Molecular Cloning, Expression, and Functional Analysis of Glycosyltransferase (TbUGGT) Gene from Trapa bispinosa Roxb.
Abstract
1. Introduction
2. Results
2.1. TbUGGT Gene Cloning and Sequence Analysis
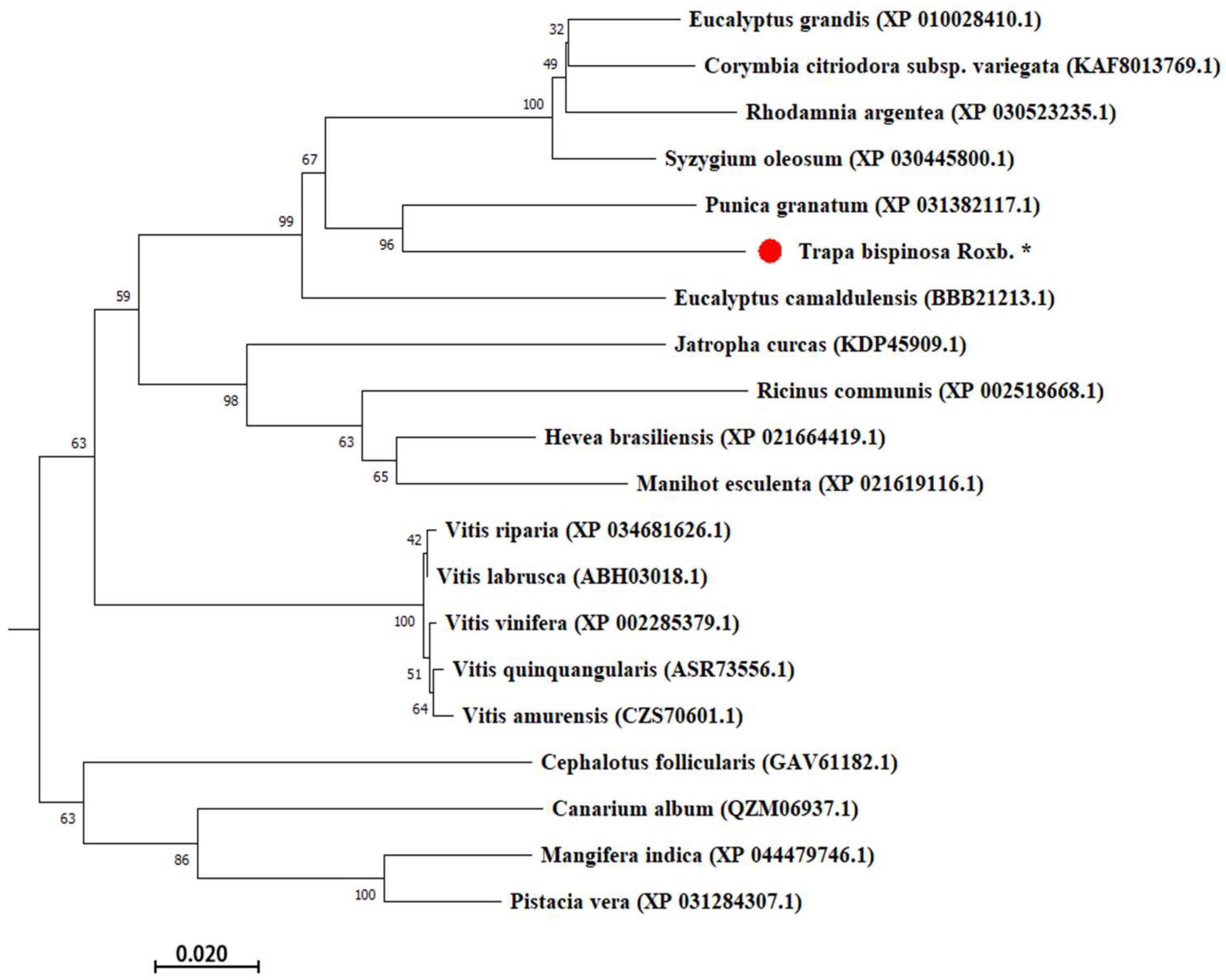
2.2. Structure and Phylogenetic Analyses
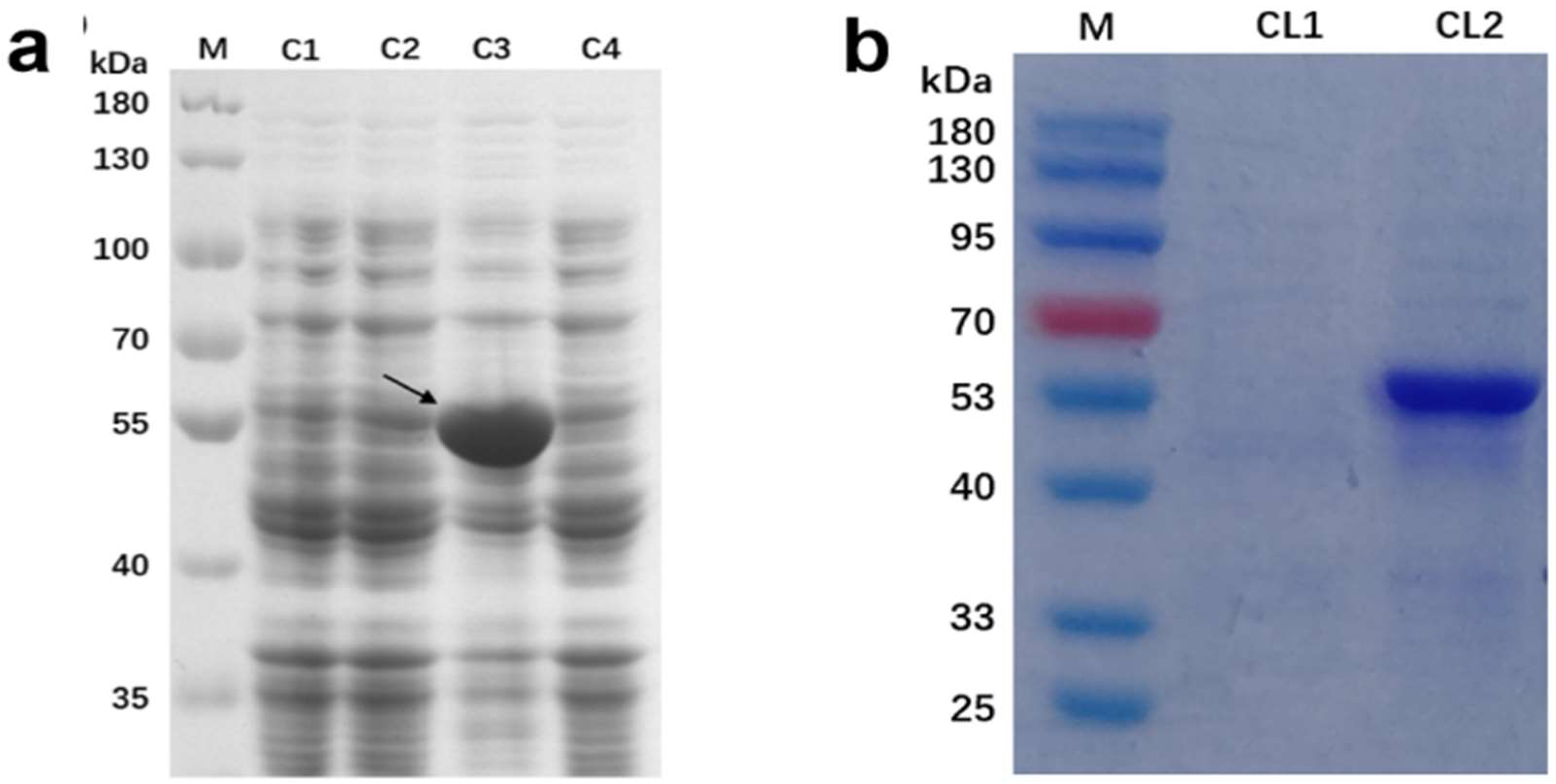
2.3. Prokaryotic Expression of TbUGGT
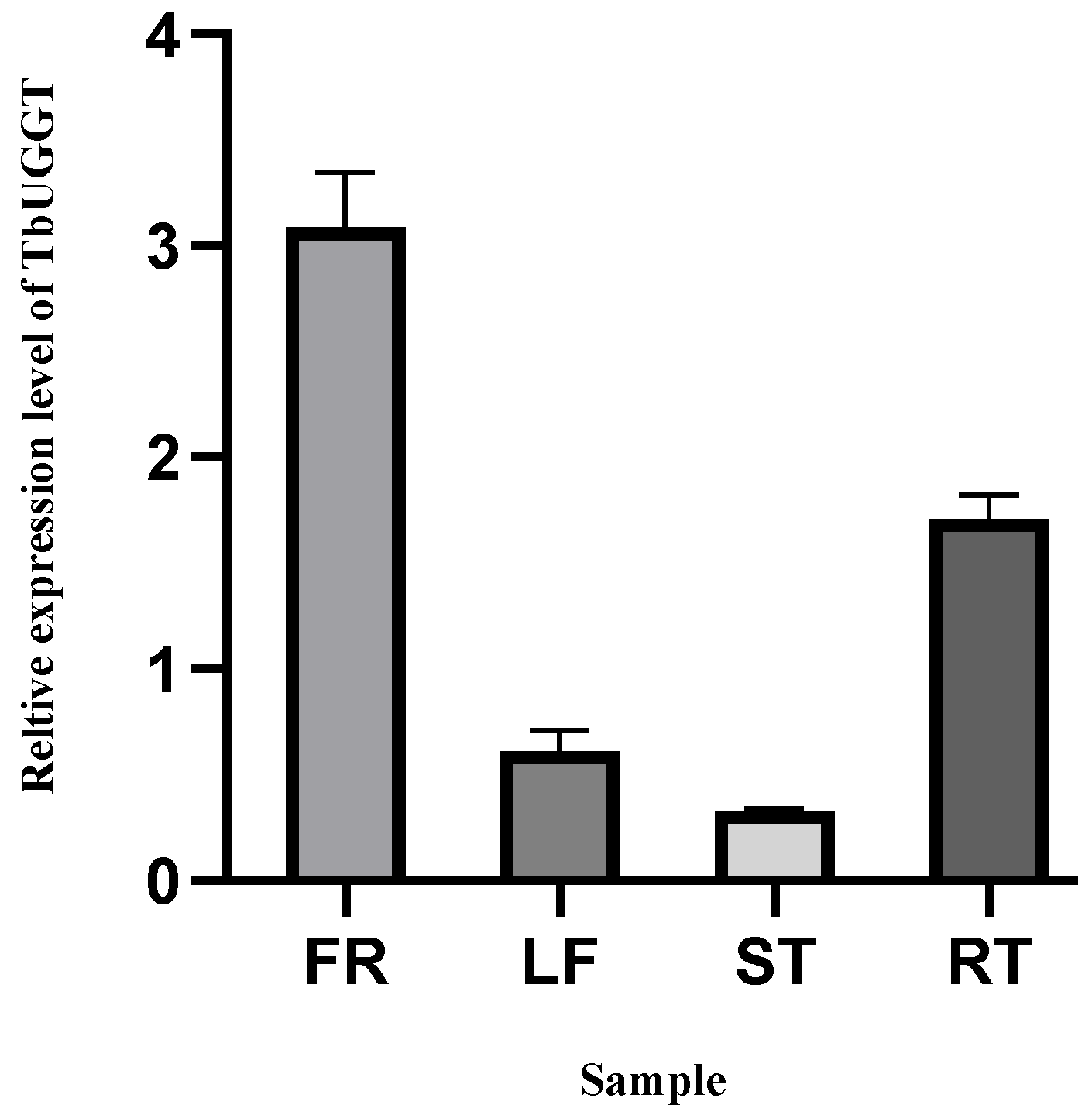
2.4. Determination of Enzyme Activity of TbUGGT Protein In Vitro
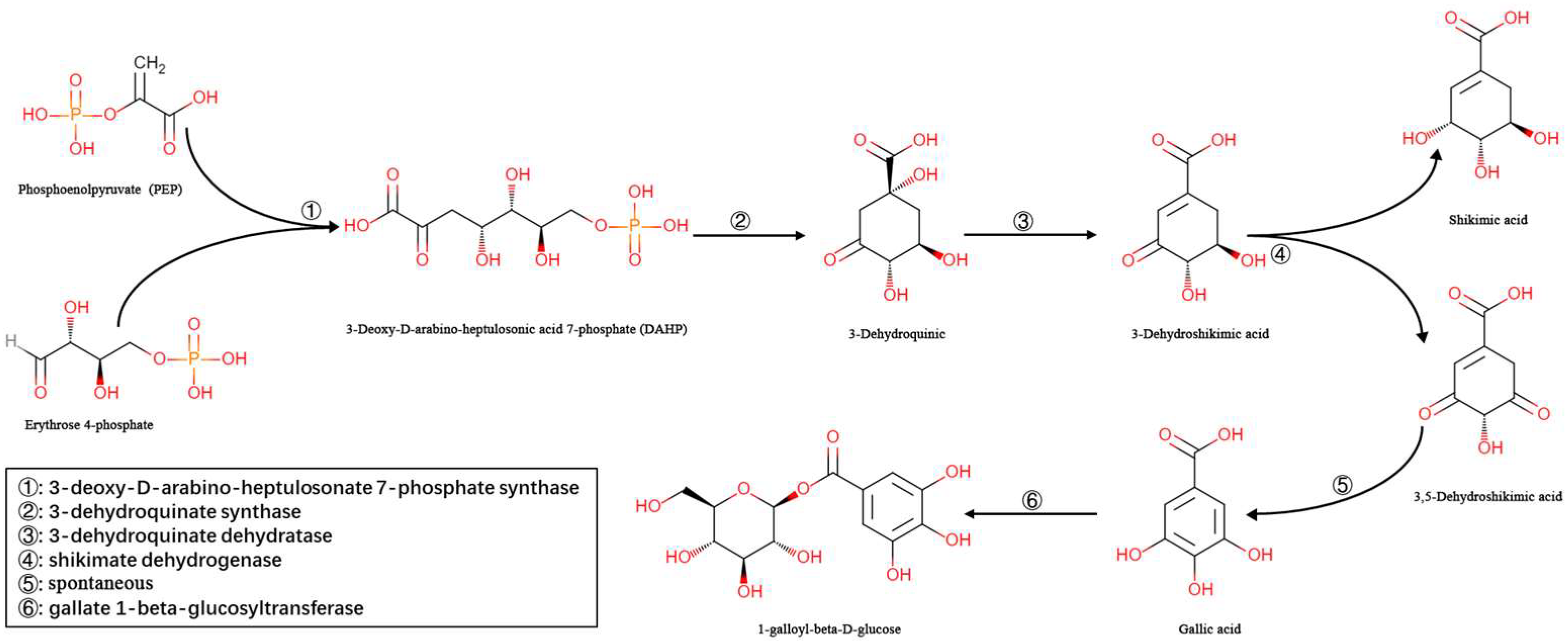
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. RNA Extraction and TbUGGT Enzyme Gene Cloning
4.3. TbUGGT Sequence Analysis and Phylogenetic Prediction
4.4. TbUGGT Prokaryotic Expression
- (1)
- Vector construction and small-scale expression
- (2)
- Expression and purification of large amounts of protein
4.5. Enzyme Activity Detection of TbUGGT Protein
4.6. TbUGGT Expression Pattern
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lim, B.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dodrecht, The Netherlands, 2012. [Google Scholar]
- Nayik, G.A.; Gull, A. Antioxidants in Vegetables and Nuts—Properties and Health Benefits; Springer: Singapore, 2020. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: New York, NY, USA, 2008. [Google Scholar]
- Huang, H.C.; Chao, C.L.; Liaw, C.C.; Hwang, S.Y.; Kuo, Y.H.; Chang, T.C.; Chao, C.H.; Chen, C.J.; Kuo, Y.H. Hypoglycemic Constituents Isolated from Trapa natans L. Pericarps. J. Agric. Food Chem. 2016, 64, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Sugawa, H.; Nanri, T.; Ohno, R.-I.; Shirakawa, J.-I.; Sato, H.; Katsuta, N.; Sakake, S.; Nagai, R. Trapa bispinosa Roxb. and lutein ameliorate cataract in type 1 diabetic rats. J. Clin. Biochem. Nutr. 2020, 66, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, I.; Popescu, A.; Sirbu, R.; Bala, C. Simultaneous Determination of Phenolic Acids in Water Caltrop by HPLC-DAD. Anal. Lett. 2012, 45, 2519–2529. [Google Scholar] [CrossRef]
