In Vitro Anti-Influenza A Virus H1N1 Effect of Sesquiterpene-Rich Extracts of Carpesium abrotanoides
Abstract
1. Introduction
2. Results and Discussion
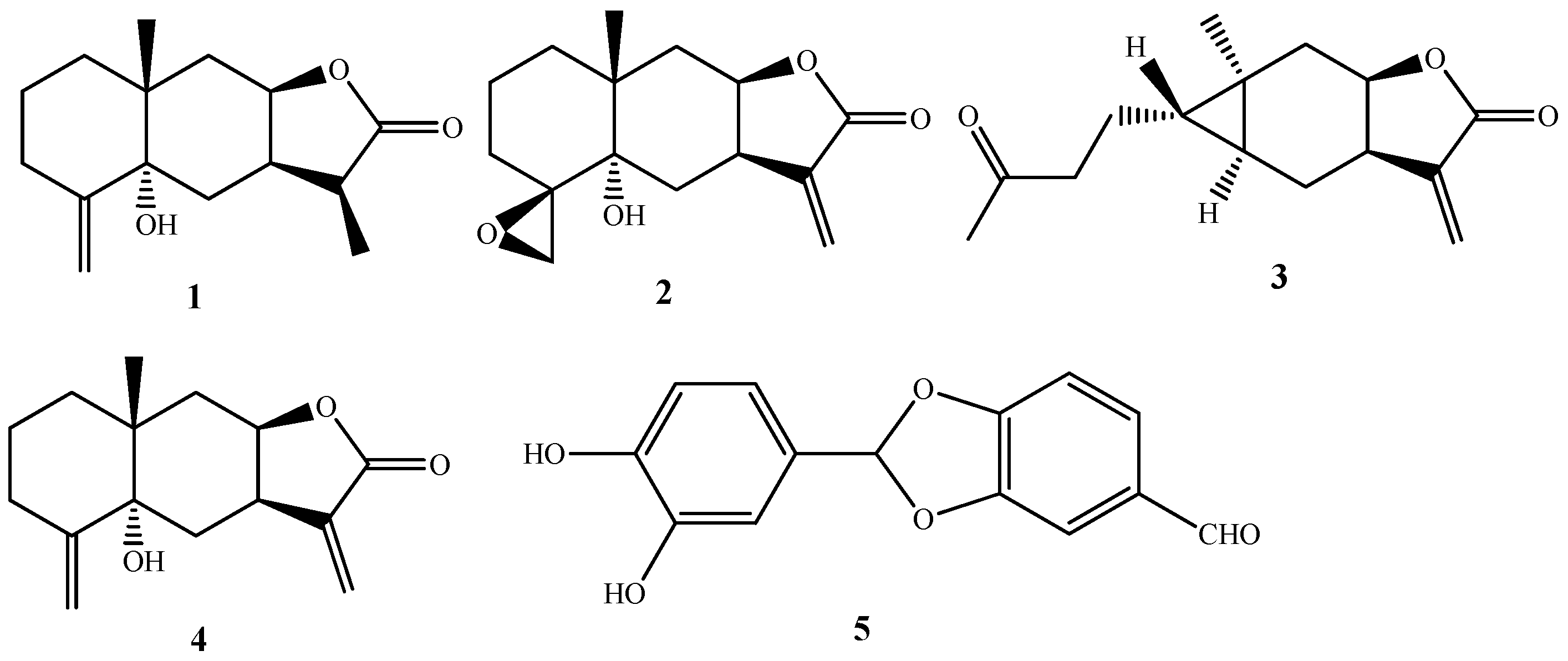
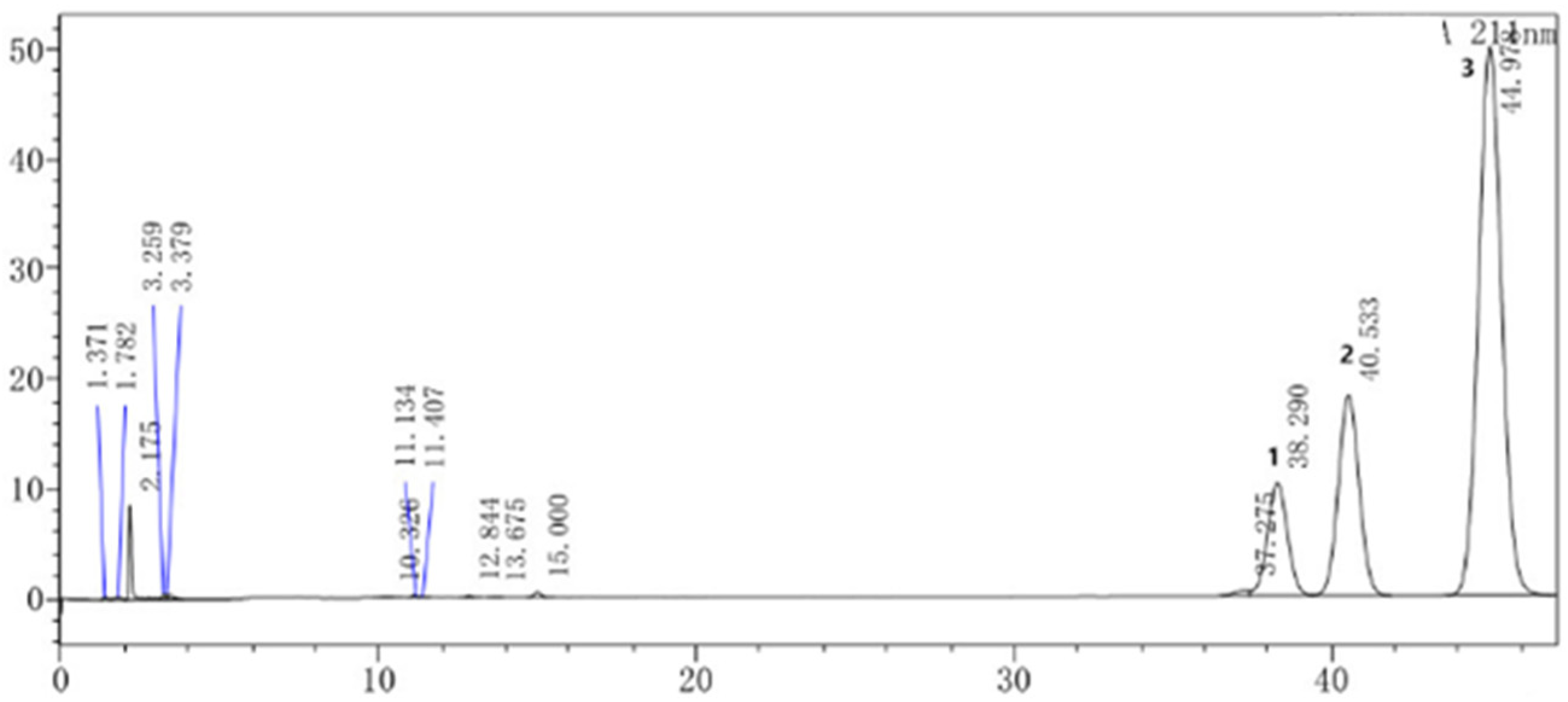
2.1. Chemical Composition of SECA
2.2. Cell Cytotoxicity of SECA on MDCK Cells
2.3. Determination of Median Tissue Culture Infective Dose (TCID50) of Influenza A Virus (H1N1)
2.4. In Vitro Anti-Influenza A Virus H1N1 Effect of SECA
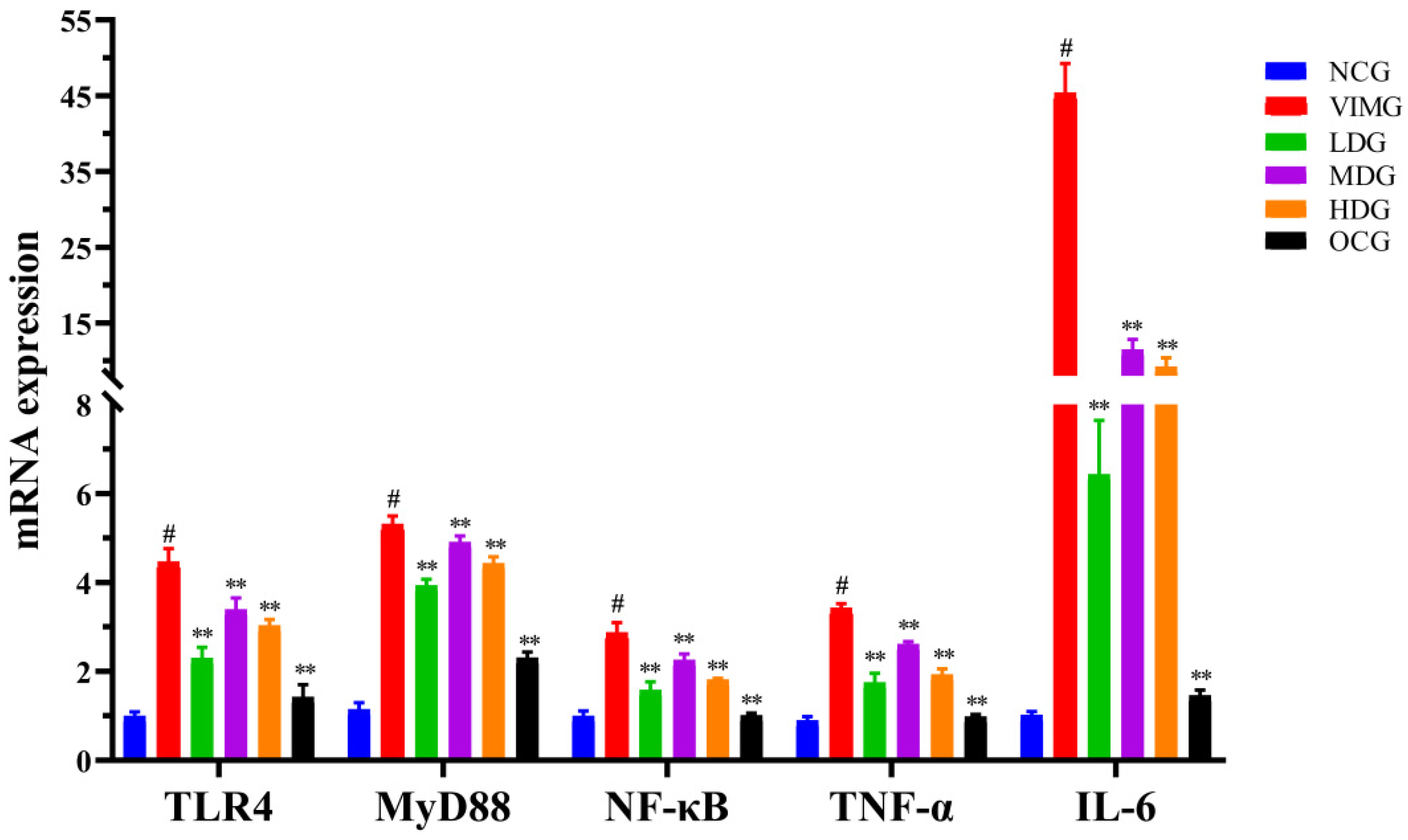
2.5. mRNA Expressions of TLR4, MyD88, NF-кB, TNF-α and IL-6 in MDCK Cells Infected by Influenza A Virus H1N1
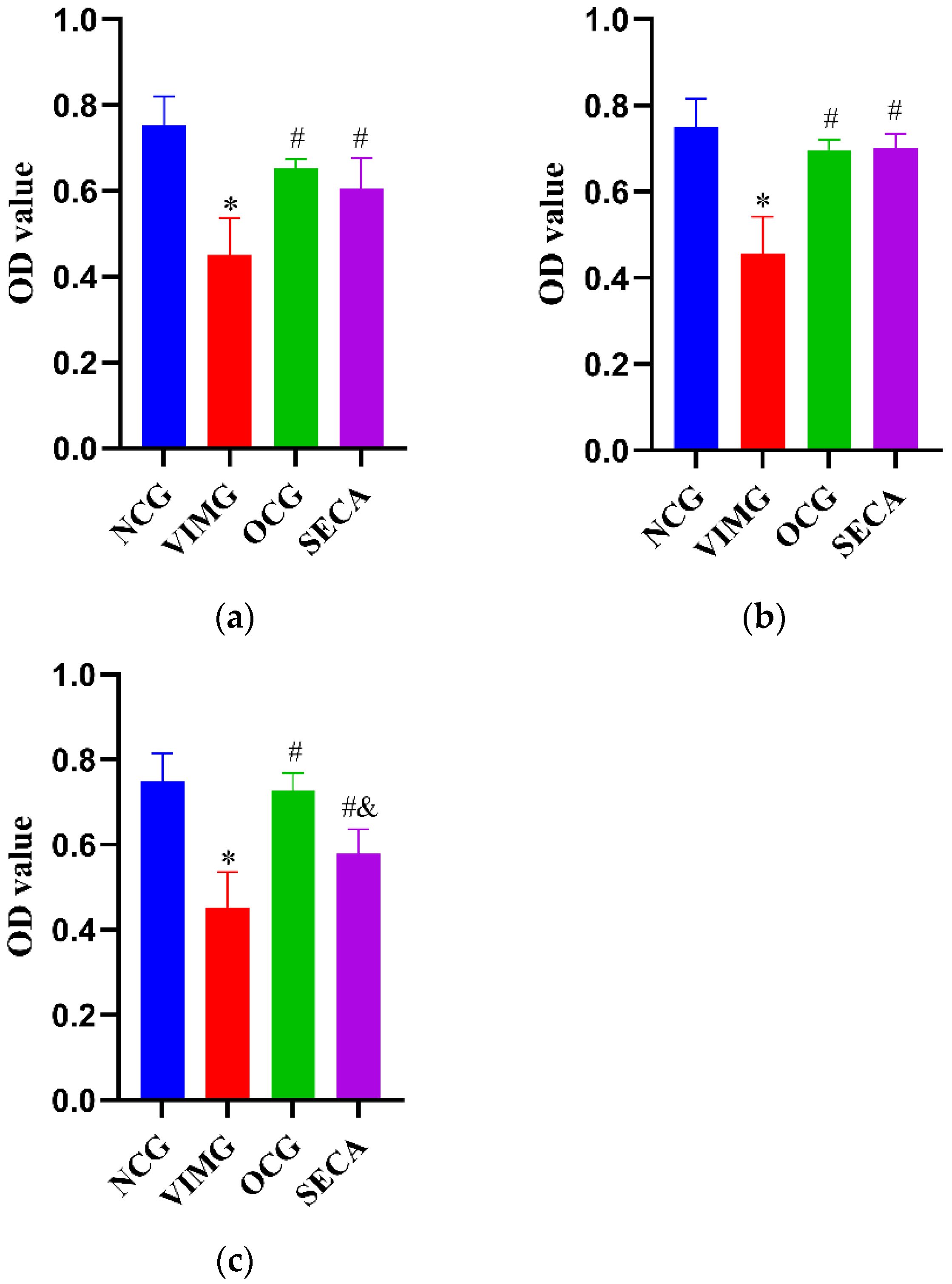
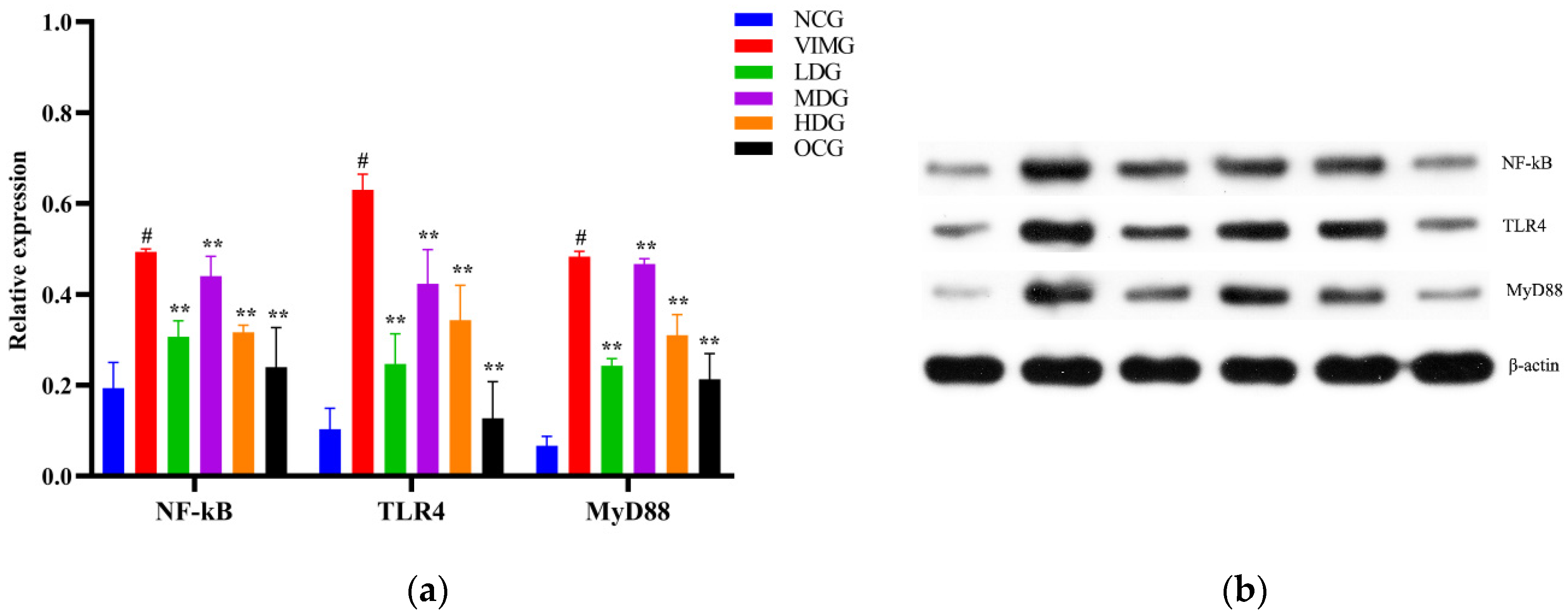
2.6. Protein Expressions of TLR4, MyD88, and NF-кB in MDCK Cells Infected by Influenza A Virus H1N1
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of SECA
3.3. Isolation of SECA
3.4. Structural Elucidation of Compounds
3.5. HPLC Assay
3.6. Drug Preparation for Antiviral Assay
3.7. Cytotoxicity Assay of SECA on MDCK Cells
3.8. Determination of Median Tissue Culture Infective Dose (TCID50) of Influenza A Virus (H1N1) Using Cytopathic Effect (CPE) Assay
3.9. Determination of Anti-Influenza A Virus H1N1 Effect of SECA In Vitro
3.10. Effect of SECA on Expressions of mRNA and Protein of TLR4, MyD88, NF-кB, TNF-α, and IL-6 in MDCK Cells Infected by Influenza A Virus H1N1
