Production of Bioactive Peptides from Baltic Herring (Clupea harengus membras): Dipeptidyl Peptidase-4 Inhibitory, Antioxidant and Antiproliferative Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of the Hydrolysates
2.2. Antioxidant and DPP4 Inhibitory Capacity In Vitro
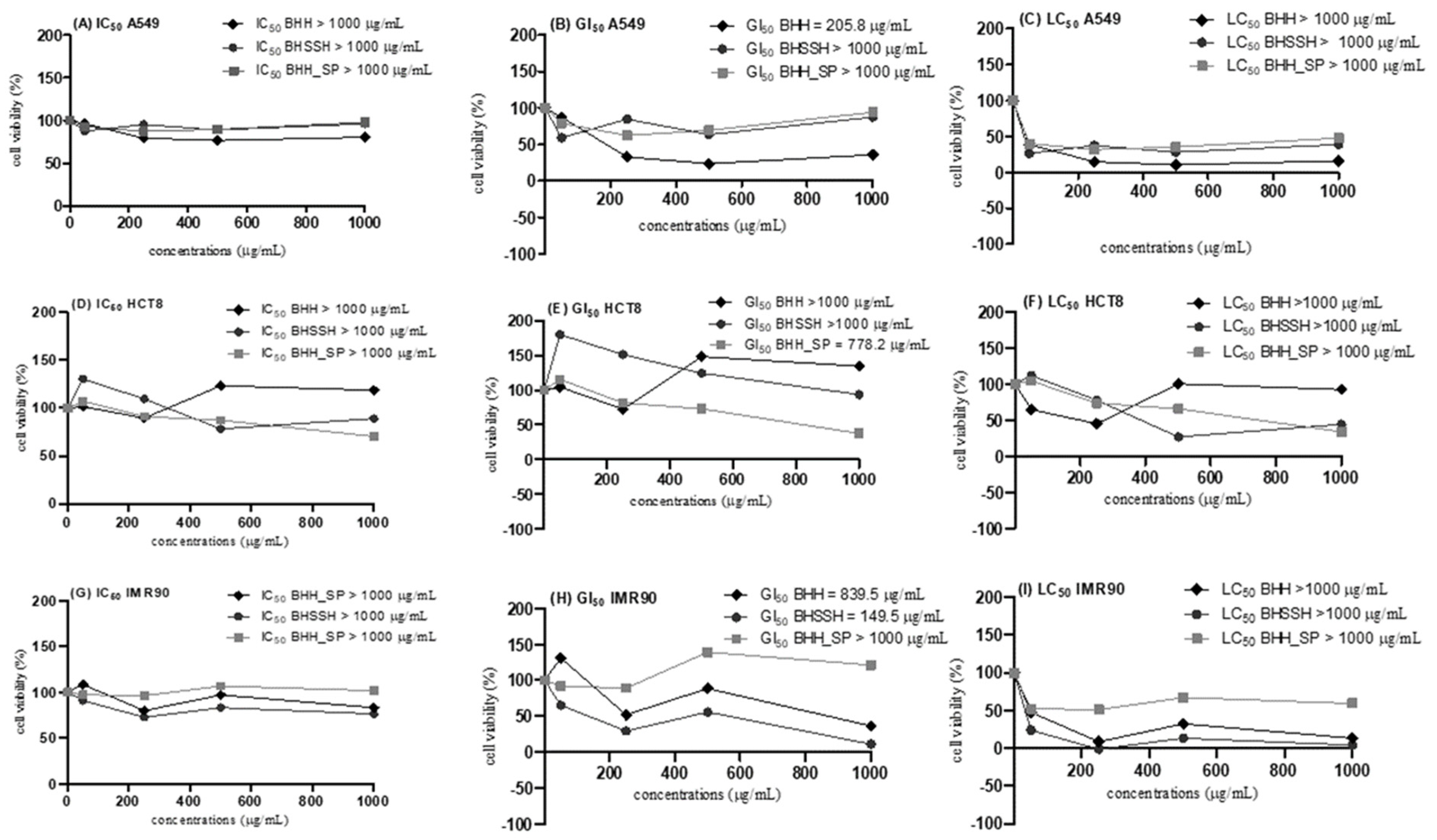
2.3. In Vitro Assays of Cytotoxicity and Proliferation
3. Materials and Methods
3.1. Chemical Reagents and Cell Lines
3.2. Fish Samples
3.3. Proximate Composition
