Effect of Cordyceps spp. and Cordycepin on Functions of Bones and Teeth and Related Processes: A Review
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Ergothioneine
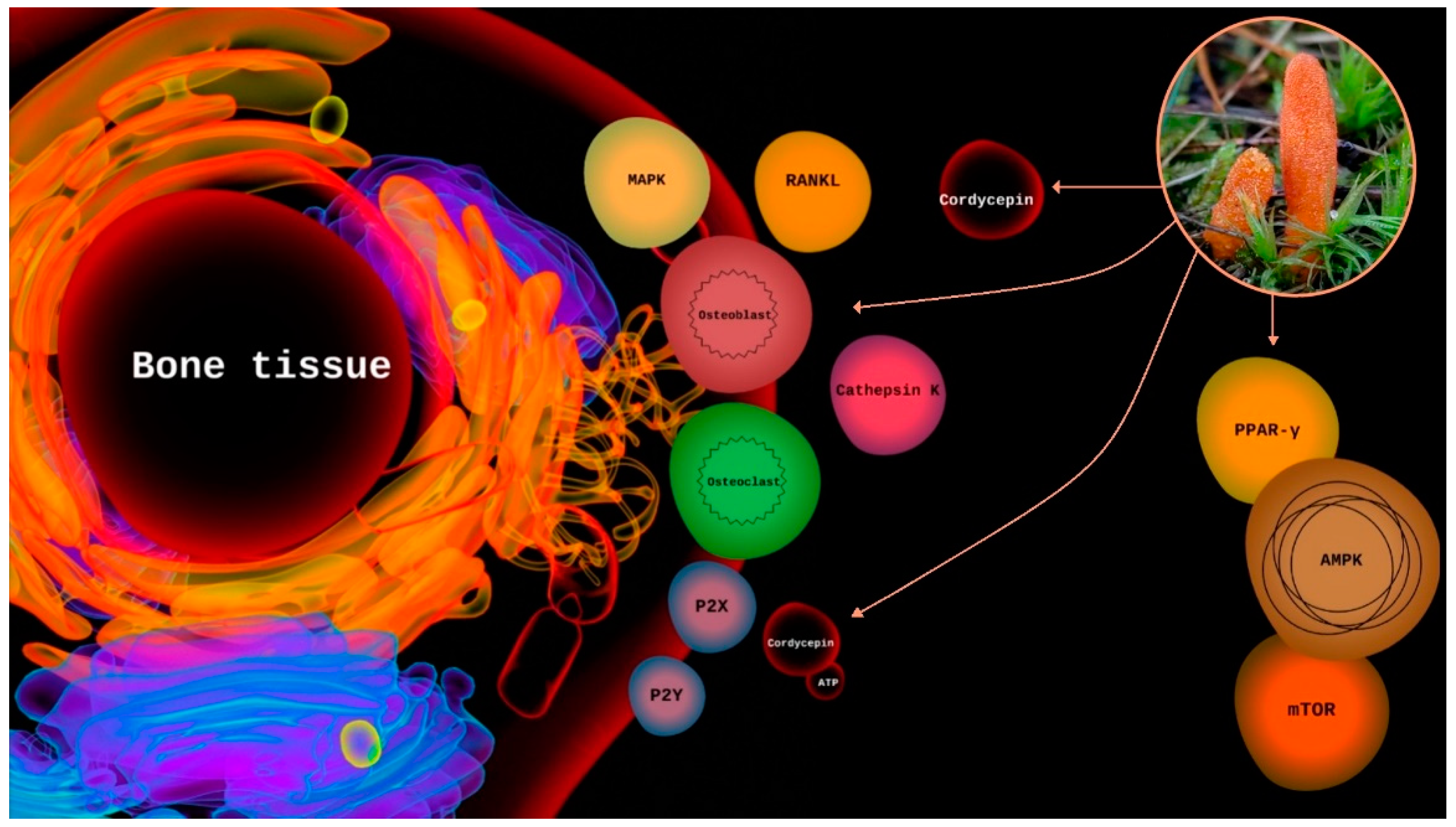
3.2. Purinergic Signaling
3.3. Cordyceps spp. as a Source of Calcium, Phosphorus, Fluoride, and Vitamin D
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khosla, S.; Hofbauer, L.C. Osteoporosis Treatment: Recent Developments and Ongoing Challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, J.; Buckland, G.; Chan, P.E.; Ong, A.; Ramos, A.S.; Baxter, M.; Laskou, F.; Dennison, E.M.; Cooper, C.; Patel, H.P. Pathophysiology and Treatment of Osteoporosis: Challenges for Clinical Practice in Older People. Aging Clin. Exp. Res. 2021, 33, 759–773. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.v.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden: A report prepared in collaboration with the international osteoporosis foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 6 October 2022).
- Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html (accessed on 6 October 2022).
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Yi, X.; Wang, W.; Xie, Q. Adenosine Receptors enhance the atp-induced odontoblastic differentiation of human dental pulp cells. Biochem. Biophys. Res. Commun. 2018, 497, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Binderman, I.; Gadban, N.; Yaffe, A. Extracellular ATP Is a Key modulator of alveolar bone loss in periodontitis. Arch. Oral. Biol. 2017, 81, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.R. Role of the Purinergic P2X Receptors in Osteoclast Pathophysiology. Curr. Opin. Pharmacol. 2019, 47, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, Y.M.; Lin, M.M.; Wang, Y.; Zhang, X.; Liang, L.; He, X. P2X7Rs: New Therapeutic Targets for Osteoporosis. Purinergic Signal. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Haertel, B. Medicinal mushrooms for prevention and therapy of osteoporosis. Int. J. Med. Mushrooms 2021, 23, 13–22. [Google Scholar] [CrossRef]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A Review. Phytother. Res. 2021, 35, 1284–1297. [Google Scholar] [CrossRef]
- Jędrejko, K.J.; Lazur, J.; Muszyńska, B. Cordyceps militaris: An overview of its chemical constituents in relation to biological activity. Foods 2021, 10, 2634. [Google Scholar] [CrossRef]
- Jędrejko, K.; Kała, K.; Sułkowska-Ziaja, K.; Krakowska, A.; Zięba, P.; Marzec, K.; Szewczyk, A.; Sękara, A.; Pytko-Polończyk, J.; Muszyńska, B. Cordyceps militaris—Fruiting bodies, mycelium, and supplements: Valuable component of daily diet. Antioxidants 2022, 11, 1861. [Google Scholar] [CrossRef]
- Boontiam, W.; Wachirapakorn, C.; Phaengphairee, P.; Wattanachai, S. Effect of Spent Mushroom (Cordyceps militaris) on Growth Performance, Immunity, and Intestinal Microflora in Weaning Pigs. Animals 2020, 10, 2360. [Google Scholar] [CrossRef]
- Boontiam, W.; Wachirapakorn, C.; Wattanachai, S. Growth performance and hematological changes in growing pigs treated with Cordyceps militaris spent mushroom substrate. Vet. World 2020, 13, 768. [Google Scholar] [CrossRef]
- European Commission (EC). Register of Nutrition and Health Claims. Available online: https://ec.europa.eu/search/ (accessed on 16 September 2022).
- European Commission (EC). Rapid Alert System for Food and Feed (RASFF). Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 6 October 2022).
- Nutraveris. Database. Available online: https://www.nutraveris.com/en/solutions/nol-data-solution/ (accessed on 16 September 2022).
