Abstract
Traditional Chinese medicine considers Lonicerae japonicae flos to have antibacterial detoxification, liver protection, and gallbladder protection. At present, studies have proven that Lonicerae japonicae flos has a good therapeutic effect on liver injury. Therefore, to confirm the clinical applicability of Lonicerae japonicae flos in the treatment of liver injury, we were the first to compare the pharmacokinetics of an oral ethanol extract of Lonicerae japonicae flos in normal rats and carbon tetrachloride-induced liver injury model rats. A method was developed for the simultaneous determination of 3-caffeoylquinic acid, 4-caffeoylquinic acid, 5-caffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, protocatechuic acid, Sweroside, and Secoxyloganin in rat plasma by ultra-performance liquid chromatography tandem mass spectrometry. The results show that the method is reliable and reproducible and can be used for quantitative determination of biological samples. The pharmacokinetic parameters showed that the area under the concentration–time curve of eight compounds in the model group was significantly increased. The results showed that the total absorption of the active components of Lonicerae japonicae flos in the blood increased, the clearance rate slowed down, and the bioavailability of Lonicerae japonicae flos increased in liver injury diseases.
1. Introduction
Lonicerae japonicae flos (LJF) is the dried flower bud or the flower with the first opening of Lonicera japonica Thunb [1]. As a national plant medicine, it is widely distributed in Asian countries and has great medicinal value (www.theplantlist.org (accessed on 24 May 2022)). In China, LJF is known as an ancient medicine used to treat fever and in detoxification [2] due to its ventilation and heat dissipation abilities [3]. Modern research has shown that LJF contains a large number of flavonoids [4], iridoids [5], triterpenoid saponins [6], organic acids [7,8], and other compounds [9]. Based on the effective material compounds, modern pharmacology has confirmed that LJF has anti-inflammatory [10], antibacterial [11], antiviral [12,13], and antioxidation [14] properties, as well as it increases immune function [15] and protects the liver and gallbladder [16,17]. Clinically, LJF was a commonly used antipyretic, often used to treat acute upper respiratory tract infections, rashes, a selection of wounds, tonsillitis in children, and other diseases [18,19,20], but the clinical preparation of LJF in protecting the liver and gallbladder has not been found.
The liver is one of the largest organs in the human body, and it is also the main place for metabolism and excretion. It plays a vital role in maintaining bodily functions and regulating the balance of the human body. Current research shows that there are about 2 million new cases of liver disease worldwide each year, and millions of deaths from liver disease related to chemical liver injury, accounting for about 3.5% of all deaths [21]. If liver damage is not promptly treated, it can lead to many forms of liver disease, such as acute and chronic hepatitis, granulomatous hepatitis, cholestasis due to bile duct damage, cholestasis with or without hepatitis, steatohepatitis, vascular disease, and tumors [22,23]. However, at present, chemical drugs are mostly used in the clinical treatment of liver injury, which have obvious side effects and are relatively expensive [24,25]. The curative effect of natural medicine on liver injury-related diseases is better, with less side effects, a range of sources, and a low price, and the experimental and clinical research results show the broad application prospects [26].
At present, the research on the liver and gallbladder protection properties of LJF is mostly pharmacological research. Hu et al. believed that the total flavonoids of LJF had a significant effect on the treatment of immunological liver injury in mice, which could effectively reduce the liver and spleen indexes of mice, and effectively improve the pathological conditions [27]. Chen et al. believed that the extract of LJF could significantly reduce the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum, showing a certain hepatoprotective effect [28]. Wang et al. believe that LJF can inhibit the occurrence of lipid peroxidation and reduce the liver index [29]. The study of pharmacokinetics is helpful to explain the material basis of the actual treatment which is closely related to liver protection. Therefore, studying the pharmacokinetics of LJF against liver injury is of practical significance for the clinical development of new drugs to protect the liver.
So far, most of the studies on the pharmacokinetics of silver anthers have focused on the pharmacokinetics of 3-caffeoylquinic acid (3-CQA) in rats after oral administration [30], but there is no pharmacokinetic study of LJF in the pathological model of liver injury. In this study, the ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method was used to study the pharmacokinetics of 3-CQA, 4-caffeoylquinic acid (4-CQA), 5-caffeoylquinic acid (5-CQA), 3,5-dicaffeoylquinic acid (3,5-diCQA), 4,5-dicaffeoylquinic acid (4,5-diCQA), protocatechuic acid (PA), Sweroside, and Secoxyloganin in the rats with acute liver injury induced by CCl4 [31,32,33]. The purpose of this study was to explain and compare the absorption and metabolism of the active ingredients of LJF in normal rats and liver injury model rats, and to provide a theoretical basis for the clinical development and application of new preparations for the liver protection properties of LJF.
