Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract Promotes Human Dermal Papilla Cell Cytoprotective Effect and Hair Regrowth Rate in C57BL/6 Mice
Abstract
1. Introduction
2. Results
2.1. Cytoprotective Effect of HY7015 and/or LT Extract in HFDPC
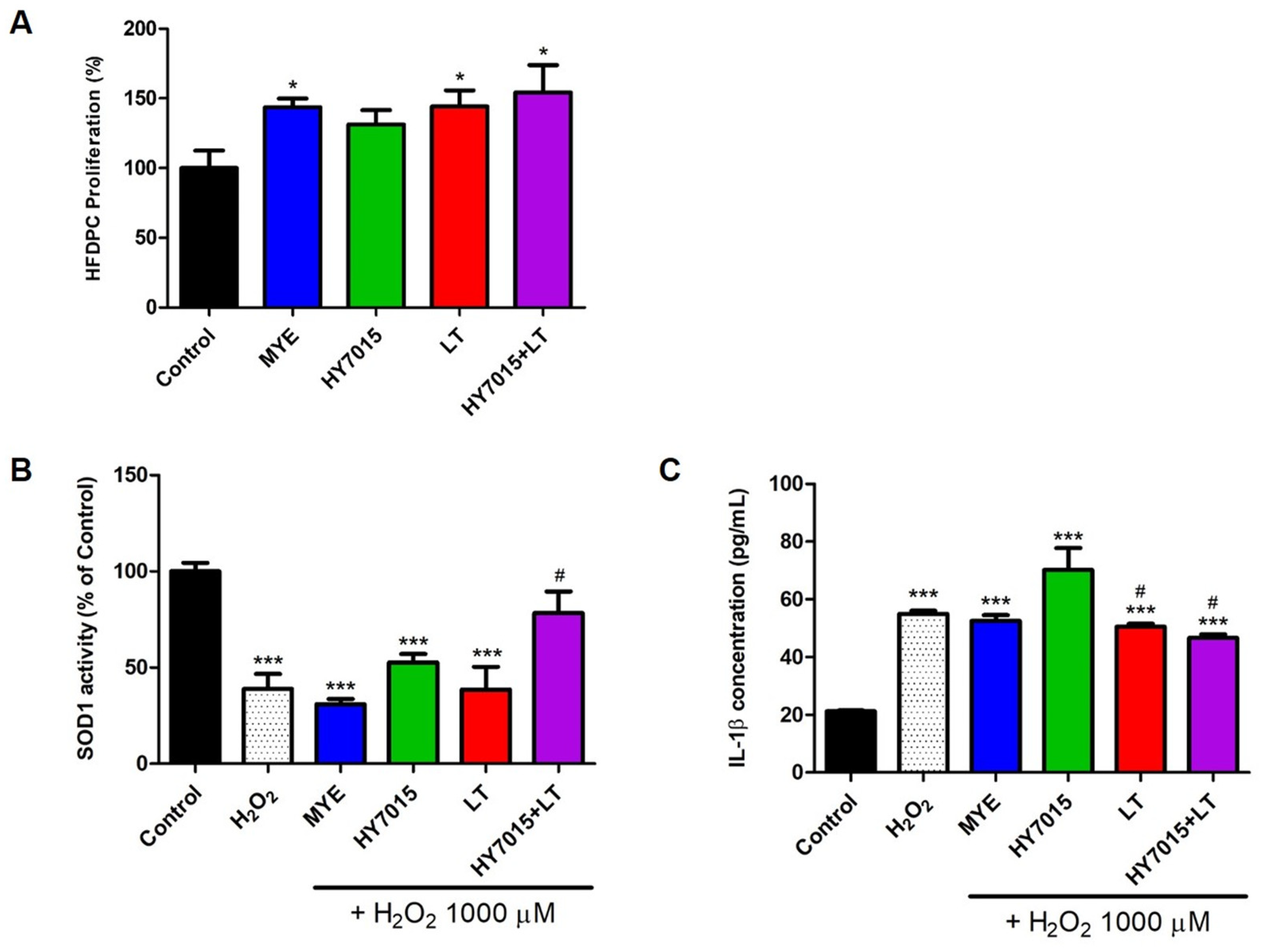
2.1.1. Effect of HY7015 and/or LT Extract on HFDPC Proliferations
2.1.2. Antioxidants and Anti-Inflammatory Effects in HFDPC
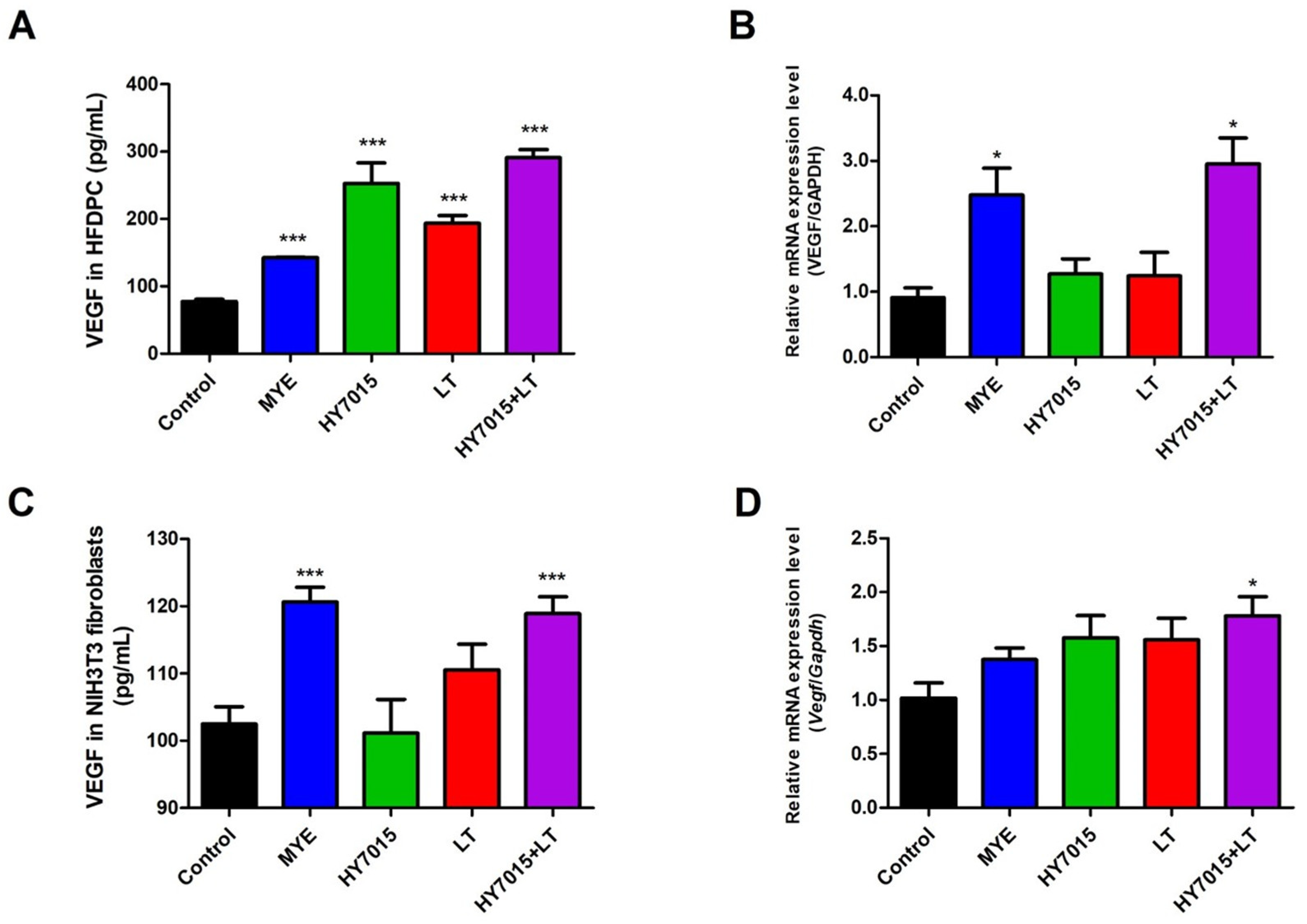
2.2. HY7015 and/or LT Affected on VEGF Secretion by HFDPC and NIH3T3 Fibroblasts
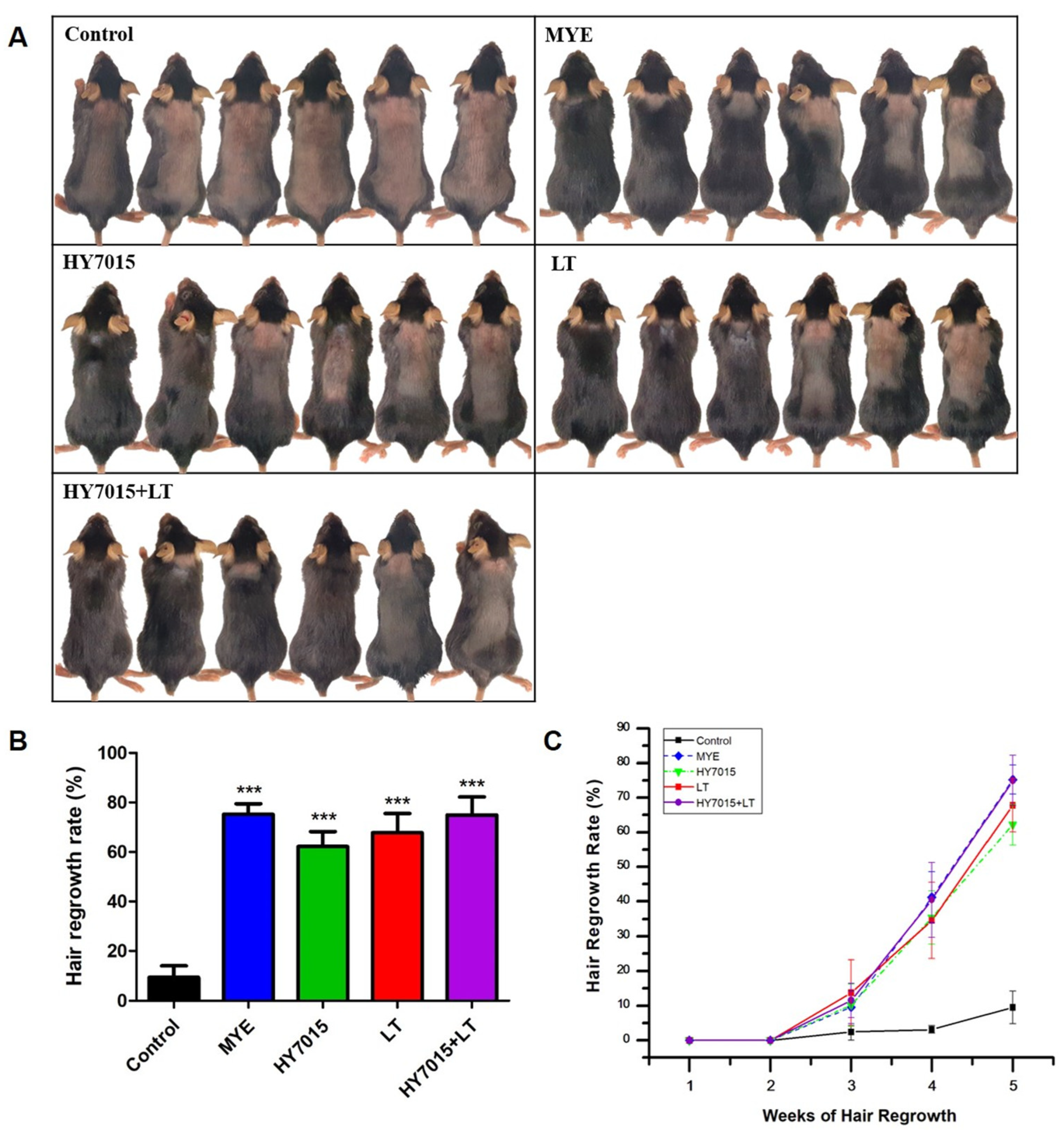
2.3. Effect of HY7015 and/or LT on Hair Regrowth in C57BL/6 Mice
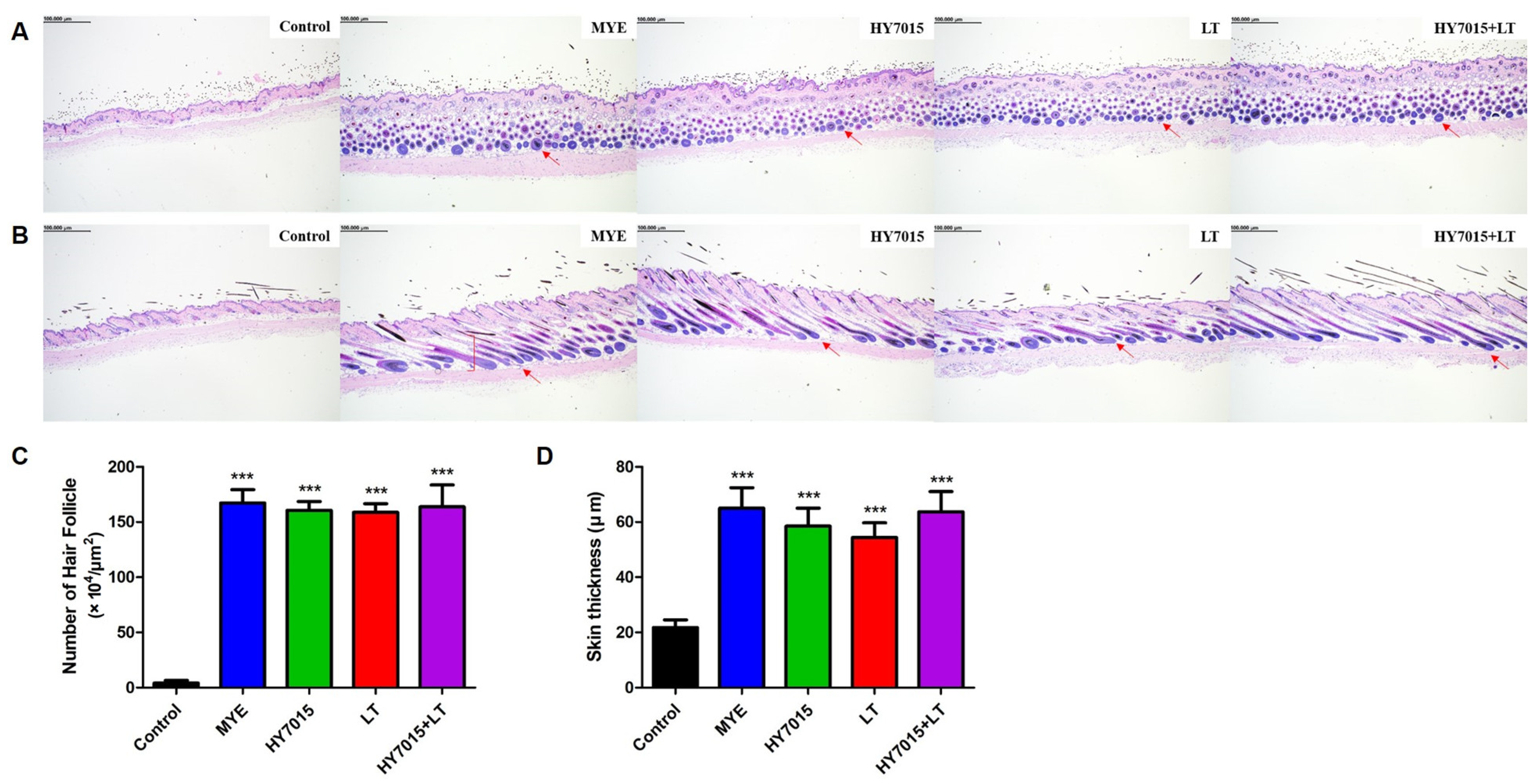
2.4. Effect of HY7015 and/or LT on Hair Follicles and Dermal Skin Thickness in C57BL/6 Mice
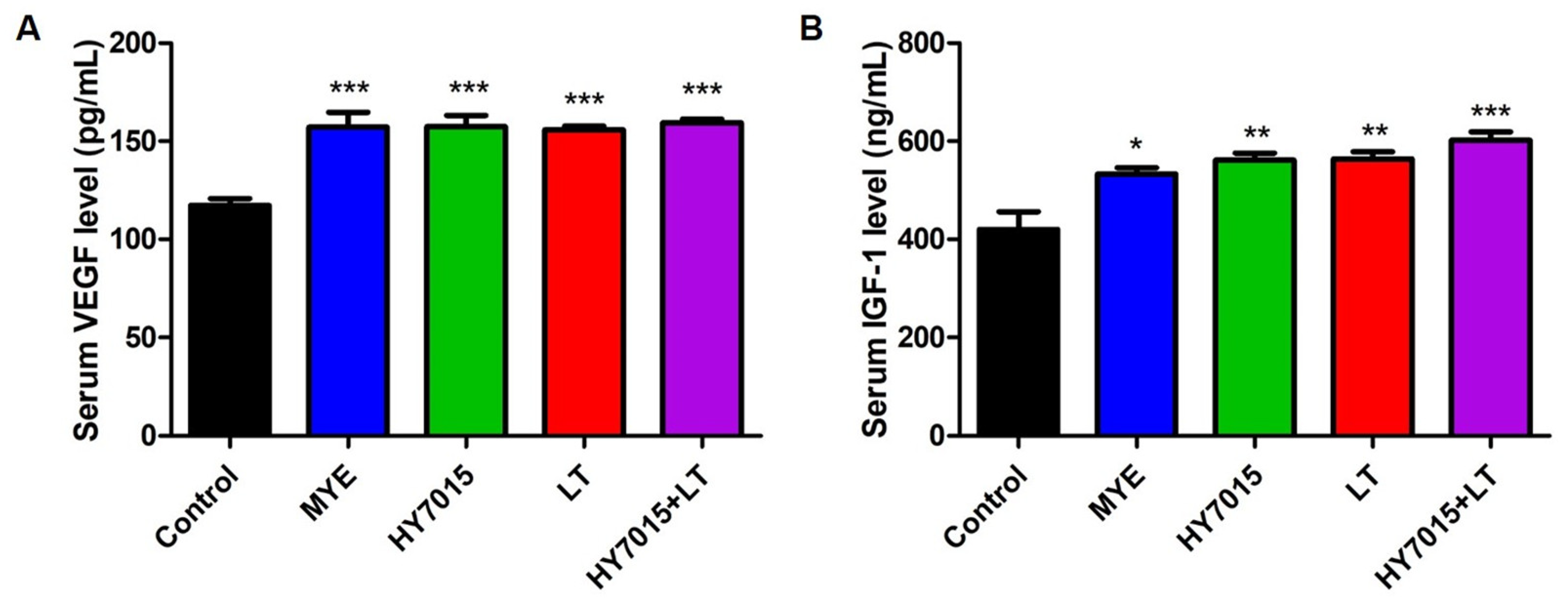