- Long, H.; Qiu, X.; Cao, L.; Liu, G.; Rao, Z.; Han, R. Toxicological safety evaluation of the cultivated chinese Cordyceps. J. Ethnopharmacol. 2021, 268, 113600. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Jędrejko, K.; Sułkowska-Ziaja, K.; Ziaja, M.; Kała, K.; Muszyńska, B. Edible mushrooms as a potential component of dietary interventions for major depressive disorder. Foods 2022, 11, 1489. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Jiang, D. Effect of Cordyceps Sinensis on Erythropoiesis in Mouse Bone Marrow. Chin. Med. J. 1993, 106, 313–316. [Google Scholar]
- Kim, G.Y.; Ko, W.S.; Lee, J.Y.; Lee, J.O.; Ryu, C.H.; Choi, B.T.; Park, Y.M.; Jeong, Y.K.; Lee, K.J.; Choi, K.-S.; et al. Water extract of Cordyceps militaris enhances maturation of murine bone marrow-derived dendritic cells in vitro. Biol. Pharm. Bull. 2006, 29, 354–360. [Google Scholar] [CrossRef][Green Version]
- Liu, W.C.; Wang, S.C.; Tsai, M.L.; Chen, M.C.; Wang, Y.C.; Hong, J.H.; McBride, W.H.; Chiang, C.S. Protection against radiation-induced bone marrow and intestinal injuries by Cordyceps sinensis, a chinese herbal medicine. Radiat. Res. 2006, 166, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Mizuha, Y.; Yamamoto, H.; Sato, T.; Tsuji, M.; Masuda, M.; Uchida, M.; Sakai, K.; Taketani, Y.; Yasutomo, K.; Sasaki, H.; et al. Water extract of Cordyceps sinensis (WECS) inhibits the RANKL-induced osteoclast differentiation. BioFactors 2007, 30, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Chuang, W.L.; Tsai, M.L.; Hong, J.H.; McBride, W.H.; Chiang, C.S. Cordyceps sinensis health supplement enhances recovery from taxol-induced leukopenia. Exp. Biol. Med. 2008, 233, 447–455. [Google Scholar] [CrossRef]
- Qi, W.; Yan, Y.B.; Wang, P.J.; Lei, W. The Co-Effect of Cordyceps Sinensis and Strontium on Osteoporosis in Ovariectomized Osteopenic Rats. Biol. Trace Elem. Res. 2011, 141, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, P.; Guo, W.; Yan, Y.; Zhang, Y.; Lei, W. The mechanism of Cordyceps sinensis and strontium in prevention of osteoporosis in rats. Biol. Trace Elem. Res. 2011, 143, 302–309. [Google Scholar] [CrossRef]
- Qi, W.; Yan, Y.B.; Lei, W.; Wu, Z.X.; Zhang, Y.; Liu, D.; Shi, L.; Cao, P.C.; Liu, N. Prevention of disuse osteoporosis in rats by Cordyceps sinensis extract. Osteoporos. Int. 2012, 23, 2347–2357. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, Y.; Yan, Y.B.; Lei, W.; Wu, Z.X.; Liu, N.; Liu, S.; Shi, L.; Fan, Y. The protective effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, on diabetic osteopenia in alloxan-induced diabetic rats. Evid. Based Complement. Altern. Med. 2013, 2013, 985636. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, Z.L.; Qi, W.; Zhao, G.Y. The effects of Cordyceps sinensis phytoestrogen on estrogen deficiency-induced osteoporosis in ovariectomized rats. BMC Complement. Altern. Med. 2014, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Qu, H.X.; Wang, J.G.; Yan, Y.F.; Zhang, J.L.; Yang, L.; Zhang, M.; Cheng, Y.H. Effects of fermentation products of Cordyceps militaris on growth performance and bone mineralization of broiler chicks. J. Appl. Anim. Res. 2015, 43, 236–241. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, Z.L.; Qi, W.; Lei, W.; Zhao, G.Y. Cordycepin (3′-Deoxyadenosine) down-regulates the proinflammatory cytokines in inflammation-induced osteoporosis model. Inflammation 2014, 37, 1044–1049. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Y.; Lv, Z.; Jiang, T.; Wang, L.; Li, Z. Cordycepin Protected against the TNF-α-induced inhibition of osteogenic differentiation of human adipose-derived mesenchymal stem cells. Int. J. Immunopathol. Pharmacol. 2015, 28, 296–307. [Google Scholar] [CrossRef]
