New Meroterpenoid and Isocoumarins from the Fungus Talaromyces amestolkiae MST1-15 Collected from Coal Area
Abstract
1. Introduction
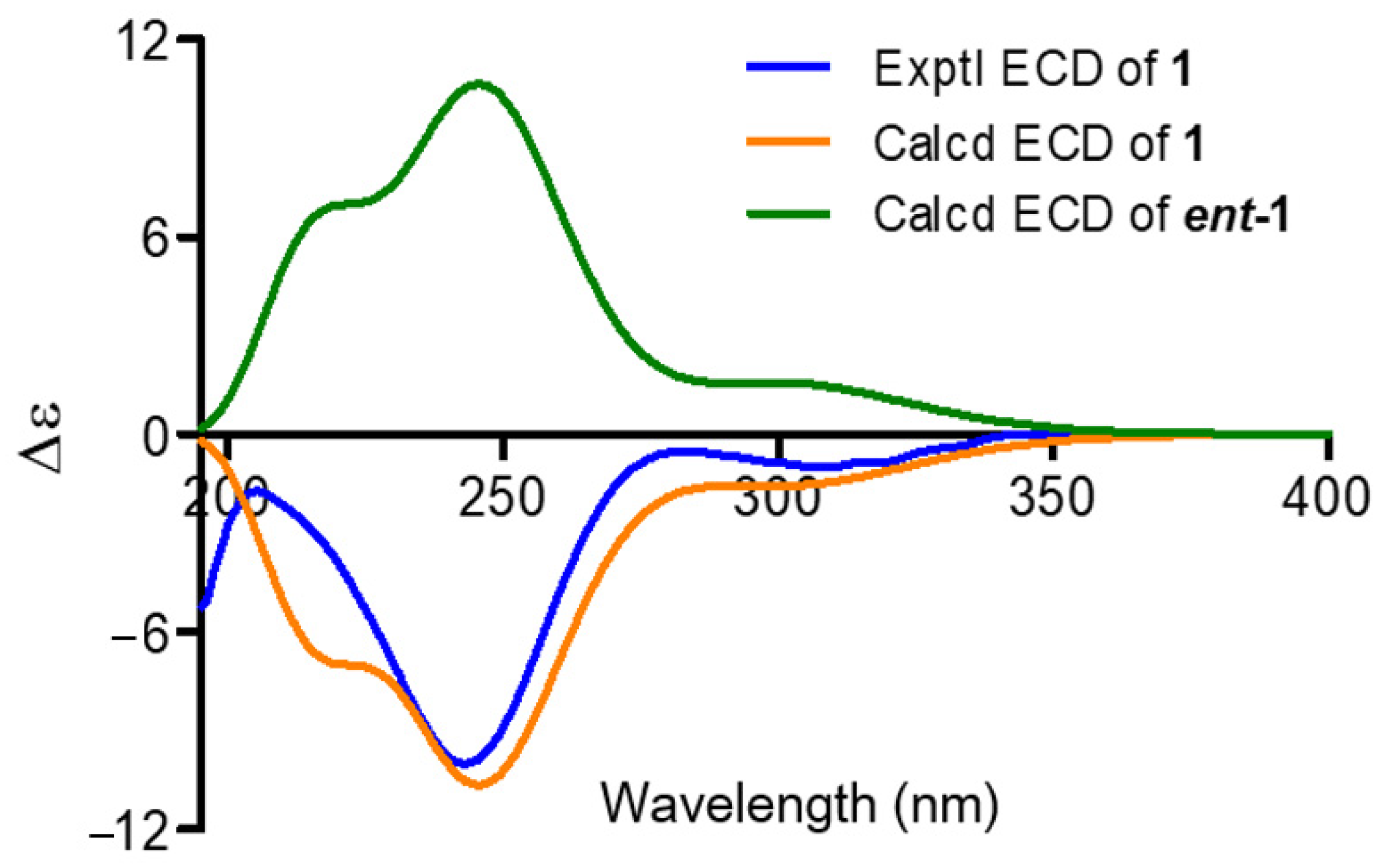
2. Results and Discussion
Structures Elucidation
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. Spectral and Physical Data of Compounds 1, 8, and 9
3.5. Antibacterial Assay
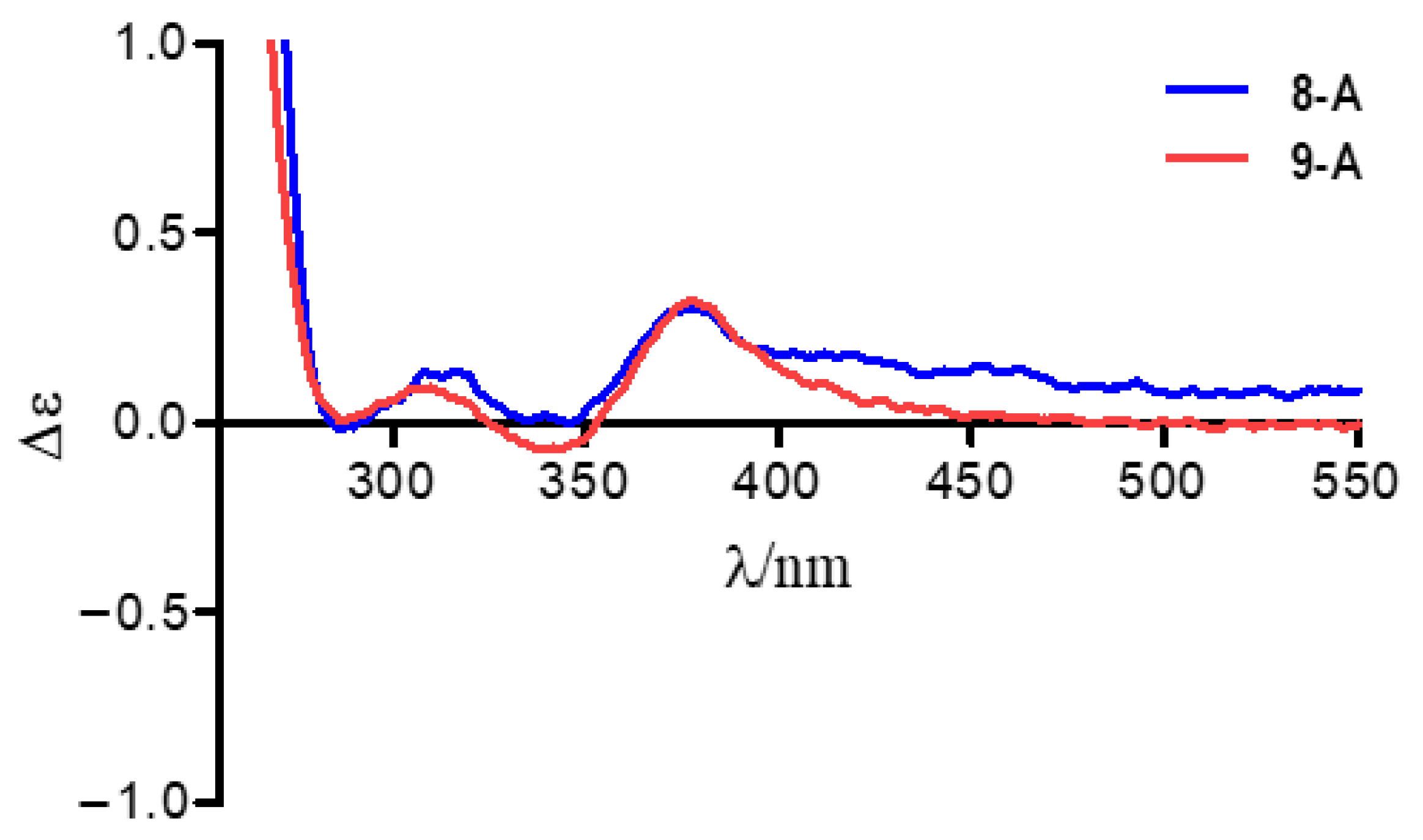
3.6. Preparation of Acetonides of 8a and 9a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Benjamin, C.R. Ascocarps of Aspergillus and Penicillium. Mycologia 1955, 47, 669–687. [Google Scholar] [CrossRef]
- Samson, R.A.; Yilmaz, N.; Houbraken, J.; Spierenburg, H.; Seifert, K.A.; Peterson, S.W.; Varga, J.; Frisvad, J.C. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Genta-Jouve, G.; Escargueil, A.E.; Larsen, A.K.; Guedon, L.; Nay, B.; Prado, S. Antimicrobial oligophenalenone dimers from the soil fungus Talaromyces stipitatus. J. Nat. Prod. 2016, 79, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, W.; Liu, X.; Wei, M.; Liang, Y.; Wang, J.; Zhu, H.; Zhang, Y. Anti-inflammatory spiroaxane and drimane sesquiterpenoids from Talaromyces minioluteus (Penicillium minioluteum). Bioorg. Chem. 2019, 91, 103166. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Liu, Z.; Cai, R.; Lu, Y.; Huang, X.; She, Z. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv. 2016, 6, 26412. [Google Scholar] [CrossRef]
