Performance Optimization of a Developed Near-Infrared Spectrometer Using Calibration Transfer with a Variety of Transfer Samples for Geographical Origin Identification of Coffee Beans
Abstract
1. Introduction
2. Results and Discussion
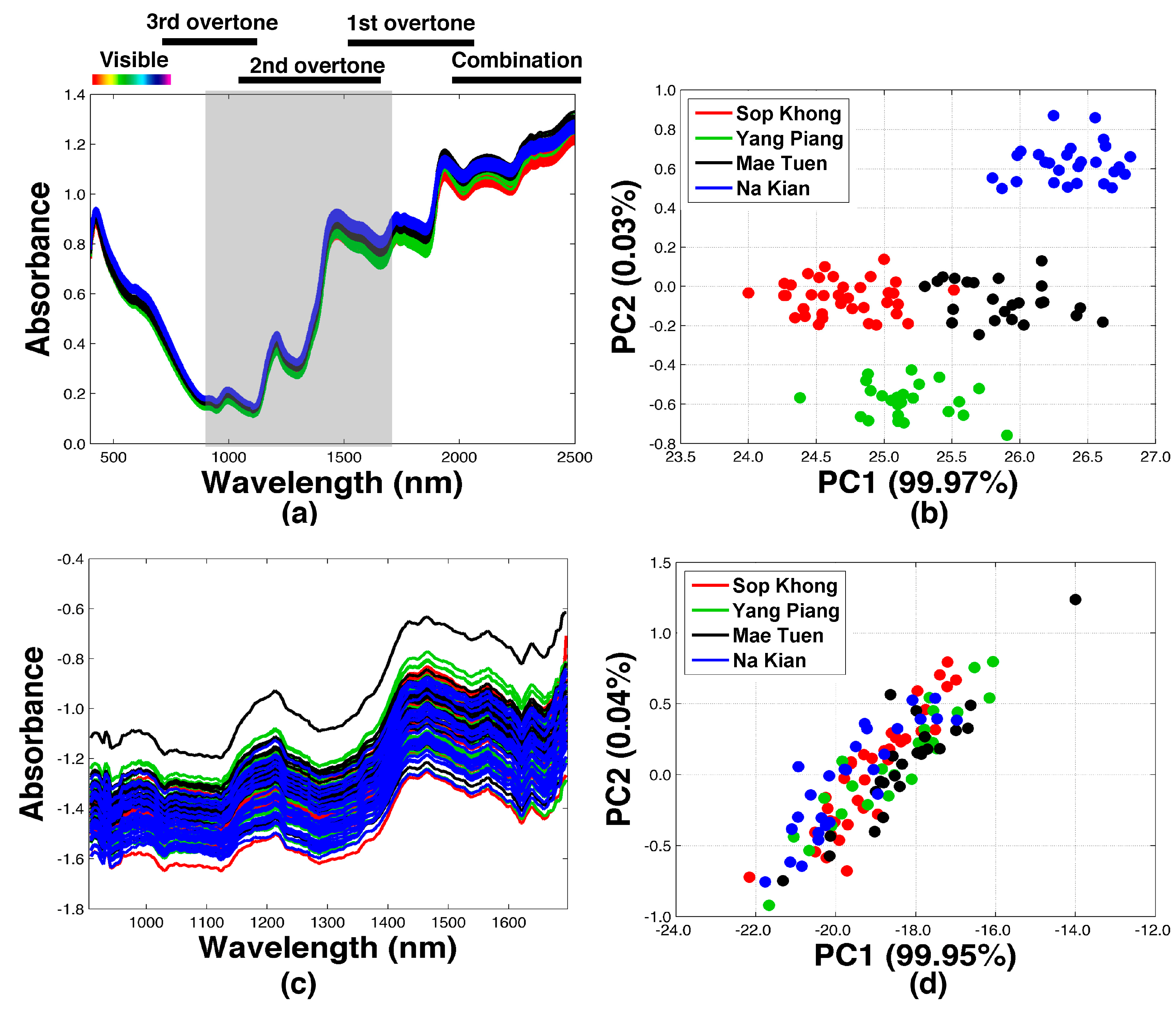
2.1. Exploratory Data Analyses of NIR Spectra
2.2. Transformation of NIR Spectra Using Different Agricultural Samples
2.3. Comparison of Classification Performance
2.3.1. Classification Results Using Uncalibrated Spectra
2.3.2. Classification Results Using Transformed Spectra
2.3.3. Evaluation of Improvement after Spectral Transformation
3. Materials and Methods
3.1. Green Coffee Bean Samples
3.2. Near-Infrared Instruments (NIRs)
3.3. Chemometric Analysis
3.3.1. Piecewise Direct Standardization (PDS)
Transfer Samples
3.3.2. Self-Organizing Map (SOM) for Classification
3.3.3. Davies–Bouldin Index (DBI)
3.3.4. Model Statistics and Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giraudo, A.; Grassi, S.; Savorani, F.; Gavoci, G.; Casiraghi, E.; Geobaldo, F. Determination of the geographical origin of green coffee beans using NIR spectroscopy and multivariate data analysis. Food Control 2019, 99, 137–145. [Google Scholar] [CrossRef]
- Luykx, D.M.; van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Cortés, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Lin, M.; Mousavi, M.; Al-Holy, M.; Cavinato, A.G.; Rasco, B.A. Rapid Near Infrared Spectroscopic Method for the Detection of Spoilage in Rainbow Trout (Oncorhynchus mykiss) Fillet. J. Food Sci. 2006, 71, S18–S23. [Google Scholar] [CrossRef]
