Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs)
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of CCl4-Induced Liver Toxicity on Liver Enzymes
2.2. The Effect of CCl4 on Total Protein and Bilirubin
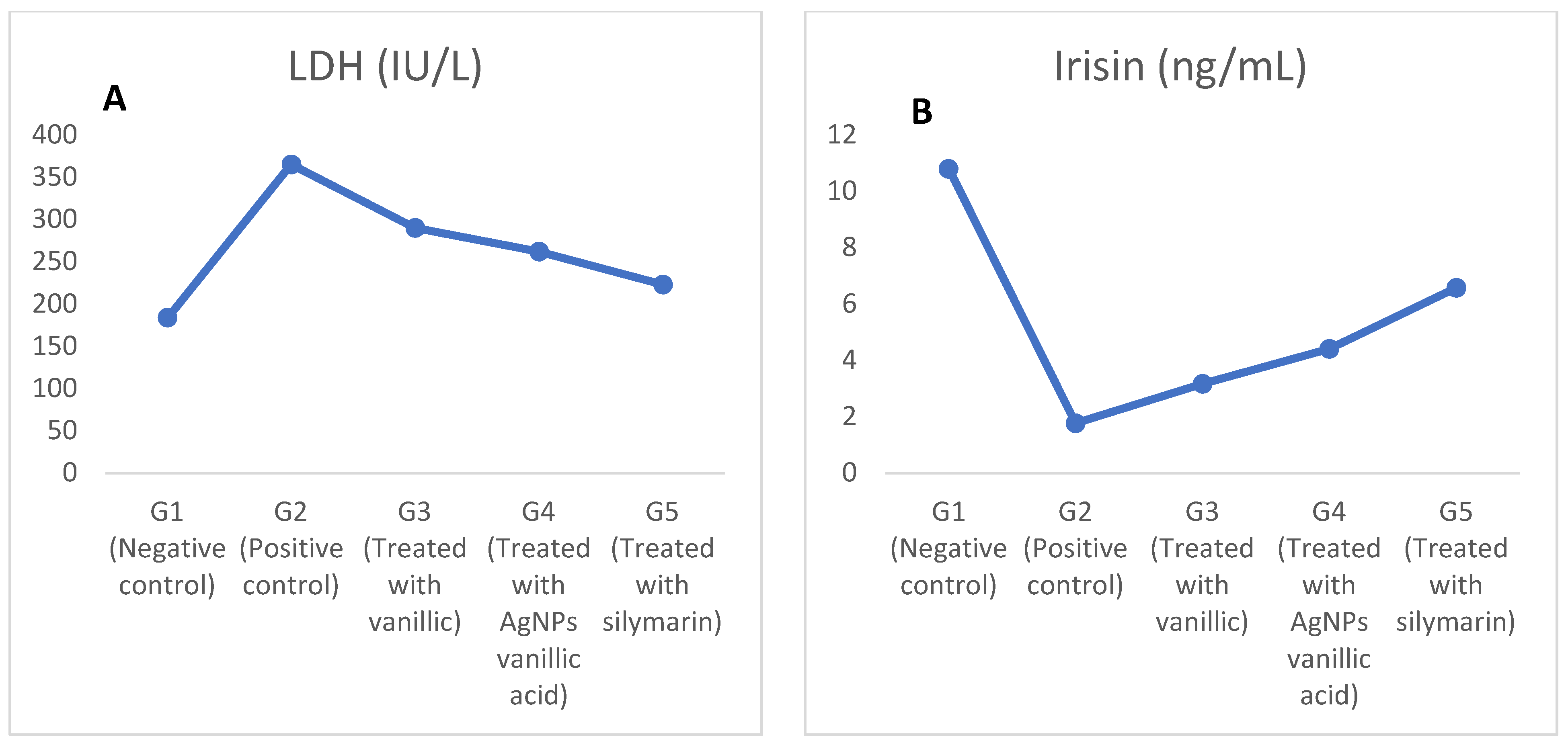
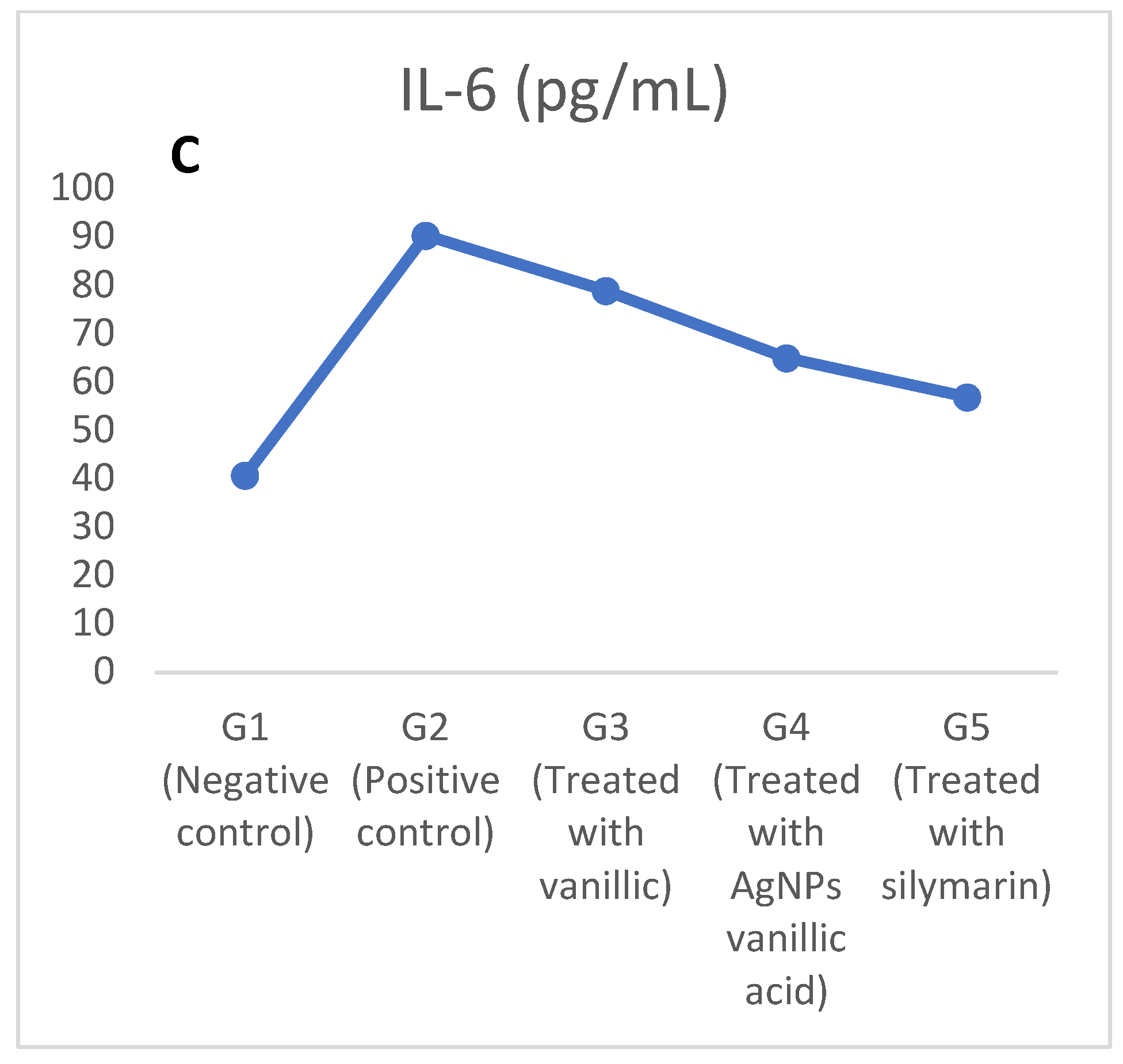
2.3. The Effect of CCl4 on Lactate Dehydrogenase (LDH), Interleukin-6 (IL-6), and Irisin
2.4. The Effect of CCl4-Induced