- Claudia, D.P.; Mario, C.H.; Arturo, N.O.; Noel, M.C.O.; Antonio, N.C.; Teresa, R.A.; Gerardo, L.-T.Z.; Margarita, D.M.; Alejandra, L.I.M.; Rosalina, C.M.Y. Phenolic Compounds in Organic and Aqueous Extracts from Acacia farnesiana Pods Analyzed by ULPS-ESI-Q-oa/TOF-MS. In Vitro Antioxidant Activity and Anti-Inflammatory Response in CD-1 Mice. Molecules 2018, 23, 2386. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Rezq, S.; Sabry, O.M.; Abdelfattah, M.; Raey, M.E.; El-Kashak, W.A.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. Albizia anthelmintica: HPLC-MS/MS profiling and in vivo anti-inflammatory, pain killing and antipyretic activities of its leaf extract. Biomed. Pharmacother. 2019, 115, 108882. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Morreel, K.; Darrah, C.; Oyarce, P.; Grabber, J.H.; Ralph, J.; Boerjan, W. Metabolic engineering of novel lignin in biomass crops. New Phytol. 2012, 196, 978–1000. [Google Scholar] [CrossRef]
- Xian-Hong, O.U.; Deng, J.G.; Xin, Y.U.; Yuan, Y.F.; University, S.M. Study on the Anti-Inflammatory Active Constituents of Mangifera indica L.Seed Kernel. Chin. Pharm. J. 2015, 19, 1673–1677. [Google Scholar]
- Gupta, A.; Sahu, T.R.; Sahu, B.K. Cultivation of Trapa crop based on indigenous knowledge. J. Trop. For. 2010, 26, 50–52. [Google Scholar]
- Li, L.; Chang, K.-C.; Zhou, Y.; Shieh, B.; Ponder, J.; Abraham, A.D.; Ali, H.; Snow, A.; Petrash, J.M.; LaBarbera, D.V. Design of an Amide N-Glycoside Derivative of β-Glucogallin: A Stable, Potent, and Specific Inhibitor of Aldose Reductase. J. Med. Chem. 2013, 57, 71–77. [Google Scholar] [CrossRef]
- Cao, T.; Wang, J.; Wu, Y.; Wang, L.; Zhang, H. Antiglaucoma Potential of beta-Glucogallin Is Mediated by Modulating Mitochondrial Responses in Experimentally Induced Glaucoma. Neuroimmunomodulation 2020, 27, 142–151. [Google Scholar] [CrossRef]