- Farres, S.; Srata, L.; Fethi, F.; Kadaoui, A. Argan oil authentication using visible/near infrared spectroscopy combined to chemometrics tools. Vib. Spectrosc. 2019, 102, 79–84. [Google Scholar] [CrossRef]
- Hespanhol, M.C.; Pasquini, C.; Maldaner, A.O. Evaluation of a low-cost portable near-infrared spectrophotometer for in situ cocaine profiling. Talanta 2019, 200, 553–561. [Google Scholar] [CrossRef]
- Modroño, S.; Soldado, A.; Martínez-Fernández, A.; de la Roza-Delgado, B. Handheld NIRS sensors for routine compound feed quality control: Real time analysis and field monitoring. Talanta 2017, 162, 597–603. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; da Silva Medeiros, M.; Barbin, D.F. On-line monitoring of egg freshness using a portable NIR spectrometer in tandem with machine learning. J. Food Eng. 2021, 306, 110643. [Google Scholar] [CrossRef]
- Sedjoah, R.-C.A.-A.; Ma, Y.; Xiong, M.; Yan, H. Fast monitoring total acids and total polyphenol contents in fermentation broth of mulberry vinegar using MEMS and optical fiber near-infrared spectrometers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119938. [Google Scholar] [CrossRef]
- Sharififar, A.; Singh, K.; Jones, E.; Ginting, F.I.; Minasny, B. Evaluating a low-cost portable NIR spectrometer for the prediction of soil organic and total carbon using different calibration models. Soil Use Manag. 2019, 35, 607–616. [Google Scholar] [CrossRef]
