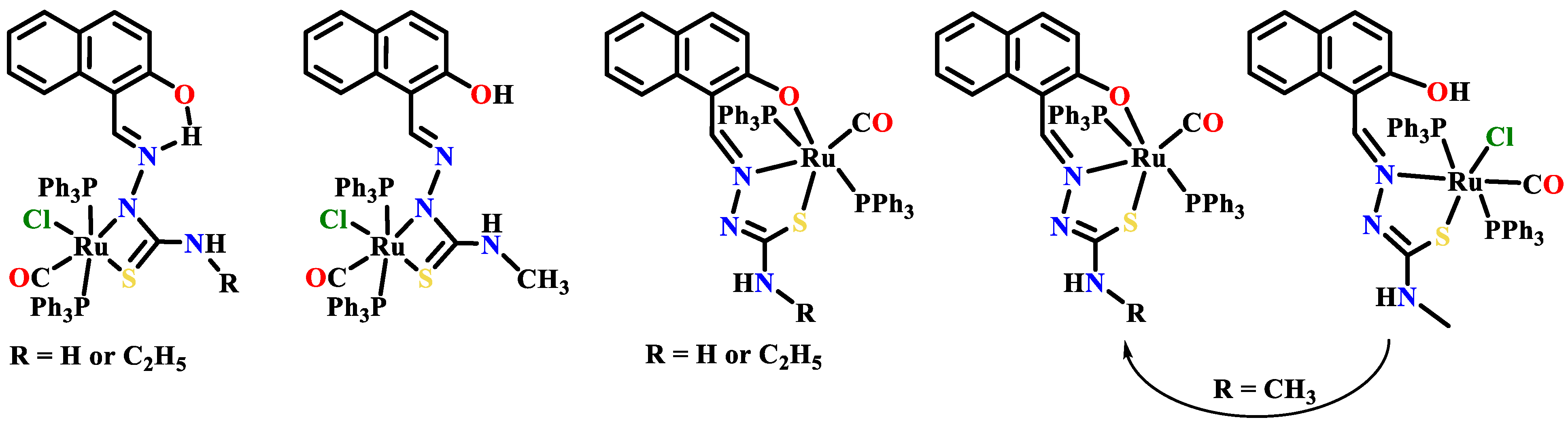
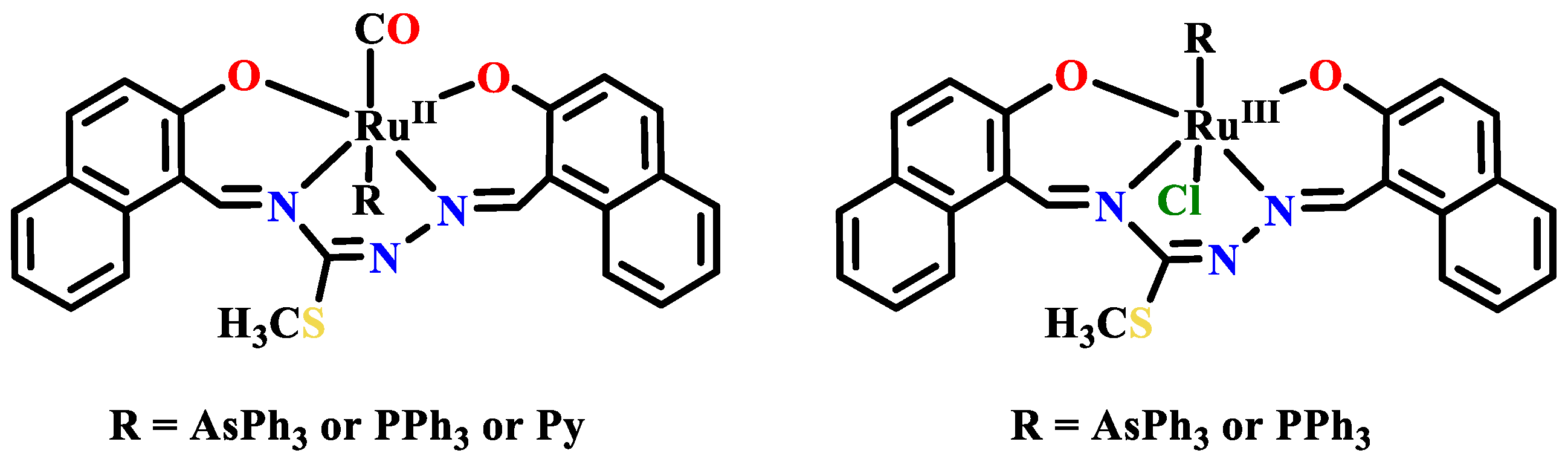
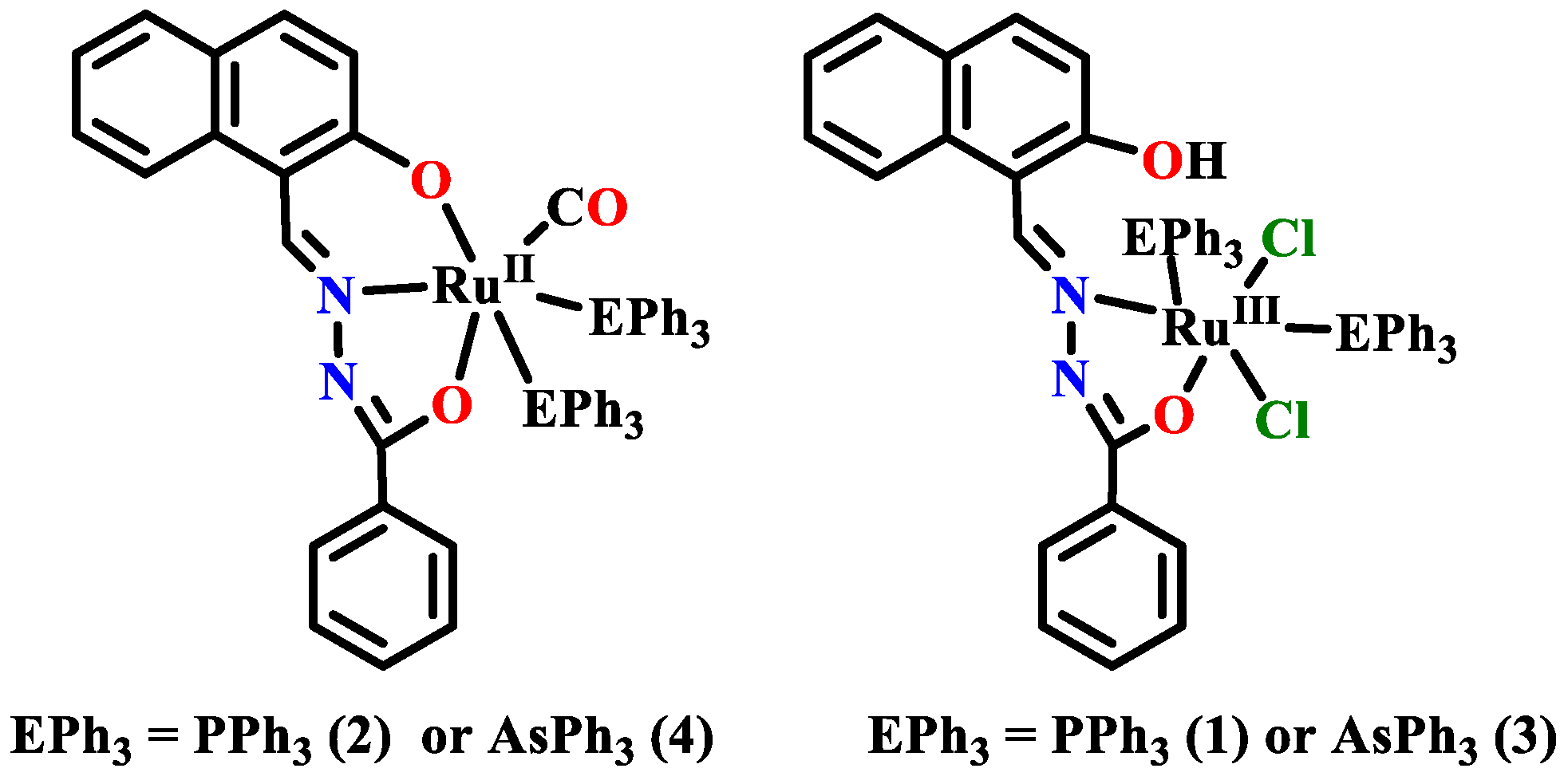


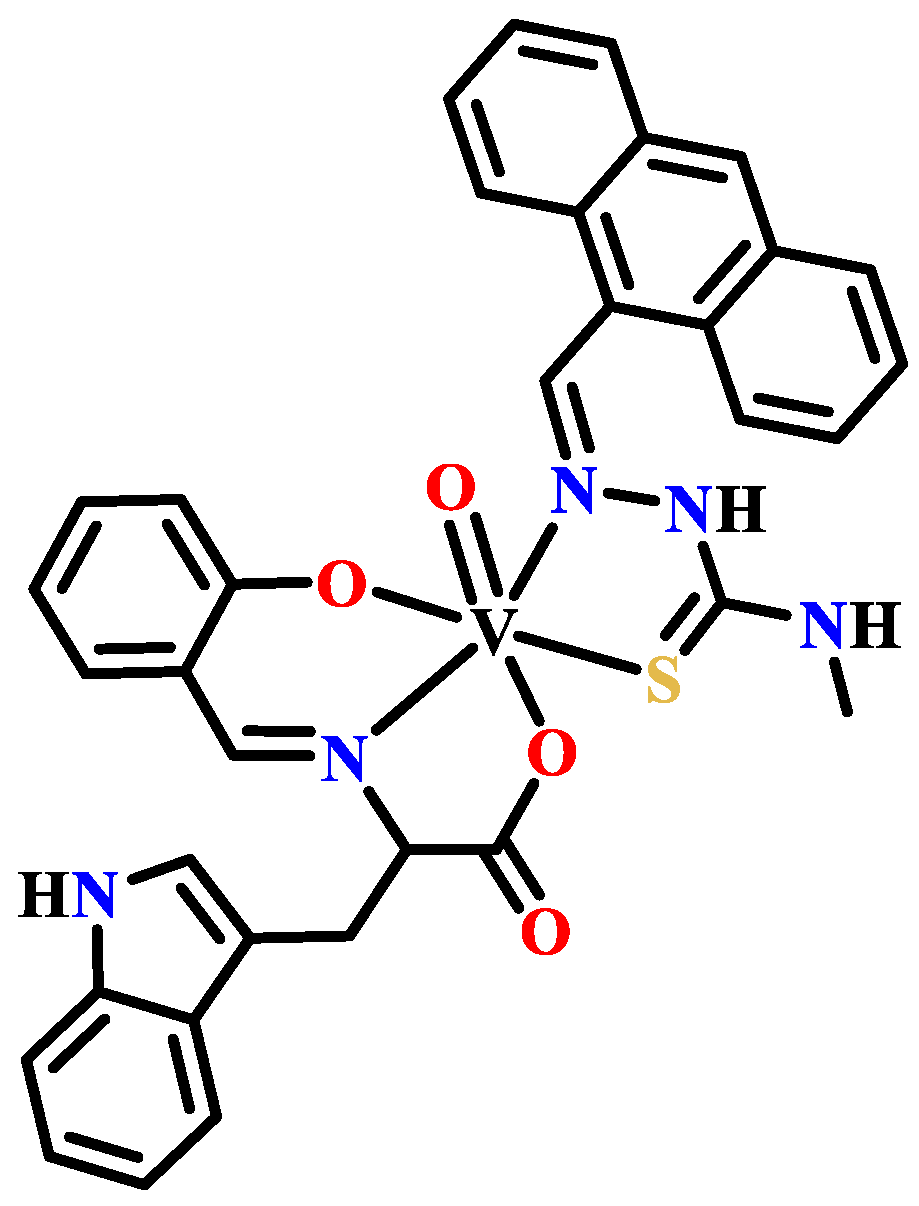