2. Results
2.1. Optimization of Chromatographic Conditions
The selection and optimization of mass spectrum parameters is very important for the quantification of analytes. The results showed that the response values of 3-CQA, 4-CQA, 5-CQA, 3,5-diCQA, 4,5-diCQA, PA, and Secoxyloganin (the structures are shown in Figure 1) under negative ion mode were higher than those under positive ion mode, and the response values of Sweroside under positive ion mode were better (MS/MS detection parameters for eight compounds in Table 1). To improve the chromatographic peak shape, enhance the sensitivity, and shorten the running time, the liquid phase conditions were optimized. Several mobile phase systems were studied, and the best reactivity, sensitivity, and separation efficiency were selected. Methanol–water and acetonitrile–water mobile phase systems with 0.1% formic acid were compared. The results showed that the methanol−0.1% formic acid system had the best chromatographic performance.
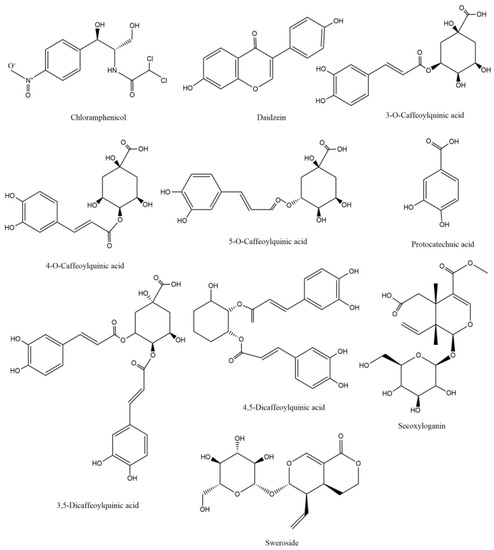
Figure 1.
Chemical structures of the eight compounds and two internal standards (ISs).

Table 1.
Quantitative ions and MS parameters of nine analytes and ISs.
2.2. Optimization of Sample Preparation
Plasma samples were treated with methanol/acetonitrile and ethyl acetate. The results showed that the recovery of protein precipitated with methanol was higher than that with other solvents, the matrix effect was lower, and the reproducibility was good.
2.3. Results of Rat Model of Liver Injury
Intraperitoneal injection of CCl4 was used to induce liver injury in rats, and the successful establishment of the model was measured by detecting ALT and AST in rat plasma. ALT and AST in the model group were significantly increased (as shown in Figure 2, p < 0.01). The results showed that the model of acute liver injury was successfully established.
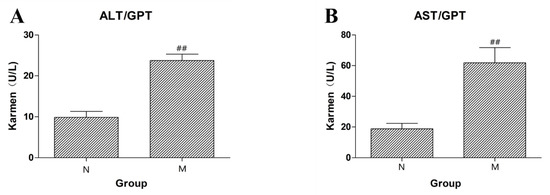
Figure 2.
(A) The content of ALT in rat serum of the normal control (N) group and the liver injury model (M) group. (B) The content of AST in rat serum of the normal control (N) group and the liver injury model (M) group (## p < 0.01 compared to N).
2.4. Methodological Verification
2.4.1. Specificity
The chromatograms of blank plasma samples, blank plasma samples with 8 added analytes and IS1 and IS2, and plasma samples taken orally for 5 min with ethanol extract of LJF are shown in Figure 3. The results showed that the eight active components and the internal standards were not disturbed by endogenous substances in plasma under the corresponding retention time. The method established in this study had good specificity.
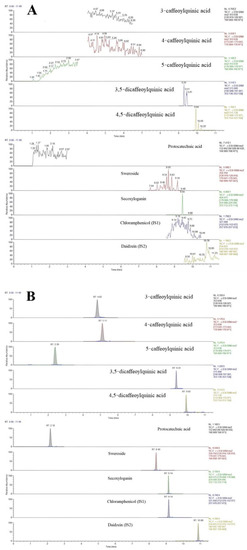
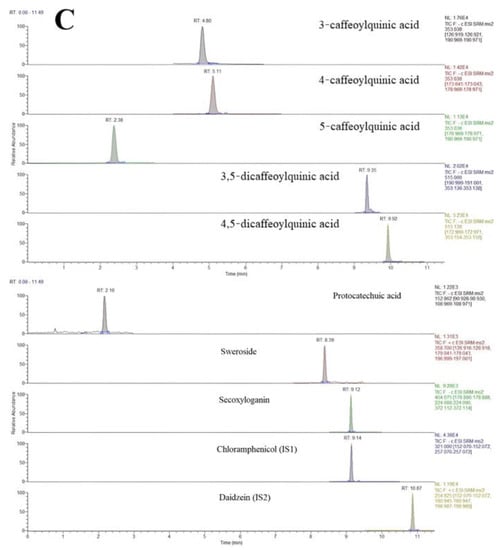
Figure 3.
Typical MRM chromatograms of eight analytes and the ISs: (A) blank plasma, (B) blank plasma spiked with the analytes and IS, and (C) plasma samples 5 min after oral administration of the LJF aqueous extract.
2.4.2. Linearity and Lower LLOQ
The eight analytes showed a good linear relationship in rat plasma (r > 0.990). The linear regression equation, linear range, linear regression coefficient, and LLOQ of the active components are shown in Table 2.