3.11. Quantitative Real-Time Reverse Transcriptase PCR (qRT-PCR)
3.12. Western Blot Analysis
3.13. Statistical Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dunning, J.; Baillie, J.K.; Cao, B.; Hayden, F.G. Antiviral combinations for severe influenza. Lancet Infect. Dis. 2014, 14, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.E.; Wen, J.; Zhao, S.; Zhang, K.; Zhou, Y. Prophylaxis and therapy of pandemic H1N1 virus infection using egg yolk antibody. J. Virol. Methods 2014, 206, 19–26. [Google Scholar] [CrossRef]
- Roberts, N.R.J.; Leonard, R.K. The continued threat of influenza A viruses. Viruses 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.L. Evolution of Influenza A Virus by Mutation and Re-Assortment. Int. J. Mol. Sci. 2015, 18, 1650. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Nguyen, H.T.; Kniss, K.; Mishin, V.P.; Gubareva, L.V. Cluster of Oseltamivir-Resistant and Hemagglutinin Antigenically Drifted Influenza A (H1N1) pdm09 Viruses, Texas, USA, January 2020. Emerg. Infect. Dis. 2021, 27, 1953–1957. [Google Scholar] [CrossRef]
- Chen, W.; Lim, C.E.; Kang, H.J.; Liu, J. Chinese herbal medicines for the treatment of type A H1N1 influenza: A systematic review of randomized controlled trials. PLoS ONE 2011, 6, e28093. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chi, X.J.; Bai, Q.L.; Chen, J.L. Chanisms underlying interferon-mediated host innate immunity during influenza a virus infection. Chin. J. Biotechnol. 2015, 31, 1671–1681. [Google Scholar]
- Robertson, S.A.; Hutchinson, M.R.; Rice, K.C.; Chin, P.Y.; Moldenhauer, L.M.; Stark, M.J.; Olson, D.M.; Keelan, J.A. Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury. Clin. Transl. Immunol. 2020, 9, e1121. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.; Singhera, G.K.; Utokaparch, S.; Hackett, T.L.; Boyd, J.H.; Luo, Z.; Si, X.; Dorscheid, D.R.; McManus, B.M.; Hegele, R.G. Toll like receptor 4 mediated p38 mitogen-activated protein kinase activation is a determinant of respiratory virus entry and tropism. J. Virol. 2010, 84, 11359–11373. [Google Scholar] [CrossRef]
- Dai, J.P.; Wang, Q.W.; Su, Y.; Gu, L.M.; Zhao, Y.; Chen, X.X.; Chen, C.; Li, W.Z.; Wang, G.F.; Li, K.S. Emodin inhibition of influenza A virus replication and influenza viral pneumonia via the Nrf2, TLR4, p38/JNK and NF-kappa B pathways. Molecules 2017, 22, 1754. [Google Scholar] [CrossRef]
- Pires-Neto, O.S.; de Sá, K.S.G.; Santana, B.B.; Gomes, S.T.M.; Amoras, E.S.G.; Conde, S.R.S.; Demachki, S.; Azevedo, V.N.; Martins-Feitosa, R.N.; Ishak, M.O.G.; et al. Lack of association between polymorphisms of the TLR4 gene and infection with the hepatitis B and C viruses. Mediat. Inflamm. 2015, 2015, 150673. [Google Scholar] [CrossRef] [PubMed]