Hepatotoxicity on Antioxidant Enzymes and Total Antioxidant Capacity
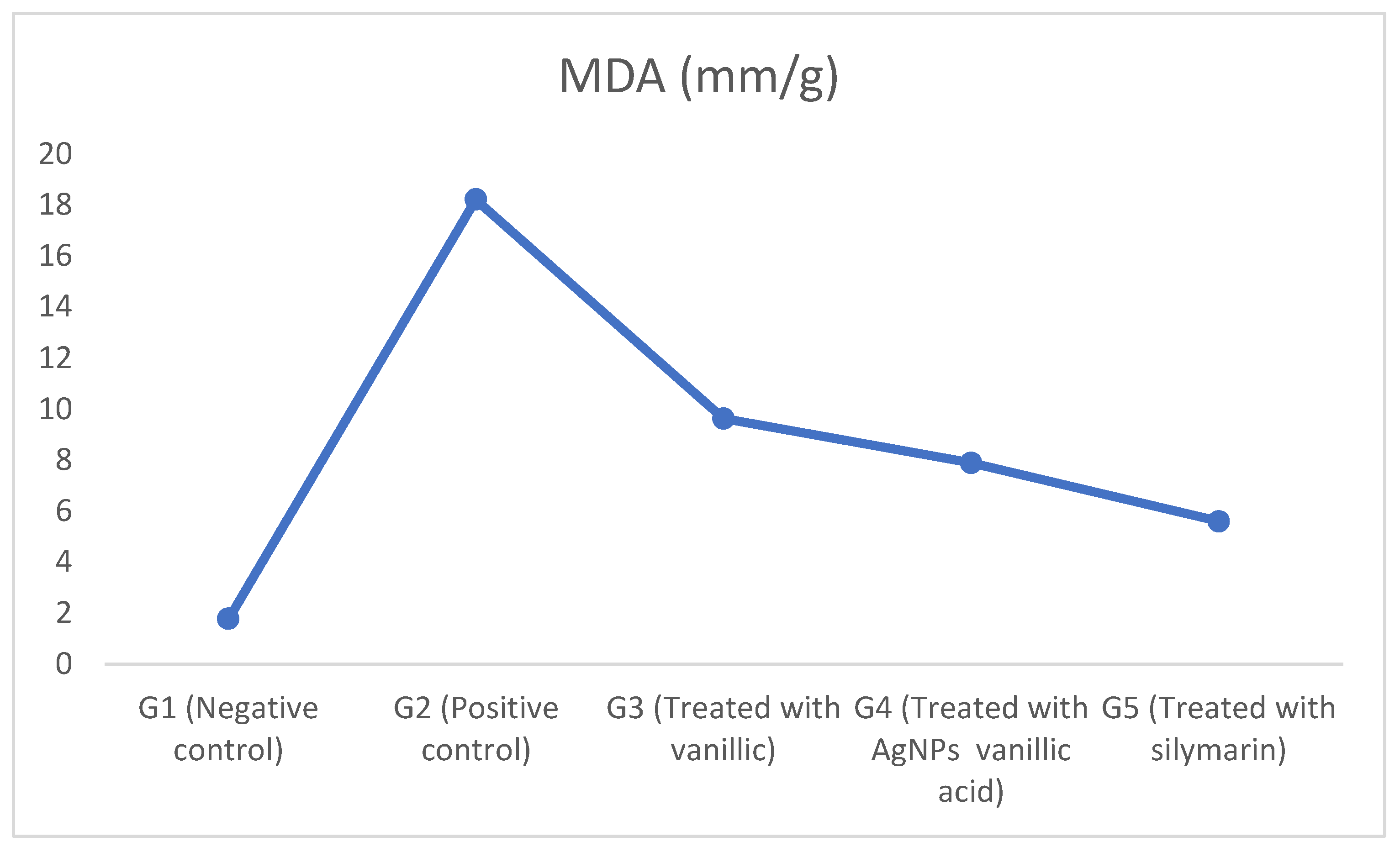
2.5. The Effect of CCl4-Induced Hepatotoxicity on Lipid Peroxidation as Revealed by Malondialdehyde (MDA)