- Zhang, D.W.; Deng, H.; Qi, W.; Zhao, G.Y.; Cao, X.R. Osteoprotective effect of cordycepin on estrogen deficiency-induced osteoporosis in vitro and in vivo. Biomed Res. Int. 2015, 2015, 423869. [Google Scholar] [CrossRef]
- Wang, F.; Yin, P.; Lu, Y.; Zhou, Z.; Jiang, C.; Liu, Y.; Yu, X. Cordycepin prevents oxidative stress-induced inhibition of osteogenesis. Oncotarget 2015, 6, 35496. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Kang, K.S.; Chun, K.H.; Hwang, G.S. Cordyceps militaris mushroom and cordycepin inhibit RANKL-induced osteoclast differentiation. J. Med. Food 2015, 18, 446–452. [Google Scholar] [CrossRef]
- Dou, C.; Cao, Z.; Ding, N.; Hou, T.; Luo, F.; Kang, F.; Yang, X.; Jiang, H.; Xie, Z.; Hu, M.; et al. Cordycepin prevents bone loss through inhibiting osteoclastogenesis by scavenging ros generation. Nutrients 2016, 8, 231. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhu, D.Y.; Xu, Z.L.; Yin, J.H.; Yu, X.W.; Mei, J.; Gao, Y.S.; Zhang, C.Q. The protective effect of cordycepin on alcohol-induced osteonecrosis of the femoral head. Cell. Physiol. Biochem. 2017, 42, 2391–2403. [Google Scholar] [CrossRef]
- Yu, S.-B.; Kim, H.J.; Kang, H.M.; Park, B.S.; Lee, J.H.; Kim, I.R. Cordycepin accelerates osteoblast mineralization and attenuates osteoclast differentiation in vitro. Evid.-Based Complement. Altern. Med. 2018, 2018, 5892957. [Google Scholar] [CrossRef]
- Li, Z.; Gu, Y.; Lin, Z.; Ma, H.; Zhang, S. Cordycepin promotes osteogenesis of bone marrow-derived mesenchymal stem cells and accelerates fracture healing via hypoxia in a rat model of closed femur fracture. Biomed. Pharmacother. 2020, 125, 109991. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, J.K.; Su, Z.X.; Jin, Q.L.; Deng, L.W.; Huang, G.; Shen, J.N. Cordycepin augments the chemosensitivity of osteosarcoma to cisplatin by activating ampk and suppressing the AKT signaling pathway. Cancer Cell Int. 2021, 21, 706. [Google Scholar] [CrossRef]
- Patil, S.; Reda, R.; Boreak, N.; Taher, H.A.; Melha, A.A.; Albrakati, A.; Vinothkumar, T.S.; Mustafa, M.; Robaian, A.; Alroomy, R.; et al. Adipogenic stimulation and pyrrolidine dithiocarbamate induced osteogenic inhibition of dental pulp stem cells is countered by cordycepin. J. Pers. Med. 2021, 11, 915. [Google Scholar] [CrossRef]
- Boreak, N.; Alkahtani, A.; Alzahrani, K.; Kenani, A.H.; Faqehi, W.H.; Faqehi, H.H.; Ageeli, R.E.; Moafa, W.N.; Baeshen, H.A.; Bhandi, S.; et al. Dose-dependent effect of cordycepin on viability, proliferation, cell cycle, and migration in dental pulp stem cells. J. Pers. Med. 2021, 11, 718. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, Y.; Sun, C.; Li, W.; Qiu, J.; Qiao, Y.; Wu, F.; Huo, X.; An, Y. Nucleosides and amino acids, isolated from Cordyceps sinensis, protected against cyclophosphamide-induced myelosuppression in mice. Nat. Prod. Res. 2022, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Weibiao, P.; Ting, W.; Xu, F.; Jiachun, W.; Zhenzhen, Z.; Zhiyong, C.H.U. Cordyceps sinensis extract protects against the ovariectomy-induced bone loss via the action on osteoclasts. J. Pharm. Pract. Serv. 2022, 40, 1–9. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of synthetic L-ergothioneine (Ergoneine®) as a novel food pursuant to regulation (EC) No 258/97. EFSA J. 2016, 14, e04629. [Google Scholar]
