The Mitochondrial Protein MitoNEET as a Probe for the Allostery of Glutamate Dehydrogenase
Abstract
1. Introduction
2. Results and Discussion
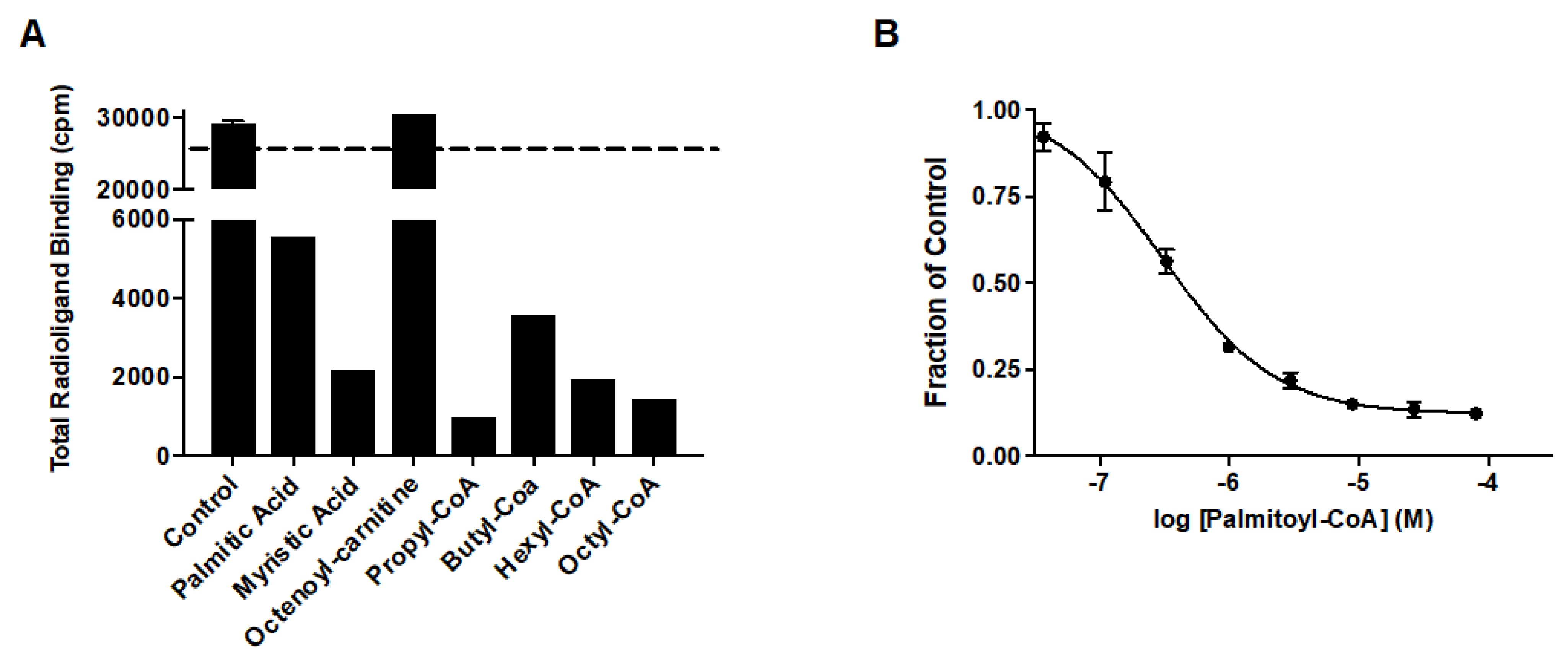
2.1. MitoNEET Rescues GDH from Palmitoyl-CoA Inhibition
2.2. MitoNEET Binds Palmitoyl-CoA with High Affinity
2.3. MitoNEET Rescues GDH from Inhibition by the Natural Product EGCG
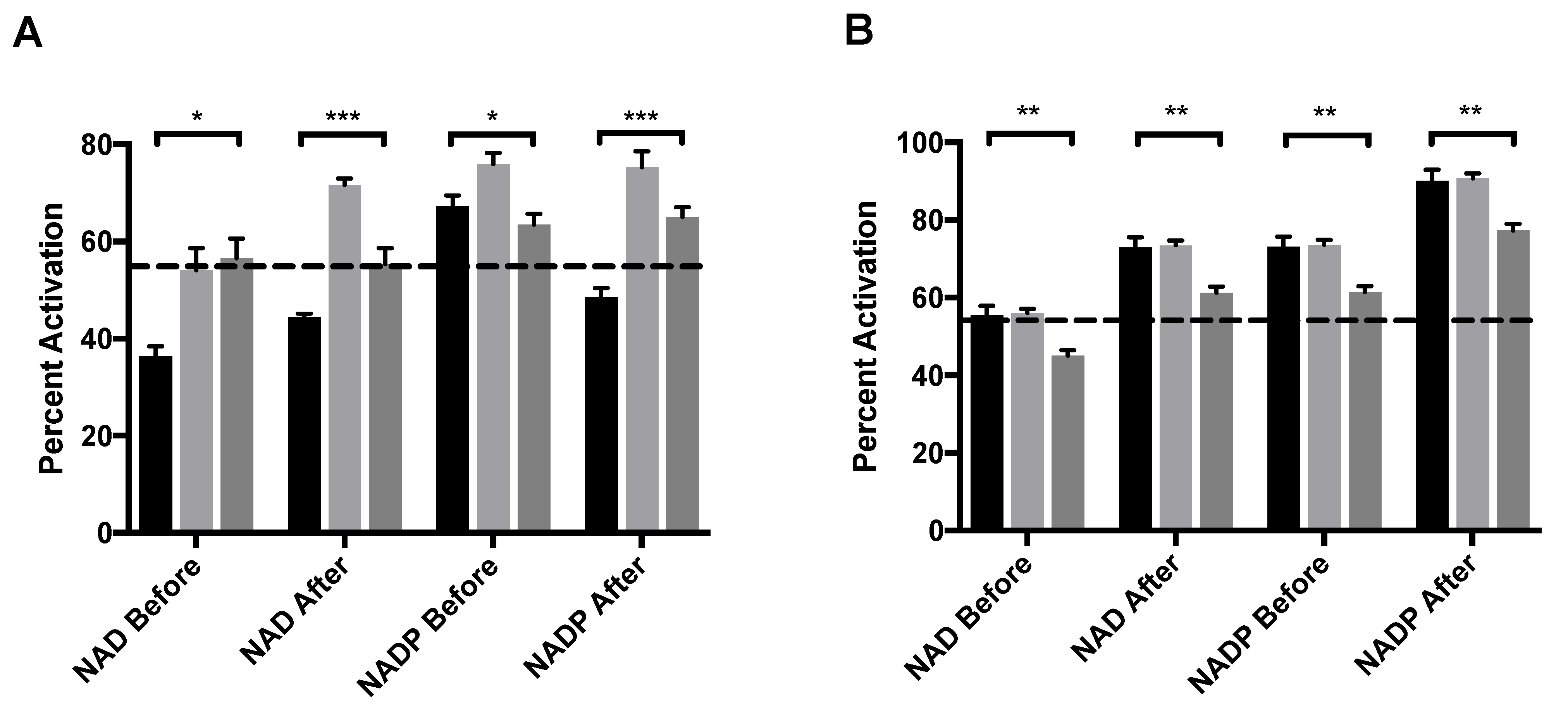
2.4. MitoNEET Rescues GDH from GTP Inhibition under Selective Conditions
2.5. MitoNEET Enhances ADP Activation of GDH
2.6. Leucine and mitoNEET Do Not Synergistically Activate GDH
3. Materials and Methods
3.1. Human MitoNEET Protein Expression and Purification
3.2. Bovine GDH Protein Preparation
3.3. Kinetic Analysis of GDH Activity Producing NADPH
3.4. Data Treatment of Inhibitory Ligands
3.5. Data Treatment of Activating Ligands
3.6. Radioligand Binding Assays
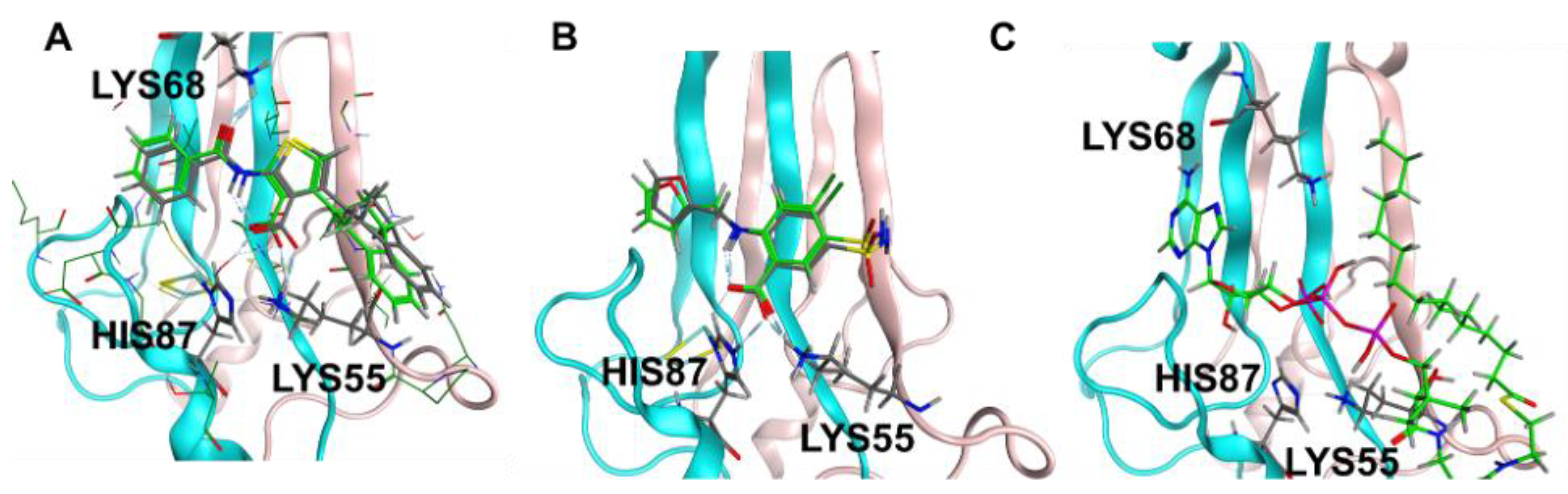
3.7. Molecular Modeling Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Banerjee, S.; Schmidt, T.; Fang, J.; Stanley, A.; Smith, J.T. Structural studies on ADP activation of mammalian glutamate dehydrogenase and the evolution of regulation. Biochemistry 2003, 42, 3446–3456. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Stanley, C.A. Untangling the glutamate dehydrogenase allosteric nightmare. Trends Biochem. Sci. 2008, 33, 557–564. [Google Scholar] [CrossRef]
- Grimaldi, M.; Karaca, M.; Latini, L.; Brioudes, E.; Schalch, T.; Maechler, P. Identification of the molecular dysfunction caused by glutamate dehydrogenase S445L mutation responsible for hyperinsulinism/hyperammonemia. Hum. Mol. Genet. 2017, 26, 3453–3465. [Google Scholar] [CrossRef] [PubMed]
- Borgnia, M.J.; Banerjee, S.; Merk, A.; Matthies, D.; Bartesaghi, A.; Rao, P.; Pierson, J.; Earl, L.A.; Falconieri, V.; Subramaniam, S.; et al. Using Cryo-EM to Map Small Ligands on Dynamic Metabolic Enzymes: Studies with Glutamate Dehydrogenase. Mol. Pharmacol. 