- Chen, S.; Ding, M.; Liu, W.; Huang, X.; Liu, Z.; Lu, Y.; Liu, H.; She, Z. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1. Phytochemistry 2018, 146, 8–15. [Google Scholar] [CrossRef]
- Oliveira, F.; Rocha, I.L.D.; Pinto, D.C.G.A.; Ventura, S.P.M.; Santos, A.G.; Crevelin, E.J.; Ebinuma, V.C.S. Identification of azaphilone derivatives of Monascus colorants from Talaromyces amestolkiae and their halochromic properties. Food Chem. 2022, 372, 131214. [Google Scholar] [CrossRef]
- Prieto, A.; de Eugenio, L.; Méndez-Líter, J.A.; Nieto-Domínguez, M.; Murgiondo, C.; Barriuso, J.; Bejarano-Muñoz, L.; Martínez, M.J. Fungal glycosyl hydrolases for sustainable plant biomass valorization: Talaromyces amestolkiae as a model fungus. Int. Microbiol. 2021, 24, 545–558. [Google Scholar] [CrossRef]
- Fu, Y.; Li, C.; Zhu, J.; Zhang, L.; Wang, Y.; Chen, Q.; Xu, L.; Zhang, S.; Fang, Y.; Liu, T. A new meroterpenoid from endophytic fungus Talaromyces amestolkiae CS-O-1. Biochem. Syst. Ecol. 2020, 100, 104377. [Google Scholar] [CrossRef]
- EI-Elimat, T.; Figueroa, M.; Raja, H.A.; Alnabulsi, S.; Oberlies, N.H. Coumarins, dihydroisocoumarins, a dibenzo-α-pyrone, a meroterpenoid, and a merodrimane from Talaromyces amestolkiae. Tetrahedron Lett. 2021, 72, 153067. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Yogabaanu, U.; Faizal Weber, J.-F.; Peter Convey, P.; Rizman-Idid, M.; Alias, S.A. Antimicrobial properties and the influence of temperature on secondary metabolite production in cold environment soil fungi. Polar Sci. 2017, 14, 60–67. [Google Scholar] [CrossRef]