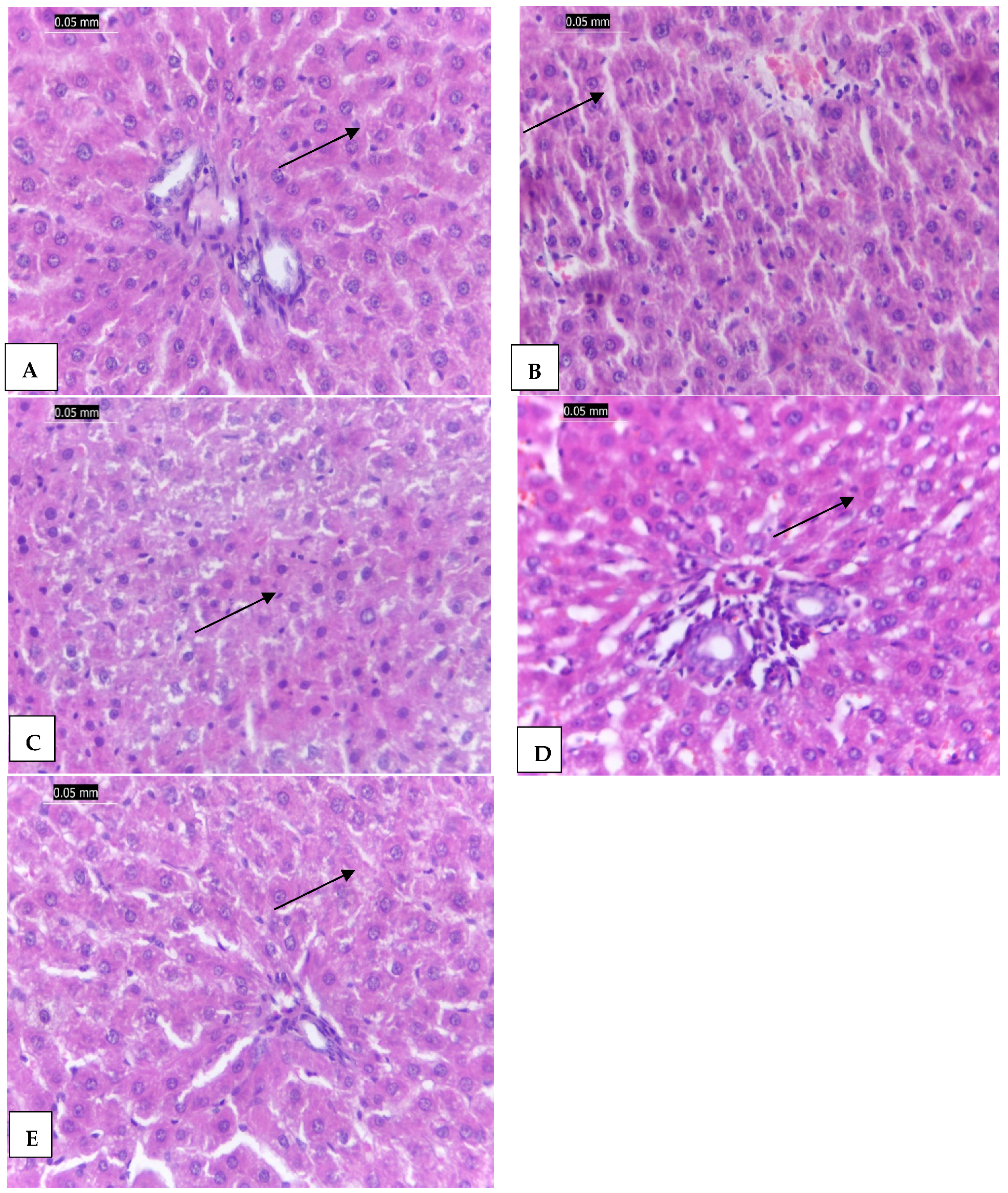
2.6. The Effect of CCl4-Induced Hepatotoxicity on Liver Histopathology
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Silver Nanoparticles from Vanillic Acid