Table 2.
The regression equations, linear range, and LLOQs for the eight compounds.
2.4.3. Accuracy and Precision
In this experiment, six duplicate QC samples of the eight analytes at three concentration levels (LQC, MQC, HQC) were tested on the same day and on three different days. The intra- and inter-day accuracy and precision of the 8 analytes to be analyzed were less than 15%, respectively, indicating that the established method has good precision and accuracy. The results are shown in Table 3.

Table 3.
Intra- and inter-day precisions and accuracies for the determination of the eight compounds from the assay samples (mean ± SD, n = 6).
2.4.4. Recovery and Matrix Effect
As shown in Table 4, the eight active ingredients and internal standard compounds had good extraction recovery, and plasma had no significant matrix effect on the eight active ingredients and internal standard compounds, which further verified the accuracy and reliability of the established method.

Table 4.
Matrix effects and extraction recoveries for the analytes and three internal standards in rat plasma (mean ± SD, n = 6).
2.4.5. Stability
As shown in Table 5, the eight active ingredients had good stability after being placed at room temperature (25 °C) for 4 h, at −80 °C for 30 days, at −80 °C/room temperature for 3 freeze/thaw cycles, and at 4 °C for 24 h in the sample chamber.

Table 5.
The stability of the eight compounds in rat plasma under different storage conditions.
2.5. Application in a Pharmacokinetic Study in Rats
This method has been successfully applied to study the pharmacokinetics of LJF orally in normal rats and acute liver injury rats. The pharmacokinetic parameters were analyzed using the non-atrioventricular model analysis on the DAS 2.0 platform. The mean plasma concentration–time curves of 3-CQA, 4-CQA, 5-CQA, 3,5-diCQA, 4,5-diCQA, PA, Secoxyloganin, and Sweroside are shown in Figure 4. Pharmacokinetic parameters included: Cmax, Tmax, area under the concentration–time curve (AUC0–t), AUC0–∞, MRT0–t, and MRT0–∞, as shown in Table 6 and Figure 4.
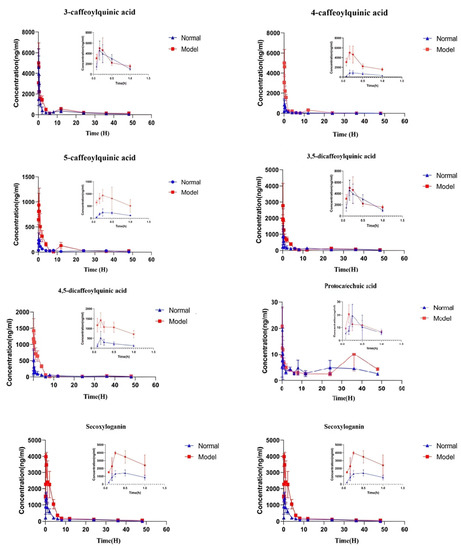
Figure 4.
Mean concentration–time curves of the eight compounds in N (normal rats group) and M (liver injury model rat group) rat plasma after oral administration of the LJF aqueous extract. Values are presented as mean ± SD of 6 rats.

Table 6.
Pharmacokinetic parameters of the eight compounds after oral administration of LJF extract in rats (mean ± standard deviation, n = 6).
2.5.1. The Pharmacokinetic Characteristics of Phenolic Acids
The pharmacokinetic characteristics of phenolic acids of LGF in plasma of the rat liver injury model were studied for the first time. Compared with the normal group, after oral administration of Lonicerae japonicae flos, Cmax absorption of 4-CQA and 5-CQA in plasma of rats in the model group increased, and AUC0–t and AUC0–∞ also increased (p < 0.01). Tmax of 5-CQA was not significantly different from that of the normal group and t1/2 was prolonged, but there was no significant difference. Compared with the normal group, the Tmax of 4-CQA was significantly decreased and the Tmax was prolonged, but there was no significant difference. AUC0–t and AUC0–∞ of 3-CQA were significantly increased compared with the normal group (p < 0.01), and the Cmax value was increased but there was no significant difference. There was no significant difference between Tmax and t1/2 compared with the normal group. The Cmax, AUC0–t, and AUC0–∞ of 3,5-diCQA and 4,5-diCQA increased significantly compared with the normal group. The t1/2 of 4,5-diCQA was prolonged, with a significant difference, and the value of MRT0–∞ was significantly increased compared with the normal group. The AUC0–t of 3,5-diCQA was significantly increased compared with the normal group (p < 0.05). Compared with the normal group, the Tmax, AUC0–∞, and MRT0–t of the PA model group reached the peak earlier, the area under the curve of the drug duration increased, the retention time was prolonged, and the clearance rate decreased.
The results showed that under the condition of liver injury, the value of AUC0–∞ of phenolic acids in LJF increased (p < 0.05), and the amount of exposure in vivo was significantly increased compared with the normal group.