- Palache, A.M.; Scheepers, H.S.; Regt, V.D.; Ewijk, P.V.; Baljet, M.; Brands, R.; Scharrenburg, G.J. Safety, reactogenicity and immunogenicity of Madin Darby Canine Kidney cell-derived inactivated influenza subunit vaccine. A meta-analysis of clinical studies. Dev. Biol. Stand. 1999, 98, 115–125. [Google Scholar] [PubMed]
- Kistner, O.; Barrett, P.N.; Mundt, W.; Reiter, M.; Schober-Bendixen, S.; Dorner, F. Development of a mammalian cell (Vero) derived candidate influenza virus vaccine. Vaccine 1998, 16, 960–968. [Google Scholar] [CrossRef]
- Youil, R.; Su, Q.; Toner, T.J.; Szymkowiak, C.; Kwan, W.S.; Rubin, B.; Petrukhin, L.; Kiseleva, I.; Shaw, A.R.; DiStefano, D. Comparative study of influenza virus replication in Vero and MDCK cell lines. J. Virol. Methods 2004, 120, 23–31. [Google Scholar] [CrossRef]
- Ye, Q.; Phan, T.; Hu, W.S.; Liu, X.; Fan, L.; Tan, W.S.; Zhao, L. Transcriptomic characterization reveals attributes of high influenza virus productivity in MDCK cells. Viruses 2021, 13, 2200. [Google Scholar] [CrossRef]
- Wang, J.F.; He, W.J.; Zhang, X.X.; Zhao, B.Q.; Liu, Y.H.; Zhou, X.J. Dicarabrol, a new dimeric sesquiterpene from Carpesium abrotanoides L. Bioorg. Med. Chem. Lett. 2015, 25, 4082–4084. [Google Scholar] [CrossRef]