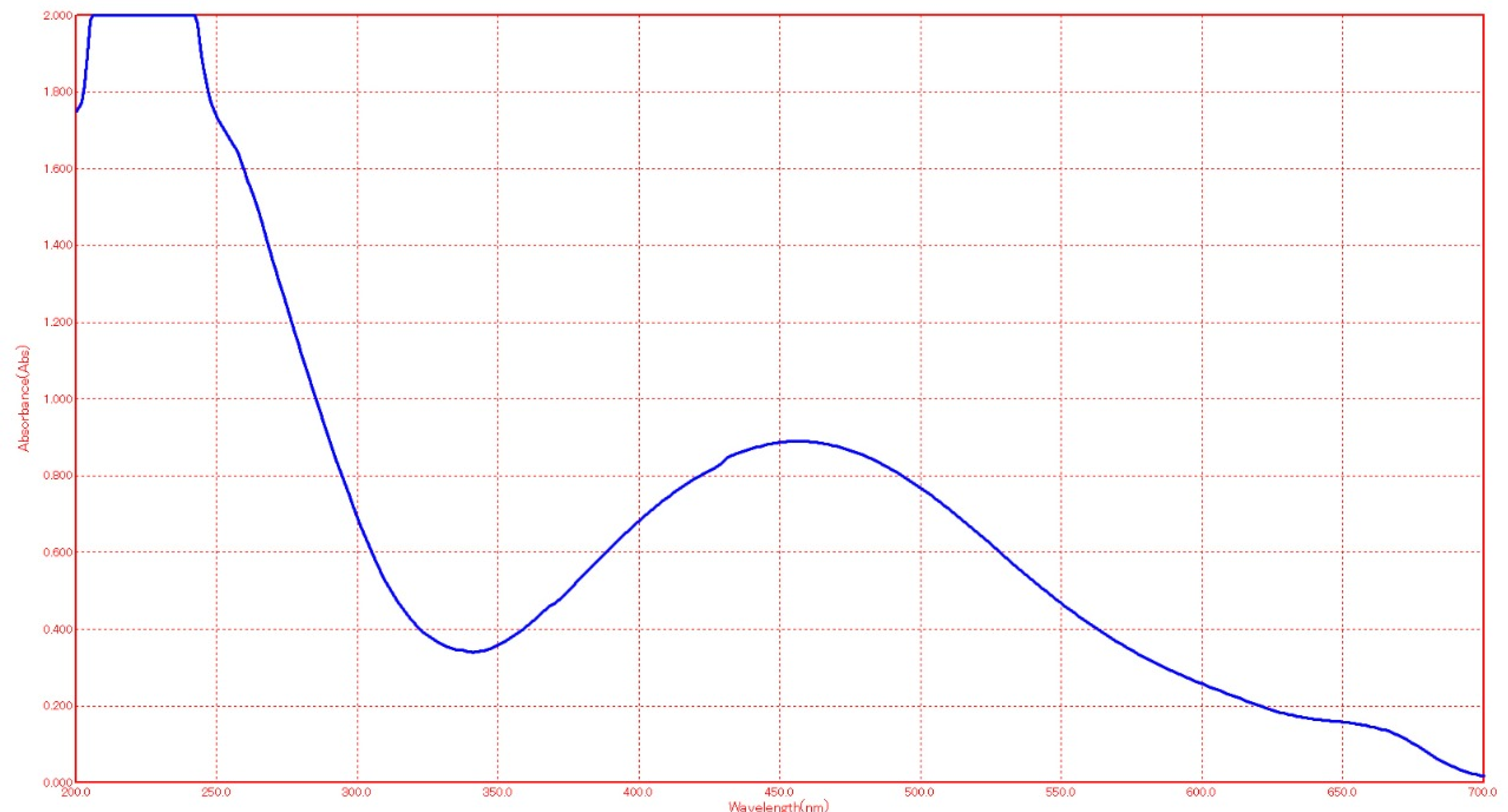
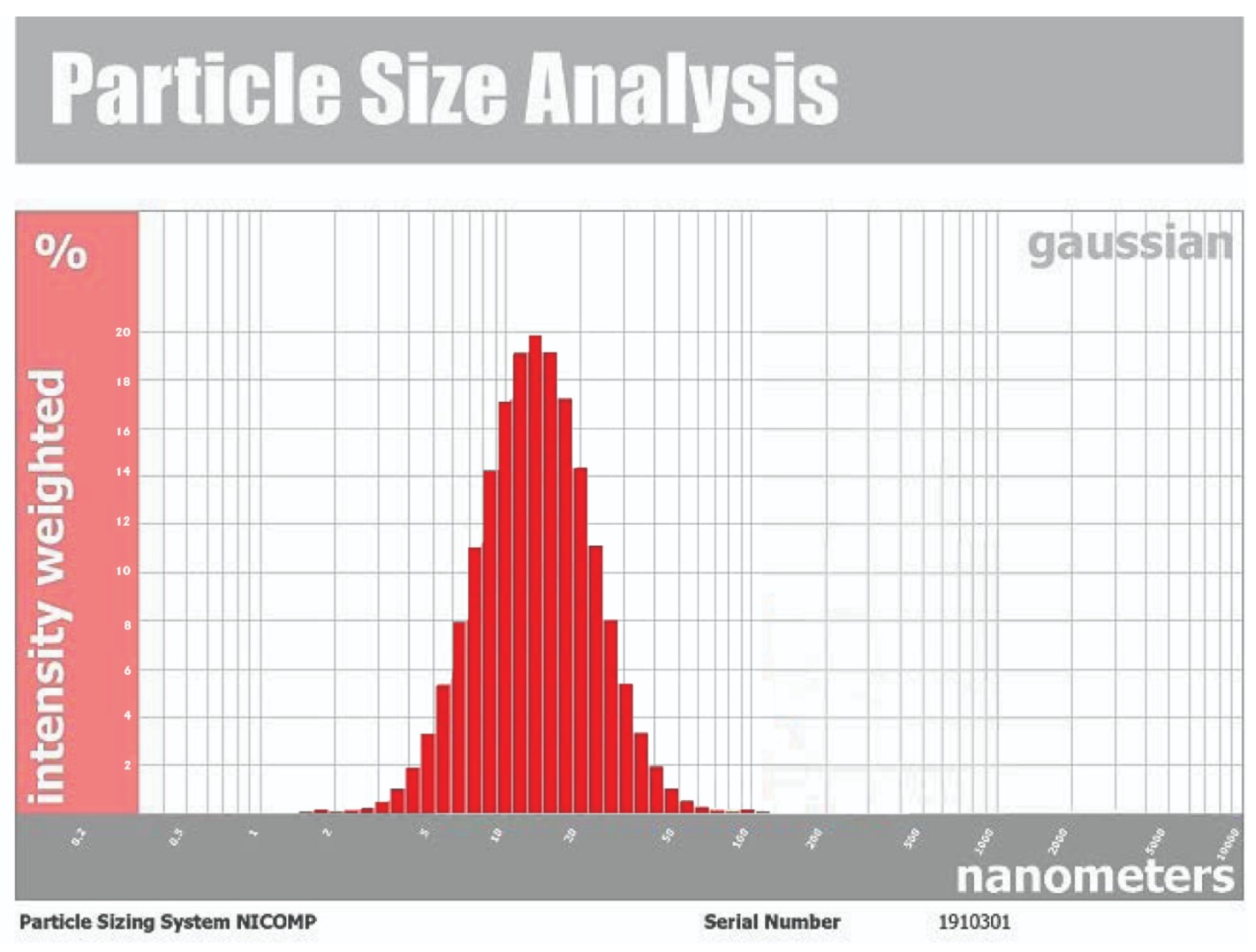
Characterization of Silver Nanoparticles
- Ultraviolet visible spectroscopy
- 2.
- Transmission electron microscope (TEM)
- 3.