2.5.2. Pharmacokinetic Characteristics of Iridoid Glycosides
The AUC0–∞ value of Sweroside in the model group was significantly higher than that in the normal group (p < 0.01) and the Cmax value was increased compared with that in the normal group, but there was no significant difference. Cmax and MRT0–∞ of Secoxyloganin were significantly higher than those in the normal group (p < 0.01), but Tmax was significantly lower than that in the normal group (p < 0.05).
The results showed that compared with the normal group, the clearance rate of iridoid glycosides in the model group was lower and the absorption amount was increased, which may be beneficial for the treatment of liver injury.
3. Discussion
Based on the hepatoprotective effect of LJF, it is of great significance to investigate the pharmacokinetics of LJF in vivo in normal and liver injury model groups. In this study, the UPLC-MS/MS method was established to study the pharmacokinetic parameters in normal rats and rats with liver injury. The experimental results showed that the pharmacokinetic parameters in normal rats and model rats were significantly different after a single administration of LJF extract (p < 0.01, p < 0.05). Combined with the current research status, the reasons may be as follows.
First, it may be related to the effect of liver injury on CYP450 enzyme activity and abnormal biliary excretion in vivo [34,35,36,37]. The liver CYP450 system metabolic enzyme is the main pathway of drug metabolism. Liver injury caused by CCl4 will affect the liver CYP450 system metabolic enzyme, resulting in decreased activity of drug metabolism enzymes, which leads to increased drug absorption, a slow elimination rate in the body, and increased total drug absorption into the blood. In addition, bile acids (BAS) are closely related to the metabolic function of the liver. In the pathological state of the liver, BAS metabolism disorder is manifested as an increased BAS level in patients’ serum and an abnormal composition proportion of BAS metabolites, which can feedback-regulate the enterohepatic circulation of BAS and the progress of liver disease by affecting the functions of immune cells and liver cells, thus affecting the activity of drug metabolism enzymes.
Secondly, based on the enteric–hepatic axis theory, the drug absorption and metabolism under the liver injury disease model were explained to provide new ideas and strategies for the treatment of liver injury [38]. On the one hand, the gut-liver axes directly affects through the entero-liver circulation [39]. Due to ecological imbalance in vivo, intestinal barrier damage, and immune state change, bacterial products can reach the liver through the portal vein, where they are recognized by specific receptors, activate the immune system, lead to a pro-inflammatory reaction, and further aggravate the disease. On the other hand, intestinal flora, as a regulator of bile acid metabolism, also plays an important role in the process of fibrosis. It can metabolize primary bile acids into secondary bile acids, and further induce inflammation through the farnesoid X receptor (FXR), affect the immune response, and promote the process of liver fibrosis.
Thirdly, the liver–renal axis theory was used to explain the slowing of drug metabolism in the liver injury disease model [40]. Studies have shown that related factors produced in the process of chronic liver disease can lead to liver fibrosis, and cause kidney damage leading to chronic kidney disease. Chronic kidney disease (CKD) causes renal dysfunction, resulting in varying degrees of increase in the AUC of some drugs cleared by the kidney and a decrease in the drug clearance rate.
4. Materials and Methods
4.1. Plants, Chemicals, and Reagents
HPLC-grade formic acid was provided by DIKMA (Lake Forest, CA, USA). HPLC-grade methanol and acetonitrile were supplied by Thermo Fisher Scientific (Pittsburgh, PA, USA) and the water was purchased from Wahaha Group (Hangzhou, China).
Dried and ripped LJF were bought from Bozhou’s herbal market (Haozhou, China) and were stockpiled at the Heilongjiang University of Chinese Medicine. A representative specimen was deposited in the laboratory, and plant materials were authenticated by Prof. Lianjie Su from Heilongjiang University of Chinese Medicine, Harbin, China. The LJF sample was refluxed with ethanol:water (1:1, v/v) at a solid:liquid ratio of 1:10 for 2 h and then filtered. The residue was extracted again under the same conditions. The combined filtrates were concentrated under reduced pressure to remove the ethanol solvent. The aqueous solution was then freeze-dried under a vacuum. The dried powders were finally redissolved and dispersed in water for administration to rats.
The reference standards 3-CQA, 4-CQA, 5-CQA, 3,5-diCQA, 4,5-diCQA, Sweroside, Secoxyloganin, and daidzein (IS2) were purchased from Chengdu Must Biotechnology (Chengdu, China). The purity of each standard substance was higher than 98%. Furthermore, heparin sodium (Shanghai Pharmaceutical, Shanghai, China) was used for the mobile phase of chromatographic analysis and blood sample preparation. The chloramphenicol (IS1) was obtained from Sigma (St. Louis, MO, USA) with a purity of 99.99%.
4.2. Instrumentation
4.2.1. Chromatographic Conditions
Eight compounds were separated on a Thermo Hypersil GOLD C18 column (100 mm × 2.1 mm, 1.9 µm) with the mobile phase consisting of (A) menthol and (B) 0.1% formic acid in water. The UPLC elution condition was optimized as follows: 0–6 min, 90% B–83% B, 6–7 min, 83% B–60% B, 7–12 min, 60% B–50% B, and 12–13 min, 50% B–90% B. The injection volume was set to 2 µL, and the column temperature was maintained at 45 °C.
