Anticonvulsant Activity of Bombyx batryticatus and Analysis of Bioactive Extracts Based on UHPLC-Q-TOF MS/MS and Molecular Networking
Abstract
1. Introduction
2. Results and Discussion
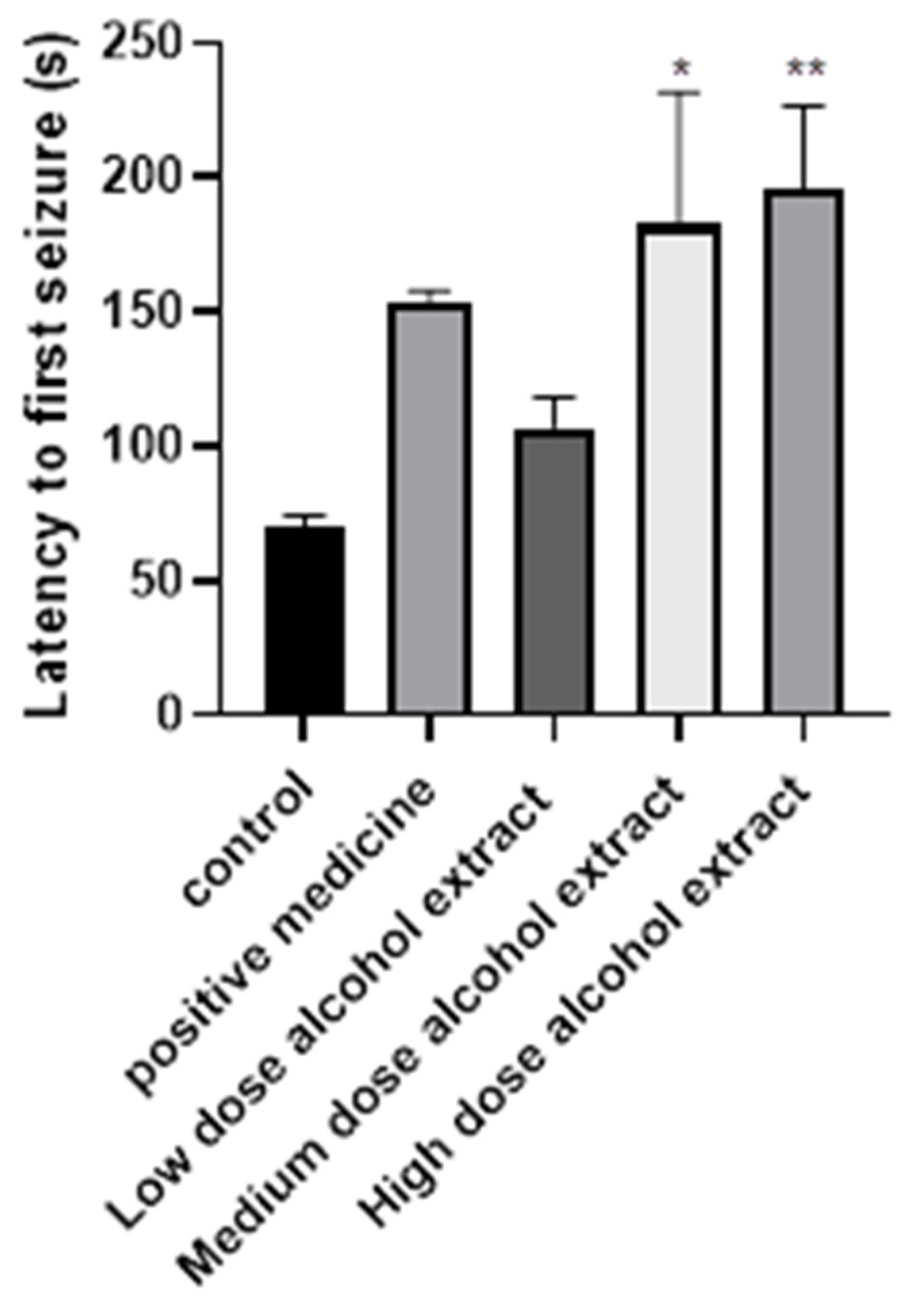
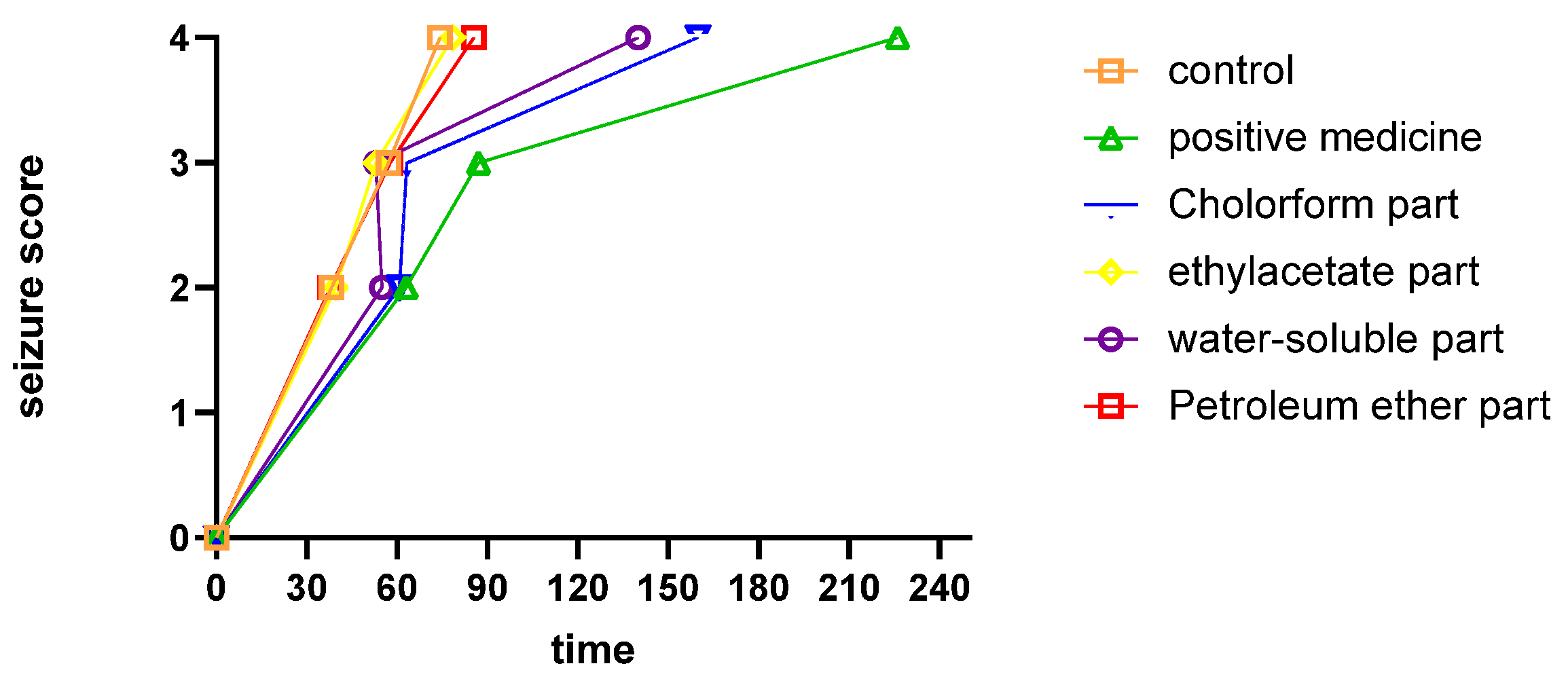
2.1. Anticonvulsant Effects of Different Fractions on PTZ-Induced Seizures
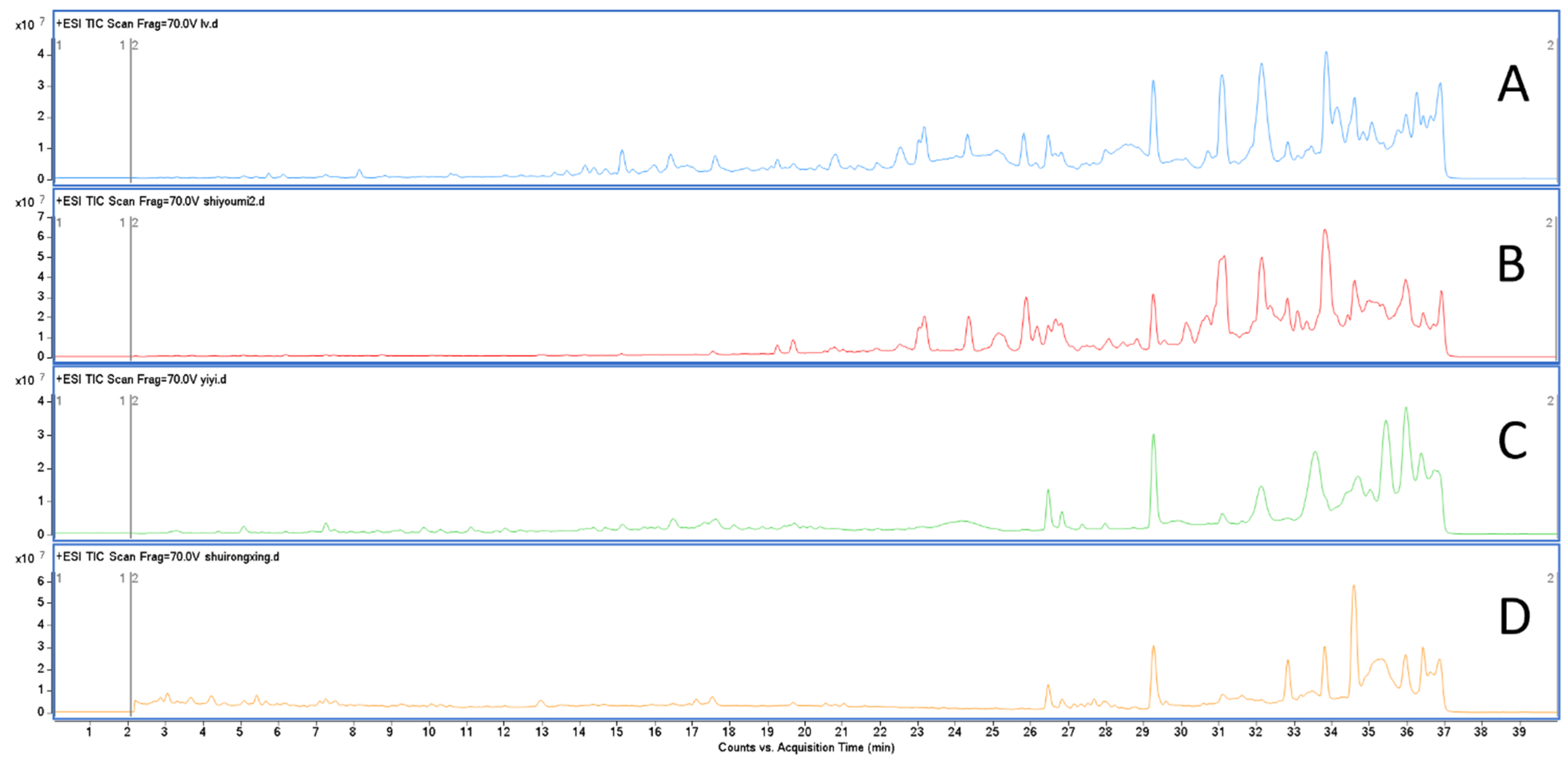
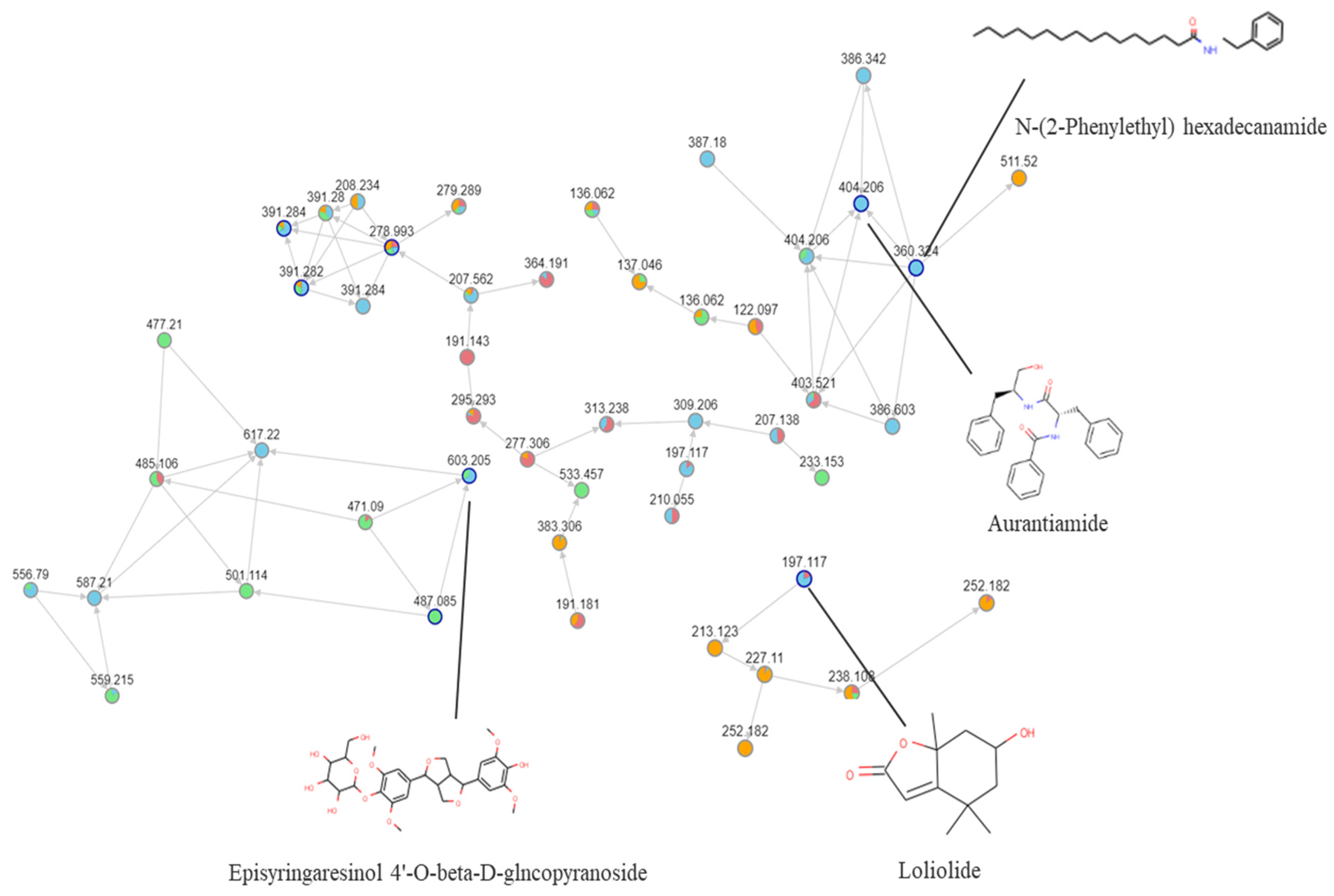
2.2. Molecular Network Based on the Compounds from Different Polarity Fractions
2.3. UHPLC Q-TOF-MS Profiling of Chloroform Extracts of BB
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction of Bombyx Batryticatus
3.3. Animals
3.4. Experiment
3.4.1. Effects of the Low, Middle, and High Doses of Crude Extracts on PTZ-Induced Seizures
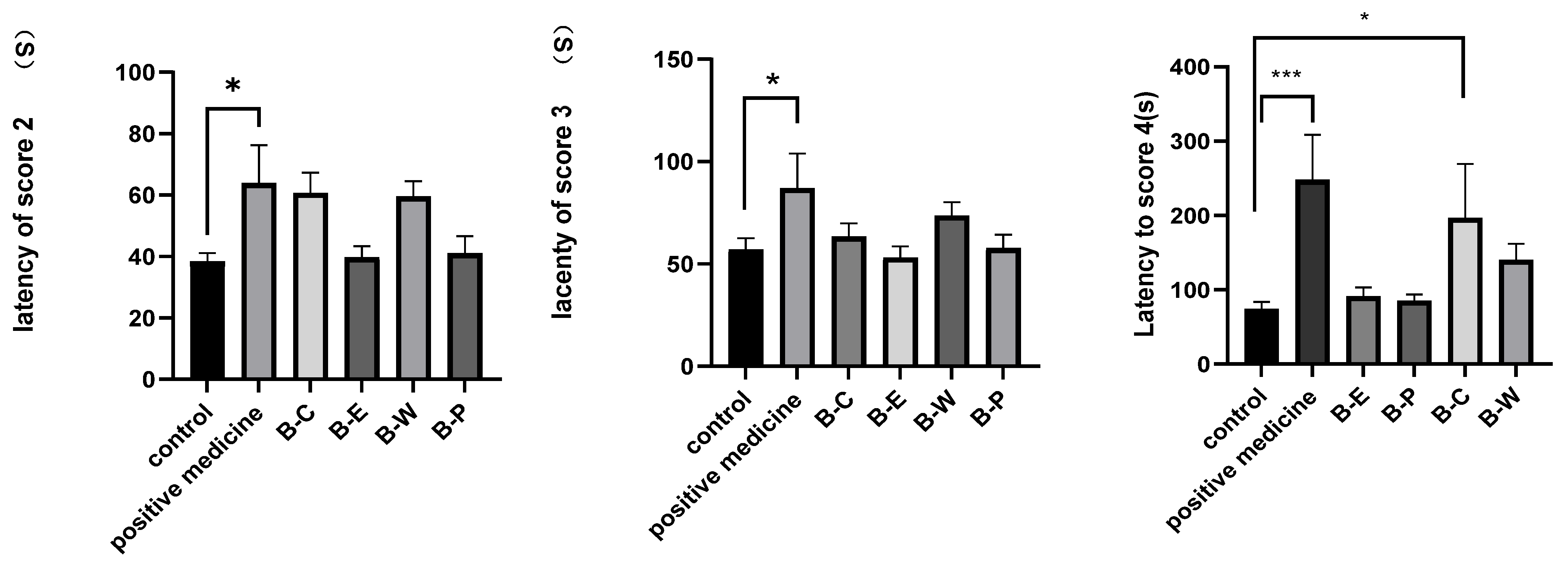
3.4.2. Effect of Different Fractions on PTZ-Induced Seizures
3.5. UHPLC-Q-TOF MS Analysis
3.6. Identification of the Fractions’ Components
3.6.1. Molecular Network
3.6.2. Other Spectral Libraries and Literature
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Marson, A.; Burnside, G.; Appleton, R.; Smith, D.; Leach, J.P.; Sills, G.; Tudur-Smith, C.; Plumpton, C.; Hughes, D.A.; Williamson, P.; et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: An open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 2021, 397, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, A.; Labate, A.; Mumoli, L.; Lopes-Cendes, I.; Cendes, F. Role of Pharmacogenomics in Antiepileptic Drug Therapy: Current Status and Future Perspectives. Curr. Pharm. Des. 2017, 23, 5760–5765. [Google Scholar] [CrossRef]