3.4. Antioxidant Activity In Vitro
3.5. DPP4 Inhibition In Vitro
3.6. Surface Hydrophobicity of the Fish Hydrolysates
3.7. In Vitro Assays of Cytotoxicity and Proliferation
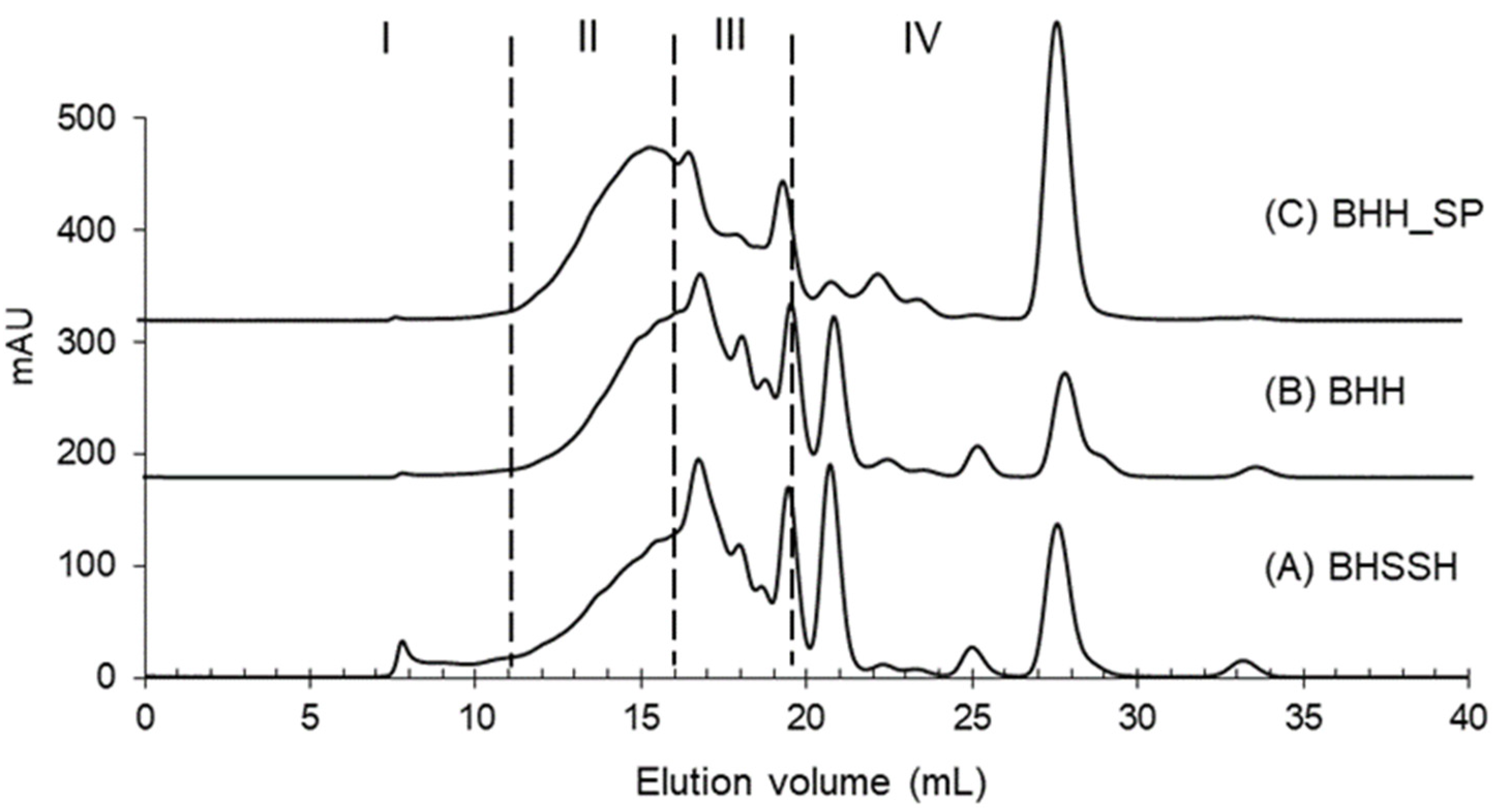
3.8. Evaluation of Molecular Weight Distribution by Size Exclusion Chromatography (SEC)
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Available online: https://www.marinefinland.fi/en-US/Nature_and_how_it_changes/Species/Fish/Baltic_herring (accessed on 17 November 2021).