- Orwa, P.; Mugambi, G.; Wekesa, V.; Mwirichia, R. Isolation of haloalkaliphilic fungi from Lake Magadi in Kenya. Heliyon 2020, 6, e02823. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, N.; Kavya, I.K.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the microorganism of extreme environments and their applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, Z.X.; Song, Y.L.; Dong, D.; Zheng, J.; Liu, T.; Zhao, Y.-F.; Ferreira, D.; Zjawiony, J.K.; Tu, P.F.; et al. Nitric oxide inhibitory meroterpenoids from the fungus Penicillium purpurogenum MHZ 111. J. Nat. Prod. 2016, 79, 1415–1422. [Google Scholar] [CrossRef]
- Stierle, D.B.; Stierle, A.A.; Patacini, B. The berkeleyacetals, three meroterpenes from a deep water acid mine waste Penicillium. J. Nat. Prod. 2007, 70, 1820–1823. [Google Scholar] [CrossRef]
- Li, S.D.; Wei, M.Y.; Chen, G.Y.; Lin, Y.C. Two new dihydroisocoumarins from the endophytic fungus Aspergillus sp. Collected from the south China sea. Chem. Nat. Compd. 2012, 48, 371–373. [Google Scholar] [CrossRef]
- Xu, L.; He, Z.; Xue, J.; Chen, X.; Wei, X. β-Resorcylic Acid Lactones from a Paecilomyces Fungus. J. Nat. Prod. 2010, 73, 885–889. [Google Scholar] [CrossRef]
- Di Bari, L.; Pescitelli, G.; Carmela, P.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar] [CrossRef]