- Antill-O’Brien, N.; Bourke, J.; O’Connell, C.D. Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models. Materials 2019, 12, 3218. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, S.S.; Wong, M. Therapeutic role of mammalian target of rapamycin (mTOR) inhibition in preventing epileptogenesis. Neurosci. Lett. 2011, 497, 231–239. [Google Scholar] [CrossRef]
- Liu, L.; He, L.; Yin, C.; Huang, R.; Shen, W.; Ge, H.; Sun, M.; Li, S.; Gao, Y.; Xiong, W. Effects of palmatine on BDNF/TrkB-mediated trigeminal neuralgia. Sci. Rep. 2020, 10, 4998. [Google Scholar] [CrossRef]
- Yao, H.; He, X.; He, Q.; Yang, L.; Ma, Y. Comparative study on pharmacodynamics of anticonvulsant effects of alcohol extracts from bombyx batryticatus and centipedes. Chin. Remedies Clin. 2006, 6, 221–223. (In Chinese) [Google Scholar]
- Hu, M.; Yu, Z.; Wang, J.; Fan, W.; Liu, Y.; Li, J.; Xiao, H.; Li, Y.; Peng, W.; Wu, C. Traditional Uses, Origins, Chemistry and Pharmacology of Bombyx batryticatus: A Review. Molecules 2017, 22, 1779. [Google Scholar] [CrossRef]
- Zhu, M.M.; Tao, J.; Tan, M.; Yang, H.T.; Ji, Y.H. U-shaped dose-dependent effects of BmK AS, a unique scorpion polypeptide toxin, on voltage-gated sodium channels. Br. J. Pharmacol. 2009, 158, 1895–1903. [Google Scholar] [CrossRef]
- Yan, Z.; Li, X.; Chen, X.; Peng, C.; Liu, Y.; Xiang, C. A primary study on anticonvulsant parts of BombyxmoriL. Lishizhen Med. Mater. Med. Res. 2006, 17, 696–697. (In Chinese) [Google Scholar]