- Fu, T.T.; Shen, L. Ergothioneine as a natural antioxidant against oxidative stress-related diseases. Front. Pharmacol. 2022, 13, 850813. [Google Scholar] [CrossRef]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.M.Y.; Yew, T.S.Z.; Lim, K.H.C.; Halliwell, B. Administration of pure ergothioneine to healthy human subjects: Uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Ottosson, F.; Hellstrand, S.; Ericson, U.; Orho-Melander, M.; Fernandez, C.; Melander, O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 2020, 106, 691–697. [Google Scholar] [CrossRef]
- Sotgia, S.; Zinellu, A.; Mangoni, A.A.; Pintus, G.; Attia, J.; Carru, C.; McEvoy, M. Clinical and biochemical correlates of serum l-ergothioneine concentrations in community-dwelling middle-aged and older adults. PLoS ONE 2014, 9, e84918. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, recent developments. Redox. Biol. 2021, 42, 101868. [Google Scholar] [CrossRef]
- Gründemann, D.; Hartmann, L.; Flögel, S. The Ergothioneine Transporter (ETT): Substrates and locations, an inventory. FEBS Lett. 2022, 596, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- D’Alimonte, I.; Nargi, E.; Lannutti, A.; Marchisio, M.; Pierdomenico, L.; Costanzo, G.; di Iorio, P.; Ballerini, P.; Giuliani, P.; Caciagli, F.; et al. Adenosine A1 receptor stimulation enhances osteogenic differentiation of human dental pulp-derived mesenchymal stem cells via wnt signaling. Stem Cell Res. 2013, 11, 611–624. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Zhang, L.; Tian, Z.; Dong, S. P2X7 Receptor Acts as an efficient drug target in regulating bone metabolism system. Biomed. Pharmacother. 2020, 125, 110010. [Google Scholar] [CrossRef]
- Xu, X.Y.; He, X.T.; Wang, J.; Li, X.; Xia, Y.; Tan, Y.Z.; Chen, F.M. Role of the P2X7 Receptor in inflammation-mediated changes in the osteogenesis of periodontal ligament stem cells. Cell Death Dis. 2019, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.Y.; Choi, Y.-H.; Lee, S.Y.; Jin, M.H.; Kim, C.D.; Kang, N.-G.; Lee, S. Adenosine and cordycepin accelerate tissue remodeling process through adenosine receptor mediated Wnt/β-catenin pathway stimulation by regulating GSK3b activity. Int. J. Mol. Sci. 2021, 22, 5571. [Google Scholar] [CrossRef]
- Zuccarini, M.; Giuliani, P.; Ronci, M.; Caciagli, F.; Caruso, V.; Ciccarelli, R.; di Iorio, P. Purinergic Signaling in Oral Tissues. Int. J. Mol. Sci. 2022, 23, 7790. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, L.S.; Penolazzi, L.; Lambertini, E.; Falzoni, S.; Sarti, A.C.; Molle, C.M.; Gendron, F.; de Bonis, P.; di Virgilio, F.; Piva, R. Expression and function of the P2X7 receptor in human osteoblasts: The role of NFATc1 transcription factor. J. Cell. Physiol. 2021, 236, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004, 7, 227–243. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Overview on Tolerable Upper Intake Levels as Derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Available online: https://www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf (accessed on 6 October 2022).