- Chang, K.C.; Laffin, B.; Ponder, J.; Enzsoly, A.; Nemeth, J.; LaBarbera, D.V.; Petrash, J.M. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem. Biol. Interact. 2013, 202, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Snow, A.; LaBarbera, D.V.; Petrash, J.M. Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells. Chem. Biol. Interact. 2015, 234, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandel, S.; Ghosh, A.; Matta, T.; Gautam, A.; Bhattacharya, A.; Babu, S.S.; Sukla, S.; Nag, D.; Ravichandiran, V.; et al. Glucogallin Attenuates the LPS-Induced Signaling in Macrophages and Protects Mice against Sepsis. Int. J. Mol. Sci. 2022, 23, 11254. [Google Scholar] [CrossRef]
- Mackenzie, P.I.; Owens, I.S.; Burchell, B.; Bock, K.W.; Bairoch, A.; Belanger, A.; Gigleux, S.F.; Green, M.; Hum, D.W.; Iyanagi, T.; et al. The UDP glycosyltransferase gene superfamily: Recommended nomenclature update based on evolutionary divergence. Pharm. Genom. 1997, 7, 255–269. [Google Scholar] [CrossRef]
- Kleczkowski, L.; Kunz, S.; Wilczynska, M. Mechanisms of UDP-Glucose Synthesis in Plants. Crit. Rev. Plant Sci. 2010, 29, 191–203. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, A.; Fu, C.H.; Gui, B.H.; Zhi, C.Z. Functional analysis of the UDP glucose: Flavonoid-3-Oglucosyltransferase (UFGT) promoter from litchi (Litchi chinesis Sonn.) and transient expression in onions (Allium cepa Linn.). Afr. J. Plant Sci. 2015, 9, 244–249. [Google Scholar] [CrossRef]
- Ossipov, V.; Salminen, J.P.; Ossipova, S.; Haukioja, E.; Pihlaja, K. Gallic acid and hydrolysable tannins are formed in birch leaves from an intermediate compound of the shikimate pathway. Biochem. Syst. Ecol. 2003, 31, 3–16. [Google Scholar] [CrossRef]