- Dynamic light scattering (DLS)
3.3. Test Animals and the Experiment Design
3.4. Blood Collection
3.5. Liver Tissue Homogenate Preparation
3.6. Biochemical Tests
3.7. Histological Study
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Scott, E.; James, H.; Kenneth., R. Current Diagnosis & Treatment in Gastroenterology; Lang Medical Books/McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- El Rabey, H.; Rezk, S.M.; Sakran, M.I.; Mohammed, G.M.; Bahattab, O.; Balgoon, M.J.; Elbakry, M.A.; Bakry, N. Green coffee methanolic extract and silymarin protect against CCl4-induced hepatotoxicity in albino male rats. BMC Complement. Med. Ther. 2021, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, J.M.; Liu, S.; Liu, A.; Gogate, A.; Nair, S.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X. Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs. Drug Metab. Dispos. 2022, 50, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, R.R. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J. Ethnopharmacol. 2003, 89, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Elbakry, M.A.; El Rabey, H.A.; Elremaly, W.; Sakran, M.I.; Almutairi, F.M. The methanolic extract of Moringa oleifera attenuates CCl4 induced hepatonephro toxicity in the male rat. Biomed. Res. 2019, 30, 23–31. [Google Scholar] [CrossRef]
- Al-Seeni, M.N.; El Rabey, H.A.; Zamzami, M.A.; Alnefayee, A.M. The hepatoprotective activity of olive oil and Nigella sativa oil against CCl4 induced hepatotoxicity in male rats. BMC Complement. Altern. Med. 2016, 16, 438. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K. Imbalance in antioxidant defense and human diseases: Multiple approaches of natural antioxidants therapy. Curr. Sci. 2001, 81, 1179–1187. [Google Scholar]
- Kulichová, K.; Sokol, J.; Nemeček, P.; Maliarová, M.; Maliar, T.; Havrlentová, M.; Kraic, J. Phenolic compounds and biological activities of rye (Secale cereale L.) grains. Open Chem. 2019, 17, 988–999. [Google Scholar] [CrossRef]
- Ingole, A.S.; Megha, K.P.; Aishwarya, P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.K.V.; Nagrik, S.U.; et al. A Review of the Pharmacological Characteristics of Vanillic Acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Ito, H. Antioxidative Properties of Vanillic Acid Esters in Multiple Antioxidant Assays. Biosci. Biotechnol. Biochem. 2012, 76, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prahalathan, P.; Raja, B. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in l-NAME-induced hypertensive rats: A dose-dependence study. Redox Rep. 2013, 16, 208–215. [Google Scholar] [CrossRef]
- Tlili, N.; Feriani, A.; Saadoui, E.; Nasri, N.; Khaldi, A. Capparis spinosa leaves extract: Source of bioantioxidants with nephroprotective and hepatoprotective effects. Biomed. Pharmacother. 2017, 87, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Khatun, A.; Nesa, M.; Hossain, H.; Jahan, I.A. Bioactive polyphenols from the methanol extract of Cnicus arvensis (L.) Roth demonstrated antinociceptive and central nervous system depressant activities in mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 794729. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tang, X.; Zhang, W.; Wei, J.; Tang, S.; Wu, H.; Yang, H. Exploration of the mechanism of traditional Chinese medicine by AI approach using unsupervised machine learning for cellular functional similarity of compounds in heterogeneous networks, XiaoErFuPi granules as an example. Pharmacol. Res. 2020, 160, 105077. [Google Scholar] [CrossRef]
- Chuang, H.W.; Wei, I.H.; Lin, F.Y.; Li, C.T.; Chen, K.T.; Tsai, M.H.; Huang, C.C. Roles of Akt and ERK in mTOR-Dependent Antidepressant Effects of Vanillic Acid. ACS Omega 2020, 5, 3709–3716. [Google Scholar] [CrossRef]
- Saravanakumar, M.; Raja, B.; Manivannan, J.; Silambarasan, T.; Prahalathan, P.; Kumar, S.; Mishra, S.K. Oral administration of veratric acid, a constituent of vegetables and fruits, prevents cardiovascular remodelling in hypertensive rats: A functional evaluation. Br. J. Nutr. 2015, 114, 1385–1394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef]
- Velli, S.K.; Sundaram, J.; Murugan, M.; Balaraman, G.; Thiruvengadam, D. Protective effect of vanillic acid against benzo (a) pyrene induced lung cancer in Swiss albino mice. J. Biochem. Mol. Toxicol. 2019, 33, 22382. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lin, J.T.; Liu, S.C.; Hu, C.C.; Shyu, Y.S.; Yang, D.J. Protective role of litchi (Litchi chinensis Sonn.) flower extract against cadmium-and lead-induced cytotoxicity and transforming growth factor β1-stimulated expression of smooth muscle α-actin estimated with rat liver cell lines. J. Funct. Foods. 2013, 5, 698–705. [Google Scholar] [CrossRef]