2.5. Effect of HY7015 and/or LT on Growth Factor Levels in Mouse Serum
2.6. Rosmarinic Acid Content of LT Extract
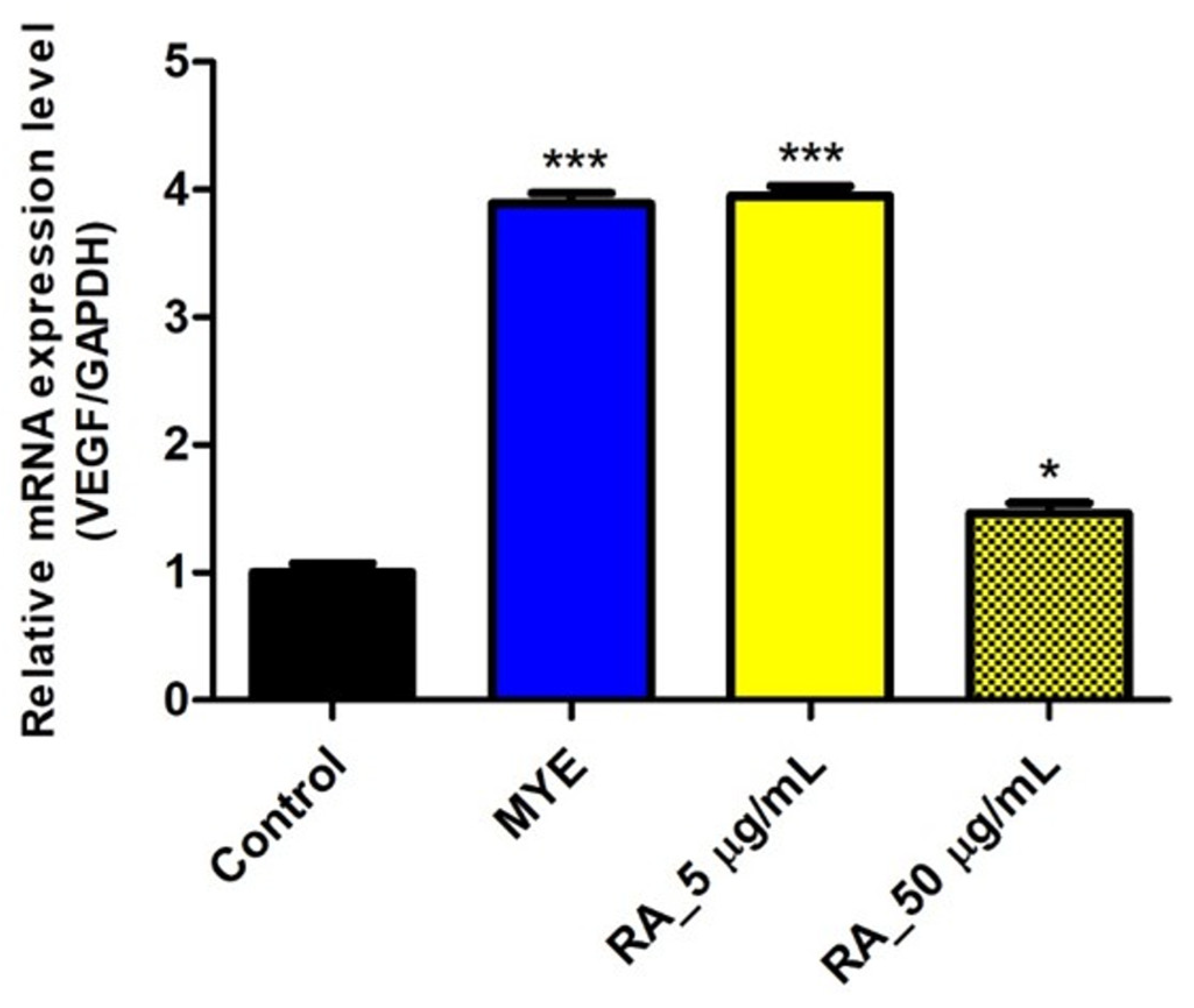
2.7. Rosmarinic Acid Affected on VEGF Expression by HFDPC
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Cell Culture and Treatments
4.3. Proliferation Analysis
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) In Vitro
4.5. Total RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR Analysis
4.6. Animal Experiments
4.7. Histological Analysis of Dorsal Skin Tissue
4.8. Analysis of Growth Factors in Mouse Serum
4.9. Measurement of Rosmarinic Acid Content
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Horev, L. Exogenous factors in hair disorders. Exog. Dermatol. 2004, 3, 237–245. [Google Scholar] [CrossRef]