- van Kollenburg, G.H.; van Manen, H.-J.; Admiraal, N.; Gerretzen, J.; Jansen, J.J. Low-cost handheld NIR spectroscopy for identification of organic solvents and low-level quantification of water contamination. Talanta 2021, 223, 121865. [Google Scholar] [CrossRef]
- Workman, J.J. A Review of Calibration Transfer Practices and Instrument Differences in Spectroscopy. Appl. Spectrosc. 2018, 72, 340–365. [Google Scholar] [CrossRef] [PubMed]
- Hernández, N.; Gracia, A.; León, L.; Barreiro, P.; Herrero, D. Calibration transfer between portable and laboratory NIR spectrophotometers. Acta Hortic. 2008, 802, 373–378. [Google Scholar] [CrossRef]
- da Silva, N.C.; Cavalcanti, C.J.; Honorato, F.A.; Amigo, J.M.; Pimentel, M.F. Standardization from a benchtop to a handheld NIR spectrometer using mathematically mixed NIR spectra to determine fuel quality parameters. Anal. Chim. Acta 2017, 954, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Milanez, K.D.T.M.; Silva, A.C.; Paz, J.E.M.; Medeiros, E.P.; Pontes, M.J.C. Standardization of NIR data to identify adulteration in ethanol fuel. Microchem. J. 2016, 124, 121–126. [Google Scholar] [CrossRef]
- Abdelkader, M.F.; Cooper, J.B.; Larkin, C.M. Calibration transfer of partial least squares jet fuel property models using a segmented virtual standards slope-bias correction method. Chemom. Intell. Lab. Syst. 2012, 110, 64–73. [Google Scholar] [CrossRef]