- Silvenius, F.; Grönroos, J.; Kankainen, M.; Kurppa, S.; Mäkinen, T.; Vielma, J. Impact of feed raw material to climate and eutrophication impacts of Finnish rainbow trout farming and comparisons on climate impact and eutrophication between farmed and wild fish. J. Clean. Prod. 2017, 164, 1467–1473. [Google Scholar] [CrossRef]
- Setälä, J.; Virtanen, J.; Nielsen, R.; Hoff, A.; Waldo, S.; Hammarlund, C. Determining the economic value of nitrogen and phosphorus removal from the Baltic Sea: Derived as a positive externality from fisheries and aquaculture activities. In IFRO Report; No. 287; Department of Food and Resource Economics, University of Copenhagen: Copenhagen, Denmark, 2019; 24p. [Google Scholar]
- Ghaly, A.E.; Ramakrishnan, V.V.; Brooks, M.S.; Budge, S.M.; Dave, D. Fish processing wastes as a potential source of proteins. Amino acids and oils: A critical review. J. Microb. Biochem. Technol. 2013, 5, 107–129. [Google Scholar]
- Jin, R.; Teng, X.; Shang, J.; Wang, D.; Liu, N. Identification of novel DPP–IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res. Int. 2020, 133, 109161. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Lee, C.W.; Yun, H.; Eun, J.B. Structure–function engineering of novel fish gelatin-derived multifunctional peptides using high-resolution peptidomics and bioinformatics. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Suwal, S.; Huang, J.Y.; Liceaga, A.M. Selective separation and characterisation of dual ACE and DPP-IV inhibitory peptides from rainbow trout (Oncorhynchus mykiss) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 1062–1073. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded Sardine pilchardus protein. Food Chem. 2020, 328, 127096. [Google Scholar] [CrossRef]
- Martinelli, E.; Granato, D.; Azevedo, L.; Gonçalves, J.E.; Lorenzo, J.M.; Munekata, P.E.S.; Simal-Gandara, J.; Barba, F.J.; Carrillo, C.; Riaz Rajoka, M.S.; et al. Current perspectives in cell-based approaches towards the definition of the antioxidant activity in food. Trends Food Sci. Technol. 2021, 116, 232–243. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A. Exploration of bioactive peptides from the various origin as promising nutraceutical treasures: In vitro, in silico and in vivo studies. Food Chem. 2022, 373, 131395. [Google Scholar] [CrossRef]
- Acquah, C.; Di Stefano, E.; Udenigwe, C.C. Role of hydrophobicity in food peptide functionality and bioactivity. J. Food Bioact. 2018, 4, 88–98. [Google Scholar] [CrossRef]
- Available online: https://biosolutions.novozymes.com/en/animal-protein/products/flavourzyme (accessed on 17 November 2021).