- EFSA. Dietary Reference Values for Nutrients Summary Report; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar]
- Chan, J.S.L.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P. Chemical composition and medicinal value of fruiting bodies and submerged cultured mycelia of caterpillar medicinal fungus Cordyceps militaris CBS-132098 (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 649–659. [Google Scholar] [CrossRef]
- Xiao, J.H.; Xiao, D.M.; Sun, Z.H.; Xiong, Q.; Liang, Z.Q.; Zhong, J.J. Chemical compositions and antimicrobial property of three edible and medicinal Cordyceps species. J. Food Agric. Environ. 2009, 7, 91–100. [Google Scholar]
- Zunsheng, W.; Yuxiang, G.; Li, Z.; Qinsheng, Y.; Yongxin, Y. Chemical constituents of Cordyceps sinensis mycelial fermentation preparations in solid media. NPRD 2005, 17, 331–336. [Google Scholar]
- Dong, J.Z.; Ding, J.; Pei, Z.Y.; Lei, C.; Zheng, X.J.; Wang, Y. Composition and distribution of the main active components in selenium-enriched fruit bodies of Cordyceps militaris Link. Food Chem. 2013, 137, 164–167. [Google Scholar] [CrossRef]
- Sakai, T.; Kobashi, K.; Tsunezuka, M. Studies on dental caries prevention by traditional chinese medicines-6-on the fluoride contents in crude drugs. Jpn. J. Pharmacol. 1985, 39, 165–169. [Google Scholar]
- Li, X.; Wang, J.; Zhang, H.; Xiao, L.; Lei, Z.; Kaul, S.C.; Wadhwa, R.; Zhang, Z. Low dose of fluoride in the culture medium of Cordyceps militaris promotes its growth and enhances bioactives with antioxidant and anticancer properties. J. Fungi 2021, 7, 342. [Google Scholar] [CrossRef] [PubMed]
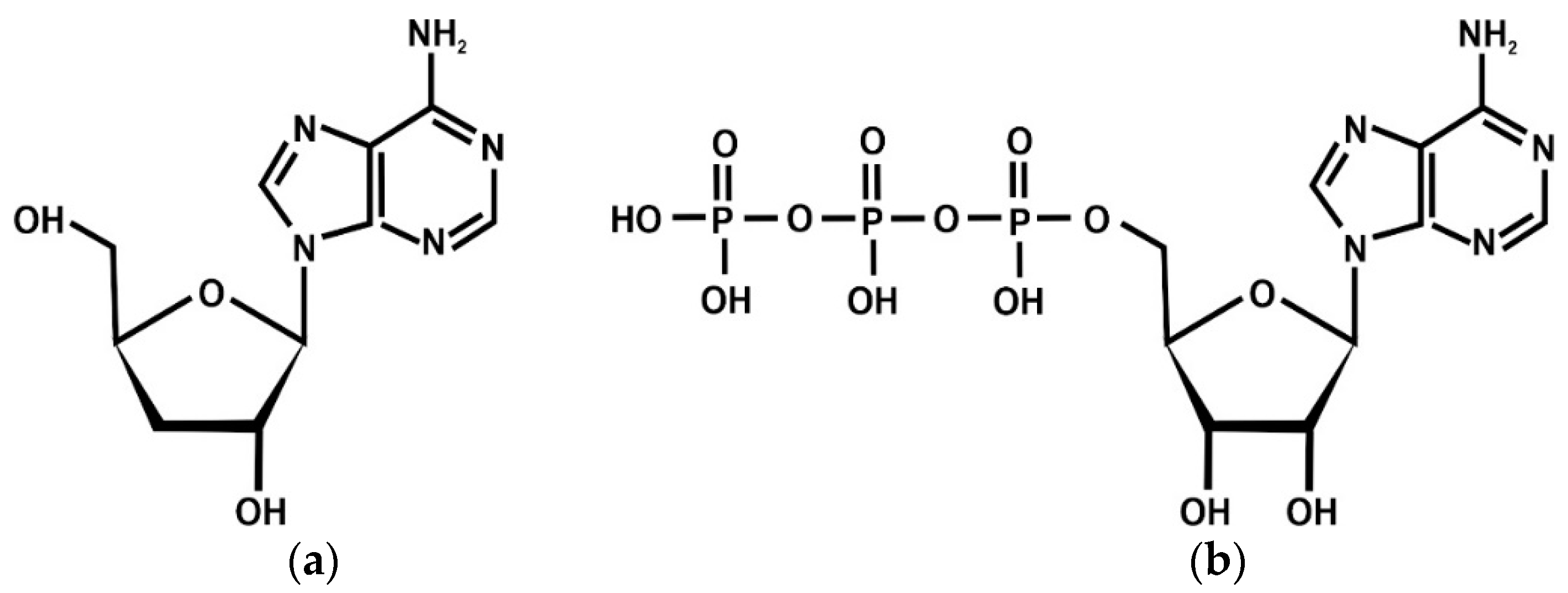

| Type of Study | Investigated Subject | Investigated Material | Dose/Concentration | Results/Outcome | Comment | References |
|---|---|---|---|---|---|---|
| In vitro | CFU-E; BFU-E | C. sinensis | 100, 150, 200 µg/mL | Increases the number of erythroid cells CFU-E and BFU-E | Content of cordycepin not determined | [24] |
| In vivo | Mice | C. sinensis | 50, 100, 150, 200, 250, 300 mg/kg/day; intraperitoneal injection for 5 days | Stimulates erythropoiesis; increases the number of CFU-E and BFU-E in mouse bone marrow | Content of cordycepin not determined | |
| In vitro | BMCs | C. militaris water extract | Up to 250 µg/mL | Induced maturation of murine BMCs | Content of cordycepin not determined | [25] |
| In vitro | BMCs: BMMSCs; BMHSCs | C. sinensis water extract | 500 µg/mL | Stimulate the proliferation of BMCs; protection of BMCs from radiation cytotoxicity | Content of cordycepin not determined | [26] |
| In vivo | Mice | C. sinensis water extract | 50 mg/kg/day; oral administration for 1 week | Protection of mice bone marrow after TBI; increase a survival of mice receiving TBI | Content of cordycepin not determined | |
| In vitro | Mouse BMCs and monocyte macrophage RAW 264.7 cells | C. sinensis water extract | 10, 50, 100 µg/mL | Inhibition of RANKL-induced osteoclast differentiation via NF-κB pathway | Content of cordycepin not determined | [27] |
| In vitro | BMMSCs; BMHSCs | C. sinensis extract | 500 µg/mL | Stimulate the differentiation of BMCs | Content of cordycepin not determined | [28] |
| In vivo | Mice with leukopenia induced by paclitaxel | C. sinensis extract | 50 mg/kg/day for 3 weeks | Enhancement recovery of mice from leukopenia induced by paclitaxel | Content of cordycepin not determined | |