- Muir, R.M.; Ibá?Ez, A.; Uratsu, S.L.; Ingham, E.S.; Leslie, C.A.; Mcgranahan, G.H.; Batra, N.; Goyal, S.; Joseph, J.; Jemmis, E.D. Mechanism of gallic acid biosynthesis in bacteria (Escherichia coli) and walnut (Juglans regia). Plant Mol. Biol. 2011, 75, 555–565. [Google Scholar] [CrossRef]
- Tahara, K.; Nishiguchi, M.; Funke, E.; Miyazawa, S.I.; Miyama, T.; Milkowski, C. Dehydroquinate dehydratase/shikimate dehydrogenases involved in gallate biosynthesis of the aluminum-tolerant tree species Eucalyptus camaldulensis. Planta 2021, 253, 3. [Google Scholar] [CrossRef]
- Werner, R.A.; Rossmann, A.; Schwarz, C.; Bacher, A.; Schmidt, H.L.; Eisenreich, W. Biosynthesis of gallic acid in Rhus typhina: Discrimination between alternative pathways from natural oxygen isotope abundance. Phytochemistry 2004, 65, 2809–2813. [Google Scholar] [CrossRef]
- Yin, D.J.; Ye, S.J.; Sun, X.Y.; Chen, Q.Y.; Min, T.; Wang, H.X.; Wang, L.M. Integrative Analysis of the Transcriptome and Metabolome Reveals Genes Involved in Phenylpropanoid and Flavonoid Biosynthesis in the Trapa bispinosa Roxb. Front. Plant Sci. 2022, 13, 913265. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Xiang, G.; Man, A.; Qinmei, W.; Dan, Y.; Meng, W.; Yang, F.; Li, W. cDNA cloning and characterization of UDP-glucose: Anthocyanidin 3-O-glucosyltransferase in Freesia hybrida. Plant Cell Rep. 2011, 30, 1209–1218. [Google Scholar]
- Dodd, L.L.; Harms, N.E.; Schad, A.N. Reciprocal competitive effects of congeneric invaders, Trapa natans L. and Trapa bispinosa Roxb. var. iinumai Nakano, in established freshwater plant cultures. Aquat. Bot. 2021, 174, 103419. [Google Scholar] [CrossRef]
- Aiwen, Z.; Xin, S.; Jing, Z.; Liang, H.E.; Chunlong, Y.I.; Leyi, N.I.; Te, C.; Garden, L.B.; Amp, J.P. Diversity and Distribution of Aquatic Plants in Lake Donghu in Wuhan in 2014. Res. Environ. Sci. 2017, 30, 398–405. [Google Scholar]
- Adhikari, B.; Shrestha, O.K. Effect of Processing Variables on Anthocyanin and Total Polyphenol Extraction from Water Caltrop (Trapa bispinosa) Hull. Himal. J. Sci. Technol. 2018, 2, 76–83. [Google Scholar] [CrossRef]