- Xu, G.-B.; Yang, F.-Y.; Wu, X.-Y.; Li, R.; Zhou, M.; Wang, B.; Yang, X.-S.; Zhang, T.-T.; Liao, S.-G. Two new dihydroisocoumarins with antimicrobial activities from the fungus Penicillium sp. XR046 collected from Xinren coal area. Nat. Prod. Res. 2021, 35, 1445–1451. [Google Scholar] [CrossRef]
- Qi, B.; Liu, X.; Mo, T.; Zhu, Z.; Li, J.; Wang, J.; Shi, X.; Zeng, K.; Wang, X.; Tu, P.; et al. 3,5-Dimethylorsellinic acid derived meroterpenoids from Penicillium chrysogenum MT-12, an endophytic fungus isolated from Huperzia serrata. J. Nat. Prod. 2017, 80, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Zheng, C.; Niu, Z.-G.; Chen, G.-Y. Bioactive meroterpenoids and isocoumarins from the mangrove-derived fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shao, C.-L.; Li, Z.-Y.; Gan, L.-S.; Fu, X.-M.; Bian, W.-T.; Zhao, H.-Y.; Wang, C.-Y. Isocoumarin derivatives and benzofurans from a sponge-derived Penicillium sp. fungus. J. Nat. Prod. 2013, 76, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-J.; Li, X.-A.; Jin, M.-Y.; Guo, W.-X.; Lei, L.-R.; Liu, R.; Zhang, M.-Z.; Guo, D.-L.; Wang, D.; Zhou, Y.; et al. Two previously undescribed phthalides from Talaromyces amestolkiae, a symbiotic fungus of Syngnathus acus. J. Asian Nat. Prod. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef]
- Matsuda, Y.; Abe, I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2015, 33, 26–53. [Google Scholar] [CrossRef]