- Pissard, A.; Marques, E.J.N.; Dardenne, P.; Lateur, M.; Pasquini, C.; Pimentel, M.F.; Pierna, J.A.F.; Baeten, V. Evaluation of a handheld ultra-compact NIR spectrometer for rapid and non-destructive determination of apple fruit quality. Postharvest Biol. Technol. 2021, 172, 111375. [Google Scholar] [CrossRef]
- Gonjo, T.; Futami, Y.; Morisawa, Y.; Wojcik, M.J.; Ozaki, Y. Hydrogen Bonding Effects on the Wavenumbers and Absorption Intensities of the OH Fundamental and the First, Second, and Third Overtones of Phenol and 2,6-Dihalogenated Phenols Studied by Visible/Near-Infrared/Infrared Spectroscopy. J. Phys. Chem. A 2011, 115, 9845–9853. [Google Scholar] [CrossRef]
- Krongchai, C.; Funsueb, S.; Jakmunee, J.; Kittiwachana, S. Application of multiple self-organizing maps for classification of soil samples in Thailand according to their geographic origins. J. Chemom. 2017, 31, e2871. [Google Scholar] [CrossRef]
- Phuangsaijai, N.; Theanjumpol, P.; Muenmanee, N.; Kittiwachana, S. Fabrication of a low-cost NIR spectrometer for detection of agricultural product quality. Chiang Mai J. Sci. 2021, 48, 332–340. [Google Scholar]
- Kaewpangchan, P.; Phuangsaijai, N.; Seehanam, P.; Theanjumpol, P.; Maniwara, P.; Kittiwachana, S. Screening of coffee impurity using a homemade NIR sensor system. Chiang Mai J. Sci. 2021, 48, 292–300. [Google Scholar]