- Arias-Santiago, S.; Arrabal-Polo, M.A.; Buendía-Eisman, A.; Arrabal-Martín, M.; Gutiérrez-Salmerón, M.T.; Girón-Prieto, M.S.; Jimenez-Pacheco, A.; Calonje, J.E.; Naranjo-Sintes, R.; Zuluaga-Gomez, A.; et al. Androgenetic alopecia as an early marker of benign prostatic hyperplasia. J. Am. Acad. Dermatol. 2012, 66, 401–408. [Google Scholar] [CrossRef]
- Ahouansou, S.; Le Toumelin, P.; Crickx, B.; Descamps, V. Association of androgenetic alopecia and hypertension. Eur. J. Dermatol. 2007, 17, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Chen, T.H. Association of androgenetic alopecia with metabolic syndrome in men: A community-based survey. Br. J. Dermatol. 2010, 163, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.K.; Rogers, N.; McMichael, A.; Kogan, S. A Novel Multi-Targeting Approach to Treating Hair Loss, Using Standardized Nutraceuticals. J. Drugs Dermatol. 2017, 16, 141–148. Available online: https://jddonline.com/articles/a-novel-multi-targeting-approach-to-treating-hair-loss-using-standardized-nutraceuticals-S1545961617S0141X/ (accessed on 1 November 2017).
- Chatterjee, A.; Bhattacharya, H.; Kandwal, A. Probiotics in periodontal health and disease. J. Indian Soc. Periodontol. 2011, 15, 23–28. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed]
- Tien, M.T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.A.; Coppee, J.Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pedron, T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Foxx-Orenstein, A.E. The role of probiotics in inflammatory bowel disease. Dig. Dis. Sci. 2007, 52, 607–611. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.M.; Kim, O.J.; Jo, E.S.; Jo, M.Y.; Ahn, M.Y.; Lee, Y.-H.; Li, C.-r.; Lee, S.-H.; Choi, J.-S.; Ha, J.M. The effect of Lactobacillus plantarum hydrolysates promoting VEGF production on vascular growth and hair growth of C57BL/6 mice. J. Anal. Sci. Technol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Joo, S.-S. In vitro and in vivo hair growth promotion effects of Lactobacillus plantarum-fermented plant extracts (MBN). Korean J. Food Sci. Technol. 2011, 43, 381–386. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, J.-S. The promoting effect of Pleuropterus cilinervis extracts fermented with Lactobacillus rhamnosus on hair growth. Microbiol. Biotechnol. Lett. 2011, 39, 345–349. [Google Scholar] [CrossRef]
- Nam, W.; Kim, H.; Bae, C.; Kim, J.; Nam, B.; Kim, J.; Park, S.; Lee, J.; Sim, J. Lactobacillus paracasei HY7015 Promotes Hair Growth in a Telogenic Mouse Model. J. Med. Food 2021, 24, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Yang, H.J.; Park, K.I.; Ma, J.Y. Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice. Phytomedicine 2019, 55, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.R.; Piao, M.S. Antioxidative constituents from Lycopus lucidus. Arch. Pharmacal Res. 2004, 27, 173–176. [Google Scholar] [CrossRef]
- Tian, Z.; Gao, N.; Li, L.; Yu, J.; Luo, X. Effect of two extracted fraction from Lycopus lucidus on coagulation function. Zhong Yao Cai 2001, 24, 507–508. Available online: http://open.oriprobe.com/articles/3943842/Effect_of_Two_Extracted_Fraction_from_Lycopus_luci.htm (accessed on 1 July 2001).