- Mazumder, A.; Majee, C.; Mazumder, R. Determination of total phenolic content and total antioxidant activity of various parts of Trapa bispinosa. Int. J. Pharm. Sci. Res. 2020, 11, 3625–3627. [Google Scholar]
- Dularia, C.; Sinhmar, A.; Thory, R.; Pathera, A.K.; Nain, V. Development of starch nanoparticles based composite films from non-conventional source—Water chestnut (Trapa bispinosa). Int. J. Biol. Macromol. 2019, 136, 1161–1168. [Google Scholar] [CrossRef]
- Lutfi, Z. Freeze-thaw stabilization of water chestnut (Trapa bispinosa) starch in the presence of gums and salts. Trakia J. Sci. 2013, 11, 163–169. [Google Scholar]
- Wang, J.; Liu, T.; Bia, X.; Hua, Z.; Wu, X. Structural characterization and physicochemical properties of starch from four aquatic vegetable varieties in China. Int. J. Biol. Macromol. 2021, 172, 542–549. [Google Scholar] [CrossRef]
- Thakkar, A.B.; Kurtkoti, S.K.; Sastry, N.V. Phytochemical screening, antibacterial and free radical scavenging activity of the fruit and peel extracts of Trapa bispinosa (water chestnut). Int. J. Pharma Bio Sci. 2018, 9, 4. [Google Scholar] [CrossRef]
- Adkar, P.P.; Dongare, A.; Ambavade, S.D.; Bhaskar, V.H. Research journal of pharmaceutical, biological and chemical sciences: Effect of Trapa bispinosa on HDAC level in animal tissues for its anti-arthritic activity. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1404–1415. [Google Scholar]
- Bowles, D.; Isayenkova, J.; Lim, E.K.; Poppenberger, B. Glycosyltransferases: Managers of small molecules—ScienceDirect. Curr. Opin. Plant Biol. 2005, 8, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.H.; Xu, Z.S.; Peng, R.H.; Tian, Y.S.; Zhao, W.; Han, H.J.; Yao, Q.H.; Wu, A.Z. Phytoremediation of Trichlorophenol by Phase II Metabolism in Transgenic Arabidopsis Overexpressing a Populus Glucosyltransferase. Environ. Sci. Technol. 