- He, Y.Q.; Cai, L.; Qian, Q.G.; Yang, S.H.; Chen, D.L.; Zhao, B.Q.; Zhong, Z.P.; Zhou, X.J. Anti-influenza A (H1N1) viral and cytotoxic sesquiterpenes from Carpesium abrotanoides. Phytochem. Lett. 2020, 35, 41–45. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Shi, Y.P.; Jia, Z.J. Chemical constituents of the aerial parts of Carpesium cernuun. J. Lanzhou Univ. 2002, 38, 61–67. [Google Scholar]
- Liu, P.A.; Liu, M.; Pan, W.W.; He, W.J.; Peng, S.; Zhou, X.J. Study on chemical constituents from Carpesium abrotanoides. J. Chin. Med. Mat. 2014, 37, 2213–2215. [Google Scholar]
- Mutax, H.; Zhang, T.; Si, J.G.; Fu, L.; Serikjan, S.; Hong, Y.Q.; Ma, X.D.; Zou, Z.M. Chemical constituents of Carpesium divaricatum. J. Int. Pharm. Res. 2018, 45, 449–454. [Google Scholar]
- Wang, L.; Tian, L.; Cheng, F.; Zou, K.; Peter, P. Terpenes from Carpesium abrotanoides and their cytotoxic activities. Chin. Tradit. Herb. Drugs 2018, 49, 530–535. [Google Scholar]
- Zhang, L.; Xiang, L.L.; Yang, L.Y.; Li, R.T.; Zhang, J.D. Chemical constituents from Miao herbal medicine Elsholtzia penduliflora. J. Chin. Med. Mat. 2019, 42, 785–789. [Google Scholar]