- Yu, J.Q.; Lei, J.C.; Zhang, X.Q.; Yu, H.D.; Tian, D.Z.; Liao, Z.X.; Zou, G.L. Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chem. 2011, 126, 1593–1598. [Google Scholar] [CrossRef]
- Beer, A.M.; Wiebelitz, K.R.; Schmidt-Gayk, H. Lycopus europaeus (Gypsywort): Effects on the thyroidal parameters and symptoms associated with thyroid function. Phytomedicine 2008, 15, 16–22. [Google Scholar] [CrossRef]
- Liu, J.; Bhuvanagiri, S.; Qu, X. The protective effects of lycopus lucidus turcz in diabetic retinopathy and its possible mechanisms. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2900–2908. [Google Scholar] [CrossRef]
- Lu, Y.H.; Huang, J.H.; Li, Y.C.; Ma, T.T.; Sang, P.; Wang, W.J.; Gao, C.Y. Variation in nutritional compositions, antioxidant activity and microstructure of Lycopus lucidus Turcz. root at different harvest times. Food Chem. 2015, 183, 91–100. [Google Scholar] [CrossRef]
- Kim, M.W. The antidiabetic properties of fractions of Lycopus Lucidic Turcz in streptozotocin diabetic rats. Korean J. Soc. Food Sci. 2000, 16, 644–651. [Google Scholar]
- Michael, M.; Phebus, R.K.; Schmidt, K.A. Plant extract enhances the viability of Lactobacillus delbrueckii subsp. bulgaricus and Lactobacillus acidophilus in probiotic nonfat yogurt. Food Sci. Nutr. 2015, 3, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jeung, W.; Nam, W.; Kim, H.; Kim, J.; Nam, B.; Jang, S.; Lee, J.; Sim, J. Oral administration of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 with Cinnamomi Ramulus extract reduces diet-induced obesity and modulates gut microbiota. Prev. Nutr. Food Sci. 2019, 24, 136–143. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Guan, M.; Zhou, X.; Liang, X.; Lv, Y.; Bai, L.; Zhang, J.; Gong, P.; Liu, T.; et al. Lactobacillus casei YRL577 combined with plant extracts reduce markers of non-alcoholic fatty liver disease in mice. Br. J. Nutr. 2021, 125, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.; Proserpio, V.; Lichtenberger, B.M.; Natsuga, K.; Sinclair, R.; Fujiwara, H.; Watt, F.M. Epidermal Wnt/beta-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. Proc. Natl. Acad. Sci. USA 2014, 111, E1501–E1509. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Spencer, J.P.E.; Rossi, R.; Aeschbach, R.; Khan, A.; Mahmood, N.; Munoz, A.; Murcia, A.; Butler, J.; Halliwell, B. An evaluation of the antioxidant and antiviral action of extracts of Rosemary and provencal herbs. Food Chem. Toxicol. 1996, 34, 449–456. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Rathi, V.; Rathi, J.; Tamizharasi, S.; Pathak, A. Plants used for hair growth promotion: A review. Pharmacogn. Rev. 2008, 2, 185. Available online: https://www.phcogrev.com/article/2008/2/3-19 (accessed on 1 January 2008).
- Jin, G.R.; Zhang, Y.L.; Yap, J.; Boisvert, W.A.; Lee, B.H. Hair growth potential of Salvia plebeia extract and its associated mechanisms. Pharm. Biol. 2020, 58, 400–409. [Google Scholar] [CrossRef]
- Nabahin, A.; Abou Eloun, A.; Abu-Naser, S.S. Expert system for hair loss diagnosis and treatment. Int. J. Eng. Technol. 2017, 1, 160–169. Available online: https://dstore.alazhar.edu.ps/xmlui/handle/123456789/374/IJEAIS170614.pdf?sequence=1&isAllowed=y (accessed on 1 June 2017).
- Kaler, S.G.; Patrinos, M.E.; Lambert, G.H.; Myers, T.F.; Karlman, R.; Anderson, C.L. Hypertrichosis and congenital anomalies associated with maternal use of minoxidil. Pediatrics 1987, 79, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Arca, E.; Acikgoz, G.; Tastan, H.B.; Kose, O.; Kurumlu, Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology 2004, 209, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.S.; Nandhini, T.; Vasanth, V.; Sriram, R.; Navale, S. Topical minoxidil fortified with finasteride: An account of maintenance of hair density after replacing oral finasteride. Indian Dermatol. Online J. 2015, 6, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Naccarato, T.; Situm, M.; Kovacevic, M.; Lotti, T.; McCoy, J. Mechanism of action of minoxidil in the treatment of androgenetic alopecia is likely mediated by mitochondrial adenosine triphosphate synthase-induced stem cell differentiation. J. Biol. Regul. Homeost. Agents 2017, 31, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Taghiabadi, E.; Nilforoushzadeh, M.A.; Aghdami, N. Maintaining hair inductivity in human dermal papilla cells: A review of effective methods. Ski. Pharmacol. Physiol. 2020, 33, 280–292. [Google Scholar] [CrossRef]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37, 25–30. [Google Scholar] [CrossRef]
- Trüeb, R.M.; Henry, J.P.; Davis, M.G.; Schwartz, J.R. Scalp condition impacts hair growth and retention via oxidative stress. Int. J. Trichol. 2008, 10, 262. [Google Scholar] [CrossRef]