| In vivo | OVX osteopenic rats | C. sinensis rich in strontium | 10 mL a mixture of C. sinensis rich in strontium; oral administration for 8 weeks | Decreases bone resorption, increases bone formation; increase the estradiol level | Content of cordycepin not determined | [29] |
| In vivo | OVX osteopenic rats | C. sinensis rich in strontium | 10 mL a mixture of C. sinensis rich in strontium; oral administration for 8 weeks | Decrease of ALP and TRAP activity; decrease of CTX and IFN-γ level; increase the OC and estradiol level | Content of cordycepin not determined | [30] |
| In vivo | Rats | C. sinensis extract | 100, 300, 500 mg/kg/day; oral administration for 8 weeks | Increase the bone mineral density; prevent disuse-induced bone loss | Content of cordycepin 5.27 µg/g | [31] |
| In vivo | Model diabetic osteopenic rats | Cordymin from C. sinensis | 20, 50, 100 mg/kg/day; intraperitoneal injection for 5 weeks | Decrease of ALP and TRAP activity | — | [32] |
| In vivo | OVX rats | Isoflavones from C. sinensis | 20, 50, 100 mg; oral administration for 8 weeks | Prevent of bone loss induced by estrogen deficiency; decrease of urinary Ca excretion; decrease of ALP and TRAP activity; decrease of CTX and IFN-γ level; increase the OC and estradiol level | Performed a histological examination of bone | [33] |
| In vivo | Broiler chicken | Fermentation products of C. militaris | 1–4 g fermentation products of C. militaris per 1 kg of feed | Increased calcium content in tibia | Content of cordycepin 5.09 mg/g. High dose at 4 g/kg of fermentation products of C. militaris had a negative impact on bone mineralization | [34] |
| In vivo | Rats with IMO | Cordycepin | 5, 10, 20 mg/kg; oral administration two times daily for 3 weeks | Prevent of bone loss; decrease of CTX, MDA, IL-1β, TNF-α level in the serum; increase the OC level | Performed a histological examination of liver, not bone | [35] |
| In vitro | ADMSCs | Cordycepin | 10–40 µg/mL | Low concentration of cordycepin 10 µg/mL promoted osteogenic differentiation | High concentration of cordycepin 20–40 µg/mL induce cell death | [36] |
| In vitro | Murine mesenchymal stem cells | Cordycepin | 10, 20, 50 µg/mL | Decrease of ALP and TRAP activity | — | [37] |
| In vivo | OVX osteopenic rats | Cordycepin | 5, 10, 20 mg/kg; oral administration two times daily for 3 weeks | Prevention of bone loss; increase the OC level; decrease the CTX level | Performed a histological examination of bone | |
| In vitro | BMMSCs | Cordycepin from C. militaris | 0.1, 0.2, 0.5, 1, 2 mM; 1, 5, 10, 20, 40, 80 μg/mL | Promoted osteogenic differentiation; decrease of ALP and TRAP activity | — | [38] |
| In vivo | OVX mice | Cordycepin from C. militaris | 1, 5, 10 and 20 mg/kg; intraperitoneal injection for 8 weeks | Increase of calcium content | Did not perform a histological examination of bone | |
| In vitro | Mouse monocyte macrophage RAW 264.7 cells | C. militaris and cordycepin | 1, 10 µg/mL | Inhibition of osteoclast differentiation | — | [39] |
| In vivo | Mouse model of lipopolysaccharide-mediated bone loss | C. militaris (content cordycepin) | 100 µg/g; oral administration for 8 days | Prevention of bone loss | Did not perform a histological examination of bone; performed a micro-CT analysis | |