- Ge, Z.Y.; Tong, J.; Wang, X.Y.; Na, J.J.; Lu, F.G. An update about effect of influenza virus on cellular signal transduction. Chin. Arch. Tradit. Chin. Med. 2014, 32, 2393–2396. [Google Scholar]
- Heil, F.; Ahmad-Nejad, P.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Gellert, T.; Dietrich, H.; Lipford, G.; Takeda, K.; Akira, S.; et al. The Toll-like receptor 7(TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 2003, 33, 2987–2997. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathway. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.Z.; Wu, Y.H.; Gu, Z.Q.; Li, H.F.; Li, J.J.; Guo, B.Y.; Liao, Z.; Yan, X.J. A novel molluscan TLR molecule engaged in inflammatory response through MyD88 adapter recruitment. Dev. Comp. Immunol. 2022, 131, 104373. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sahu, K.; Singh, C.; Singh, A. Lipopolysaccharide induced altered signaling pathways in various neurological disorders. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Alan, A.; Alan, E.; Arslan, K.; Daldaban, F.; Aksel, E.; Çınar, M.; Akyüz, B. LPS- and LTA-induced expression of TLR4, MyD88, and TNF-α in lymph nodes of the Akkaraman and Romanov lambs. Microsc. Microanal. 2022, 28, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.G.; Banner, D.; Zhao, Z.; Fang, Y.; Huang, S.S.; León, A.J.; Ng, D.C.; Almansa, R.; Martin-Loeches, L.; Ramirez, P.; et al. Interleukin-6 is a potential biomarker for severe pandemic H1N1 influenza A infection. PLoS ONE 2012, 7, e38214. [Google Scholar] [CrossRef]