- Guo, X.; Yan, Z.; Liu, T.; Song, D.; Li, X. Anticonvulsive activity of three compounds isolated from Beauveria bassiana. Chin. J. Exp. Tradit. Med. 2013, 19, 248–250. (In Chinese) [Google Scholar]
- Guo, X.; Wu, Y.; Song, D.; Yan, Z.; Liu, T. Compounds isolated and purified from chloroform active part of Bombyx batryticatus and their anticonvulsive activities. Chin. J. Pharm. 2014, 45, 431–433. (In Chinese) [Google Scholar]
- Cheng, S.M.; Huang, J.; Wang, H.Y.; Li, G.Y.; Lin, R.C.; Wang, J.H. Two new compounds from Bombyx batryticatus. J. Asian Nat. Prod. Res. 2014, 16, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, X.; Wang, W.; Zhang, Y.; Li, T.; Zhao, L.; Bao, Y.; Meng, X. Interpretation of the absorbed constituents and pharmacological effect of Spica Schizonepetae extract on non-small cell lung cancer. PLoS ONE 2021, 16, e0248700. [Google Scholar] [CrossRef] [PubMed]
- Costantini, S.; Guerriero, E.; Teta, R.; Capone, F.; Caso, A.; Sorice, A.; Romano, G.; Ianora, A.; Ruocco, N.; Budillon, A.; et al. Evaluating the Effects of an Organic Extract from the Mediterranean Sponge Geodia cydonium on Human Breast Cancer Cell Lines. Int. J. Mol. Sci. 2017, 18, 2112. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.A.; Nothias, L.F.; Vining, O.; Meehan, M.; Esquenazi, E.; Dorrestein, P.C. Molecular Networking As a Drug Discovery, Drug Metabolism, and Precision Medicine Strategy. Trends Pharmacol. Sci. 2017, 38, 143–154. [Google Scholar] [CrossRef]