4.2.2. MS Conditions
MS analysis was adopted with a TSQ QuantisTM Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific, USA) via selected reaction monitoring (SRM). A negative-ion scan was used to obtain the precursor ion and production spectra of phenolic acids, Secoxyloganin, and IS1, and a positive-ion scan was used to obtain the precursor ion and production spectra of Sweroside and IS2. Simultaneously, the data were acquired using Trace Finder TM software. The optimized parameters for 10 analytes (including IS1 and IS2) are shown in Table 1. Other optimized MS parameters were as follows: spray voltage 3500 V, the temperatures of the ion transfer tube and vaporizer were set at 325 and 350 °C, respectively, and the sheath gas and auxiliary gas pressures were 30 and 10 Arb, respectively.
4.3. Experiments on Animals
Specific acid-free male Sprague-Dawley rats with body weights of 250 ± 20 g were purchased from the Experimental Animal Center of Heilongjiang University of Chinese Medicine and fed in the SPF animal laboratory, under a standard room temperature of 25 ± 2 °C, relative humidity of 50 ± 15%, and a 12 h light/12 h dark cycle. The experiment followed the ethical norms of experimental animals of Heilongjiang University of Chinese Medicine (License number: SCXK (Hei) 2021-004). Twelve rats were randomly divided into normal and model groups of six rats each. After intraperitoneal injection of CCl4 (0.06 mL/kg) for 24 h, blood samples were collected into heparinized tubes via the orbital vein, centrifuged at 2000 r/min for 20 min, and the levels of AST and ALT in serum were determined by the microplate method.
4.4. Pharmacokinetic Studies
The pharmacokinetic properties and parameters of 3-CQA, 4-CQA, 5-CQA, 3,5-diCQA, 4,5-diCQA, PA, Secoxyloganin, and Sweroside in rats were studied after oral administration of the LJF extract. After the model was successfully induced, 0.83, 0.17, 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 36, and 48 h after administration of the ethanol extract of LJF (15.75 g/kg), 0.3 mL of blood was collected from the fundus venous plexus of rats and placed into a 1.5 mL centrifuge tube containing heparin sodium. The supernatant plasma was then transferred to a 1.5 mL centrifuge tube and stored at −20 °C for later use.
4.5. Correction Curves and Quality Control Samples Were Prepared
The 3-CQA, 4-CQA, 5-CQA, 3,5-diCQA, 4,5-diCQA, PA, Secoxyloganin, Sweroside, chloramphenicol, and daidzein were dissolved in methanol and added into the original standard solution at a concentration of 1 mg/mL. The original solution was continuously diluted with methanol:water (1:1, v/v). The internal standard solution of 500 ng/mL of chloramphenicol (IS1) and daidzein (IS2) and the gradient working solution of the analyte were obtained.
The standard curve samples were obtained by spiking the series standard solutions (100 μL) into blank rat plasma (100 μL) to yield the concentration ranges of 3-CQA, 4-CQA, 5-QCA, 3,5-diCQA, 4,5-diCQA, PA, Sweroside, and Secoxyloganin. The linearity ranges were: 25–13,500 ng/mL for 3-CQA, 7–3500 ng/mL for 4-CQA, 2.5–2100 ng/mL for 5-CQA, 30–5000 ng/mL for 3,5-diCQA, 7.5–2500 ng/mL for 4,5-diCQA, 3–300 ng/mL for PA, 15–6000 ng/mL for Sweroside, and 25–4500 ng/mL for Secoxyloganin.
4.6. Sample Preparation
Here, 100 μL aliquots of the plasma sample were thawed and pipetted into centrifuge tubes and 10 μL of chloramphenicol solution (500 ng/mL) and daidzein solution (500 ng/mL) were added, respectively, and 780 μL of methanol was added to precipitate for 5 min. Then, the supernatant was centrifuged for 5 min at 12,000 rp/min. The supernatant was transferred to a nitrogen blowing machine and dried at 40 °C. The residue was reconstituted in 100 μL of 50% methanol, and the sample was filtered through the membrane and then injected into the UPLC-MS/MS system.
4.7. Data Analysis
DAS 2.0 software was used to calculate the pharmacokinetic parameters of the non-atrioventricular model, including Cmax, Tmax, AUC0–t, AUC0–∞, MRT0–t, MRT0–∞, and t1/2. The statistical software GraphPad Prism 5.0 was used for graph plotting and statistical analysis of drug duration curves. The results are shown in Figure 3 and Table 1.
4.8. Method Validation
4.8.1. Specificity
To assess obvious sources of interference at the retention times of interest in blank samples under the same operating conditions, the specificity of the values of the current method was investigated. The differences between the target analytes and interference were analyzed by comparing chromatograms from blank plasma and plasma spiked with target analytes and IS1 and IS2. The specificity was assessed by analyzing the chromatograms of blank plasma and plasma samples spiked with the eight components, IS1, and IS2, along with real plasma samples collected from LJF extract-treated groups.