| The Concentration of SECA (μg/mL) | Inhibition Rate (%) |
|---|---|
| 50 | 66% |
| 25 | 71% |
| 12.5 | 31% |
| 6.25 | 21% |
| 3.125 | 7% |
| 1.5625 | 4% |
| Dilution of Virus | Number of CPE Wells | Number of Non-CPE Wells | Cumulative Number of CPE Wells | Cumulative Number of Non-CPE Wells | Percentage of CPE Wells |
|---|---|---|---|---|---|
| 10−1 | 8 | 0 | 21 | 0 | 100% |
| 10−2 | 8 | 0 | 13 | 0 | 100% |
| 10−3 | 3 | 5 | 5 | 5 | 50% |
| 10−4 | 2 | 6 | 2 | 11 | 15.3% |
| 10−5 | 0 | 8 | 0 | 19 | 0% |
| 10−6 | 0 | 8 | 0 | 27 | 0% |
| Name | Primer Sequence(5′→3′) | Product Length |
|---|---|---|
| TNF-α | Forward: CGAACCCCAAGTGACAAGCC | 119 bp |
| Reverse: TCTGTCAGCTCCACGCCGTTG | ||
| IL-6 | Forward: TGACCCAACCACAGACGCCAG | 178 bp |
| Reverse: AGGAATGCCCATGAACTACAGC | ||
| TLR4 | Forward: TGCCAGAATGATGTCTCCTACCC | 192 bp |
| Reverse: CTCAGGTCCAGTTTCTCGGTT | ||
| MyD88 | Forward: CCTGAGCGTTTTGATGCCTT | 100 bp |
| Reverse: ACTTCAGCCGATAGTTTGTCT | ||
| NF-κB | Forward: GCACAGACACCACCAAGACCCAC | 136 bp |
| Reverse: CGGCAGTCTTTCCCCACAAGCTC | ||
| Actin | Forward: ACTTTAGTTGCGTTACACCCT | 189 bp |
| Reverse: TAAATCCTGAGTCAAGCGCCAA |
| Protein | Catalog Number | Species | Dilution | Company |