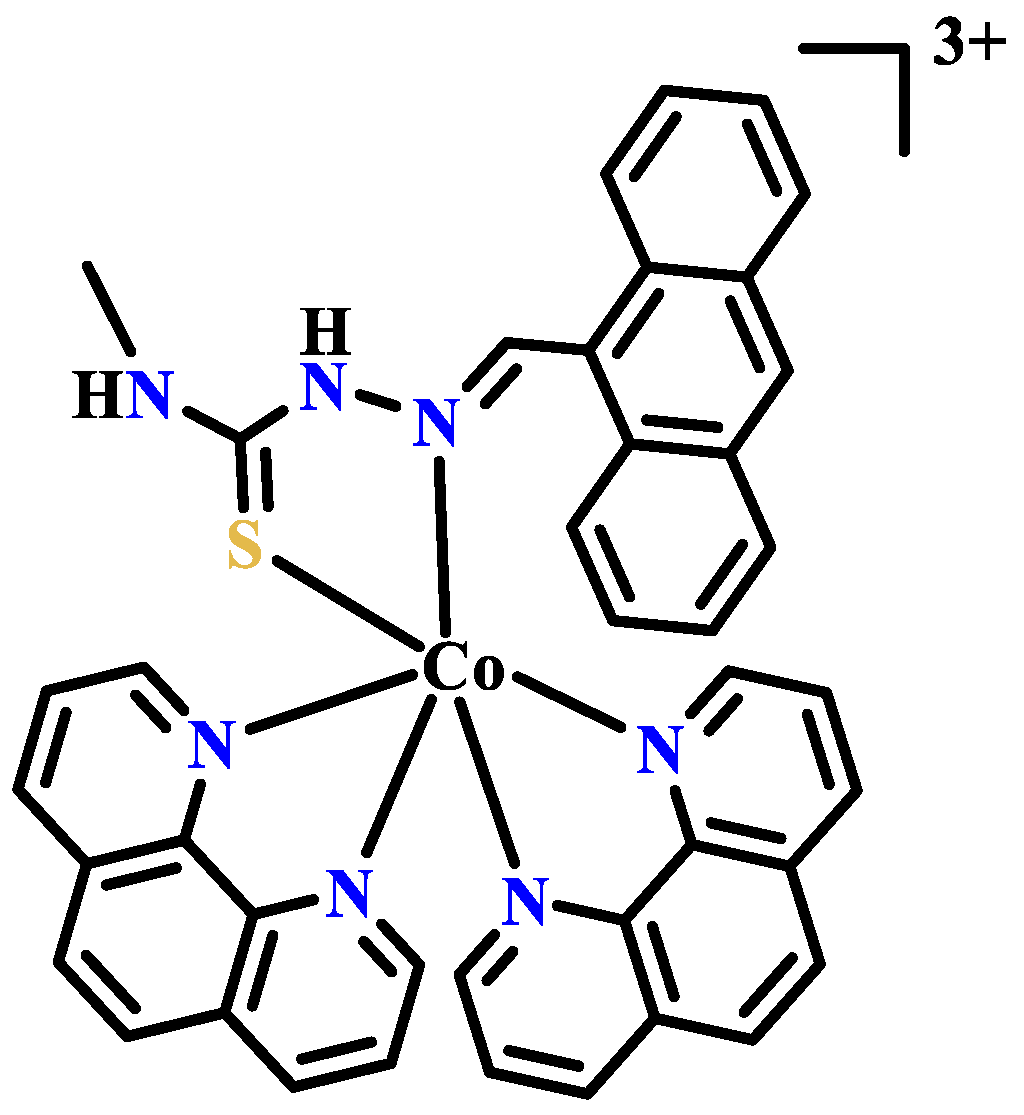






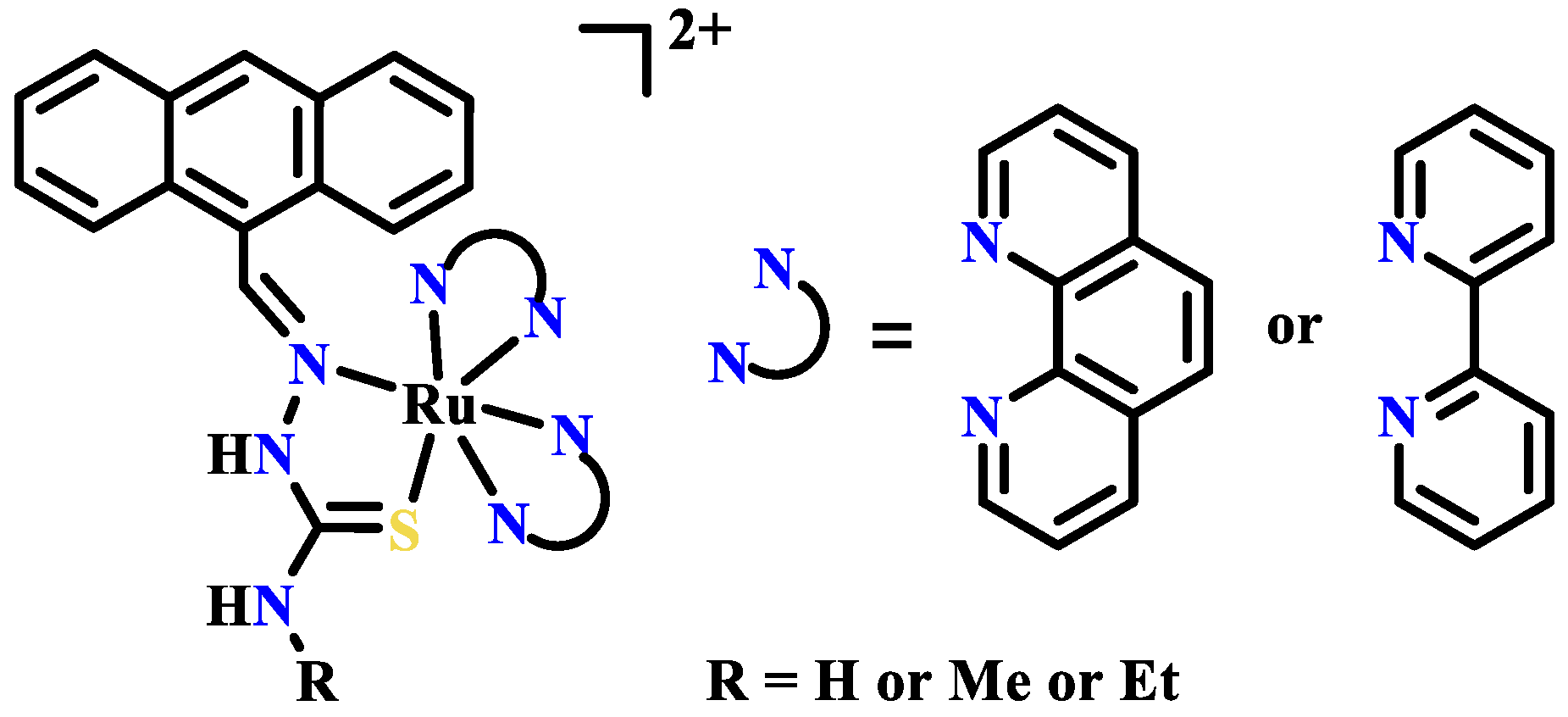
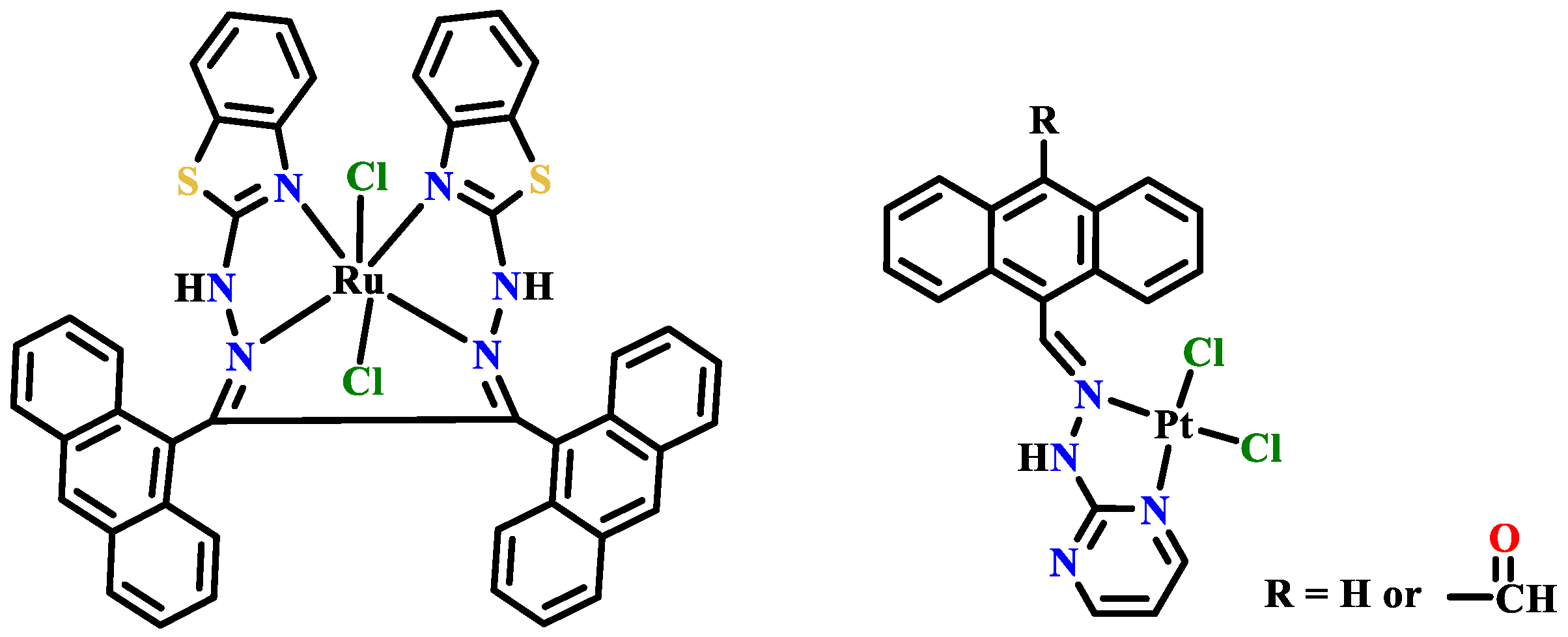
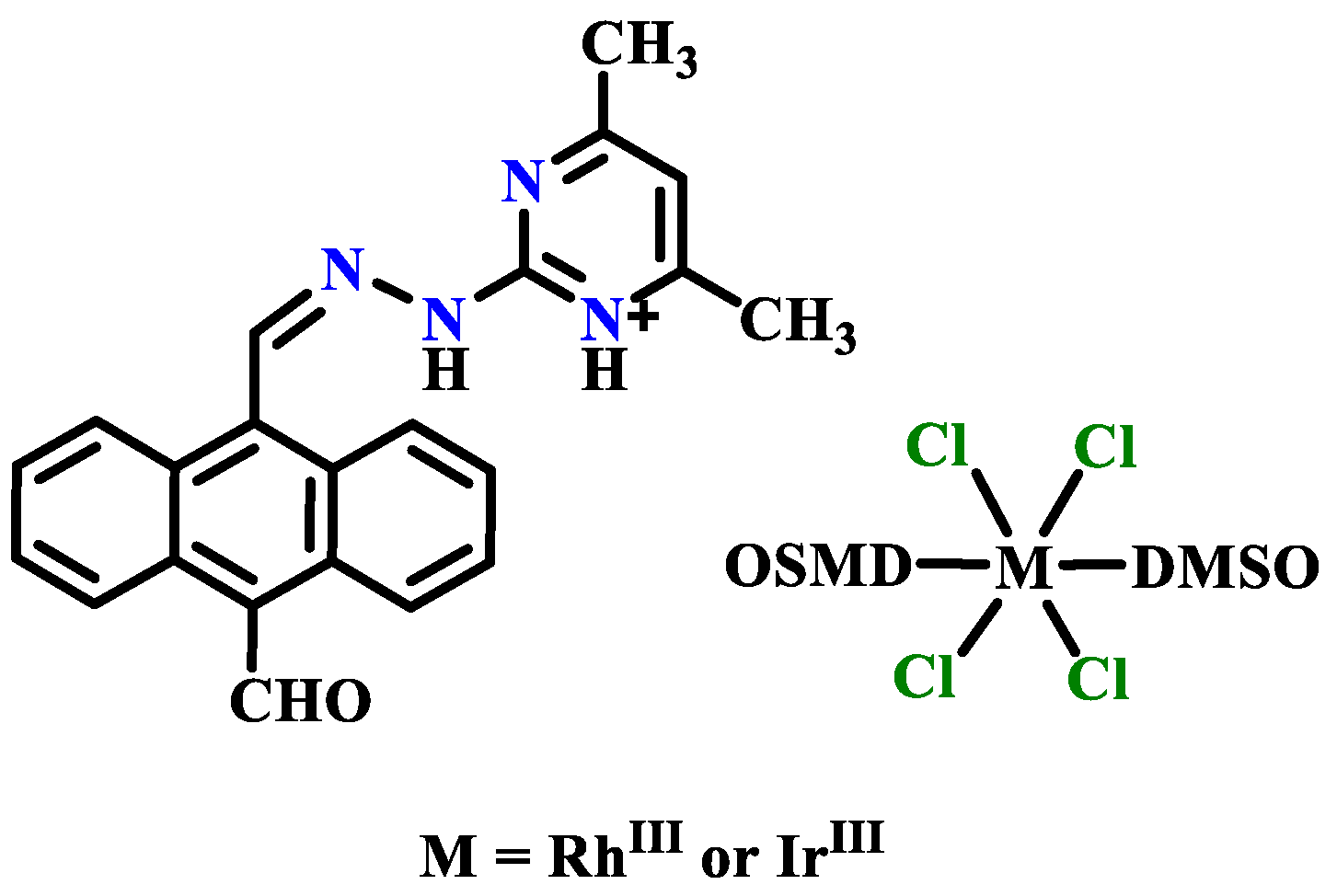







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Cui, J.; Ding, T.; Liu, Y.; Liang, H. Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones. Molecules 2022, 27, 8393. https://doi.org/10.3390/molecules27238393
Liu R, Cui J, Ding T, Liu Y, Liang H. Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones. Molecules. 2022; 27(23):8393. https://doi.org/10.3390/molecules27238393
Chicago/Turabian StyleLiu, Ruixue, Jingbo Cui, Tongyan Ding, Yancheng Liu, and Hong Liang. 2022. "Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones" Molecules 27, no. 23: 8393. https://doi.org/10.3390/molecules27238393
APA StyleLiu, R., Cui, J., Ding, T., Liu, Y., & Liang, H. (2022). Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones. Molecules, 27(23), 8393. https://doi.org/10.3390/molecules27238393