4.8.2. Linearity and Lower Limits of Quantification
Weighted least-square linear regression (1/x2) was used to plot the ratio of the peak area of the analyte to IS to the plasma concentration, and the correction curves of the eight analytes were constructed. The linear correlation coefficient should be greater than 0.99. Lower limits of quantification (LLOQ) were detected on the minimum analysis of six repeated plasma samples on the calibration curve. The signal-to-noise ratio of LLOQ should be greater than 5. The precision (RSD) and accuracy (RE) required by the LLOQ should be within 20% and 15%, respectively.
4.8.3. Accuracy and Precision
Three different concentrations of QC samples were prepared. For each concentration, five samples were analyzed and measured for three consecutive days. The concentration of QC samples was calculated according to the standard curve of daily travel. The intra-day and intra-day precision values (expressed by RSD %) was not more than 15%, and accuracy (expressed as Re %) is required to be within ±15%.
4.8.4. Recovery and Matrix Effect
Three QC samples of different concentrations were prepared for each compound. Comparing analytical results of analytes in extracted samples with corresponding extracts of blanks spiked with the analytes post-extraction. The matrix effect was measured by comparing peak areas of analytes in post-extracted samples with those acquired from pure solutions. The effect of the plasma matrix on the measured compounds and internal standard compounds was determined by comparing the peak areas of the standard solution and the standard solution with the same concentration after plasma samples’ treatment, as to ensure that the coefficient of variation was within 15%.
4.8.5. Stability
For the measurement of stability of the eight analytes and IS in rat plasma, three QC samples of each concentration at low, medium, and high levels were prepared for analysis under different storage conditions. Stability in plasma was assessed by analyzing samples kept at room temperature for 8 h, samples stored at 80 °C for 30 days, and samples after 3 freeze–thaw cycles. The stability of the stock solutions was determined 24 h after storage at 4 °C. RE was used to express stability.
5. Conclusions
In this study, a rapid, sensitive, and selective method for simultaneous determination of two iridoid glycosides and six phenolic acids in rat plasma was established by UPLC-MS/MS. In addition, this method was successfully used for the first time in the pharmacokinetic study of eight analytes in normal rats and liver injury model rats after oral administration of LJF. There were significant differences in some pharmacokinetic parameters between the two groups, which might be related to the pathology of acute liver injury induced by CCl4 and the pharmacological effects of analytes. In addition, this study also provides a reference for further drug development and clinical application of LJF.
Author Contributions
Conceptualization, H.J. and L.Y. (Liu Yang); methodology, X.W.; software and validation, J.D., S.Z. and J.L.; formal analysis, S.L.; data curation, X.W.; writing—original draft preparation, X.W.; writing—review and editing, X.W.; visualization and supervision, H.J. and L.Y. (Liu Yang); project administration, L.Y. (Lin Yang); funding acquisition, H.J. and L.Y. (Liu Yang). All authors have read and agreed to the published version of the manuscript.
Funding
The following financial support was provided for this article: The National Natural Science Foundation of China, Grant/Award Numbers: 81703684 and 81973604. The Heilongjiang Touyan Innovation Team Program. The National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Construction Program of the National Administration of TCM (No. [2022], No. 75). The Seventh Batch of National Old Traditional Chinese Medicine Experts Academic Experience Inheritance Program (No. [2022], No. 76). The Traditional Chinese Medicine Processing Technology Inheritance Base Project. Central Finance to Support the Development of Local Universities. The Graduate Innovative Research Project Foundation of Heilongjiang University of Chinese Medicine (No. 2022yjscx059).