2012, 46, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Hu, Z.; Zhang, T.; Gong, T.; Chen, J.; Zhu, P.; Li, Y.; Yang, J. Production of a bioactive unnatural ginsenoside by metabolically engineered yeasts based on a new UDP-glycosyltransferase from Bacillus subtilis. Metab. Eng. 2017, 44, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Pandey, R.P.; Shin, J.Y.; Jung, H.J.; Sohng, J.K. Synthetic analog of anticancer drug daunorubicin from daunorubicinone using one-pot enzymatic UDP-recycling glycosylation. J. Mol. Catal. B Enzym. 2016, 124, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Baldauf, S.; Lim, E.-K.; Bowles, D.J. Phylogenetic Analysis of the UDP-glycosyltransferase Multigene Family of Arabidopsis thaliana *210. J. Biol. Chem. 2001, 276, 4338–4343. [Google Scholar] [CrossRef]
- Cwab, C.; Jing, D.; Zcb, C.; Wtbc, E.; Yan, Y.; Hai, Y.D.; Jian, Z.A.; Wei, H. Comprehensive analysis and expression profiles of cassava UDP-glycosyltransferases (UGT) family reveal their involvement in development and stress responses in cassava. Genomics 2021, 113, 3415–3429. [Google Scholar]
- López-Jimenez, A.; Frusciante, S.; Niza, E.; Ahrazem, O.; Rubio-Moraga, N.; Diretto, G.; Gómez-Gómez, L. A New Glycosyltransferase Enzyme from Family 91, UGT91P3, Is Responsible for the Final Glucosylation Step of Crocins in Saffron (Crocus sativus L.). Int. J. Mol. Sci. 2021, 22, 8815. [Google Scholar] [CrossRef]
- Hou, B.; Lim, E.K.; Higgins, G.S.; Bowles, D.J. N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 47822–47832. [Google Scholar] [CrossRef]
- Poppenberger, B.; Fujioka, S.; Soeno, K.; George, G.L.; Vaistij, F.E.; Hiranuma, S.; Seto, H.; Takatsuto, S.; Adam, G.; Yoshida, S. The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proc. Natl. Acad. Sci. USA 2005, 102, 15253–15258. [Google Scholar] [CrossRef]