- Park, J.; Cho, S.Y.; Kang, J.; Park, W.Y.; Lee, S.; Jung, Y.; Kang, M.W.; Kwak, H.J.; Um, J.Y. Vanillic Acid Improves Comorbidity of Cancer and Obesity through STAT3 Regulation in High-Fat-Diet-Induced Obese and B16BL6 Melanoma-Injected Mice. Biomolecules 2020, 10, 1098. [Google Scholar] [CrossRef]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern. Med. 2016, 16, 330. [Google Scholar] [CrossRef]
- Molaahmadibahraseman, N.; Farhoosh, R.; Jhony, S.; Sharif, A. Antioxidant Activity of Syringic and Vanillic Acids in Kilka Fish Oil And Its Oil-In-Water Emulsion. Iran Food Sci. Technol. Res. J. 2017, 12, 588–595. [Google Scholar]
- Taner, G.; Vardar, D.Ö.; Aydin, S.; Aytaç, Z.; Başaran, A.; Başaran, N. Use in vitro assays to assess the potential cytotoxic, genotoxic and antigenotoxic effects of vanillic and cinnamic acid. Drug Chem. Toxicol. 2016, 40, 183–190. [Google Scholar] [CrossRef]
- Yao, X.; Jiao, S.; Qin, M.; Hu, W.; Yi, B.; Liu, D. Vanillic Acid Alleviates Acute Myocardial Hypoxia/Reoxygenation Injury by Inhibiting Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 8348035. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Ikram, M.; Park, T.J.; Ahmad, R.; Saeed, K.; Alam, S.I.; Rehman, I.U.; Khan, A.; Khan, I.; Jo, M.G.; et al. Vanillic Acid, a Bioactive Phenolic Compound, Counteracts LPS-Induced Neurotoxicity by Regulating c-Jun N-Terminal Kinase in Mouse Brain. Int. J. Mol. Sci. 2021, 22, 361. [Google Scholar] [CrossRef] [PubMed]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Ioana, G.; Cristea, D. Silver Nanoparticles for Delivery Purposes. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 347–371. [Google Scholar]
- Almutairi, F.A.; El Rabey, H.A.; Alalawy, A.I.; Salama, A.M.; Tayel, A.A.; Mohammed, G.M.; Keshk, A.A.; Aljuhani, M.M.; Abbas, N.H.; Zayed, M.M. Application of Chitosan/Alginate Nanocomposite Incorporated with Phycosynthesized Iron Nanoparticles for Efficient Remediation of Chromium. Polymers 2021, 13, 2481. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.M.; Saafan, A.E.; Salem, E.H.; El Rabey, H.A.; Alsieni, M.A.; Alatawi, F.A.; Alalawy, A.I.; Mohammed, A.B. Inhibition of the Vancomycin Resistance in Staphylococcus aureus in Egypt Using Silver Nanoparticles. BioMed Res. Int. 2022, 2022, 7380147. [Google Scholar] [CrossRef]
- Zidane, N.S.; Tayel, A.A. Augmented control of drug-resistant Candida spp. Via fluconazole loading into fungal chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 141, 511–516. [Google Scholar]
- Alalawy, A.I.; El Rabey, H.A.; Almutairi, F.M.; Tayel, A.A.; Al-Duais, M.A.; Zidan, N.S.; Sakran, M.I. Effectual Anticancer Potentiality of Loaded Bee Venom onto Fungal Chitosan Nanoparticles. Int. J. Polym. Sci. 2020, 2020, 2785304. [Google Scholar] [CrossRef]
- Burdușel, A.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M.; Moghadam, M.T.; Mirnejad, R.; Mirkalantari, S.; Mirzaei, M. The Efficacy of AgNO3 Nanoparticles Alone and Conjugated with Imipenem for Combating Extensively Drug-Resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2020, 15, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.; Moradshahi, A. Synthesis and coating of nanosilver by vanillic acid and its effects on Dunaliella salina Teod. Mol. Cell Biol. Res. Commun. 2013, 2, 47–55. [Google Scholar]
- Vivek, R.; Ramar, T.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic Light Scattering as a Powerful Tool for Gold Nanoparticle Bioconjugation and Biomolecular Binding Studies. Anal. Chem. 2009, 81, 9425–9432. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, Y.; Mazani, M.; Rezagholizadeh, L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol. Rep. 2017, 4, 455–462. [Google Scholar] [CrossRef]
- Freitag, A.F.; Cardia, G.F.E.; da Rocha, B.A.; Aguiar, R.P.; Silva-Comar, F.M.D.S.; Spironello, R.A.; Grespan, R.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Nakamura Cuman, R.K. Hepatoprotective Effect of Silymarin (Silybum marianum) on Hepatotoxicity Induced by Acetaminophen in Spontaneously Hypertensive Rats. Evid.-Based Complement. Alternat. Med. 2015, 2015, 538317. [Google Scholar] [CrossRef]
- Baradaran, A.; Samadi, F.; Ramezanpour, S.S.; Yousefdoust, S. Hepatoprotective effects of silymarin on CCl4-induced hepatic damage in broiler chickens model. Toxicol. Rep. 2019, 6, 788–794. [Google Scholar] [CrossRef]