Institutional Review Board Statement
Specific-acid free male Sprague-Dawley rats with body weights of 250 ± 20 g were purchased from the Experimental Animal Center of Heilongjiang University of Chinese Medicine. The experiment followed the ethical norms of experimental animals of Heilongjiang University of Chinese Medicine (License number: SCXK (Hei) 2021-004).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, T.L.; Dong, C.M.; Gao, Q.G.; Zhang, J.S.; Qi, D.M.; Xu, X.L.; Li, J.H. Brief discussion on textual research ideas of ancient books and textual collection of predecessors:A case study of Lonicera japonica. Chin. Tradit. Herb. Drugs 2021, 52, 15. [Google Scholar]
- Wang, Y.D.; Yang, J.B.; Zhong, Z.; Ma, S.C. Research progress on Lonicerae japonicae flos. Chin. J. Pharm. Anal. 2014, 16, 1928–1935. [Google Scholar]
- Liu, S.T.; Yang, L.; Wang, S.; Wang, X.J.; Zhang, J.X.; Hou, A.J.; Jiang, H. Study on Chemical Components of Honeysuckle. Inf. Tradit. Chin. Med. 2020, 37, 18–21. [Google Scholar]
- Ehrman, T.M.; Barlow, D.J.; Hylands, P.J. In silico search for multi-target anti-inflammatories in Chinese herbs and formulas. Bioorganic Med. Chem. 2010, 18, 2204–2218. [Google Scholar] [CrossRef]
- Lou, H.X.; Lang, W.J.; Lv, M.J. Separation and structure determination of water-soluble chemical compounds in Lonicerae japonicae flos. Chin. Tradit. Herb. Drugs 1996, 27, 195–199. [Google Scholar]
- Zhang, R.; Wang, T.T.; Su, P.W.; Li, M.M.; Huang, P.; Li, H.G. Study on Quality Standard of Organic Acid Effective Part of Honeysuckle. Asia-Pac. Tradit. Med. 2020, 16, 76–80. [Google Scholar]
- Huang, L.J. Study on Synthesis of Bisantins Analogues from Lonicerae japonicae flos; Peking Union Medical College of China: Beijing, China, 2006. [Google Scholar]
- Chen, X.Y.; Qin, S.S.; Li, C.; Wu, Q.H.; Jiang, C.; Yang, J.; Guo, X.Y.; Ou, C.L. Differential Gene Expressions and Phytohormone Changes Altered Lonicera japonica Quality after Plant Introduction. World J. Tradit. Chin. Med. 2019, 60, 18–23. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.Y.; Xu, H.L.; Wu, W.B.; He, X.; Wang, X.Y.; Jiang, M.; Hou, Y.Y.; Bai, G. A natural AKT inhibitor swertiamarin targets AKT-PH domain, inhibits downstream signaling, and alleviates inflammation. FEBS J. 2020, 287, 1816–1829. [Google Scholar] [CrossRef]
- Rahman, A.; Kang, S.C. In vitro control of food-borne and food spoilage bacteria by essential oil and ethanol extracts of Lonicera japonica Thunb. Food Chem. 2009, 116, 670–675. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.B.; Shen, J.; Zhang, Z.F. Screening of active components from Chinese materia medica against SARS-CoV-2 based on literature mining and molecular docking. Chin. Tradit. Herb. Drugs 2020, 51, 845–850. [Google Scholar]
- Liu, J.; Yan, B.F.; Zeng, M.Y.; Zeng, Q.Q.; Yang, H.J.; Zhang, J.Z. Potential Mechanism of Couplet Medicines Baical Skullcap Root Honeysuckle for Coronavirus Disease 2019 Based on Network Pharmacology. World Chin. Med. 2020, 15, 502–511. [Google Scholar]
- Fu, M.R.; Qu, Q.L.; Dai, H.F. Variation in antioxidant properties and metabolites during flower maturation of Flos Lonicerae Japonicae flowers. Eur. Food Res. Technol. 2015, 240, 735–741. [Google Scholar] [CrossRef]
- Yin, H.M.; Lv, X.Y. Study on preparation technology optimization and immune activity of Polysaccharide from Flos Lonicerae Japonicae flowers. China J. Chin. Mater. Med. 2010, 35, 453–455. [Google Scholar]
- Miao, H.; Zhang, Y.; Huang, Z.L.; Lu, B.; Ji, L.L. Lonicera japonica Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in Mice: Molecular Mechanisms of Action. Am. J. Chin. Med. 2019, 42, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.F.; Deng, L.H.; Mao, Z.H.; Zhang, X.R. Jinyinghua Oral Liquid in Combination with Interferon Spray for Treatment of Hand Foot and Mouth Disease in 40 Children with Pathogens Invading Lung-Spleen Pattern. J. Tradit. Chin. Med. 2021, 62, 687–690. [Google Scholar]
- Wang, S.; Yang, L.; Hou, A.J.; Liu, S.T.; Yang, L.; Jiang, H.; Kuang, H.X. Screening hepatoprotective effective components of Lonicerae japonica Flos based on the spectrum-effect relationship and its mechanism exploring. Food Sci. Hum. Wellness 2022, 12, 283–294. [Google Scholar] [CrossRef]
- Qiao, Y.R.; Wang, Z.H.; Wang, Y.F. Effect of pneumonia No.3 combined with honeysuckle granules on lung function, serum inflammatory factors and immune function in children with pneumonia. J. Guangxi Med. Univ. 2021, 38, 132–137. [Google Scholar]