- Vincenti, F.; Montesano, C.; Di Ottavio, F.; Gregori, A.; Compagnone, D.; Sergi, M.; Dorrestein, P. Molecular Networking: A Useful Tool for the Identification of New Psychoactive Substances in Seizures by LC-HRMS. Front. Chem. 2020, 8, 572952. [Google Scholar] [CrossRef] [PubMed]
- Azizah, M.; Pripdeevech, P.; Thongkongkaew, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. UHPLC-ESI-QTOF-MS/MS-Based Molecular Networking Guided Isolation and Dereplication of Antibacterial and Antifungal Constituents of Ventilago denticulata. Antibiotics 2020, 9, 606. [Google Scholar] [CrossRef]
- Rajabzadeh, A.; Bideskan, A.E.; Fazel, A.; Sankian, M.; Rafatpanah, H.; Haghir, H. The effect of PTZ-induced epileptic seizures on hippocampal expression of PSA-NCAM in offspring born to kindled rats. J. Biomed. Sci. 2012, 19, 56. [Google Scholar] [CrossRef]
- Quintans Júnior, L.J.; Almeida, J.R.; Lima, J.T.; Nunes, X.P.; Siqueira, J.S.; Oliveira, L.E.G.D.; Almeida, R.N.; Athayde-Filho, P.F.D.; Barbosa-Filho, J.M. Plants with anticonvulsant properties: A review. Rev. Bras. Farmacogn. 2008, 18, 798–819. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Guan, S.H.; Tang, R.N.; Tao, S.J.; Guo, D.A. Simultaneous determination of atractylenolide II and atractylenolide III by liquid chromatography-tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of Atractylodes Macrocephala Rhizoma extract. Biomed. Chromatogr. 2012, 26, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Rants’o, T.A. Establishment and Optimization of the LC-MS-Based Strategy for Screening of Passively Absorbed Açaí and Maca Constituents for CYP3A4 Inhibition. Master’s Thesis, Auburn University, Auburn, AL, USA, 2017. [Google Scholar]
