Substituent Effects on the Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone
Abstract
1. Introduction
2. Experimental Section
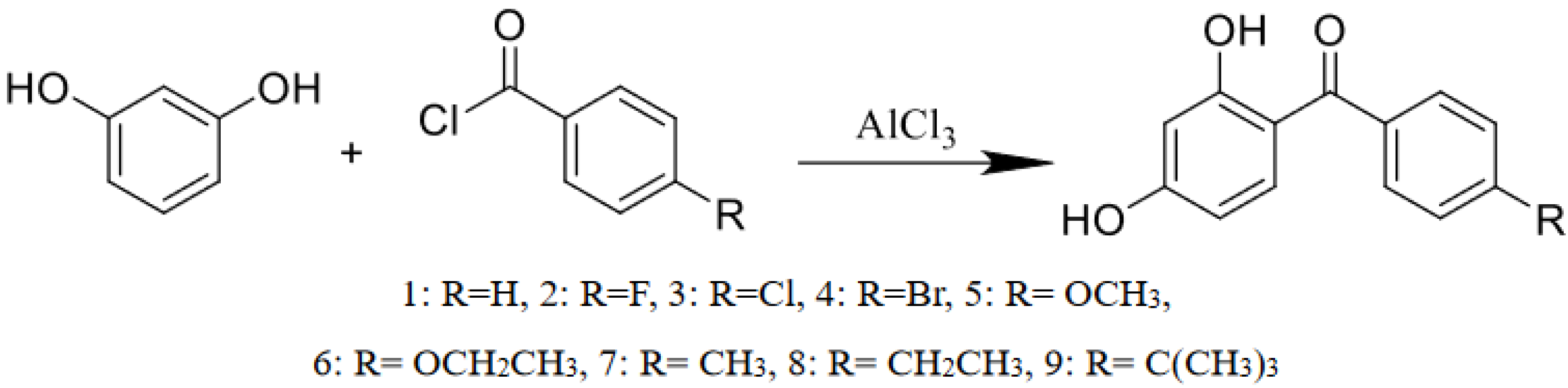
2.1. Sample Preparation
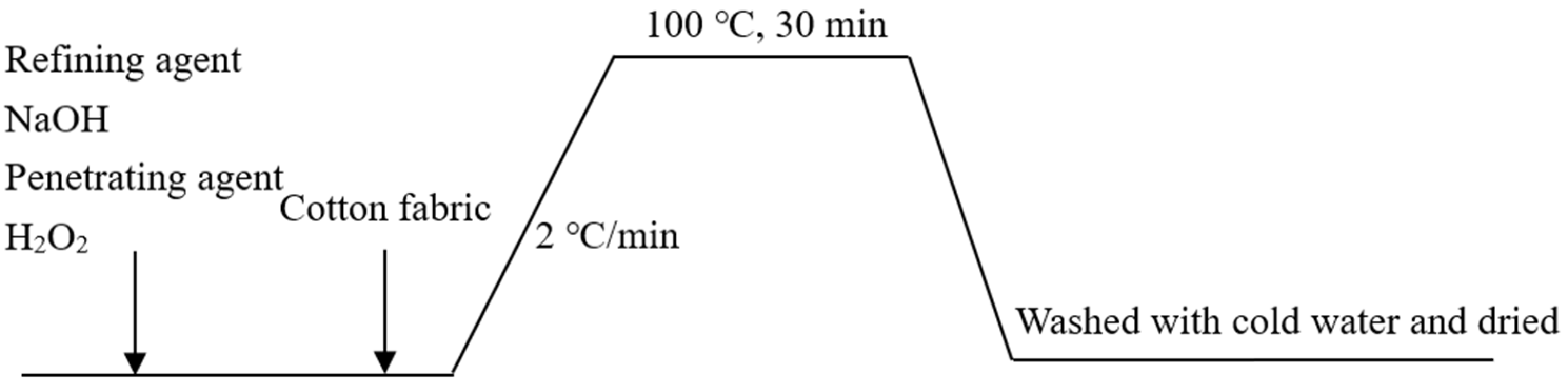
2.2. Pretreatment of Fabrics
2.3. UV Resistance Test
2.4. Dyeing Rate Test
3. Results and Discussion
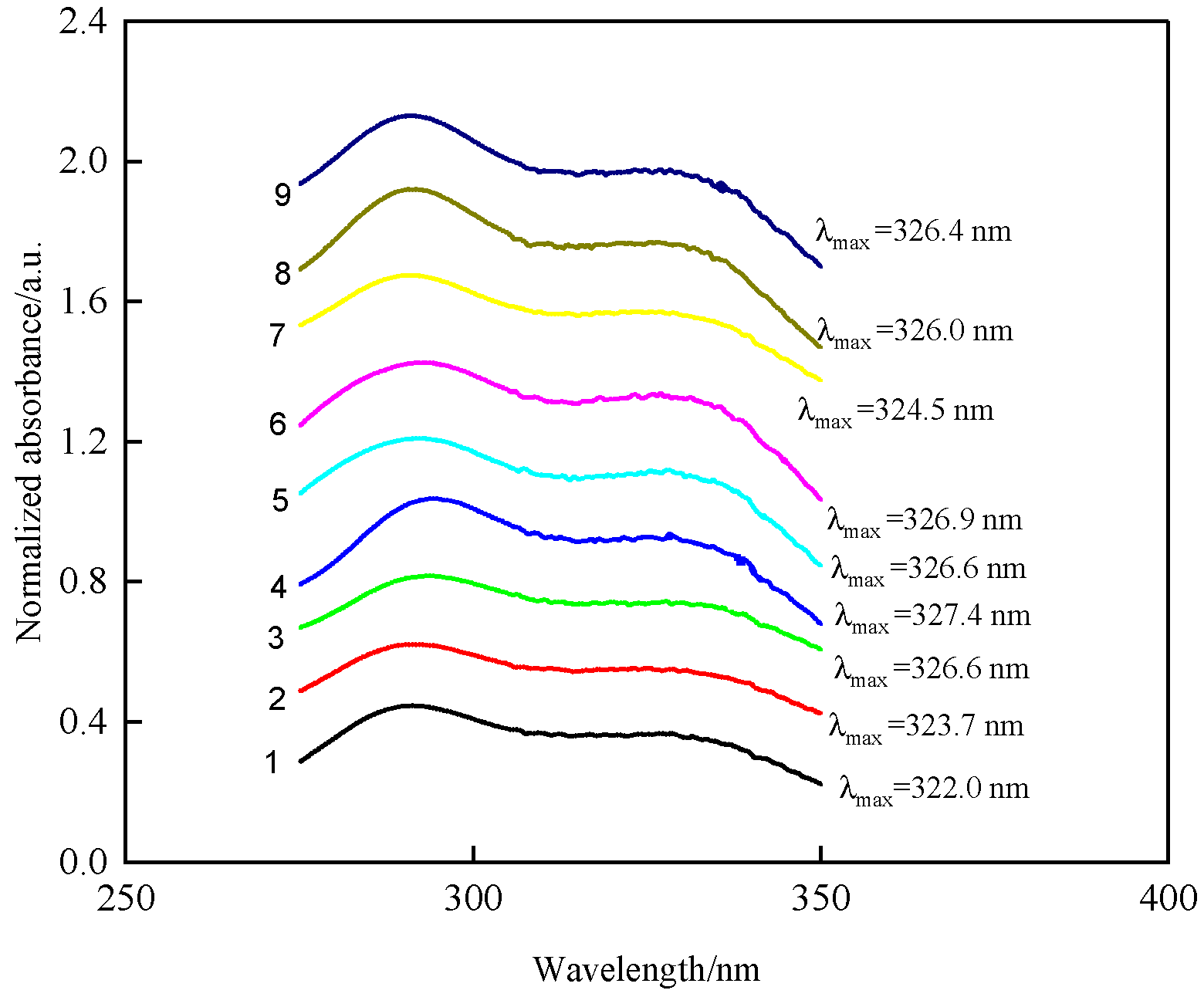
3.1. Effects of Substituents on UV Spectra of 2,4-Dihydroxy Dibenzophenone
3.2. Effects of Substituents on UV Resistance of 2,4-Dihydroxydibenzophenone
3.3. Dyeing Rate of 2,4-Dihydroxybenzophenone UV Absorbent Coated on Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.Q.; Tang, Z.; Wu, M.D.; Zhou, Z.H. Design, preparation and application of conjugated microporous polymers. Polym. Int. 2014, 63, 381–392. [Google Scholar] [CrossRef]
- Lei, H.B.; He, D.L.; Guo, Y.N.; Tang, Y.N.; Huang, H.Q. Synthesis and characterization of UV-absorbing fluorine-silicone acrylic resin polymer. Appl. Surf. Sci. 2018, 442, 71–77. [Google Scholar] [CrossRef]
- Gawryszewski, F.M.; Birch, D.; Kemvisible, D.J.; Herberstein, M.E. Dissecting the variation of a visual trait: The proximate basis of UV-reflectance in crab spiders (Thomisidae). Funct. Ecol. 2015, 29, 44–54. [Google Scholar] [CrossRef]
- Wu, G.J.; Zhu, C.; Weng, X.D.; Sun, X.D.; Lu, X.L. Preparation of poly (vinyl chloride)-based UV absorbents and their resistance property of photoaging. Chin. J. Org. Chem. 2016, 36, 1963–1969. [Google Scholar] [CrossRef][Green Version]
- Li, J.Y.; Zhao, C.X.; Lan, F.J.; Chen, F.; Teng, C.L.; Yan, Q.Y.; Tang, J.T. An efficient CeGeO4 catalyst for degradation of organic dyes without light irradiation at room temperature. Catal. Commun. 2016, 77, 26–31. [Google Scholar] [CrossRef]