- Wang, X. Structure, mechanism and engineering of plant natural product glycosyltransferases. Febs Lett. 2009, 583, 3303–3309. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, P.T.; Palmer, I.; Liang, S. Folding and Purification of Insoluble (Inclusion Body) Proteins from Escherichia coli. Curr. Protoc. Protein Sci. 2014, 78, 6.5.1–6.5.30. [Google Scholar]
- Palmer, I.; Wingfield, P.T. Preparation and Extraction of Insoluble (Inclusion-Body) Proteins from Escherichia coli. Curr. Protoc. Protein Sci. 2012, 70, 6.3.1–6.3.20. [Google Scholar] [CrossRef] [PubMed]
- Chew, F.N.; Abidin, N.; Yusof, N.; Rafi, N.M.; Chua, G.K. Recovery of inclusion body protein in Escherichia coli: Effects of solubilization methods and process condition. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022120. [Google Scholar] [CrossRef]
- Jong, W.; Hagen-Jongman, C.; Vikstrm, D.; Dontje, W.; Luirink, J. Mutagenesis-Based Characterization and Improvement of a Novel Inclusion Body Tag. Front. Bioeng. Biotechnol. 2020, 7, 442. [Google Scholar] [CrossRef]
- Jones, O.P.; Hatfield, S.G.S. Root Initiation in Apple Shoots Cultured In Vitro with Auxins and Phenolic Compounds. J. Hortic. Sci. 1976, 51, 495–499. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–586. [Google Scholar] [CrossRef]
- Rotenberg, D.; Thompson, T.S.; German, T.L.; Willis, D.K. Methods for effective real-time RT-PCR analysis of virus-induced gene silencing. J. Virol. Methods 2006, 138, 49–59. [Google Scholar] [CrossRef]





| Primer Name | Sequence (5′-3′) |
|---|---|
| TbUGGT_F | CGGAATTCATGGGTTCCGAGTCCTCGC |
| TbUGGT_R | TAAAGCGGCCGCTCACGGGACCGGCTCTACC |
| TbUGGT_F | ggatccGAATTCATGGGAAGTGAATC |
| TbUGGT_R | ataagaatGCGGCCGCTTAGG |
| T7 F | TAATACGACTCACTATAGGG |
| T7 R | GCTAGTTATTGCTCAGCGG |
| q-TbUGGT_F | GTTTCAGATGGGAACGGCACTAGG |
| q-TbUGGT_R | TCTGCGATGCTGTGGGTTCAAAG |
| C1168.2 F | GCTTGAAGATATTGTCCCCTCATCCC |