- Ji, W.; Viscarra Rossel, R.A.; Shi, Z. Improved estimates of organic carbon using proximally sensed vis-NIR spectra corrected by piecewise direct standardization. Eur. J. Soil Sci. 2015, 66, 670–678. [Google Scholar] [CrossRef]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Bouveresse, E.; Massart, D. Improvement of the piecewise direct standardisation procedure for the transfer of NIR spectra for multivariate calibration. Chemom. Intell. Lab. Syst. 1996, 32, 201–213. [Google Scholar] [CrossRef]
- Wongsaipun, S.; Theanjumpol, P.; Kittiwachana, S. Development of a Universal Calibration Model for Quantification of Adulteration in Thai Jasmine Rice Using Near-infrared Spectroscopy. Food Anal. Methods 2021, 14, 997–1010. [Google Scholar] [CrossRef]
- Astel, A.; Tsakovski, S.; Barbieri, P.; Simeonov, V. Comparison of self-organizing maps classification approach with cluster and principal components analysis for large environmental data sets. Water Res. 2007, 41, 4566–4578. [Google Scholar] [CrossRef]
- Kittiwachana, S.; Wangkarn, S.; Grudpan, K.; Brereton, R.G. Prediction of liquid chromatographic retention behavior based on quantum chemical parameters using supervised self organizing maps. Talanta 2013, 106, 229–236. [Google Scholar] [CrossRef]
- Brereton, R.G. Chemometrics for Pattern Recognition; John Wiley & Sons Inc.: New York, NY, USA, 2009. [Google Scholar]