- Tang, J.T.; Li, D.T.; Feng, Z.X.; Tan, Z.; Ou, B.L. A novel AgIO4 semiconductor with ultrahigh activity in photodegradation of organic dyes: Insights into the photosensitization mechanism. RSC Adv. 2014, 4, 2151–2154. [Google Scholar] [CrossRef]
- Zhan, C.C.; Chen, F.; Yang, J.T.; Zhong, M.Q.; Song, J.P.; Jiang, X.P. Enhanced UV/H2O2 process by expanded graphite: An effective method for rhodamine B dye decolorization. Res. Chem. Intermediat. 2018, 44, 2425–2437. [Google Scholar]
- Aparicio, F.J.; Alcaire, M.; Borras, A.; Gonzalez, J.C.; Lopez-Arbeloa, F.; Blaszczyk-Lezak, I.; Gonzalez-Elipe, A.R.; Barranco, A.J. Luminescent 3-hydroxyflavone nanocomposites with a tuneable refractive index for photonics and UV detection by plasma assisted vacuum deposition. Mater. Chem. C 2014, 2, 6561–6573. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Carotenoids and protection against solar UV radiation. Skin Pharmacol. Physiol. 2002, 15, 291–296. [Google Scholar] [CrossRef]
- Li, G.; Liu, Q.Q.; Xia, B.J.; Huang, J.; Li, S.; Guang, Y.Z.; Zhou, H.; Liao, B.; Zhou, Z.H.; Liu, B. Synthesis of stable metal-containing porous organic polymers for gas storage. Eur. Polym. J. 2017, 91, 242–247. [Google Scholar] [CrossRef]
- Ou, B.L.; Chen, M.L.; Huang, R.; Zhou, H. Preparation and application of novel biodegradable polyurethane copolymer. RSC Adv. 2016, 6, 47138–47144. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in Human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Jeon, J.H.; Lee, Y.H.; Lee, D.J.; Park, H.; Chun, H.H.; Kim, H.D. Synthesis and properties of UV-curable polyurethane acrylates containing fluorinated acrylic monomer/ vinyltrimethoxysilane. Polym. Bull. 2015, 72, 1921–1936. [Google Scholar] [CrossRef]