2016, 89, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Allen, A.; Kwagh, J.; Doliba, N.M.; Qin, W.; Najafi, H.; Collins, H.W.; Matschinsky, F.M.; Stanley, C.; Smith, T.J. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J. Biol. Chem. 2006, 281, 10214–10221. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.E.; Smith, T.J. The structure of bovine glutamate dehydrogenase provides insights into the mechanism of allostery. Structure 1999, 7, 769–782. [Google Scholar] [CrossRef]
- Wilson, D.F.; Cember, A.T.J.; Matschinsky, F.M. Glutamate dehydrogenase: Role in regulating metabolism and insulin release in pancreatic β-cells. J. Appl. Physiol. 2018, 125, 419–428. [Google Scholar] [CrossRef]
- Colca, J.R.; McDonald, W.G.; Waldon, D.J.; Leone, J.W.; Lull, J.M.; Bannow, C.A.; Lund, E.T.; Mathews, W.R. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Endocrinol. Metab. 2004, 286, 252–260. [Google Scholar] [CrossRef]
- Paddock, M.L.; Wiley, S.E.; Axelrod, H.L.; Cohen, A.E.; Roy, M.; Abresch, E.C.; Capraro, D.; Murphy, A.N.; Nechushtai, R.; Dixon, J.E.; et al. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc. Natl. Acad. Sci. USA 2007, 104, 14342–14347. [Google Scholar] [CrossRef]
- Kunk, C.; Kruger, J.; Mendoza, G.; Markitan, J.; Bias, T.; Mann, A.; Nath, A.; Geldenhuys, W.J.; Menze, M.A.; Konkle, M.E. MitoNEET’s Reactivity of Lys55 toward Pyridoxal Phosphate Demonstrates its Activity as a Transaminase Enzyme. ACS Chem. Biol. 2022, 17, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Crail, J.P.; Laffoon, M.M.; Fernandez, W.G.; Menze, M.A.; Konkle, M.E. Identification of disulfide bond formation between MitoNEET and glutamate dehydrogenase 1. Biochemistry 2013, 52, 8969–8971. [Google Scholar] [CrossRef][Green Version]
- Smith, T.J.; Schmidt, T.; Fang, J.; Wu, J.; Siuzdak, G.; Stanley, C.A. The structure of apo human glutamate dehydrogenase details subunit communication and allostery. J. Mol. Biol. 2002, 318, 765–777. [Google Scholar] [CrossRef]