|---|---|---|---|---|
| NF-kB | 10745-1-AP | Rabbit | 1:2000 | Proteintech |
| TLR4 | 19811-1-AP | Rabbit | 1:750 | Proteintech |
| MyD88 | 23230-1-AP | Rabbit | 1:1000 | Proteintech |
| β-actin | 60008-1-Ig | Mouse | 1:5000 | Proteintech |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yang, S.; Chen, D.; Wu, Z.; Zhang, M.; Yang, F.; Qin, L.; Zhou, X. In Vitro Anti-Influenza A Virus H1N1 Effect of Sesquiterpene-Rich Extracts of Carpesium abrotanoides. Molecules 2022, 27, 8313. https://doi.org/10.3390/molecules27238313
Li L, Yang S, Chen D, Wu Z, Zhang M, Yang F, Qin L, Zhou X. In Vitro Anti-Influenza A Virus H1N1 Effect of Sesquiterpene-Rich Extracts of Carpesium abrotanoides. Molecules. 2022; 27(23):8313. https://doi.org/10.3390/molecules27238313
Chicago/Turabian StyleLi, Li, Shenghui Yang, Dilu Chen, Zhihuang Wu, Meijun Zhang, Fang Yang, Li Qin, and Xiaojiang Zhou. 2022. "In Vitro Anti-Influenza A Virus H1N1 Effect of Sesquiterpene-Rich Extracts of Carpesium abrotanoides" Molecules 27, no. 23: 8313. https://doi.org/10.3390/molecules27238313
APA StyleLi, L., Yang, S., Chen, D., Wu, Z., Zhang, M., Yang, F., Qin, L., & Zhou, X. (2022). In Vitro Anti-Influenza A Virus H1N1 Effect of Sesquiterpene-Rich Extracts of Carpesium abrotanoides. Molecules, 27(23), 8313. https://doi.org/10.3390/molecules27238313