- Qu, A.L.; Wen, X.F.; Pi, P.H.; Cheng, J.; Yang, Z.R. Synthesis and characterization of hybrid fluoro-emulsion based on silica/copolymer composite particles. Polym. Int. 2008, 57, 1287–1294. [Google Scholar] [CrossRef]
- Cao, W.Q.; Ma, X.P.; Lv, B.L. Synthesis of Water-soluble Ultraviolet Absorbent 2, 4-Dihydroxy-5-sulfo-benzophenone. Adv. Fine Petrochem. 2008, 9, 35–38. [Google Scholar]
- Tong, X.Y. Synthesis of UV absorber UV-531. Zhejiang Chem. Ind. 2003, 20, 21–22. [Google Scholar]
- Xu, R.; Xu, A.L.; Sui, Y.J. Optimization of Desizing & Scouring & Bleaching One-bath Process for Cotton Woven Fabric. Prog. Text. Sci. Technol. 2016, 7, 18–20. [Google Scholar]
- Chen, G.F.; Cao, C.Z.; Sheng, B. The substituent effect on the UV energy of 4,4–disubstituted benzylideneanilines. J. Phys. Org. Chem. 2012, 25, 327–333. [Google Scholar] [CrossRef]
- Cao, C.Z.; Fang, Z.J. Substituent effects on the UV spectra of extended benzylidene anilines p-X-PhCH=NPhCH=CHPh-p-Y. Spectrochim. Acta Part A 2013, 111, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.J.; Cao, C.Z.; Chen, J.F.; Deng, X.C. Effect of molecular conformation on spectroscopic properties of symmetrical Schiff bases derived from 1,4-phenylenediamine. J. Mol. Struct. 2013, 1036, 447–451. [Google Scholar] [CrossRef]
- Yao, H.H.; Cheng, H.H.; Cheng, C.H.; Lin, C.K.; Yang, J.S.; Chen, I.C. Charge-transfer and isomerization reactions of trans-4-(N-arylamino) stilbenes. Phys. Chem. Chem. Phys. 2016, 18, 28164–28174. [Google Scholar] [CrossRef] [PubMed]
- Din, Y.; Zhou, X.Y.; Shen, Y. Dyeing and adsorption character of kapok fiber with direct dyes. Dye. Finish. 2009, 20, 3. [Google Scholar]
- Chen, W.G.; Dai, J.J.; Wang, X.F. Kinetics and thermodynamics of UV-absorbers taken up to polyester. J. Text. Res. 2010, 31, 75–81. [Google Scholar]

| Concentration/mol·L−1 | Compound No. | Cotton Fabric | Polyester | ||||||