- Abdelaal, S.E.; Mousa, H.S.; Ahmed, S.M. Effect of Silymarin versus Silymarin and Green Coffee Extract on Thioacetamide Induced Liver Injury in Adult Male Albino Rats (Histological and Immunohistochemical Study). Egypt. J. Histol. 2019, 42, 133–146. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Karimi, G.; Vahabzadeh, M.; Lari, P.; Rashedinia, M.; Moshiri, M. Silymarin, a Promising Pharmacological Agent for Treatment of Diseases. Iran. J. Basic Med. Sci. 2011, 14, 308–317. [Google Scholar] [PubMed]
- Reichling, J.J.; Kaplan, M.M. Clinical use of serum enzymes in liver disease. Dig. Dis. Sci. 1988, 33, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Mossner, E.; Boll, M.; Pfleiderer, G. Purification of human and bovine alkaline phosphatases by affinity chromatography. Hoppe-Seyler’s Z. Physiol. Chem. 1980, 361, 543–549. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. The haematoxylin and eosin. In Theory and Practice of Histological Techniques; Churchill Livingstone: London, UK, 1996; Volume 6. [Google Scholar]
- Armitage, W.G.; Berry, G.Y. Statistical Methods, 7th ed.; Iowa State University: Ames, IA, USA, 1987; pp. 39–63. [Google Scholar]


| Parameters mg/dL | Statistics | G1 (Negative Control) | G2 (Positive Control) | G3 (Treated with Vanillic) | G4 (Treated with AgNP–Vanillic Acid) | G5 (Treated with Silymarin) |
|---|---|---|---|---|---|---|
| Total Protein | Mean ± SE | 6.53 ± 0.11 a | 3.34 ± 0.05 e | 4.84 ± 0.04 d | 5.58 ± 0.06 c | 6.07 ± 0.03 d |
| LSD0.05 = 0.259 | ||||||
| t-test | - | 1.16 *** | −1.80 *** | −1.86 *** | −0.48 *** | |
| Albumin | Mean ± SE | 4.23 ± 0.05 a | 1.43 ± 0.05 e | 2.30 ± 0.03 d | 2.80 ± 0.03 c | 3.56 ± 0.04 d |
| LSD0.05 = 0.143 | ||||||
| t-test | - | 0.56 *** | −0.55 *** | −0.82 *** | −0.21 *** | |
| Total Bilirubin | Mean ± SE | 0.43 ± 0.01 a | 1.82 ± 0.02 e | 1.14 ± 0.03 b | 0.86 ± 0.02 c | 0.66 ± 0.01 d |
| LSD0.05 = 0.065 | ||||||
| t-test | - | −1.51 *** | 1.97 *** | 2.60 *** | 1.04 *** | |
| Direct Bilirubin | Mean ± SE | 0.3 ± 0.00 a | 1.22 ± 0.80 e | 0.74 ± 0.50 b | 0.64 ± 0.40 c | 0.44 ± 0.30 d |
| LSD0.05 = 0.028 | ||||||
| t-test | - | 0.76 *** | −1.06 *** | −1.58 *** | −1.42 *** |
| Parameters Mg Liver Tissue/dL | Statistics | G1 (Negative Control) | G2 (Positive Control) | G3 (Treated with Vanillic) | G4 (Treated with AgNPs–Vanillic Acid) | G5 (Treated with Silymarin) |
|---|---|---|---|---|---|---|
| GST U/g | Mean ± SE | 317.00 ± 5.01 a | 113.00 ± 4.06 e | 185.33 ± 2.76 d | 237.00 ± 4.66 c | 281.33 ± 2.13 b |
| LSD0.05 = 12.026 | ||||||
| t-test | - | 111.73 *** | −11.48 *** | −21.87 *** | −29.49 *** | |
| GSH U/g | Mean ± SE | 310.33 ± 2.56 a | 104.00 ± 3.84 e | 154.00 ± 2.55 d | 186.66 ± 2.37 c | 253.33 ± 4.26 b |
| LSD0.05 = 10.583 | ||||||
| t-test | - | 35.43 *** | −09.85 *** | −25.25 *** | −20.72 *** | |
| TAC U/g | Mean ± SE | 5.40 ± 0.10 a | 0.87 ± 0.02 e | 1.80 ± 0.04 d | 2.53 ± 0.04 c | 3.29 ± 0.05 b |
| LSD0.05 = 0.189 | ||||||
| t-test | - | 41.60 *** | −27.21 *** | −25.69 *** | −31.28 *** | |
| SOD U/g | Mean ± SE | 342.33 ± 2.07 a | 110.33 ± 1.52 e | 238.00 ± 2.28 d | 257.66 ± 2.78 c | 293.66 ± 3.31 b |
| LSD0.05 = 5.688 | ||||||
| t-test | - | 211.78 *** | −063.48 *** | −47.44 *** | −091.16 *** | |
| CAT U/g | Mean ± SE | 7.46 ± 0.09 a | 2.01 ± 0.02 e | 2.53 ± 0.04 d | 3.70 ± 0.07 c | 4.61 ± 0.09 b |
| LSD0.05 = 0.226 | ||||||
| t-test | - | 60.25 *** | −7.85 *** | −28.41 *** | −27.67 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamri, E.S.; El Rabey, H.A.; Alzahrani, O.R.; Almutairi, F.M.; Attia, E.S.; Bayomy, H.M.; Albalwi, R.A.; Rezk, S.M. Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs). Molecules 2022, 27, 8308. https://doi.org/10.3390/molecules27238308
Alamri ES, El Rabey HA, Alzahrani OR, Almutairi FM, Attia ES, Bayomy HM, Albalwi RA, Rezk SM. Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs). Molecules. 2022; 27(23):8308. https://doi.org/10.3390/molecules27238308
Chicago/Turabian StyleAlamri, Eman S., Haddad A. El Rabey, Othman R. Alzahrani, Fahad M. Almutairi, Eman S. Attia, Hala M. Bayomy, Renad A. Albalwi, and Samar M. Rezk. 2022. "Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs)" Molecules 27, no. 23: 8308. https://doi.org/10.3390/molecules27238308
APA StyleAlamri, E. S., El Rabey, H. A., Alzahrani, O. R., Almutairi, F. M., Attia, E. S., Bayomy, H. M., Albalwi, R. A., & Rezk, S. M. (2022). Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs). Molecules, 27(23), 8308. https://doi.org/10.3390/molecules27238308