- Luna, A.S.; da Silva, A.P.; Alves, E.A.; Rocha, R.B.; Lima, I.C.A.; de Gois, J.S. Evaluation of chemometric methodologies for the classification of Coffea canephora cultivars via FT-NIR spectroscopy and direct sample analysis. Anal. Methods 2017, 9, 4255–4260. [Google Scholar] [CrossRef]
- Dixon, S.J.; Heinrich, N.; Holmboe, M.; Schaefer, M.L.; Reed, R.R.; Trevejo, J.; Brereton, R.G. Use of cluster separation indices and the influence of outliers: Application of two new separation indices, the modified silhouette index and the overlap coefficient to simulated data and mouse urine metabolomic profiles. J. Chemom. 2009, 23, 19–31. [Google Scholar] [CrossRef]
- Theanjumpol, P.; Wongzeewasakun, K.; Muenmanee, N.; Wongsaipun, S.; Krongchai, C.; Changrue, V.; Boonyakiat, D.; Kittiwachana, S. Non-destructive identification and estimation of granulation in ‘Sai Num Pung’ tangerine fruit using near infrared spectroscopy and chemometrics. Postharvest Biol. Technol. 2019, 153, 13–20. [Google Scholar] [CrossRef]
- Dixon, S.J.; Xu, Y.; Brereton, R.G.; Soini, H.A.; Novotny, M.V.; Oberzaucher, E.; Grammer, K.; Penn, D.J. Pattern recognition of gas chromatography mass spectrometry of human volatiles in sweat to distinguish the sex of subjects and determine potential discriminatory marker peaks. Chemom. Intell. Lab. Syst. 2007, 87, 161–172. [Google Scholar] [CrossRef]
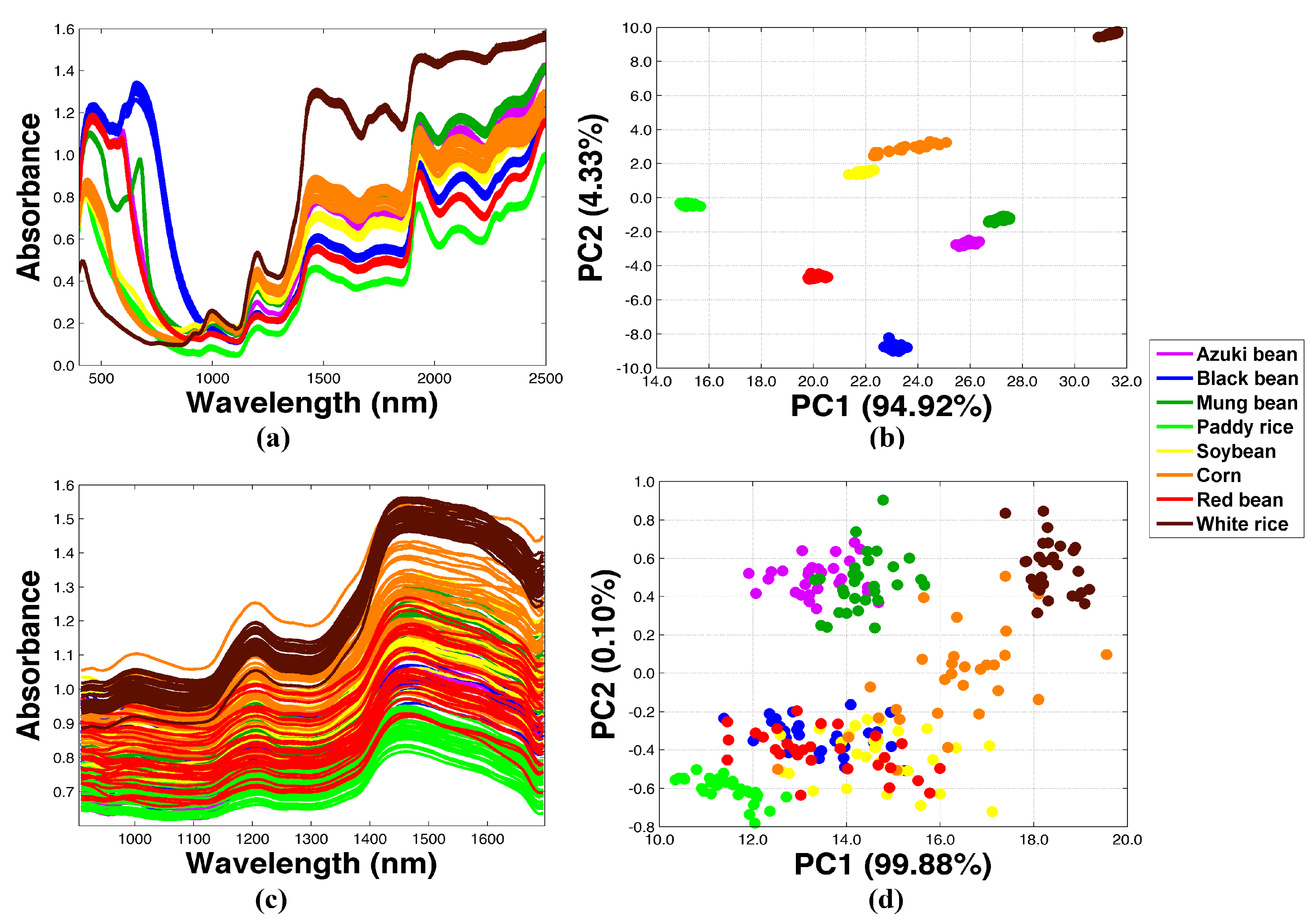
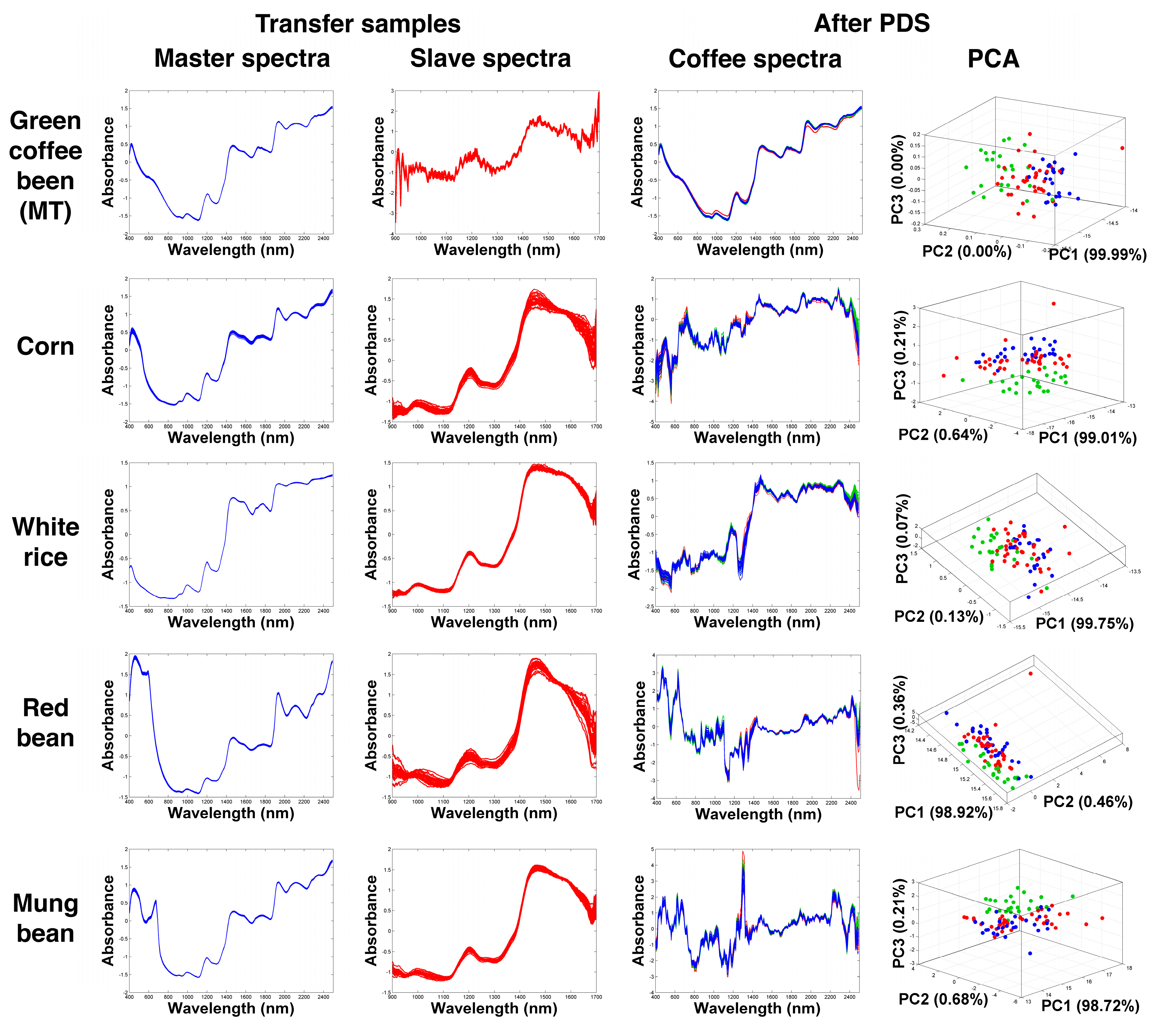
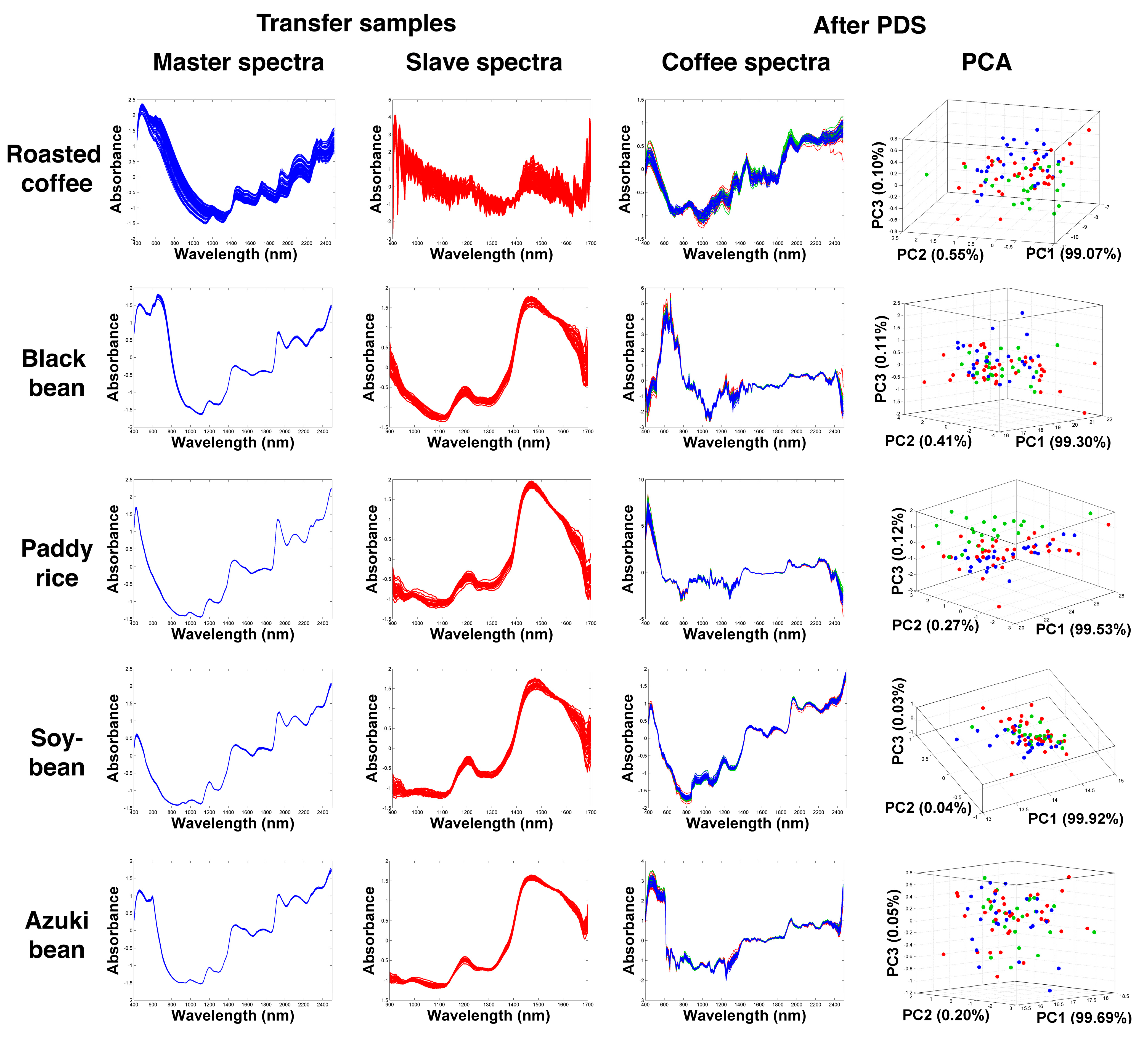
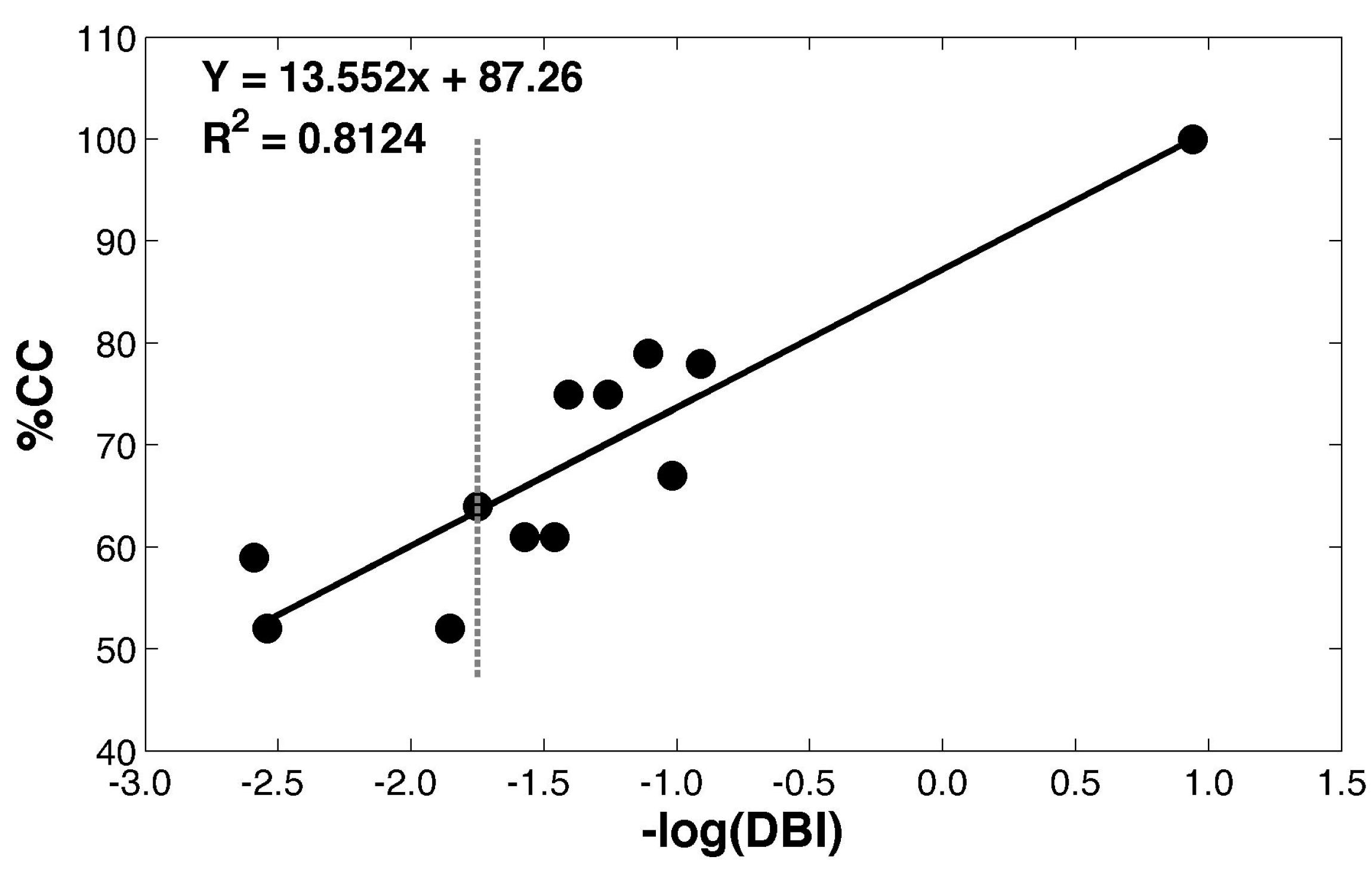