- Zhang, H.T.; Chen, J.L.; Zhu, X.Y.; Zeng, H.L.; Li, J.; Sun, X.B.; Qi, X.Y.; Zeng, J.C.; Zeng, Y.R. Effect and molecular mechanism of Honeysuckle-Rhizoma coptidis in the treatment of periprosthetic joint infection based on molecular docking and network pharmacology. Chin. J. Tissue Eng. Res. 2021, 25, 3360–3367. [Google Scholar]
- Fung, J.; Lai, C.L.; Yuen, M.F. Hepatitis B virus DNA and hepatitis B surface antigen levels in chronic hepatitis B. Expert Rev. Anti-Infect. Ther. 2010, 8, 717–726. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Zhang, J.X.; Li, N.; Xu, Q.Y.; Yang, Y.; Xie, H.B.; Shen, T.; Zhu, Q.X. Kupffer cell depletion attenuates IL-6/STAT3 mediates hepatocyte apoptosis in immunological liver injury of trichloroethylene sensitized mice. Int. Immunopharmacol. 2020, 88, 106897. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Koch, D.G.; Lee, W.M. Drug-induced acute liver failure: Results of a u.s. multicenter, prospective study. Hepatol. Off. J. Am. 2010, 52, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- El-Kamary, S.S.; Shardell, M.D.; Abdel-Hamid, M.; Ismail, S.; El-Ateek, M. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. J. Phytomed. 2009, 16, 391–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, L.; Bi, Q.; Dong, J.C.; Yang, X.X.; Yu, J. Progress in the development of natural drugs with liver protection. Biot. Resour. 2018, 02, 59–69. [Google Scholar]
- Hu, C.M.; Jiang, H.; Liu, H.F.; Li, R.; Li, J. Protective effect of Lonicera japonica total flavone (LTF)on immunological liver injury in mice. Pharmacol. Clin. Chin. Mater. Med. 2007, 23, 35. [Google Scholar]
- Chen, H.L. Effects of Flos Lonicerae Japonicae on acute liver injury induced by carbon tetrachloride in mice. Chin. J. Gerontol. 2011, 31, 3086–3087. [Google Scholar]
- Wang, D.S. Protective effect of extracts from Flos Lonicerae Japonicae on liver injury mice. Her. Med. 2011, 30, 1010–1012. [Google Scholar]
- Zhou, Y.L.; Zeng, R.; Pei, Q.; Liu, S.K. Pharmacokinetics and bioavailability of chlorogenic acid extracted from Jinyinhua in rats. Chin. J. Hosp. Pharm. 2016, 36, 164–167. [Google Scholar]
- Kanhar, S.; Sahoo, A.K. Meliorative effect of Homalium zeylanicum against carbon tetrachloride-induced oxidative stress and liver injury in rats. Biomed. Pharmacother. 2019, 111, 305–314. [Google Scholar] [CrossRef]
- Yang, C.C.; Fang, J.Y.; Hong, T.L.; Wang, T.C.; Zhou, Y.E.; Lin, T.C. Potential antioxidant properties and hepatoprotective effects of an aqueous extract formula derived from three Chinese medicinal herbs against CCl (4)-induced liver injury in rats. Int. Immunopharmacol. 2013, 15, 106–113. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, J.H.; Jeong, H.G. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem. Toxicol. 2007, 45, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.N.; Li, R.; Liu, S.H.; Qiao, Y.X.; Shi, S.; Yuan, S.X.; Zhang, J.P. Establishment of carbon tetrachloride-induced acute liver injury murine model. Chin. J. Liver Dis. (Electron. Version) 2014, 6, 27–31. [Google Scholar]
- Zhi, Y.; Song, Y.N.; Zhang, M.; Wang, J.N.; Zhao, B. Pharmacological effects on carbon tetrachloride induced acute liver injury in mice. Chin. Arch. Tradit. Chin. Med. 2022, 40, 107–109. [Google Scholar]
- Xie, Y.; Hao, H.; An, K.; Yan, L.; Xie, T.; Sun, S.; Dai, C.; Zheng, X.; Lin, X.; Li, J. Integral pharmacokinetics of multiple lignan components in normal, CCl4-induced hepatic injury and hepatoprotective agents pretreated rats and correlations with hepatic injury biomarkers. J. Ethnopharmacol. 2010, 131, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, Q.; Jiang, W.; Xue, P.; Hao, K. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed. Pharmacother. 2017, 91, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Du, G.F.; Dong, J.K.; Zhang, T.; Yang, X.R.; Lu, S.S.; Qu, J.H.; Lu, Y.Y.; Hong, Z.X. Correlation between Fyn and bile acid metabolism in liver fibrosis mice. Infect. Dis. Inf. 2018, 31, 326–330. [Google Scholar]
- Li, L.; Wang, Y.L.; Qing, H.Y.; Qu, Y.Y.; Yang, T.S.; Wang, Z.Y.; Xie, J.R. Research progress on berberine in treatment of nonalcoholic disease by regulation gut-liver axis. Chin. Tradit. Herb. Drugs 2021, 52, 1501–1509. [Google Scholar]
- Li, Y.T.; Wang, L.; Chen, Y.; Chen, Y.B.; Wang, H.Y.; Wu, Z.W.; Li, L.J. Effects of Gut Microflora on Hepatic Damage After Acute Liver Injury in Rats. J. Trauma 2010, 68, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Ding, Y.F.; Yin, X.; Shao, J.Z.; Zhuang, Z.R.; Zhang, T.; Su, P.L.; Peng, Y.R. Progress in understanding hepatic fibrosis and renal fibrosis based on the gut-liver-kidney axis. Acta Pharm. Sin. 2021, 56, 9–20. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).