- Matsuda, Y.; Iwabuchi, T.; Fujimoto, T.; Awakawa, T.; Nakashima, Y.; Mori, T.; Zhang, H.; Hayashi, F.; Abe, I. Discovery of key dioxygenases that diverged the paraherquonin and acetoxydehydroaustin pathways in Penicillium brasilianum. J. Am. Chem. Soc. 2016, 138, 12671–12677. [Google Scholar] [CrossRef]
- Barsky, E.E.; Williams, K.A.; Priebe, G.P.; Sawicki, G.S. Incident Stenotrophomonas maltophilia infection and lung function decline in cystic fibrosis. Pediatr. Pulm. 2017, 52, 1276–1282. [Google Scholar] [CrossRef]
- Assaw, S.; Mohd Amir, M.I.H.; Khaw, T.T.; Bakar, K.; Mohd Radzi, S.A.; Mazlan, N.W. Antibacterial and antioxidant activity of naphthofuranquinones from the twigs of tropical mangrove Avicennia officinalis. Nat. Prod. Res. 2020, 34, 2403–2406. [Google Scholar] [CrossRef]
- Jabrane, A.; Jannet, H.B.; Mastouri, M.; Mighri, Z.; Casanova, J. Chemical composition and in vitro evaluation of antioxidant and antibacterial activities of the root oil of Ridolfia segetum (L.) Moris from Tunisia. Nat. Prod. Res. 2010, 24, 491–499. [Google Scholar] [CrossRef]
- Jiang, X.-Z.; Yu, Z.-D.; Ruan, Y.-M.; Wang, L. Three new species of Talaromyces sect. Talaromyces discovered from soil in China. Sci. Rep. 2018, 8, 4932. [Google Scholar] [CrossRef]
- Xu, G.-B.; He, G.; Bai, H.-H.; Yang, T.; Zhang, G.-L.; Wu, L.-W.; Li, G.-Y. Indole Alkaloids from Chaetomium globosum. J. Nat. Prod. 2015, 78, 1479–1485. [Google Scholar] [CrossRef]
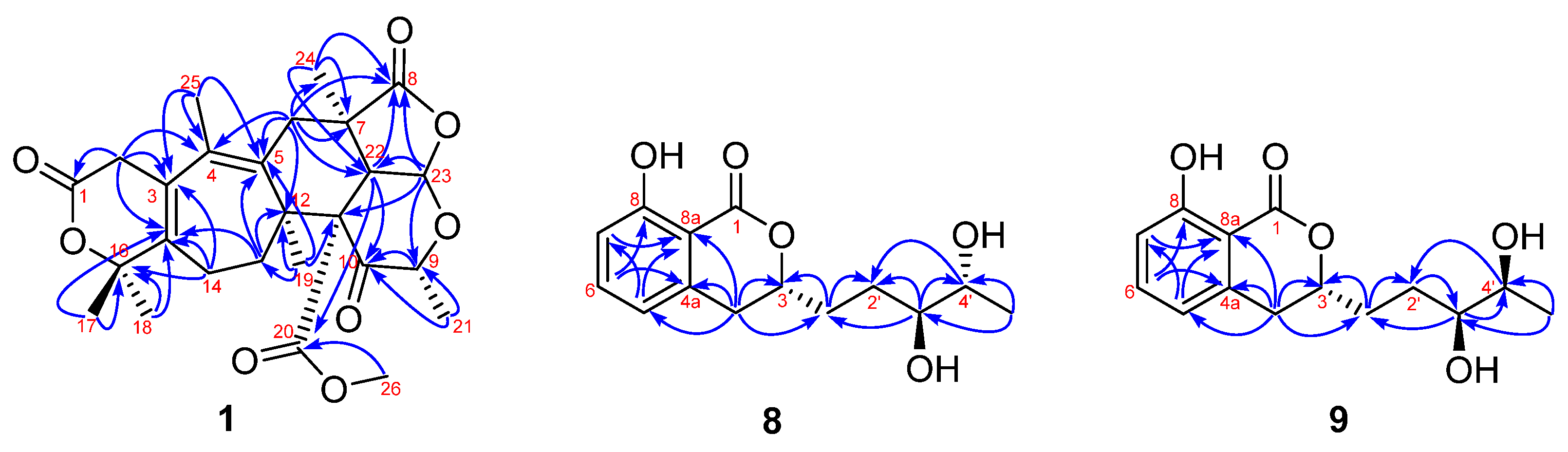
 ) and 1H-1H COSY (
) and 1H-1H COSY ( ) correlations of 1.
) correlations of 1.
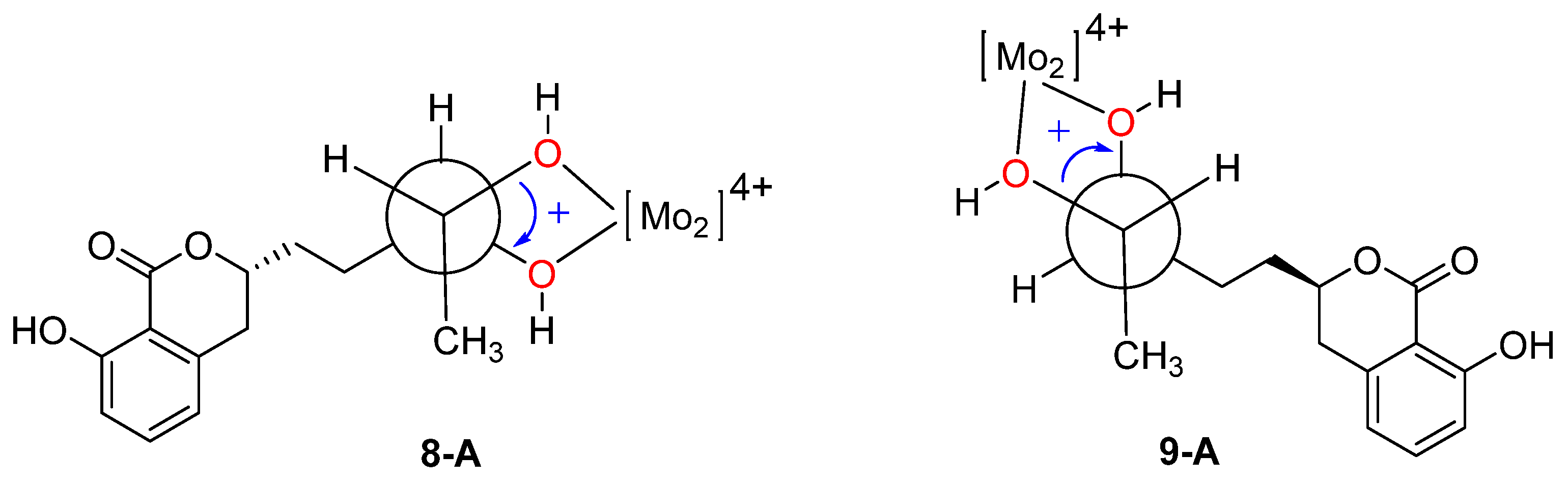
 ) of 8a and 9a.