|---|---|---|---|---|---|---|---|---|---|
| UPF | T(UVA)% | T(UVB)% | T(UVR)% | UPF | T(UVA)% | T(UVB)% | T(UVR)% | ||
| 0 | blank | 30.7 | 4.0 | 2.9 | 3.6 | 89.7 | 3.8 | 0.4 | 2.8 |
| 2 × 10−2 | 1 | 61.9 | 2.4 | 1.3 | 2.0 | 136.7 | 2.3 | 0.4 | 1.8 |
| 2 | 53.6 | 2.6 | 1.5 | 2.3 | 143.2 | 2.4 | 0.4 | 1.8 | |
| 3 | 52.5 | 2.5 | 1.5 | 2.2 | 142.9 | 2.1 | 0.4 | 1.6 | |
| 4 | 52.1 | 2.7 | 1.5 | 2.4 | 112.5 | 2.6 | 0.5 | 2.0 | |
| 5 | 48.0 | 2.8 | 1.7 | 2.4 | 132.4 | 2.7 | 0.4 | 2.0 | |
| 6 | 45.4 | 3.0 | 1.7 | 2.7 | 141.1 | 2.3 | 0.4 | 1.7 | |
| 7 | 47.1 | 3.0 | 1.7 | 2.6 | 137.9 | 2.4 | 0.4 | 1.8 | |
| 8 | 46.7 | 3.1 | 1.7 | 2.7 | 138.4 | 1.8 | 0.5 | 1.4 | |
| 9 | 42.3 | 3.2 | 1.9 | 2.8 | 138.5 | 1.9 | 0.5 | 1.5 | |
| 1 × 10−2 | 1 | 51.1 | 2.8 | 1.5 | 2.4 | 115.4 | 2.6 | 0.5 | 2.0 |
| 2 | 48.9 | 2.9 | 1.6 | 2.5 | 127.0 | 2.5 | 0.4 | 1.9 | |
| 3 | 41.1 | 3.3 | 1.9 | 2.9 | 125.0 | 2.7 | 0.4 | 2.0 | |
| 4 | 37.4 | 3.4 | 2.1 | 3.0 | 93.8 | 3.5 | 0.6 | 2.6 | |
| 5 | 38.7 | 3.3 | 2.1 | 2.9 | 104.8 | 3.1 | 0.5 | 2.3 | |
| 6 | 39.2 | 3.4 | 2.0 | 3.0 | 125.3 | 2.8 | 0.4 | 2.1 | |
| 7 | 42.4 | 3.2 | 1.9 | 2. 8 | 118.4 | 2.4 | 0.5 | 1.8 | |
| 8 | 42.8 | 3.1 | 1.9 | 2.8 | 122.7 | 2.3 | 0.5 | 1.8 | |
| 9 | 40.1 | 3.5 | 2.0 | 3.1 | 126.7 | 2.2 | 0.5 | 1.7 | |
| 5 × 10−3 | 1 | 43.2 | 3.2 | 1.8 | 2.8 | 103.2 | 2.9 | 0.6 | 2.2 |
| 2 | 44.5 | 3.1 | 1.8 | 2.7 | 96.0 | 3.3 | 0.6 | 2.5 | |
| 3 | 36.4 | 3.5 | 2.2 | 3.1 | 93.3 | 3.7 | 0.6 | 2.8 | |
| 4 | 33.2 | 3.4 | 2.4 | 3.1 | 91.2 | 3.1 | 0.6 | 2.4 | |
| 5 | 35.3 | 3.6 | 2.3 | 3.2 | 98.2 | 3.4 | 0.6 | 2.6 | |
| 6 | 37.8 | 3.3 | 2.1 | 3.0 | 101.0 | 3.3 | 0.6 | 2.5 | |
| 7 | 40.8 | 3.3 | 1.9 | 2.9 | 91.8 | 3.9 | 0.7 | 2.3 | |
| 8 | 40.5 | 3.6 | 1.9 | 3.1 | 93.5 | 3.2 | 0.6 | 2.5 | |
| 9 | 36.0 | 3.2 | 2.2 | 2.9 | 96.0 | 3.2 | 0.6 | 2.4 | |