- Bera, S.; Rashid, M.; Medvinsky, A.B.; Sun, G.-Q.; Li, B.-L.; Acquisti, C.; Sljoka, A.; Chakraborty, A. Allosteric regulation of glutamate dehydrogenase deamination activity. Sci. Rep. 2020, 10, 16523. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Long, T.; Saralkar, P.; Iwasaki, T.; Nuñez, R.A.A.; Nair, R.R.; Konkle, M.E.; Menze, M.; Pinti, M.V.; Hollander, J.M.; et al. Crystal structure of the mitochondrial protein mitoNEET bound to a benze-sulfonide ligand. Commun. Chem. 2019, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Piktel, D.; Moore, J.C.; Rellick, S.L.; Meadows, E.; Pinti, M.V.; Hollander, J.M.; Ammer, A.G.; Martin, K.H.; Gibson, L.F. Loss of the redox mitochondrial protein mitoNEET leads to mitochondrial dysfunction in B-cell acute lymphoblastic leukemia. Free Radic. Biol. Med. 2021, 175, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Marjault, H.B.; Karmi, O.; Zuo, K.; Michaeli, D.; Eisenberg-Domovich, Y.; Rossetti, G.; de Chassey, B.; Vonderscher, J.; Cabantchik, I.; Carloni, P.; et al. An anti-diabetic drug targets NEET (CISD) proteins through destabilization of their [2Fe-2S] clusters. Commun. Biol. 2022, 5, 437. [Google Scholar] [CrossRef]
- Sandor, M.; Kiss, R.; Keseru, G.M. Virtual fragment docking by Glide: A validation study on 190 protein-fragment complexes. J. Chem. Inf. Model. 2010, 50, 1165–1172. [Google Scholar] [CrossRef]
- Ludzki, A.; Paglialunga, S.; Smith, B.K.; Herbst, E.A.F.; Allison, M.K.; Heigenhauser, G.J.; Neufer, P.D.; Holloway, G.P. Rapid Repression of ADP Transport by Palmitoyl-CoA Is Attenuated by Exercise Training in Humans: A Potential Mechanism to Decrease Oxidative Stress and Improve Skeletal Muscle Insulin Signaling. Diabetes 2015, 64, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, M.; Chen, P.; Narayan, S.; Matschinsky, F.M.; Bennett, M.J.; Stanley, C.; Smith, T.J. Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J. Biol. Chem. 2011, 286, 34164–341674. [Google Scholar] [CrossRef]