) of 8a and 9a.
 ) of 8a and 9a.
) of 8a and 9a.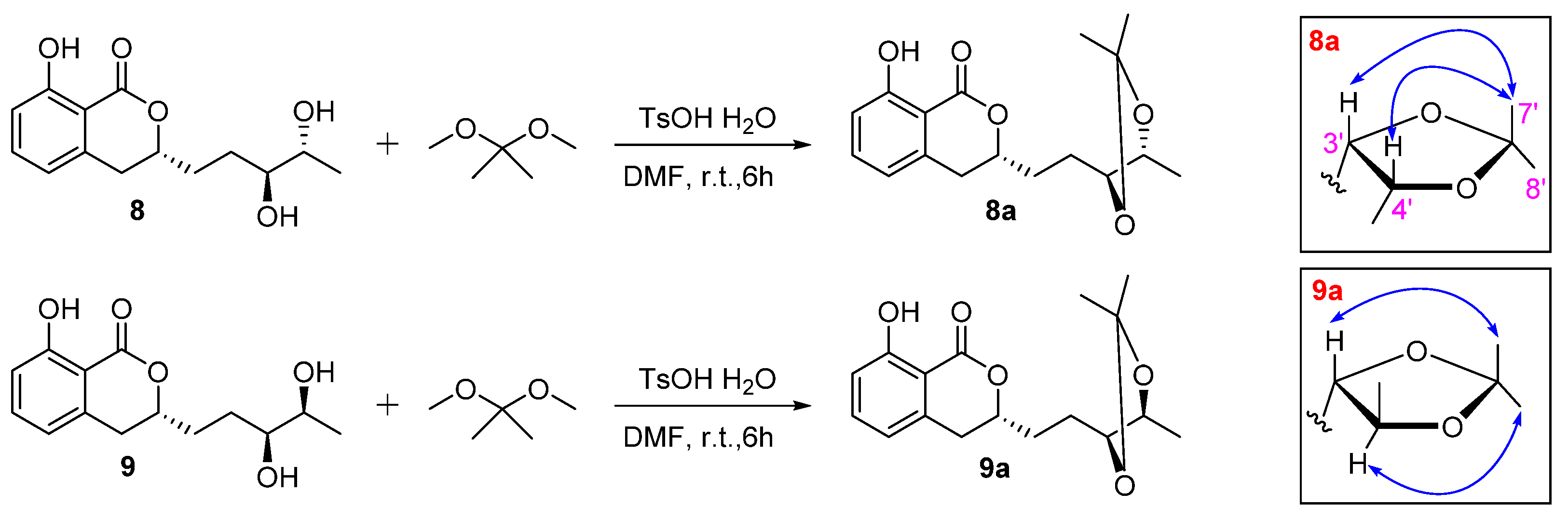


| No. | 1 (in CDCl3) a | |
|---|---|---|
| δH (J in Hz) | δC | |
| 1 | 169.4 | |
| 2 | 3.32 (1H, d, 21.1) 2.88 (1H, dd, 21.1, 1.3) | 31.6 |
| 3 | 126.9 | |
| 4 | 132.8 | |
| 5 | 137.0 | |
| 6 | 2.74 (1H, d, 16.2) 2.14 (1H, d, 16.2) | 34.0 |
| 7 | 41.0 | |
| 8 | 179.8 | |
| 9 | 4.18 (1H, q, 6.8) | 72.3 |
| 10 | 209.4 | |
| 11 | 63.2 | |
| 12 | 44.1 | |
| 13 | 3.22 (1H, dt, 13.7, 3.6) 1.54 (1H, dd, 13.7, 3.2) | 49.1 |
| 14 | 2.70 (1H, t like) 1.95 (1H, dt, 13.7, 3.2) | 26.7 |
| 15 | 136.3 | |
| 16 | 85.6 | |
| 17 | 1.57 (3H, s) | 27.4 |
| 18 | 1.48 (3H, s) | 27.9 |
| 19 | 1.14 (3H, s) | 27.3 |
| 20 | 168.2 | |
| 21 | 1.42 (3H, d, 6.8) | 17.7 |
| 22 | 3.69 (1H, d, 8.2) | 46.0 |
| 23 | 6.09 (1H, d, 8.2) | 98.5 |
| 24 | 1.33 (3H, s) | 26.5 |
| 25 | 1.77 (3H, s) | 18.3 |
| 26 | 3.80 (3H, s) | 53.2 |
| No. | δH (J in Hz) | δC | ||
|---|---|---|---|---|
| 8 | 9 | 8 | 9 | |
| 1 | 171.5 | 171.5 | ||
| 3 | 4.63 (1H, m) | 4.64 (1H, m) | 81.2 | 81.7 |
| 4 | 3.01 (1H, dd, 16.2, 3.0) 2.94 (1H, dd, 16.2, 11.2) | 3.05 (1H, dd, 16.3, 3.2) 2.96 (1H, dd, 16.3, 11.4) | 33.6 | 33.7 |
| 4a | 141.6 | 141.6 | ||
| 5 | 6.76 (1H, d, 7.3) | 6.80 (1H, d, 7.4) | 119.3 | 119.3 |
| 6 | 7.42 (1H, dd, 8.3, 7.3) | 7.45 (1H, dd, 8.4, 7.4) | 137.4 | 137.4 |
| 7 | 6.81 (1H, d, 8.3) | 6.84 (1H, d, 8.4) | 116.7 | 116.7 |
| 8 | 163.2 | 163.2 | ||
| 8a | 109.5 | 109.5 | ||