| No. | Equation | Cotton Fabric | Polyester | ||
|---|---|---|---|---|---|
| Absorbance | η% | Absorbance | η% | ||
| 1 | y = 0.0252x + 0.0475, R2 = 0.9998 | 0.053 | 77.8 | 0.052 | 80.8 |
| 2 | y = 0.0144x + 0.041, R2 = 0.9997 | 0.046 | 68.5 | 0.044 | 81.5 |
| 3 | y = 0.0171x + 0.0463, R2 = 0.9996 | 0.052 | 67.8 | 0.050 | 77.9 |
| 4 | y = 0.0246x + 0.059, R2 = 0.9999 | 0.068 | 65.3 | 0.066 | 75.3 |
| 5 | y = 0.0239x + 0.0711, R2 = 0.9999 | 0.080 | 63.2 | 0.078 | 67.4 |
| 6 | y = 0.0184x + 0.0698, R2 = 0.9998 | 0.077 | 60.9 | 0.075 | 71.0 |
| 7 | y = 0.0213x + 0.0468, R2 = 0.9999 | 0.053 | 73.2 | 0.051 | 83.6 |
| 8 | y = 0.0192x + 0.0561, R2 = 0.9999 | 0.062 | 68.9 | 0.059 | 84.1 |
| 9 | y = 0.0145x + 0.0482, R2 = 0.9999 | 0.054 | 61.0 | 0.051 | 83.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Tan, S.; Fang, Z.; Deng, J.; He, Z.; Huang, C.; Au, C.; Yi, B. Substituent Effects on the Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone. Molecules 2022, 27, 8169. https://doi.org/10.3390/molecules27238169
Wu F, Tan S, Fang Z, Deng J, He Z, Huang C, Au C, Yi B. Substituent Effects on the Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone. Molecules. 2022; 27(23):8169. https://doi.org/10.3390/molecules27238169
Chicago/Turabian StyleWu, Feng, Shengqiong Tan, Zhengjun Fang, Jiyu Deng, Zhengjie He, Chaoyi Huang, Chaktong Au, and Bing Yi. 2022. "Substituent Effects on the Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone" Molecules 27, no. 23: 8169. https://doi.org/10.3390/molecules27238169
APA StyleWu, F., Tan, S., Fang, Z., Deng, J., He, Z., Huang, C., Au, C., & Yi, B. (2022). Substituent Effects on the Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone. Molecules, 27(23), 8169. https://doi.org/10.3390/molecules27238169