- Hou, C.; Miao, Y.; Wang, J.; Wang, X.; Chen, C.Y.; Hu, Z.Q. Collagenase IV plays an important role in regulating hair cycle by inducing VEGF, IGF-1, and TGF-beta expression. Drug Des. Dev. Ther. 2015, 9, 5373–5383. [Google Scholar] [CrossRef]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Zhao, Y.; Li, J.; Cai, J.; Wang, P.; Meng, S.; Feng, J.; Miao, C.; Ding, M.; et al. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem. Biophys. Res. Commun. 2005, 330, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, D.P.; Rubin, J.S.; Ron, D.; Finch, P.W.; Florio, C.; Aaronson, S.A. Characterization of the receptor for keratinocyte growth factor. Evidence for multiple fibroblast growth factor receptors. J. Biol. Chem. 1990, 265, 12767–12770. [Google Scholar] [CrossRef] [PubMed]
- Muller-Rover, S.; Peters, E.J.; Botchkarev, V.A.; Panteleyev, A.; Paus, R. Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J. Histochem. Cytochem. 1998, 46, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.C.; Uyama, H.; Lee, C.H.; Sung, M.H. In vivo hair growth promotion effects of ultra-high molecular weight poly-gamma-glutamic acid from Bacillus subtilis (Chungkookjang). J. Microbiol. Biotechnol. 2015, 25, 407–412. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yamamoto, S.; Kato, R. Induction of anagen in telogen mouse skin by topical application of FK506, a potent immunosuppressant. J. Investig. Dermatol. 1995, 104, 523–525. [Google Scholar] [CrossRef]
- Bel-Rhlid, R.; Crespy, V.; Page-Zoerkler, N.; Nagy, K.; Raab, T.; Hansen, C.E. Hydrolysis of rosmarinic acid from rosemary extract with esterases and Lactobacillus johnsonii in vitro and in a gastrointestinal model. J. Agric. Food Chem. 2009, 57, 7700–7705. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H.; Okuda, T.; Hatano, T.; Arichi, S. Studies on the activities of tannins and related compounds, X. Effects of caffeetannins and related compounds on arachidonate metabolism in human polymorphonuclear leukocytes. J. Nat. Prod. 1987, 50, 392–399. [Google Scholar] [CrossRef]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1–dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohto, Y.; Murakami, A.; Ohigashi, H. Superoxide scavenging activity of rosmarinic acid from Perilla frutescens Britton var. acuta f. viridis. J. Agric. Food Chem. 1998, 46, 4545–4550. [Google Scholar] [CrossRef]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Therapeutic and nutraceutical potential of rosmarinic acid—Cytoprotective properties and pharmacokinetic profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Fernando, P.M.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.; Chae, S.W.; Hyun, J.W. Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells. Biomol. Ther. 2016, 24, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, S.; Hajnos, M.; Skalicka-Woźniak, K.; Matkowski, A. Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Nam, B.; Kim, S.A.; Park, S.D.; Kim, H.J.; Kim, J.S.; Bae, C.H.; Kim, J.Y.; Nam, W.; Lee, J.L.; Sim, J.H. Regulatory effects of Lactobacillus plantarum HY7714 on skin health by improving intestinal condition. PLoS ONE 2020, 15, e0231268. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.; Handjiski, B.; Hagen, E.; Pincus, M.; Bruenahl, C.; Bienenstock, J.; Paus, R. Is there a ‘gut–brain–skin axis’? Exp. Dermatol. 2010, 19, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant extracts rich in polyphenols as potent modulators in the growth of probiotic and pathogenic intestinal microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Liu, J.; Wan, Y.; Zhao, Z.; Chen, H. Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.) Britt. Chem. Cent. J. 2013, 7, 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]


| Sample | Rosmarinic Acid Content (μg/mL) |
|---|---|
| Water Extract of Lycopus lucidus Turcz. | 67.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, H.; Kim, J.-H.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract Promotes Human Dermal Papilla Cell Cytoprotective Effect and Hair Regrowth Rate in C57BL/6 Mice. Molecules 2022, 27, 8235. https://doi.org/10.3390/molecules27238235
Lee H, Kim H, Kim J-H, Park S-D, Shim J-J, Lee J-L. Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract Promotes Human Dermal Papilla Cell Cytoprotective Effect and Hair Regrowth Rate in C57BL/6 Mice. Molecules. 2022; 27(23):8235. https://doi.org/10.3390/molecules27238235
Chicago/Turabian StyleLee, Hayera, Hyeonji Kim, Ji-Hyun Kim, Soo-Dong Park, Jae-Jung Shim, and Jeong-Lyoul Lee. 2022. "Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract Promotes Human Dermal Papilla Cell Cytoprotective Effect and Hair Regrowth Rate in C57BL/6 Mice" Molecules 27, no. 23: 8235. https://doi.org/10.3390/molecules27238235
APA StyleLee, H., Kim, H., Kim, J.-H., Park, S.-D., Shim, J.-J., & Lee, J.-L. (2022). Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract Promotes Human Dermal Papilla Cell Cytoprotective Effect and Hair Regrowth Rate in C57BL/6 Mice. Molecules, 27(23), 8235. https://doi.org/10.3390/molecules27238235