- Poerschmann, J.; Gorecki, T. Molecular Level-based Analysis of Organosolv Wastewater. Curr. Chromatogr. 2017, 4, 140–155. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Bifulco, E.; Caboni, P.; Sarais, G.; Cottiglia, F.; Floris, I. Lumichrome and phenyllactic acid as chemical markers of thistle (Galactites tomentosa Moench) honey. J. Agric. Food Chem. 2011, 59, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Jia, D.; Yang, J.; Zhao, J.; Chen, C.; Liu, H.; Liang, X. Pharmacokinetics and Biodistribution of Aurantiamide and Aurantiamide Acetate in Rats after Oral Administration of Portulaca oleracea L. Extracts. J. Agric. Food Chem. 2016, 64, 3445–3455. [Google Scholar] [CrossRef]
- Chang, L.W.; Hou, M.L.; Tsai, T.H. Pharmacokinetics of dibutyl phthalate (DBP) in the rat determined by UPLC-MS/MS. Int. J. Mol. Sci. 2013, 14, 836–849. [Google Scholar] [CrossRef]
- Barciela-Alonso, M.C.; Otero-Lavandeira, N.; Bermejo-Barrera, P. Solid phase extraction using molecular imprinted polymers for phthalate determination in water and wine samples by HPLC-ESI-MS. Microchem. J. 2017, 132, 233–237. [Google Scholar] [CrossRef]