- Aluko, R.E. Functional Foods and Nutraceuticals; Springer: New York, NY, USA, 2012; pp. 37–61. [Google Scholar]
- Durand, R.; Fraboulet, E.; Marette, A.; Bazinet, L. Simultaneous double cationic and anionic molecule separation from herring milt hydrolysate and impact on resulting fraction bioactivities. Sep. Purif. Technol. 2019, 210, 431–441. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Preparation and Identification of Antioxidative Peptides from Pacific Herring (Clupea pallasii) Protein. Molecules 2019, 24, 1946. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Yasemi, M.; Gavlighid, H.A.; Xu, X. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing byproduct with different pretreatments: Antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. LWT—Food Sci.Technol. 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biocem. 2019, 43, e12451. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.C.Y.; Huang, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from Atlantic salmon skin gelatin as dipeptidylpeptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.; Luo, H.; Yang, Z. Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty). J. Func. Foods 2014, 10, 104–111. [Google Scholar] [CrossRef]
- Sierra, L.; Fan, H.; Zapata, J.; Wu, J. Antioxidant peptides derived from hydrolysates of red tilapia (Oreochromis sp.) scale. LWT—Food Sci. Technol. 2021, 146, 111631. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ngo, D.-H.; Bomi, R.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydrolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Dinesh Kumar, B. A Antiproliferative, ACE-inhibitory and functional properties of protein hydrolysates from rohu (Labeo rohita) roe (egg) prepared by gastrointestinal proteases. J. Food Sci. Technol. 2015, 52, 8300–8307. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.; Zhang, B.; Yang, Z.; Wang, D. Antioxidant and antiproliferative activities of heated sterilized pepsin hydrolysate derived from half-fin anchovy (Setipinna taty). Marine Drugs 2011, 9, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.; Giblin, L.; Brien, N.O. Assessment of the biological activity of fish muscle protein hydrolysates using in vitro model systems. Food Chem. 2021, 359, 129852. [Google Scholar] [CrossRef] [PubMed]
- Picot, L.; Bordenave, S.; Didelot, S.; Fruitier-Arnaudin, I.; Sannier, F.; Thorkelsson, G.; Berge, J.P.; Guérard, F.; Chabeaud, A.; Piot, J.M. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Proc. Biochem. 2006, 41, 1217–1222. [Google Scholar] [CrossRef]
- International Organization for Standardization. Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 2: Block Digestion and Steam Distillation Method (ISO Standard No. 5983-2:2009). 2009. Available online: https://www.iso.org/standard/52199.html (accessed on 10 October 2020).
- International Organization for Standardization. Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method (ISO Standard No. 20483:2013). 2013. Available online: https://www.iso.org/standard/59162.html (accessed on 10 October 2020).