- Stanley, C.A. Hyperinsulinism/hyperammonemia syndrome: Insights into the regulatory role of glutamate dehydrogenase in ammonia metabolism. Mol. Genet. Metab. 2004, 81 (Suppl. 1), S45–S51. [Google Scholar] [CrossRef]
- Tomita, T.; Kuzuyama, T.; Nishiyama, M. Structural basis for leucine-induced allosteric activation of glutamate dehydrogenase. J. Biol. Chem. 2011, 286, 37406–37413. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.Q.; Li, C.; Stanley, C.A.; Smith, T.J. Glutamate Dehydrogenase, a Complex Enzyme at a Crucial Metabolic Branch Point. Neurochem. Res. 2019, 44, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.A.; Bunik, V.I.; Bruch, E.M.; Bellinzoni, M. Structural Basis for the Binding of Allosteric Activators Leucine and ADP to Mammalian Glutamate Dehydrogenase. Int. J. Mol. Sci. 2022, 23, 11306. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nnatubeugo, C.; Johnson, E.; Gisondi, S.; Roland, F.; Geldenhuys, W.J.; Menze, M.A.; Konkle, M.E. The Mitochondrial Protein MitoNEET as a Probe for the Allostery of Glutamate Dehydrogenase. Molecules 2022, 27, 8314. https://doi.org/10.3390/molecules27238314
Nnatubeugo C, Johnson E, Gisondi S, Roland F, Geldenhuys WJ, Menze MA, Konkle ME. The Mitochondrial Protein MitoNEET as a Probe for the Allostery of Glutamate Dehydrogenase. Molecules. 2022; 27(23):8314. https://doi.org/10.3390/molecules27238314
Chicago/Turabian StyleNnatubeugo, Chimere, Erica Johnson, Sarah Gisondi, Felicia Roland, Werner J. Geldenhuys, Michael A. Menze, and Mary E. Konkle. 2022. "The Mitochondrial Protein MitoNEET as a Probe for the Allostery of Glutamate Dehydrogenase" Molecules 27, no. 23: 8314. https://doi.org/10.3390/molecules27238314
APA StyleNnatubeugo, C., Johnson, E., Gisondi, S., Roland, F., Geldenhuys, W. J., Menze, M. A., & Konkle, M. E. (2022). The Mitochondrial Protein MitoNEET as a Probe for the Allostery of Glutamate Dehydrogenase. Molecules, 27(23), 8314. https://doi.org/10.3390/molecules27238314