- Lu, M.; Jiang, W.; Gao, Q.; Zhang, M.; Hong, Q. Degradation of dibutyl phthalate (DBP) by a bacterial consortium and characterization of two novel esterases capable of hydrolyzing PAEs sequentially. Ecotoxicol. Environ. Saf. 2020, 195, 110517. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Zhao, L.; Li, F.; Xiong, Z. Kidney tissue targeted metabolic profiling of glucocorticoid-induced osteoporosis and the proposed therapeutic effects of Rhizoma Drynariae studied using UHPLC/MS/MS. Biomed. Chromatogr. 2014, 28, 878–884. [Google Scholar] [CrossRef]
- Zengin, G.; Aumeeruddy, M.Z.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Yıldıztugay, A.; Yıldıztugay, E.; Mahomoodally, M.F. A comprehensive appraisal on Crocus chrysanthus (Herb.) Herb. flower extracts with HPLC-MS/MS profiles, antioxidant and enzyme inhibitory properties. J. Pharm. Biomed. Anal. 2019, 164, 581–589. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Nielsen, K.F.; Rasmussen, P.H.; Thrane, U. Development of a LC-MS/MS method for the analysis of enniatins and beauvericin in whole fresh and ensiled maize. J. Agric. Food Chem. 2008, 56, 10439–10443. [Google Scholar] [CrossRef]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ferrer, E.; Mañes, J. Identification and Quantification of Enniatins and Beauvericin in Animal Feeds and Their Ingredients by LC-QTRAP/MS/MS. Metabolites 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Serrano, Ana Belén; Capriotti, Anna Laura; Cavaliere, Chiara; Piovesana, Susy; Samperi, Roberto; Ventura, Salvatore; Laganà, Aldo, Development of a Rapid LC-MS/MS Method for the Determination of Emerging Fusarium mycotoxins Enniatins and Beauvericin in Human Biological Fluids. Toxins 2015, 7, 3554–3571.