- International Organization for Standardization. Animal Feeding Stuffs—Determination of Fat Content (ISO Standard No. 6492:1999). 1999. Available online: https://www.iso.org/standard/12865.html (accessed on 10 October 2020).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free-radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef]
- Arte, E.; Huang, X.; Nordlund, E.; Katina, K. Biochemical characterization and technofunctional properties of bioprocessed wheat bran protein isolates. Food chem. 2019, 289, 103–111. [Google Scholar] [CrossRef]
- Do Carmo, M.A.V.; Pressete, C.G.; Marques, M.J.; Granato, D.; Azevedo, L. Polyphenols as potential antiproliferative agents: Scientific trends. Curr. Opin. Food Sci. 2018, 24, 26–35. [Google Scholar] [CrossRef]
| Composition (g/100 g DM) | BHSS | BHSSH | BH | BHH |
|---|---|---|---|---|
| Protein | 60.2 ± 0.4 c | 91.2 ± 1.2 a | 67.1 ± 2.5 b | 86.1 ± 1.0 a |
| Fat | 23.3 ± 0.5 a | 0.40 ± 0.05 b | 24.0 ± 0.8 a | 0.30 ± 0.04 b |
| Ash | 16.6 ± 0.9 a | 8.1 ± 0.1 b | 8.9 ± 3.2 b | 4.2 ± 0.8 c |
| I (%) >10,000 Da | II (%) 10,000–1000 Da | III (%) 1000–200 Da | IV (%) <200 Da | |
|---|---|---|---|---|
| BHSSH | 4.0 ± 0.2 a | 29.5 ± 0.8 c | 40.1 ± 0.8 b | 26.4 ± 0.3 b |
| BHH | 0.9 ± 0.2 b | 32.0 ± 0.7 b | 43.3 ± 1.0 a | 23.6 ± 0.5 c |
| BHH_SP | 1.1 ± 0.3 b | 40.8 ± 0.9 a | 27.2 ± 1.1 c | 30.8 ± 0.4 a |
| Hydrolysates | Reducing Power (mg GAE/g) | Folin–Ciocalteu Reducing Power (mg GAE/g) | DPPH (mg AAE/g) | Cu2+ Chelating Ability (mg EDTAE/g) | DPP4 Inhibition Activity (IC50: mg/mL) | Surface Hydrophobicity (S0) |
|---|---|---|---|---|---|---|
| BHSSH | 0.07 ± 0.01 c | 28.51 ± 0.57 b | 0.29 ± 0.02 b | 21.01 ± 0.08 b | 7.50 ± 0.03 a | 4430 ± 250 a |
| BHH | 0.18 ± 0.00 a | 29.11 ± 0.32 b | 0.08 ± 0.03 c | 21.32 ± 0.09 b | 7.92 ± 0.16 a | 4530 ± 480 a |
| BHH_SP | 0.16 ± 0.01 b | 36.43 ± 0.68 a | 8.84 ± 0.53 a | 27.08 ± 0.03 a | 5.38 ± 0.27 b | 18260 ± 2130 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mäkinen, S.; Hiidenhovi, J.; Huang, X.; Lima, A.d.S.; Azevedo, L.; Setälä, J.; Välimaa, A.-L.; Mattila, P.; Granato, D. Production of Bioactive Peptides from Baltic Herring (Clupea harengus membras): Dipeptidyl Peptidase-4 Inhibitory, Antioxidant and Antiproliferative Properties. Molecules 2022, 27, 5816. https://doi.org/10.3390/molecules27185816
Mäkinen S, Hiidenhovi J, Huang X, Lima AdS, Azevedo L, Setälä J, Välimaa A-L, Mattila P, Granato D. Production of Bioactive Peptides from Baltic Herring (Clupea harengus membras): Dipeptidyl Peptidase-4 Inhibitory, Antioxidant and Antiproliferative Properties. Molecules. 2022; 27(18):5816. https://doi.org/10.3390/molecules27185816
Chicago/Turabian StyleMäkinen, Sari, Jaakko Hiidenhovi, Xin Huang, Amanda dos Santos Lima, Luciana Azevedo, Jari Setälä, Anna-Liisa Välimaa, Pirjo Mattila, and Daniel Granato. 2022. "Production of Bioactive Peptides from Baltic Herring (Clupea harengus membras): Dipeptidyl Peptidase-4 Inhibitory, Antioxidant and Antiproliferative Properties" Molecules 27, no. 18: 5816. https://doi.org/10.3390/molecules27185816
APA StyleMäkinen, S., Hiidenhovi, J., Huang, X., Lima, A. d. S., Azevedo, L., Setälä, J., Välimaa, A.-L., Mattila, P., & Granato, D. (2022). Production of Bioactive Peptides from Baltic Herring (Clupea harengus membras): Dipeptidyl Peptidase-4 Inhibitory, Antioxidant and Antiproliferative Properties. Molecules, 27(18), 5816. https://doi.org/10.3390/molecules27185816