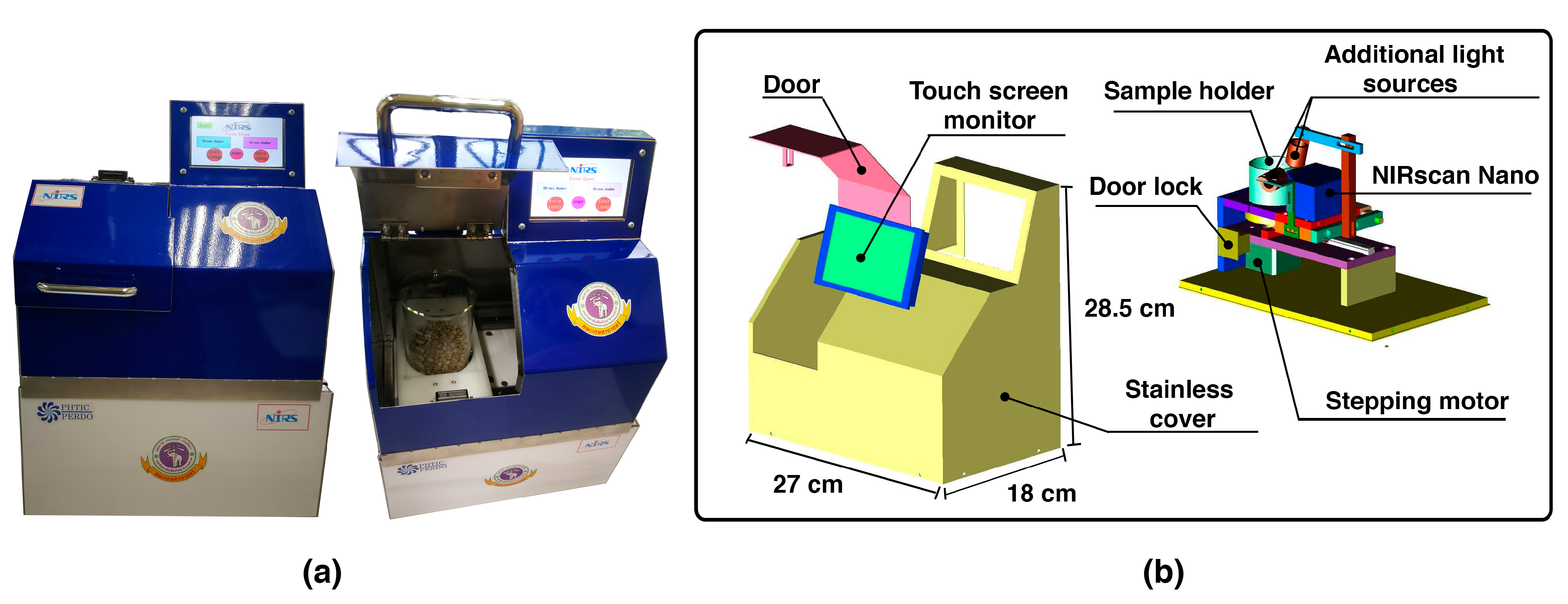
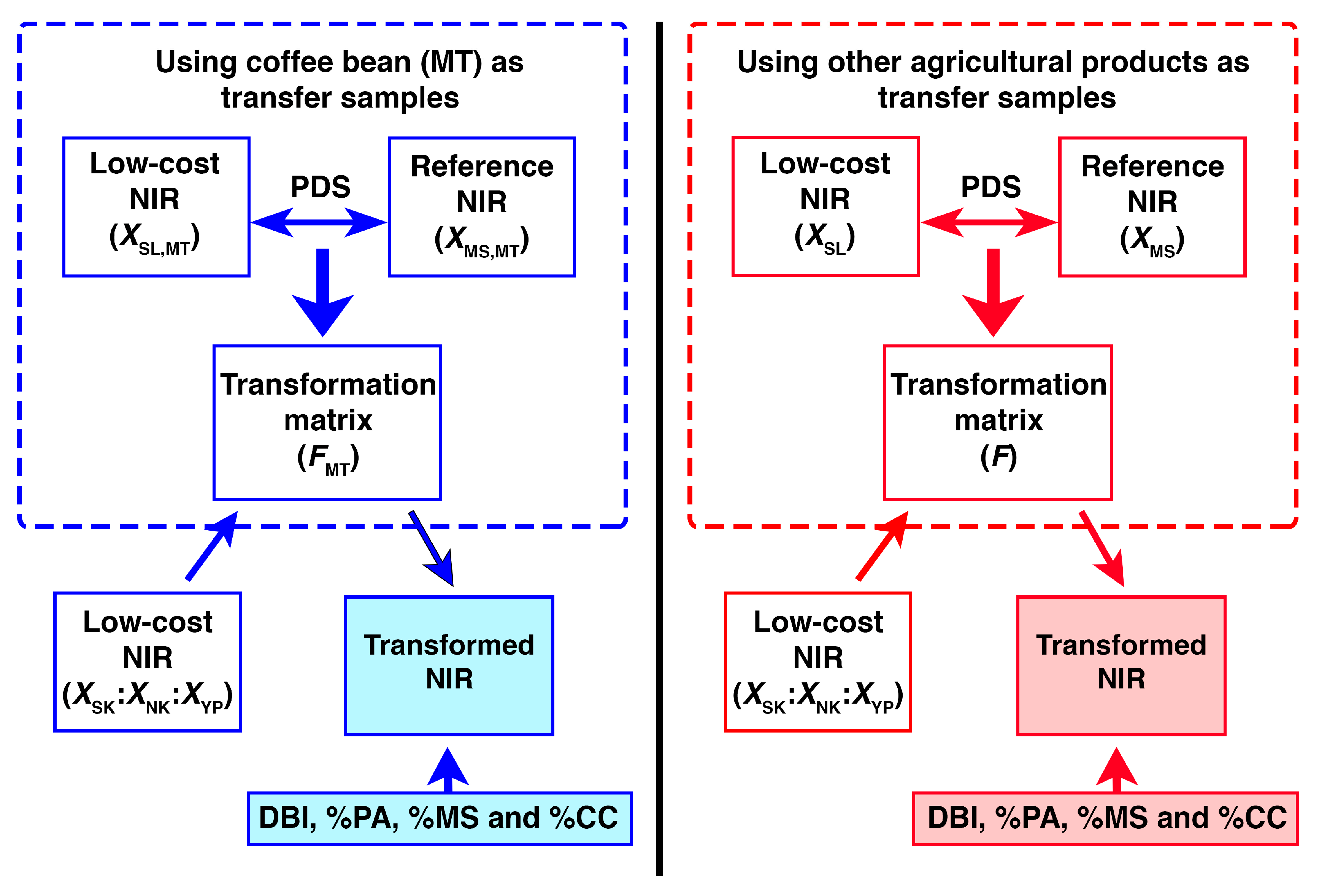
| Spectrometer | Transfer Sample | % PA | % MS | % CC | DBI | −log(DBI) | |||
|---|---|---|---|---|---|---|---|---|---|
| Tr * | Ts ** | Tr | Ts | Tr | Ts | ||||
| NIRSystem 6500 | - | 100 | 98 | 100 | 96 | 100 | 100 | 0.39 | 0.94 |
| Homemade instrument | - | 98 | 61 | 96 | 58 | 100 | 64 | 5.75 | −1.75 |
| Corn | 97 | 71 | 95 | 67 | 100 | 79 | 3.03 | −1.11 | |
| Red bean | 96 | 71 | 93 | 67 | 99 | 75 | 4.09 | −1.41 | |
| Mung bean | 97 | 59 | 93 | 60 | 100 | 67 | 2.77 | −1.02 | |
| White rice | 96 | 66 | 92 | 58 | 99 | 75 | 3.53 | −1.26 | |
| Green coffee (MT) | 99 | 70 | 99 | 65 | 100 | 78 | 2.49 | −0.91 | |
| Black bean | 96 | 59 | 92 | 66 | 100 | 61 | 4.83 | −1.57 | |
| Roasted coffee | 98 | 55 | 96 | 56 | 100 | 61 | 4.31 | −1.46 | |
| Adzuki bean | 93 | 46 | 87 | 53 | 100 | 52 | 12.72 | −2.54 | |
| Paddy rice | 92 | 51 | 85 | 52 | 99 | 52 | 6.38 | −1.85 | |
| Soybean | 91 | 52 | 82 | 57 | 99 | 59 | 13.36 | −2.59 | |
| Plantation Area | No. of Samples | Altitude (m) | Width (mm) | Length (mm) | Thickness (mm) | Weight (g) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| Sop Khong (SK) | 36 | 1107 | 7.6 ± 0.6 | 10.7 ± 0.9 | 4.6 ± 0.6 | 0.137 ± 0.03 | 0.99 ± 0.22 |
| Yang Piang (YP) | 27 | 936 | 8.0 ± 0.6 | 10.4 ± 1.1 | 4.9 ± 0.5 | 0.152 ± 0.03 | 1.01 ± 0.24 |
| Mae Tuen (MT) | 24 | 441 | 8.1 ± 0.5 | 11.1 ± 1.1 | 4.9 ± 0.5 | 0.156 ± 0.03 | 0.95 ± 0.14 |