| In vitro | Mouse monocyte macrophage RAW 264.7 cells and BMMs | Cordycepin | 0.01, 0.05, 0.1, 0.5, 1, 5, 10 μg/mL | Inhibition of RANKL-induced osteoclastogenesis | Inhbitory effects of cordycepin started at 0.1 μg/mL; concentration of cordycepin above to 5 μg/mL; cytotoxic effect started at concentration of 5 µg/mL | [40] |
| In vivo | OVX mice | Cordycepin | 10 mg/kg/day; Oral administration for 4 weeks | Prevention of bone loss | Performed a histological analysis; performed a BMD analysis | |
| In vitro | HBMSCs | Cordycepin | 0.1, 1, 10 μg/mL | Decrease of ALP activity | Even a high concentration at 10 μg/mL not suppress a proliferation of HBMSCs | [41] |
| In vivo | Rats model of alcohol-induced ONFH | Cordycepin | 10 mg/kg/day; intraperitoneal injection for 6 weeks | Cordycepin prevent on alcohol-induced ONFH | Performed a micro-CT analysis | |
| In vitro | Murine MC3T3-E1 and RAW264.7 cells | Cordycepin | 0.5, 1 µM | Inhibition of RANKL-induced osteoclast differentiation | Even a high concentration at 5 µM provide no cytotoxic effect | [42] |
| In vitro | BMMSCs | Cordycepin | 10 µg/mL | Promotes osteogenesis | — | [43] |
| In vivo | Rat model of closed femur fracture | Cordycepin | 10 mg/kg/day | Accelerate a fracture healing | Performed a histological analysis | |
| In vitro | Osteosarcoma cells and osteoblast cells | Cordycepin | 100, 200, and 400 µM | Inhibition of proliferation osteosarcoma cells; induce of osteosarcoma cell apoptosis | — | [44] |
| In vivo | Mice | Cordycepin | 40 mg/kg/day; intraperitoneal injection for 32 days | Inhibited osteosarcoma cell invasion | — | |
| In vitro | Dental pulp stem cells | Cordycepin | 0.5, 1, 2.5, 5, 10, 25, 50 µM | Increased the expression of RUNX2, COL1A1, OSX, OCN | — | [45] |
| In vitro | Dental pulp stem cells | Cordycepin | 1, 2.5, 5, 10, 25, 50 µM | Increased the migration of dental pulp stem; increase the number of stem cells | Concentration above at 10 µM resulted an cytotoxic effects | [46] |
| In vivo | Mice with myelosuppression induced by cyclophosphamide | Nucleosides and amino acids from C. sinensis (natural and artificially-cultivated) | 0.48–1.78 (contents ratio of artificially-cultivated versus natural C. sinensis) | Protection against myelosuppression induced by cyclophosphamide | Content of cordycepin not determined | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrejko, K.; Kała, K.; Sułkowska-Ziaja, K.; Pytko-Polończyk, J.; Muszyńska, B. Effect of Cordyceps spp. and Cordycepin on Functions of Bones and Teeth and Related Processes: A Review. Molecules 2022, 27, 8170. https://doi.org/10.3390/molecules27238170
Jędrejko K, Kała K, Sułkowska-Ziaja K, Pytko-Polończyk J, Muszyńska B. Effect of Cordyceps spp. and Cordycepin on Functions of Bones and Teeth and Related Processes: A Review. Molecules. 2022; 27(23):8170. https://doi.org/10.3390/molecules27238170
Chicago/Turabian StyleJędrejko, Karol, Katarzyna Kała, Katarzyna Sułkowska-Ziaja, Jolanta Pytko-Polończyk, and Bożena Muszyńska. 2022. "Effect of Cordyceps spp. and Cordycepin on Functions of Bones and Teeth and Related Processes: A Review" Molecules 27, no. 23: 8170. https://doi.org/10.3390/molecules27238170
APA StyleJędrejko, K., Kała, K., Sułkowska-Ziaja, K., Pytko-Polończyk, J., & Muszyńska, B. (2022). Effect of Cordyceps spp. and Cordycepin on Functions of Bones and Teeth and Related Processes: A Review. Molecules, 27(23), 8170. https://doi.org/10.3390/molecules27238170