| C1168.2 R | AGTCATCCTTTGTGCTGCCATTCTC |
| Tool Name | Tool Web Site | Access Date |
|---|---|---|
| ProtParam | https://web.expasy.org/protparam/ | 12 February 2022 |
| CD Search | https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi? | 12 February 2022 |
| ScanProsite | https://prosite.expasy.org/scanprosite/ | 12 February 2022 |
| Pfam | http://pfam.xfam.org/ | 12 February 2022 |
| ProtScale | https://web.expasy.org/protscale/ | 12 February 2022 |
| TMHHM | https://services.healthtech.dtu.dk/service.php?TMHMM | 20 February 2022 |
| SignalP | https://services.healthtech.dtu.dk/service.php?SignalP-6.0 | 20 February 2022 |
| SOPMA | https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.htmL | 30 July 2022 |
| AphaFold2 | https://github.com/lucidrains/alphafold2 | 5 November 2022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Yin, D.; Sun, X.; Chen, Q.; Min, T.; Wang, H.; Wang, L. Molecular Cloning, Expression, and Functional Analysis of Glycosyltransferase (TbUGGT) Gene from Trapa bispinosa Roxb. Molecules 2022, 27, 8374. https://doi.org/10.3390/molecules27238374
Ye S, Yin D, Sun X, Chen Q, Min T, Wang H, Wang L. Molecular Cloning, Expression, and Functional Analysis of Glycosyltransferase (TbUGGT) Gene from Trapa bispinosa Roxb. Molecules. 2022; 27(23):8374. https://doi.org/10.3390/molecules27238374
Chicago/Turabian StyleYe, Shijie, Dongjie Yin, Xiaoyan Sun, Qinyi Chen, Ting Min, Hongxun Wang, and Limei Wang. 2022. "Molecular Cloning, Expression, and Functional Analysis of Glycosyltransferase (TbUGGT) Gene from Trapa bispinosa Roxb." Molecules 27, no. 23: 8374. https://doi.org/10.3390/molecules27238374
APA StyleYe, S., Yin, D., Sun, X., Chen, Q., Min, T., Wang, H., & Wang, L. (2022). Molecular Cloning, Expression, and Functional Analysis of Glycosyltransferase (TbUGGT) Gene from Trapa bispinosa Roxb. Molecules, 27(23), 8374. https://doi.org/10.3390/molecules27238374