| Na Kian (NK) | 24 | 1212 | 7.9 ± 0.6 | 10.5 ± 0.9 | 4.9 ± 0.4 | 0.142 ± 0.03 | 0.92 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuangsaijai, N.; Theanjumpol, P.; Kittiwachana, S. Performance Optimization of a Developed Near-Infrared Spectrometer Using Calibration Transfer with a Variety of Transfer Samples for Geographical Origin Identification of Coffee Beans. Molecules 2022, 27, 8208. https://doi.org/10.3390/molecules27238208
Phuangsaijai N, Theanjumpol P, Kittiwachana S. Performance Optimization of a Developed Near-Infrared Spectrometer Using Calibration Transfer with a Variety of Transfer Samples for Geographical Origin Identification of Coffee Beans. Molecules. 2022; 27(23):8208. https://doi.org/10.3390/molecules27238208
Chicago/Turabian StylePhuangsaijai, Nutthatida, Parichat Theanjumpol, and Sila Kittiwachana. 2022. "Performance Optimization of a Developed Near-Infrared Spectrometer Using Calibration Transfer with a Variety of Transfer Samples for Geographical Origin Identification of Coffee Beans" Molecules 27, no. 23: 8208. https://doi.org/10.3390/molecules27238208
APA StylePhuangsaijai, N., Theanjumpol, P., & Kittiwachana, S. (2022). Performance Optimization of a Developed Near-Infrared Spectrometer Using Calibration Transfer with a Variety of Transfer Samples for Geographical Origin Identification of Coffee Beans. Molecules, 27(23), 8208. https://doi.org/10.3390/molecules27238208