- Boecker, S.; Grätz, S.; Kerwat, D.; Adam, L.; Schirmer, D.; Richter, L.; Schütze, T.; Petras, D.; Süssmuth, R.D.; Meyer, V. Aspergillus niger is a superior expression host for the production of bioactive fungal cyclodepsipeptides. Fungal Biol. Biotechnol. 2018, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Munir, I.; Zhang, M.; Liu, Y.; Moe, T.S.; Xue, J.; Zhang, X. Characterization of Endophytic Fungi, Acremonium sp., from Lilium davidii and Analysis of Its Antifungal and Plant Growth-Promoting Effects. BioMed. Res. Int. 2021, 2021, 9930210. [Google Scholar] [CrossRef]
- Nichols, K.K.; Ham, B.M.; Nichols, J.J.; Ziegler, C.; Green-Church, K.B. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Investig. Ophthalmol. Vis. Sci. 2007, 48, 34–39. [Google Scholar] [CrossRef]
- Dabur, R.; Mittal, A. Detection and qualitative analysis of fatty acid amides in the urine of alcoholics using HPLC-QTOF-MS. Alcohol 2016, 52, 71–78. [Google Scholar] [CrossRef]
- Prentice, R.N.; Younus, M.; Krittaphol-Bailey, W.; Rizwan, S.B. A sensitive LC-MS/MS method for the study of exogenously administered (13) C-oleoylethanolamide in rat plasma and brain tissue. J. Sep. Sci. 2021, 44, 2693–2704. [Google Scholar] [CrossRef]
- Matsumoto, T.; Matsuno, M.; Ikui, N.; Mizushina, Y.; Omiya, Y.; Ishibashi, R.; Ueda, T.; Mizukami, H. Identification of pheophorbide a as an inhibitor of receptor for advanced glycation end products in Mallotus japonicus. J. Nat. Med. 2021, 75, 675–681. [Google Scholar] [CrossRef]
- Peng, A.; Lin, L.; Zhao, M.; Sun, B. Identifying mechanisms underlying the amelioration effect of Chrysanthemum morifolium Ramat. ‘Boju’ extract on hyperuricemia using biochemical characterization and UPLC-ESI-QTOF/MS-based metabolomics. Food Funct. 2019, 10, 8042–8055. [Google Scholar] [CrossRef]
- Huang, J. Chemical constituents from Bombyx Batryticatus. Chin. Tradit. Herb. Drugs 2015, 24, 2377–2380. (In Chinese) [Google Scholar]
- Jiang, X.; Chen, Y.; Shi, L. Optimization of Flavonoids Extraction from Bombyx batryticatus Using Response Surface Methodology and Evaluation of Their Antioxidant and Anticancer Activities In vitro. Food Sci. Biotechnol. 2013, 22, 1707–1715. [Google Scholar] [CrossRef]
- Kikuchi, Haruhisa; Takahashi, Nahoko; Oshima, Yoshiteru, Novel aromatics bearing 4-O-methylglucose unit isolated from the oriental crude drug Bombyx Batryticatus. Tetrahedron Lett. 2004, 45, 367–370. [CrossRef]
- Kong, Y.; Xu, C.; He, Z.L.; Zhou, Q.M.; Wang, J.B.; Li, Z.Y.; Ming, X. A novel peptide inhibitor of platelet aggregation from stiff silkworm, Bombyx batryticatus. Peptides 2014, 53, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]



| Treatments | Seizure Latency of Score 2 (s) | Seizure Latency of Score 3 (s) | Seizure Latency of Score 4 (s) | Protection | Convulsion |
|---|---|---|---|---|---|
| control | 38.33 ± 9.65 | 57.09 ± 18.33 | 60.09 ± 31.19 | 0/13 (0%) | 13/13 (100%) |
| Carbamazepine (80 mg/kg) | 63.00 ± 18.53 | 94.17 ± 44.65 | 226.13 ± 34.87 *** | 12/13 (92.31%) *** | 9/13 (69.23%) ** |
| B-C | 60.71 ± 17.38 | 63.29 ± 17.41 | 159.88 ± 19.67 * | 6/13 (46.15%) * | 5/13 (61.54%) |
| B-E | 39.70 ± 11.60 | 53.00 ± 18.49 | 78.50 ± 21.87 | 3/13 (23.08%) | 12/13 (92.31%) |
| B-W | 54.90 ± 20.33 | 73.21 ± 21.30 | 140.00 ± 39.34 | 4/13 (30.77%) | 12/13 (92.31%) |
| B-P | 41.00 ± 17.83 | 57.70 ± 21.02 | 95.20 ± 30.93 | 3/13 (23.08%) | 12/13 (92.31%) |
| Peak No. | tR (Min) | m/z | Fragment Ions | Adduct | Error (ppm) | Formula | Compound | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 15.1 | 197.1173 | 133.1014, 179.1058, 161.0946 | M + H+ | 0.40 | C11H16O3 | Loliolide | [21,22] |