| 1′ | 2.05 (1H, m) 1.77 (1H, m) | 2.00 (1H, m) 1.89 (1H, m) | 32.3 | 32.6 |
| 2′ | 1.77 (1H, m) 1.57 (1H, m) | 1.82 (1H, m) 1.53 (1H, m) | 28.9 | 29.0 |
| 3′ | 3.35 (1H, m) | 3.38 (1H, m) | 76.2 | 76.3 |
| 4′ | 3.55 (1H, m) | 3.63 (1H, m) | 71.8 | 71.4 |
| 5′ | 1.16 (3H, d, 6.3) | 1.17 (3H, d, 6.4) | 18.8 | 18.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-Y.; Zhu, Q.-F.; Ao, J.-L.; Wang, F.-R.; Long, X.-M.; Liao, S.-G.; Xu, G.-B. New Meroterpenoid and Isocoumarins from the Fungus Talaromyces amestolkiae MST1-15 Collected from Coal Area. Molecules 2022, 27, 8223. https://doi.org/10.3390/molecules27238223
Li K-Y, Zhu Q-F, Ao J-L, Wang F-R, Long X-M, Liao S-G, Xu G-B. New Meroterpenoid and Isocoumarins from the Fungus Talaromyces amestolkiae MST1-15 Collected from Coal Area. Molecules. 2022; 27(23):8223. https://doi.org/10.3390/molecules27238223
Chicago/Turabian StyleLi, Kai-Yu, Qin-Feng Zhu, Jun-Li Ao, Fu-Rui Wang, Xing-Mei Long, Shang-Gao Liao, and Guo-Bo Xu. 2022. "New Meroterpenoid and Isocoumarins from the Fungus Talaromyces amestolkiae MST1-15 Collected from Coal Area" Molecules 27, no. 23: 8223. https://doi.org/10.3390/molecules27238223
APA StyleLi, K.-Y., Zhu, Q.-F., Ao, J.-L., Wang, F.-R., Long, X.-M., Liao, S.-G., & Xu, G.-B. (2022). New Meroterpenoid and Isocoumarins from the Fungus Talaromyces amestolkiae MST1-15 Collected from Coal Area. Molecules, 27(23), 8223. https://doi.org/10.3390/molecules27238223