| 2 | 15.2 | 331.1538 | 137.0594, 107.1292, 133.0642, 122.0346 | M + H+ | 0.15 | C19H22O5 | 3-(4-Hydroxy-3-Methoxyphenyl)Propyl 3-(4-Hydroxyphenyl)Propanoate | [23] |
| 3 | 15.9 | 603.2046 | 185.0466, 441.1469, 425.1227 | M + Na+ | 0.03 | C28H36O13 | Episyringaresinol 4′-O-beta-d-glucopyranose | ___ |
| 4 | 17.8 | 243.0874 | 172.0859, 198.06941, 103.0547, 170.0714 | M + H+ | −1.04 | C12H10N4O2 | LUMICHROME | [24] |
| 5 | 23.1 | 445.2135 | 194.1168, 224.1070, 105.0330, 252.1019 | M + H+ | 2.96 | C27H28N2O4 | Aurantiamide acetate | [25] |
| 6 | 26.5 | 279.1593 | 149.0238, 150.0247, 279.1593 | M + H+ | 0.77 | C16H22O4 | Dibutyl phthalate | [26,27,28] |
| 7 | 28.4 | 318.3004 | 282.2779, 270.2780, 264.2667 | M + H+ | 0.14 | C18H39NO3 | Phytosphingosine | [29,30] |
| 8 | 28.5 | 360.3240 | 105.0703, 122.0956, 360.3263, 106.0718 | M + H+ | −0.58 | C24H41NO | N-(2-Phenylethyl) hexadecanamide | ___ |
| 9 | 31.1 | 806.4002 | 134.1311, 244.1331, 784.4168, 262.1437 | M + Na+ | 1.86 | C45H57N3O9 | Beauvericin | [31,32,33] |
| 10 | 32.1 | 815.4594 | 244.1330, 134.0967, 537.2946, 262.1431 | M + NH4+ | −0.13 | C46H59N3O9 | Beauvericin A | ___ |
| 11 | 33.9 | 926.6439 | 210.1490, 228.1595, 445.3109, 909.6165 | M + NH4+ | 1.03 | C48H84N4O12 | Bassianolide | [34] |
| 12 | 34.6 | 563.5511 | 282.2783, 265.2540, 247.2422, 283.2822 | 2M + H+ | 0.17 | C20H39NO2 | 9-Octadecenamide, (Z) | [35,36] |
| 13 | 33.8 | 256.2636 | 130.1232, 144.1374, 102.0909, 158.1541 | M + H+ | 0.42 | C16H33NO | Palmitamide | [37] |
| 14 | 34.4 | 326.3060 | 308.2951, 309.2820, 121.1021, 135.1178 | M + H+ | 1.97 | C20H39NO | Oleoyl ethanolamide | [38] |
| 15 | 34.8 | 593.2761 | 533.2545, 461.2311, 505.2225, 594.2779 | M + H+ | 0.43 | C35H36N4O5 | Pheophorbide A | [39] |
| 16 | 36.4 | 284.2954 | 284.2951, 285.2980, 286.3013 | M + H+ | 2.14 | C18H37NO | Octadecanamide | [40] |
| Score | Behavior |
|---|---|
| 0 | No response |
| 1 | ear and facial twitching |
| 2 | clonic jerk with hind limb extension |
| 3 | turning over onto side position, tonic-clonic seizures |
| 4 | clonic seizure with loss of righting reflex, generalized tonic–clonic seizures. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, R.; Zheng, C.; Zhang, L.; Meng, H.; Zhang, Y.; Ma, L.; Chen, B.; Wang, J. Anticonvulsant Activity of Bombyx batryticatus and Analysis of Bioactive Extracts Based on UHPLC-Q-TOF MS/MS and Molecular Networking. Molecules 2022, 27, 8315. https://doi.org/10.3390/molecules27238315
Wang Q, Wang R, Zheng C, Zhang L, Meng H, Zhang Y, Ma L, Chen B, Wang J. Anticonvulsant Activity of Bombyx batryticatus and Analysis of Bioactive Extracts Based on UHPLC-Q-TOF MS/MS and Molecular Networking. Molecules. 2022; 27(23):8315. https://doi.org/10.3390/molecules27238315
Chicago/Turabian StyleWang, Qinglei, Rong Wang, Cheng Zheng, Linlin Zhang, Hong Meng, Yi Zhang, Linke Ma, Bilian Chen, and Juanjuan Wang. 2022. "Anticonvulsant Activity of Bombyx batryticatus and Analysis of Bioactive Extracts Based on UHPLC-Q-TOF MS/MS and Molecular Networking" Molecules 27, no. 23: 8315. https://doi.org/10.3390/molecules27238315
APA StyleWang, Q., Wang, R., Zheng, C., Zhang, L., Meng, H., Zhang, Y., Ma, L., Chen, B., & Wang, J. (2022). Anticonvulsant Activity of Bombyx batryticatus and Analysis of Bioactive Extracts Based on UHPLC-Q-TOF MS/MS and Molecular Networking. Molecules, 27(23), 8315. https://doi.org/10.3390/molecules27238315






